Abstract
Isolated pulmonic valve endocarditis is an uncommon clinical entity and is usually associated with intravenous drug abuse. We describe a case of isolated pulmonary valve endocarditis in a young woman with no apparent precipitating factors other than a history of recent normal delivery. During the clinical course she suffered a pulmonary embolism which could be managed conservatively and she was discharged after a 4-week course of antibiotic therapy. The literature on the isolated pulmonary valve endocarditis is reviewed.
<Learning objective: In right-sided endocarditis, the option for surgical management should not be based solely on the vegetation size. Large vegetation in the right-sided valves with multiple pulmonary embolisms can be managed by medical therapy if the patient is hemodynamically stable.>
Keywords: Pulmonary valve, Infective endocarditis, Pulmonary embolism, Vegetation
Introduction
Isolated pulmonary valve endocarditis is rare, affecting less than 1.5–2% of patients suffering from infective endocarditis [1]. Review of previously published data showed fewer than 90 cases of isolated pulmonary valve endocarditis [2]. Risk factors include intravenous drug abuse, sepsis, and central venous catheter or pacemaker implantation with subsequent lead infection. We describe a case of isolated pulmonary valve endocarditis in a 19-year-old female complicated with multiple septic pulmonary emboli.
Case report
A 19-year-old female was admitted to a community hospital for evaluation of fever and productive cough with scanty hemoptysis of 2 months’ duration. She had history of normal vaginal delivery 3 months previously and her complaints started 1 month after that. Physical examination revealed chest crepitatations; in particular, no heart murmur could be detected. On admission, her leukocyte count was 17,500 cells/mm3; 78% polymorphs, C-reactive protein was 86 mg/L; erythrocyte sedimentation rate (ESR) 120 mm/h, Mantoux test negative, and chest X-ray showed right upper lobe, lower lobe, left lower lobe pneumonic patches (Fig. 1A). Ultra sonogram of abdomen was normal and computerized tomography (CT) thorax showed peripheral patchy consolidation in right upper lobe posterior segment, right lower lobe basal segment, and left lower lobe basal segment (Fig. 2A). She was treated as having community-acquired pneumonia with intravenous ceftriaxone and gentamicin for 2 weeks and became afebrile after 1 week of starting antibiotics. She was referred to our hospital after 2 weeks of admission for evaluation of a newly detected cardiac murmur.
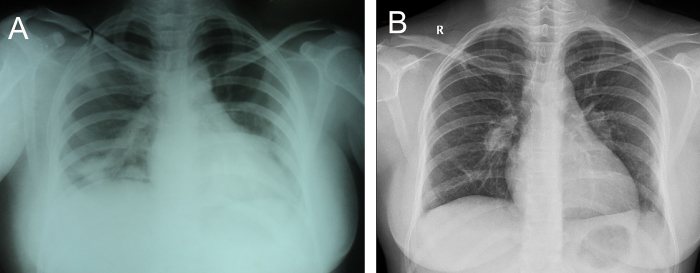
Fig. 1.
(A) Chest X-ray showing right upper lobe, lower lobe, and left lower lobe pneumonic patches. (B) Dilated right descending pulmonary artery with decreased distal vascular markings.
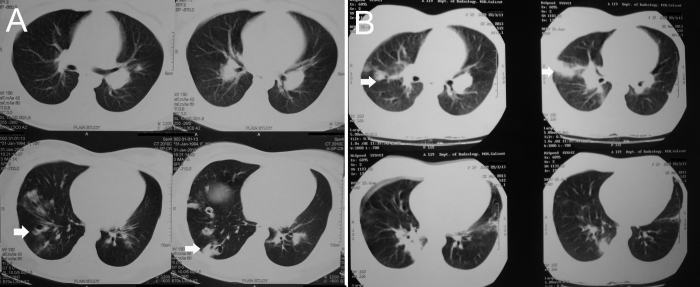
Fig. 2.
(A) Computed tomography (CT) thorax showing peripheral patchy consolidation suggestive of multiple septic emboli. (B) Repeat CT thorax showing evidence of multiple thromboembolism.
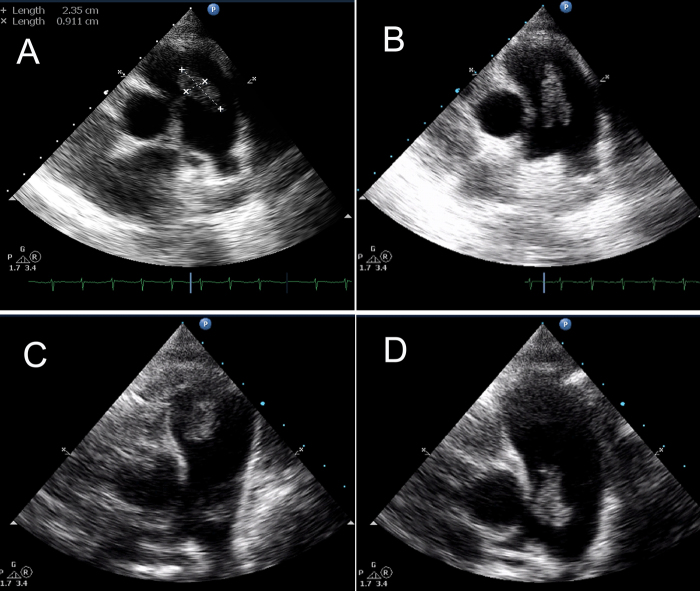
On admission to our hospital she was afebrile and there was a 3/6 systolic murmur and a short diastolic murmur in the pulmonary area. Laboratory investigation showed a hemoglobin 9 mg%, leukocyte count 11,500 cells/mm3, 70% polymorphs, ESR 60 mm/h, and C-reactive protein 20 mg/L. Transthoracic echocardiography revealed mild pulmonary regurgitation and a large vegetation of size 9 mm × 23 mm attached to the pulmonary valve (Fig. 3A and B), other valves were normal. Repeated blood cultures were sterile. She was diagnosed as having infective endocarditis. Intravenous ceftriaxone was continued as she responded to the initial treatment. After 2 additional weeks of antibiotic therapy parameters of inflammation normalized, blood culture was sterile, and chest X-ray showed clearing of pneumonic patches, however transthoracic echocardiography showed persistence of the vegetation (Fig. 3C and D). She was discharged from hospital as she was asymptomatic and advised regular follow up.
Fig. 3.
(A and B) Transthoracic echocardiography basal short axis view showing a large vegetation of size 9 mm × 23 mm attached to the pulmonary valve. (A) Diastole; (B) systole. (C and D) Transthoracic echocardiography basal short axis view showing persistence of the pulmonary valve vegetation. (C) Diastole; (D) systole.
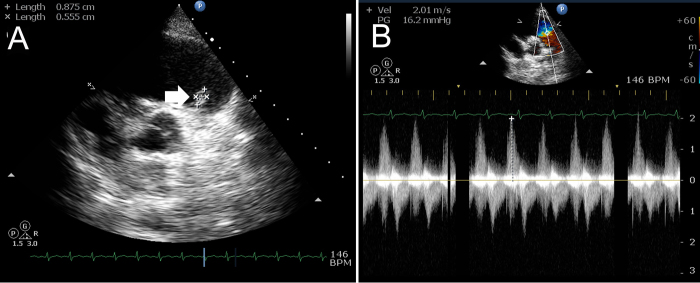
After 2 weeks, she again presented with fever and right-sided chest pain. Chest X-ray revealed dilated right descending pulmonary artery with decreased vascular markings distally (Fig. 1B). Repeat echocardiogram was showing decrease in size of the vegetation (Fig. 4A), however, the patient had hemoptysis of 300 mL fresh blood after two days of admission. CT of the thorax was repeated and revealed evidence of multiple thromboembolism (Fig. 2B). Repeat blood cultures were negative and she was put on injections of vancomycin, ciprofloxacin, and gentamicin and supportive measures. She became afebrile within four days and no further hemoptysis occurred. She was discharged after 4 weeks of therapy. At one-month follow-up, she was asymptomatic and serial echocardiogram showed diminution of vegetation size with nonhypertensive moderate pulmonary regurgitation (Fig. 4B).
Fig. 4.
(A) Follow up transthoracic echocardiography basal short axis view showing decrease in size of the vegetation. (B) Color Doppler image showing nonhypertensive moderate pulmonary regurgitation.
Discussion
The incidence of right-sided infective endocarditis ranges from 5 to 10% in different series 3, 4. The majority of cases involve the tricuspid valve. Isolated pulmonary valve endocarditis is rare. It is assumed that its rarity is due to the low pressure gradients within the right heart, the low prevalence of congenital malformations, the lower oxygen content of venous blood, and the differences in the covering and vascularization of the right heart endothelium [5]. Most cases of pulmonary valve endocarditis in children are secondary to the presence of a congenitally abnormal pulmonary valve and in adults secondary to intravenous drug abuse. Isolated pulmonary valve endocarditis has also been identified in patients undergoing chronic hemodialysis and orthotopic liver transplantation 6, 7. A significant number of patients present with primarily pulmonary symptoms such as pleuritic chest pain, cough, and dyspnea. When peripheral embolic or neurologic features occur, either left-sided endocarditis or paradoxical embolism should be considered.
The clinical presentation of isolated pulmonary valve endocarditis in our patient included prolonged fever, features of multiple septic pulmonary embolism, and a new pulmonary murmur. A conservative approach is recommended for the majority of patients with infective endocarditis affecting the tricuspid or pulmonary valve [8]. In our case, the patient responded to antibiotics and became asymptomatic within 4 weeks of initial admission, even though echocardiography showed mild increase in the size of vegetation. As she was asymptomatic and inflammatory markers were normalized, we did not consider the option of surgery and put the patient on regular follow up. A review of the published data indicated that the role of surgery in isolated pulmonic valve endocarditis is unclear. Recurrent pulmonary emboli are not an indication for surgery, which is only needed if fever persists despite 3 weeks of appropriate antibiotic treatment in the absence of a pulmonary abscess [9]. Surgical options include debridement of the infected area, vegetation excision with either valve preservation or valve repair or valve replacement. Preservation of the native pulmonary valve is recommended whenever possible, and use of a homograft or xenograft is preferred if replacement is unavoidable.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Cassling R.S., Rogler W.C., McManus B.M. Isolated pulmonic valve infective endocarditis: a diagnostically elusive entity. Am Heart J. 1985;109:558–567. doi: 10.1016/0002-8703(85)90563-0. [DOI] [PubMed] [Google Scholar]
- 2.Katsufumi N., Osamu N., Dean S.N. Pulmonary valve endocarditis caused by right ventricular outflow obstruction in association with sinus of valsalva aneurysm: a case report. J Cardiothorac Surg. 2008;3:46. doi: 10.1186/1749-8090-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delahaye F., Goulet V., Lacassin F., Ecochard R., Suty-Selton C., Hoen B., Etienne J., Briançon S., Leport C. Characteristics of infective endocarditis in France in 1991: A 1-year survey. Eur Heart J. 1995;16(3):394–401. doi: 10.1093/oxfordjournals.eurheartj.a060923. [DOI] [PubMed] [Google Scholar]
- 4.Van der Meer J.T.M., Thompson J., Valkenburg H.A., Michel M.F. Epidemiology of bacterial endocarditis in the Netherlands. Patient characteristics. Arch Intern Med. 1992;152:1863–1868. doi: 10.1001/archinte.152.9.1863. [DOI] [PubMed] [Google Scholar]
- 5.Ramadan F.B., Beanlands D.S., Burwash I.G. Isolated pulmonic valve endocarditis in healthy hearts: a case report and review of the literature. Can J Cardiol. 2000;16:1282–1288. [PubMed] [Google Scholar]
- 6.Kamaraju S., Nelson K., Williams D.N., Ayenew W., Modi K.S. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19:605–608. doi: 10.1159/000013528. [DOI] [PubMed] [Google Scholar]
- 7.Hearn C.J., Smedira N.G. Pulmonic valve endocarditis after orthotopic liver transplantation. Liver Transpl Surg. 1999;5:456–467. doi: 10.1002/lt.500050509. [DOI] [PubMed] [Google Scholar]
- 8.The Endocarditis Working Group of the International Society of Chemotherapy, Petterson G., Carbon C. Recommendations for the surgical treatment of endocarditis. Clin Microbiol Infect. 1998;4(Suppl. 3):S34–S46. [PubMed] [Google Scholar]
- 9.Moon M.R., Stinson E.B., Miller D.C. Surgical treatment of endocarditis. Prog Cardiovasc Dis. 1997;40:239–264. doi: 10.1016/s0033-0620(97)80036-9. [DOI] [PubMed] [Google Scholar]