Abstract
Background:
We aimed to investigate the correlation between Helicobacter pylori infection and the development and prognosis of gastric cancer.
Methods:
Retrospective analysis was used to collect 120 paraffin-embedded specimens and 42 paracancerous specimens of gastric cancer patients archived in Department of Pathology, Weihaiwei People’s Hospital from 2010–2012. All patients with gastric cancer were followed for 5 years. Real-time fluorescent quantitative PCR was used to detect the relative H. pylori infection in gastric cancer tissues and paracancerous tissues. The relationship between clinicopathological parameters and H. pylori relative infection was analyzed. Kaplan-Meier was used for survival analysis.
Results:
The relative amount of H. pylori infection in gastric cancer tissues was significantly higher than that in paracancerous normal tissues (P<0.001). The relative H. pylori infection was related to tumor size, lymph node metastasis, clinical stage, and depth of invasion (P<0.05). The 1-, 3-, and 5-year survival rates of gastric cancer patients were negatively correlated with the relative H. pylori infection. The relative H. pylori infection, age, tumor size, lymph node metastasis, distant metastasis, clinical stage and depth of invasion were positively correlated with the prognosis of patients with gastric cancer.
Conclusion:
The relative H. pylori infection and clinical stage of patients could increase the risk of death in gastric cancer patients. H. pylori is one of the independent risk factors for the progression and prognosis of gastric cancer. It is also an index to evaluate the development process and prognosis of gastric cancer.
Keywords: Gastric cancer, Helicobacter pylori, Clinical pathology, Prognosis, Correlation analysis
Introduction
Gastric cancer is a common tumor worldwide. The number of newly-diagnosed patients is approximately 951,000 and the death toll is approximately 723,000 (1). The burden of gastric cancer in developing countries is heavier than in developed countries (2). The occurrence and development of gastric cancer is a complex process in which multiple factors, genes, and stages interact with each other. Smoking, smoked products, high dietary salt, high sugar, and other unhealthy lifestyles can increase the risk of gastric cancer (3). At present, the main method for the treatment of gastric cancer is still the comprehensive treatment based on surgery, but the local recurrence rate is still as high as 50%, postoperative 5-year survival rate is only 20%–50% (4, 5).
Helicobacter pylori infection is one of the most important factors involved in stomach canceration. The incidence of gastric cancer and peptic ulcers increases with the increase of H. pylori infection rate (6). H. pylori has been included as the first type of carcinogen by the International Agency for Research on Cancer (7). At the same time, most scholars have recognized H. pylori’s role in screening for gastric cancer and its role in the development and prognosis of gastric cancer (8–10). At present, the specific pathogenesis of gastric cancer has not yet been clarified. There is no definitive conclusion on H. pylori infection and tumor development and prognosis. It has been reported that the occurrence of gastric cancer is closely related to H. pylori infection, and H. pylori can cause gastric mucosal damage by inducing inflammatory responses through the regulation of relevant signaling pathways, thereby affecting the prognosis of gastric cancer patients (11–13).
The purpose of this study was to investigate the relationship between H. pylori infection and the progression and prognosis of gastric cancer in patients with gastric cancer, and to guide the diagnosis, treatment and prognosis of gastric cancer.
Materials and Methods
Sample collection
A retrospective method was used to collect 120 paraffin-embedded specimens and 42 paraneoplastic tissues from patients with gastric cancer who were first surgically resected from October 2010 to October 2012 in Weihaiwei People’s Hospital.
This study was approved by the Ethics Committee of Weihaiwei People’s Hospital, Weihai, China. All patients or their families have signed the informed consent.
The average age of 120 patients was 54.24±16.48 yr, including 79 males and 41 females. The adjacent tissue was a non-cancer tissue with a margin of 5 cm from the edge of the lesion. Specimens were fixed with formaldehyde. All patients had no chemotherapy or radiotherapy before surgery. All cases were histopathologically confirmed as gastric cancer and had not received anti- H. pylori treatment. All patients were excluded from other parts and tissues of primary and malignant tumors, heart, liver, and renal insufficiency, and gastric surgery was not performed within six months before admission. All subjects and their families signed informed consent.
Main reagents and instruments
Qiagen 56404 QIAamp DNA FFPE Tissue Kit Paraffin Tissue DNA Extraction Kit and Qiagen 204054 QuantiFast SYBR Green PCR Kit were purchased from QIAGEN; Nanodrop 2000 UV spectrophotometer was purchased from Thermo Scientific, USA; Ro-tor-Gene Q-PCR instrument Purchased from QIAGEN.
DNA extraction
The paraffin tissues were sectioned into 20 pieces with a thickness of 5 μm and loaded into a sterile EP tube. Qiagen 56404 QIAamp DNA FFPE Tissue Kit was used to extract DNA from gastric cancer and paracancerous tissues. DNA purity and mass concentration were measured using a Nanodrop 2000 UV spectrophotometer.
Primer design
This experiment was designed and synthesized by Sangon Biotech (Shanghai) Co., Ltd.. using the H. pylori marker gene HPYR1 and GAPDH as an internal reference gene. The primer sequences are showed in Table 1.
Table 1:
HPYR1 primers and internal reference sequences
| Upstream primers | Downstream primers | |
|---|---|---|
| HPYR1 | 5′-GAGCCCTCAAAGAACTGCAC-3′ | 5′-AATTGGACAGCACCTTCTGG-3′ |
| GAPDH | 5′-TCAACGACCACTTTGTCAAGCTCA-3′ | 5′-GCTGGTGGTCCAGGGGTCTTACT-3′ |
Real-time PCR detection
The real-time PCR reaction system was prepared according to the instructions and a total of 25 μL was prepared: 12.5 μL of 2×Quantifast SYBR Green PCR Master Mix, 1 μL each of 10 μmol primers, and 3 μL of template DNA. Finally, RNase-free water was used to complete to 25 μL. Using the Ro-tor-Gene Q real-time fluorescence quantitative PCR instrument for PCR amplification, the reaction conditions were: 95°C 5 min, 95 °C 20 s, 60 °C 45 s, a total of 45 cycles. The PCR product was stored at 4°C. GAPDH was used as the internal control, and 2−ΔCt method was used to analyze the relative H. pylori infection in the specimens. The average value of the experiment was repeated three times.
Follow-up
The patients in this group were followed up by telephone and outpatient follow-up. All patients with gastric cancer were followed for 5 years. The relationship between the relative H. pylori infection and the clinicopathological features of gastric cancer was observed, and the relationship between the relative H. pylori infection and patient survival was analyzed.
Statistical methods
SPSS21.0 statistical software package (Cabit Information Technology Co., Ltd.) was used for statistical analysis of data. Measurement data were analyzed by t-test, Kaplan-Meier survival analysis was used, Log Rank test was used to compare survival rates among different H. pylori infections, and Cox regression was used to analyze the relationship between relevant variables and clinical prognosis. P<0.05 was considered statistically significant.
Results
Relative infections of H. pylori in two groups
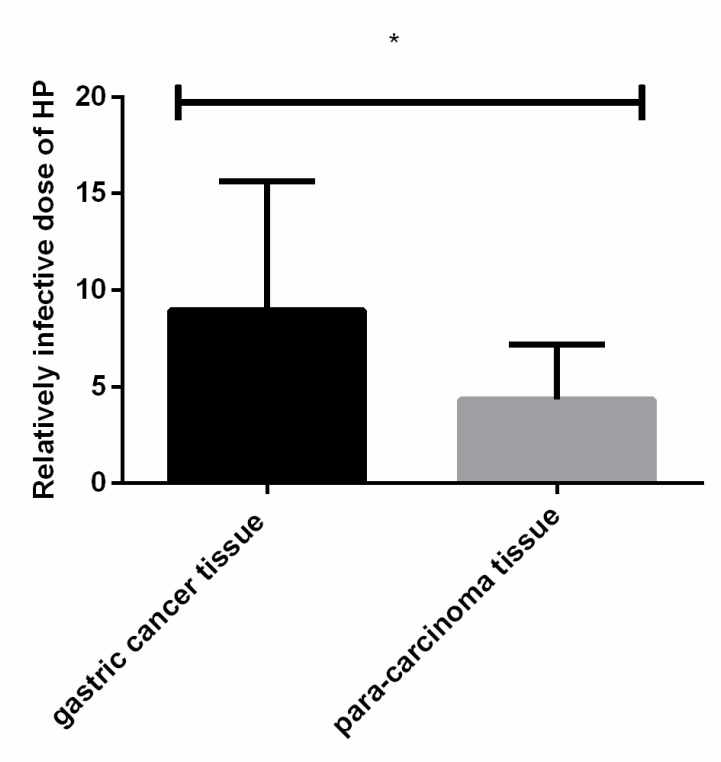
The relative amount of H. pylori infection in gastric cancer tissues (8.94±6.71) was significantly higher than that in paracancerous normal tissues (4.32±2.87). The difference was statistically significant (P<0.001) (Fig. 1 and Table 2).
Fig. 1:
Relative H. pylori infection in both groups. Real-time fluorescence quantitative PCR results showed that the relative amount of H. pylori infection in gastric cancer tissue was significantly higher than that in paracancerous normal tissues, and the difference was statistically significant (P<0.001)
Note: *<0.001 compared with paracancerous normal tissues
Table 2:
H. pylori relative infection
| Group | n | H. pylori relative infection | t | P |
|---|---|---|---|---|
| Gastric cancer tissue | 120 | 8.94±6.71 | 4.319 | < 0.001 |
| Paracancerous tissue | 42 | 4.32±2.87 |
Relationship between relative infections of H. pylori and clinicopathological features of gastric cancer
There was no significant correlation between H. pylori relative infection and age, gender, distant metastasis, and the difference was not statistically significant (P>0.05). The relative H. pylori infection was correlated with tumor size, lymph node metastasis, clinical stage, and depth of invasion. The difference was statistically significant (P<0.01) (Table 3).
Table 3:
Relationship between relative infections of H. pylori and clinicopathological features of gastric cancer
| Variety | Number of cases (n=120) | H. pylori relative infection | t | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | ||||||||||
| ≥54 | 75 | 9.56±5.46 | 0.0 | 0.985 | ||||||
| <54 | 45 | 9.58±5.84 | 19 | |||||||
| Gender | ||||||||||
| Male | 79 | 10.49±5.76 | 1.6 | 0.108 | ||||||
| Female | 41 | 8.65±6.17 | 20 | |||||||
| Tumor size | ||||||||||
| ≥5 cm | 46 | 10.73±5.24 | 4.2 | < 0.001 | ||||||
| <5 cm | 74 | 7.12±4.11 | 04 | |||||||
| Lymph node metastasis | ||||||||||
| Yes | 53 | 11.47±5.23 | 3.7 | < 0.001 | ||||||
| No | 67 | 7.89±5.12 | 68 | |||||||
| Distant metastasis | ||||||||||
| Yes | 106 | 9.64±6.46 | 0.1 | 0.905 | ||||||
| No | 14 | 9.43±3.47 | 19 | |||||||
| Clinical staging | ||||||||||
| I+II | 55 | 7.82±6.15 | 2.5 | 0.013 | ||||||
| III+IV | 65 | 10.46±5.28 | 30 | |||||||
| Infiltration depth | ||||||||||
| T1+T2 | 37 | 6.89±6.12 | 4.1 | < 0.001 | ||||||
| T3+T4 | 83 | 11.42±5.16 | 89 | |||||||
The relationship between the survival rate of gastric cancer and the relative infection of H. pylori
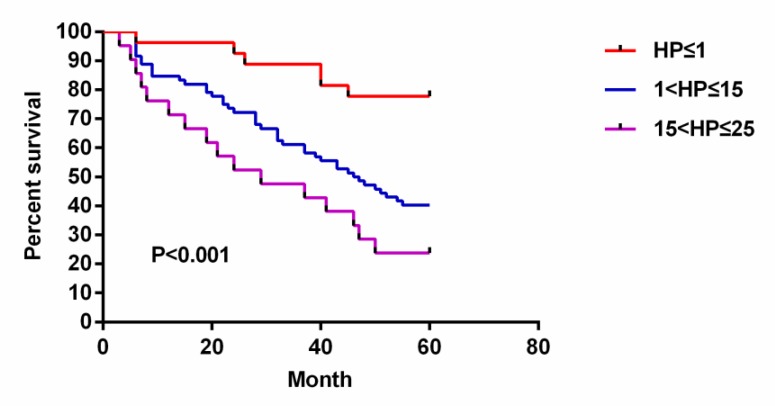
The higher the relative H. pylori infection, the lower the survival rate of gastric cancer patients. The 1-year, 3-year and 5-year survival rates of gastric cancer patients were negatively correlated with the relative H. pylori infection (Fig. 2 and Table 4).
Fig. 2:
Relationship between gastric cancer survival rate and relative H. pylori infection. Kaplan-Meier survival analysis showed that the higher the relative H. pylori infection, the lower the survival rate of gastric cancer patients, and the 1-, 3-, and 5-year survival rates of gastric cancer patients were negatively correlated with the relative H. pylori infection. The 5-year survival rate was 77.78% in the H. pylori≤1 group, 40.28% in the 1<H. pylori≤15 group, and 23.81% in the 15<H. pylori≤25 group. The difference between the three groups was statistically significant (P<0.001)
Table 4:
The relationship between the survival rate of gastric cancer and the relative infection of H. pylori
| Relative infection | n | Survival rate[(n)%] | P | ||
|---|---|---|---|---|---|
| 1-year | 3-year | 5-year | |||
| H. pylori≤1 | 27 | 26 (96.30) | 23 (85.19) | 21 (77.78) | < 0.001 |
| 1 < H. pylori≤15 | 72 | 61 (84.72) | 43 (59.72) | 29 (40.28) | |
| 15 < H. pylori≤25 | 21 | 15 (71.43) | 10 (47.62) | 5 (23.81) | |
Cox regression analysis of clinical pathological characteristics and relative H. pylori infection in gastric cancer
Cox regression univariate analysis showed that the prognosis of patients with gastric cancer was positively correlated with the relative H. pylori infection, age, tumor size, lymph node metastasis, distant metastasis, clinical stage, and depth of invasion. Multivariate analysis showed that the relative H. pylori infection and clinical stage of patients could increase the risk of death in gastric cancer patients, and it was also an independent risk factor for the development process and poor prognosis of gastric cancer (Table 5 and 6).
Table 5:
Single factor analysis of prognosis of gastric cancer patients
| Group | Regression coefficients | Relative risk | P |
|---|---|---|---|
| H. pylori relative infection | 0.089 | 1.087 | < 0.001 |
| Age | 0.031 | 1.034 | 0.016 |
| Gender | 0.021 | 0.652 | 0.421 |
| Size of tumor | 0.752 | 2.128 | < 0.001 |
| Lymph node metastasis | 0.824 | 2.408 | < 0.001 |
| Distant metastasis | 1.516 | 4.324 | < 0.001 |
| Clinical staging | 1.171 | 2.956 | < 0.001 |
| Infiltration depth | 0.957 | 2.649 | < 0.001 |
Table 6:
Multivariate analysis of prognosis of gastric cancer patients
| Group | Regression coefficients | Relative risk | P |
|---|---|---|---|
| H. pylori relative infection | 0.062 | 1.059 | 0.008 |
| Age | 0.038 | 1.039 | 0.055 |
| Gender | 0.084 | 1.043 | 0.856 |
| Size of tumor | 0.534 | 1.507 | 0.146 |
| Lymph node metastasis | 0.367 | 1.382 | 0.124 |
| Distant metastasis | 0.288 | 1.412 | 0.625 |
| Clinical staging | 0.851 | 2.456 | 0.014 |
| Infiltration depth | 0.052 | 1.125 | 0.885 |
Discussion
H. pylori can secrete more antioxidative enzymes to prevent it from being killed by gastric neutrophils, and it can hydrolyze the specific protective layer produced by urea to resist the killing by gastric acid (14). A large number of reports have reported that persistent H. pylori infection may induce gastritis, further induce gastric mucosal cell damage, infiltrate inflammatory cells, and cause deterioration of gastric mucosal cells (15–17). H. pylori can cause gastric precancerous lesions, but also can cause oncogene mutations, leading to the occurrence of gastric cancer (18). Foreign research reports show that H. pylori infection is involved in the entire process of gastric cancer development (18, 19).
This study showed that the relative amount of H. pylori infection in gastric cancer tissue was higher than that in normal tissues adjacent to the cancer. This indicates that H. pylori can promote the occurrence of gastric cancer. The results of Wroblewski's study (20) are consistent with ours. He indicates that the prevalence of gastric cancer in H. pylori -infected patients is much higher than that in non-infected patients, and total gastrectomy for gastric cancer patients and eradication of H. pylori are beneficial to gastric cancer patients.
In this study, there was no significant correlation between the relative amount of H. pylori infection and age, gender, distant metastasis. The relative amount of H. pylori infection was correlated with tumor size, lymph node metastasis, clinical stage, and depth of infiltration, and the difference was statistically significant. The results of other studies (21, 22) are basically consistent with ours. H. pylori infection is associated with gastric cancer infiltration (22).
Most patients showed H. pylori positive in the T1 and T2 staging of gastric cancer, indicating that H. pylori is involved in the metastasis of gastric cancer. H. pylori infection was associated with lymph node metastasis (22). Most patients with gastric cancer showed positive H. pylori in the N0 and N1 stages. The higher the relative H. pylori infection, the lower the survival rate of gastric cancer patients. The 1-year, 3-year and 5-year survival rates of gastric cancer patients were negatively correlated with the relative H. pylori infection.
Cox regression univariate analysis showed that the prognosis of patients with gastric cancer was positively correlated with the relative H. pylori infection, age, tumor size, lymph node metastasis, distant metastasis, clinical stage, and depth of invasion. Multivariate analysis showed that the relative H. pylori infection and clinical stage of patients could increase the risk of death in gastric cancer patients, and it was also an independent risk factor for the development process and poor prognosis of gastric cancer. For the report of the relationship between the prognosis of gastric cancer and H. pylori infection, the results of the study are mixed. Patients with advanced H. pylori and metastases have higher sensitivity to chemotherapy than patients with H. pylori-negative gastric cancer, so the prognosis is better (23). H. pylori infection plays a protective role in the prognosis of gastric cancer (24). In contrast, gastric cancer patients not infected with H. pylori had a better prognosis (25). Relative H. pylori infection was an independent risk factor for poor prognosis (26). The reason for the difference in results may be due to differences in the treatment effect, age, and clinical stage of the study subjects, or it may be due to the fact that some patients had undergone H. pylori radical resection prior to surgery.
Conclusion
H. pylori infection is related to the clinicopathological data of gastric cancer patients. H. pylori is one of the independent risk factors for the development and prognosis of gastric cancer, and it is also an index to evaluate the development process and prognosis of gastric cancer.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
No funding was received in this study.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. (2016). Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev, 25: 16–27. [DOI] [PubMed] [Google Scholar]
- 2.Ferro A, Peleteiro B, Malvezzi M, et al. (2014). Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer, 50: 1330–1344. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez CA, Agudo A. (2012). Carcinogenesis, prevention and early detection of gastric cancer: where we are and where we should go. Int J Cancer, 130: 745–753. [DOI] [PubMed] [Google Scholar]
- 4.Paoletti X, Oba K, Burzykowski T, et al. (2010). Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA, 303: 1729–1737. [DOI] [PubMed] [Google Scholar]
- 5.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. (2015). Global cancer statistics, 2012. CA Cancer J Clin, 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 6.Peek RM, Jr., Blaser MJ. (2002). Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer, 2: 28–37. [DOI] [PubMed] [Google Scholar]
- 7.Rain JC, Selig L, Reuse HD, et al. (2001). Erratum: The protein–protein interaction map of Helicobacter pylori. Nature, 409: 211–215. [DOI] [PubMed] [Google Scholar]
- 8.Uemura N. (2003). Helicobacter pylori infection and the development of gastric cancer. Nihon Rinsho, 61:25–29. [PubMed] [Google Scholar]
- 9.Talley NJ, Vakil N, Ballard ED, 2nd, Fennerty MB. (1999). Absence of benefit of eradicating Helicobacter pylori in patients with nonulcer dyspepsia. N Engl J Med, 341(15):1106–11. [DOI] [PubMed] [Google Scholar]
- 10.Group TE. (1993). An international association between Helicobacter pylori infection and gastric cancer. Lancet, 341:1359–1362. [PubMed] [Google Scholar]
- 11.Polk DB, Peek RM., Jr. (2010). Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer, 10: 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. (2000). Translocation of Helicobacter pylori CagA into Gastric Epithelial Cells by Type IV Secretion. Science, 287: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Compton CC. (2010). The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol, 17: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 14.Vaira D, Malfertheiner P, Megraud F, et al. (2000). Noninvasive antigen-based assay for assessing Helicobacter pylori eradication: a European multicenter study. The European Helicobacter pylori HpSA Study Group. Am J Gastroenterol, 95: 925–929. [DOI] [PubMed] [Google Scholar]
- 15.Poplawski T, Chojnacki C, Czubatka A, Klupinska G, Chojnacki J, Blasiak J. (2013). Helicobacter pylori infection and antioxidants can modulate the genotoxic effects of heterocyclic amines in gastric mucosa cells. Mol Biol Rep, 40: 5205–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JS, Yeom JS, Seo JH, et al. (2015). Immunohistochemical Expressions of MUC2, MUC5AC, and MUC6 in Normal, Helicobacter pylori Infected and Metaplastic Gastric Mucosa of Children and Adolescents. Helicobacter, 20: 260–268. [DOI] [PubMed] [Google Scholar]
- 17.Guindi M. (1999). Role of Helicobacter pylori in the pathogenesis of gastric carcinoma and progression of lymphoid nodules to lymphoma. Can J Gastroenterol, 13: 224–227. [DOI] [PubMed] [Google Scholar]
- 18.Parsonnet J, Friedman GD, Vandersteen DP, et al. (1991). Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med, 325: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 19.Peek RM, Jr., Blaser MJ. (2002). Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer, 2: 28–37. [DOI] [PubMed] [Google Scholar]
- 20.Wroblewski LE, Peek RM, Jr., Wilson KT. (2010). Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev, 23: 713–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meimarakis G, Winter H, Assmann I, et al. (2006). Helicobacter pylori as a prognostic indicator after curative resection of gastric carcinoma: a prospective study. Lancet Oncol, 7: 211–222. [DOI] [PubMed] [Google Scholar]
- 22.Qiu HB, Zhang L Y, Keshari R P, et al. (2010). Relationship between H. Pylori infection and clinicopathological features and prognosis of gastric cancer. BMC Cancer, 10: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi IK, Sung HJ, Lee JH, Kim JS, Seo JH. (2012). The relationship between Helicobacter pylori infection and the effects of chemotherapy in patients with advanced or metastatic gastric cancer. Cancer Chemother Pharmacol, 70: 555–558. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Sun G, Zou Y, Zhong F, Ma T, Li X. (2013). Protective role of Helicobacter pylori infection in prognosis of gastric cancer: evidence from 2,454 patients with gastric cancer. PLoS One, 8: e62440>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo JY, Jin EH, Yoon H, et al. (2015). Characteristics and prognosis of gastric cancer according to Helicobacter pylori. J Clin Oncol, 33 (3_suppl): 14–14. [Google Scholar]
- 26.Tsai KF, Liou JM, Chen MJ, et al. (2017). Distinct Clinicopathological Features and Prognosis of Helicobacter pylori Negative Gastric Cancer. PLoS One, 12: e0170942>. [DOI] [PMC free article] [PubMed] [Google Scholar]