Abstract
Both when actions are executed and observed, electroencephalography (EEG) has shown reduced alpha-band (8–12 Hz) oscillations over sensorimotor cortex. This ‘μ-alpha’ suppression is thought to reflect mental simulation of action, which has been argued to support internal representation of others’ emotional states. Despite the proposed role of simulation in emotion perception, little is known about the effect of emotional content on μ-suppression. We recorded high-density EEG while participants viewed point-light displays of emotional vs neutral body movements in ‘coherent’ biologically plausible and ‘scrambled’ configurations. Although coherent relative to scrambled stimuli elicited μ-alpha suppression, the comparison of emotional and neutral movement, controlling for basic visual input, revealed suppression effects in both alpha and beta bands. Whereas alpha-band activity reflected reduced power for emotional stimuli in central and occipital sensors, beta power at frontocentral sites was driven by enhancement for neutral relative to emotional actions. A median-split by autism-spectrum quotient score revealed weaker μ-alpha suppression and beta enhancement in participants with autistic tendencies, suggesting that sensorimotor simulation may be differentially engaged depending on social capabilities. Consistent with theories of embodied emotion, these data support a link between simulation and social perception while more firmly connecting emotional processing to the activity of sensorimotor systems.
Keywords: action simulation, emotional body movement, time-frequency EEG, mu suppression, beta enhancement
Introduction
We can rapidly infer emotions from nonverbal face and body cues. Although this process could arise from high-level theorizing and reasoning, a growing body of evidence suggests that we understand others through our own sensorimotor systems, specifically by internally simulating their external movements (Niedenthal, 2007; Barsalou, 2008; Wood et al., 2016). Consistent with this idea, research suggests that body movements provide a rich and immediate source of social information (Beall et al., 2008) that can be extracted rapidly and automatically (de Gelder, 2006).
Behavioral evidence for simulation of emotion comes from emotional mimicry, that is, quick and spontaneous matching of another’s expressions (Dimberg and Thunberg, 1998). More than a simple motor matching process, mimicry appears to arise from mental simulations of others’ emotions (Moody et al.,2007; Wood et al., 2016) and has been shown to be impaired in conditions associated with social processing deficits such as autism spectrum disorder (ASD) (McIntosh et al., 2006; Moody and McIntosh, 2006; Beall et al., 2008; Oberman et al., 2009). Likewise, emotional contagion, in which individuals ‘catch’ others’ emotional states automatically and unconsciously, has been associated with facial, vocal and postural mimicry (Hatfield et al., 1993).
In interpreting such ‘embodied emotion’ phenomena, researchers have drawn on an extensive literature documenting neural simulation within sensorimotor systems during observation of others’ actions (Decety, 1996; Gallese and Goldman, 1998; Jeannerod, 2001, 2006). Both when actions are actually executed and when they are merely imagined or observed, there are visible decreases in the power, or magnitude, of oscillatory neural activity over sensorimotor cortex in electroencephalography (EEG; for a review, see Fox et al., 2016). Known as the μ-rhythm, this oscillatory activity is typically measured over central sensors in the alpha (8–12 Hz) frequency band (Niedermeyer and da Silva, 2005). Paralleling the report of mirror neurons (Rizzolatti et al., 2001), which respond to both observed and executed actions, μ-suppression has been argued to reflect the active engagement of sensorimotor areas during action simulation (Niedermeyer and da Silva, 2005; Ulloa and Pineda, 2007).
Differential μ-suppression has been associated with action understanding (Rizzolatti et al., 2001; Ulloa and Pineda, 2007), empathy and social processes (Kilner et al., 2006; Pineda and Hecht, 2009) and social processing impairments in ASD (Oberman et al., 2005; Oberman and Ramachandran, 2007). Yet, despite these claims, the link between μ-suppression, action simulation and the role of sensorimotor systems in perceiving others’ emotions has not been directly tested. Although previous studies have examined the effect of emotion on μ-suppression (Perry et al., 2010; Moore et al., 2012; Moore and Franz, 2017), the stimuli used in these experiments are not associated with body movements and/or are unlikely to directly initiate mental reenactment: for example, static faces (Moore et al., 2012; Moore and Franz, 2017) or situational information about the painfulness of a stimulus applied to another individual (Perry et al., 2010).
In contrast, body movements form an ideal stimulus category to test whether action simulation plays a role in perceiving others’ emotions. Body movements can be rapidly perceived and categorized even from relatively sparse visual information, as in point-light displays (PLDs; Blake and Shiffrar, 2007). By ‘scrambling’ the global stimulus structure of the PLDs, we can preserve low-level motion energy while disrupting the perception of coherent, biologically plausible movement. Finally, and most importantly, PLDs have been shown to carry motion information allowing differentiation of emotions such as happiness, sadness and anger (Dittrich et al., 1996; Atkinson et al., 2004), enabling the direct comparison of simulation processes for emotional vs emotionally neutral actions (e.g. ‘jumping for joy’ vs ‘jumping jacks’).
In this study, we measured μ-suppression elicited by emotional and neutral body movements in order to elucidate the role of action simulation in perceiving others’ emotions. We predicted that greater μ-suppression would be elicited by biologically plausible coherent PLDs than by biologically implausible scrambled stimuli, in line with previous studies (Ulloa and Pineda, 2007). Furthermore, if action simulation contributes to understanding others’ emotions, we should find increased μ-suppression for emotional, as compared to neutral, movements. Finally, we assessed whether action simulation of body movements relates to behavioral measurements of social perception ability. Previous works examining individuals with ASD have found reductions in μ-suppression (Oberman et al., 2005) and rapid facial mimicry (McIntosh et al., 2006; Beall et al., 2008) in this population, suggesting that ASD may be associated with impairments in emotion and action simulation. Therefore, we conducted an exploratory analysis comparing μ-suppression for individuals rated high vs low in autistic tendencies on the Autism-Spectrum Quotient (AQ) scale (Baron-Cohen et al., 2001).
Notably, recent discussions have raised methodological concerns about the reliability and specificity of μ-suppression effects (Fox et al., 2016; Hobson and Bishop, 2016; Bowman et al., 2017; Hobson and Bishop, 2017). In the comparison of emotional and neutral body movements, one particular concern is that emotional postures are known to capture selective attention (Bannerman et al., 2009), which is associated with alpha suppression over occipitoparietal sites (Sadaghiani and Kleinschmidt, 2016). Since effects of selective attention and action simulation overlap in frequency and scalp topography, studies that look at a small number of electrode sites rather than the whole-scalp topographic distribution run the risk of conflating processes related to attention and action simulation (Hobson and Bishop, 2017).
Additionally, many studies limit their measurements to the alpha band, potentially ignoring sensorimotor activity in other frequency ranges (Hobson and Bishop, 2016). In particular, activity in the central beta band (16–20 Hz) is suppressed by imagined, observed and executed movement, similar to μ-suppression (McFarland et al., 2000; Babiloni et al., 2002; Zaepffel et al., 2013). Beta activity has also been reported to increase during the first 500 ms of viewing emotional stimuli such as angry faces (Guntekin and Basar, 2014).
To address these issues, we measured EEG power spectra over 128 electrode locations in a dense whole-head electrode array across a broad range of frequencies between 4 and 20 Hz. Directly comparing biologically plausible coherent vs scrambled PLDs allowed us to more rigorously control for brain activity related to low-level visual stimulation. By implementing these more rigorous standards, our study provides a novel and meticulous approach to the link between μ-suppression, action simulation and social perception.
Methods
Participants
Forty-six undergraduate participants were recruited for either monetary compensation or partial course credit. Eight participants were excluded from the analyses for the following reasons: (i) technical issues with EEG recording (n = 5); (ii) failure to comply with experiment instructions (n = 1); and (iii) performance 2.5 s.d. lower than the average d-prime (d′) score (n = 2). Thus, a total of 38 participants (ages 18–23; 16 females) were submitted for data analysis. This final sample size complied with recent recommendations for adequate power to measure μ-suppression (Hobson and Bishop, 2017). All experimental procedures were reviewed and approved by Claremont McKenna College’s Institutional Review Board, and informed consent was obtained in writing from all participants prior to the experiment.
Stimuli
Stimuli consisted of whole-body actions depicted in PLD videos, which control for irrelevant visual body shape information (Figure 1A). The PLDs were 3 s video clips used in Atkinson et al. (2012), adapted from a larger database developed by Atkinson et al. (2004).
Fig. 1.
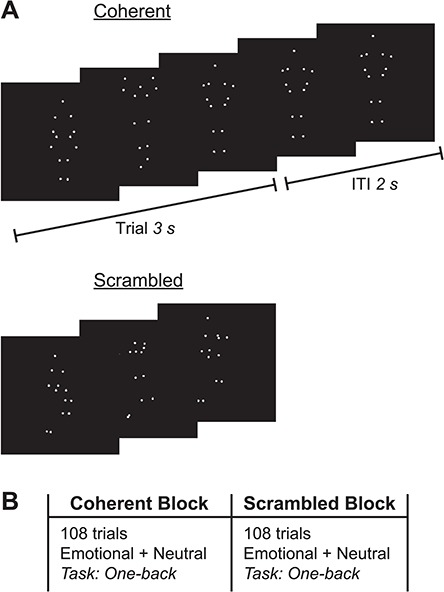
Experimental stimuli and procedure. (A) Still frames taken from biological PLDs of coherent (top) and scrambled (bottom) body movement. Each PLD was 3 s in duration, after which the final frame was maintained on the screen for an ITI of 2 s. (B) The experiment comprised two blocks, a coherent block and a scrambled block, consisting of 108 trials of emotional and neutral actions, pseudo-randomly interleaved. Participants monitored for the immediate repetition of individual movies (one-back task). Block order was counterbalanced across participants.
PLD videos consisted of 11 different actors of both genders (5 males, 6 females) portraying whole-body expressions of different emotions or of emotionally neutral common everyday actions. The emotional content condition contained PLDs of actors’ expressions of happiness, sadness and anger. Atkinson et al. (2004) found that actors’ expressions could be correctly categorized by participants at above chance levels (71–84% correct). The neutral content condition contained PLDs of actors’ portrayal of three different emotionally neutral common actions: walking, jumping on one foot and touching toes. There were six different videos (i.e. six performances) of each of the three emotions and two different videos of each of the three neutral actions. Fewer versions of the neutral actions were used because only two versions of each neutral action were available in the Atkinson et al. (2012) database.
For each coherent movement PLD, we also used a corresponding ‘scrambled’ PLD (Figure 1A, bottom), created by independently randomizing the starting location of each dot of the point-light agent within the bounds of the original viewing frame (Atkinson et al., 2012). Scrambled PLDs are often used in visual studies of biological motion because they preserve the individual motions of the dots but disrupt the spatial relations among them, thereby reducing or eliminating form-from-motion cues (e.g. Grossman and Blake, 1999). For our purposes, these scrambled PLDs contain the same basic visual inputs as coherent PLDs, but do not represent biologically plausible movement that could be simulated by the human body.
In summary, there were four types of stimuli constituting a 2 × 2 design with content (emotional/neutral) and action coherency (coherent/scrambled) as within-subject factors. There were 48 distinct PLDs in total: 24 coherent and 24 scrambled PLDs. Each action coherency condition included 18 distinct emotional and 6 distinct neutral PLDs.
Procedure
During the experiment, participants viewed two separate blocks of coherent and scrambled biological motion stimuli (Figure 1B) while EEG recordings were taken. The order of the two blocks was counterbalanced across participants. Each individual trial consisted of a single, 3 s PLD, followed by an intertrial interval (ITI) during which the last frame was presented as a static image for another 2 s. To ensure attentive viewing, participants performed a continuous one-back task in which they monitored for the immediate repetition of each video (i.e. the same configuration and movement of the dots, presented twice in a row); participants were explicitly instructed to look for an exact repetition of the dot configurations and their local motion, rather than just the general gist. Given the dynamic nature of the PLDs, the duration of each stimulus and the high number of non-immediate stimulus repetitions across the experiment, this task was substantially more challenging than a traditional one-back paradigm and required participants to pay attention throughout the experiment.
In each block, the number of presentations of the emotional and neutral PLDs were equated by presenting each of 18 emotional PLDs 3 times and each of 6 neutral PLDs 9 times, respectively, for a total of 54 trials per content type (emotional vs neutral). Thus, each action coherency block (coherent vs scrambled) consisted of 108 (2 ×54) trials for each content type. Across the two experimental blocks, PLDs were repeated following 12 randomly selected trials within each block to create one-back trials for the task, generating a total of 240 trials (216 stimulus presentations plus 24 one-back trials). Aside from the 24 one-back trials, all stimuli within each action coherency block were pseudo-randomly interleaved to ensure that there were no consecutive repetitions of each video. Participants completed 10 practice trials (5 coherent and 5 scrambled, each containing a one-back trial) before beginning the main experiment.
After EEG data acquisition, participants answered two multiple-choice questions where they were asked to select all emotions and actions that they thought they saw during the experiment, out of six word choices for emotion (sadness, happiness, anger, fear, disgust and surprise) and action (touching toes, jumping on one foot, walking, jumping jacks, kicking and kneeling down). They then completed an online version of the autism quotient questionnaire (Baron-Cohen et al., 2001).
EEG data acquisition and analysis
EEG data was collected using a BioSemi ActiveTwo system (Biosemi B.V., Amsterdam, the Netherlands) with 128 channels of active electrodes inserted in fitted headcaps. Two additional electrodes were adhered bilaterally to participants’ mastoids to serve as reference channels for data import. Recorded signals were digitized continuously at 512 Hz with a hardware low-pass at one-fifth of the sampling rate. Before beginning data collection, electrodes were adjusted until offsets were between –20 and 20 μV and there were no obvious slow drifts in the online data output.
Data preprocessing was performed offline using the EEGLAB toolbox (Delorme and Makeig, 2004) for MATLAB (Mathworks, Natick, MA). Following import into EEGLAB, data were re-sampled at 500 Hz. Linear detrending was conducted on continuous data to remove direct current offsets. A high-pass filter of 0.5 Hz was applied to remove slow voltage drifts via a two-way least-squares finite impulse response filter. Data were not re-referenced and no low-pass filters were applied in order to avoid distorting the power spectra (Luck, 2014). For each trial, 3600 ms epochs were extracted around the 3000 ms stimulus, –400 ms pre- to 3200 ms post-stimulus onset. Data were baseline-corrected to the pre-stimulus period to correct for initial electrode offsets.
All one-back trials and other trials where participants made a motor response were removed from data analyses to exclude potential motor preparatory activity. To identify and remove EEG artifacts (e.g. head, eye and jaw movements as well as electrical and sensor noise), we conducted an independent components analysis on the remaining trials for each participant using the second-order blind identification method (Belouchrani et al., 1997; Tang et al., 2005). The remaining task-related components were projected back onto the scalp (Jung et al., 2000).
Event-related potentials (ERPs) for each condition were compared using threshold-free cluster enhancement (Mensen and Khatami, 2013), with default parameters of extent of 0.666 and height of 1. Grand average waveforms for each subject and condition were entered into a 2 × 2 analysis of variance (ANOVA) with content (emotional/neutral) and action coherency (coherent/scrambled) as factors. Resulting clusters of significant activity were corrected for multiple comparisons using a permutation approach (2500 permutations).
Time-frequency analysis was performed using the FieldTrip toolbox (Oostenveld et al., 2011) for MATLAB. For each participant and condition (EmotionalCoherent, EmotionalScrambled, NeutralCoherent and NeutralScrambled), power was computed averaging across trials at each electrode location using a Morlet wavelet (width = 7) for a conservatively large frequency range of 4–20 Hz and an analysis window centered on –0.2 to 3 s, sliding in steps of 0.01 s. Time-frequency data was log10 transformed to normalize the frequency distribution. We compared power across conditions using a dependent sample two-tailed t-test with a nonparametric cluster-based Monte Carlo permutation test (1000 repetitions) to correct for multiple comparisons. Significance was assessed using an overall threshold of P = 0.05, with significance of P = 0.025 at each tail. Effects before 1 s and beyond 2 s from stimulus onset were excluded as a conservative measure due to potential confounds in alpha-band activity associated with stimulus onset and offset (Fox et al., 2016; Hobson and Bishop, 2017).
Results
Behavioral results
As a manipulation check following EEG recording, we asked participants to indicate which emotions and actions they had seen during the task. Participants were highly accurate at identifying the emotions portrayed by the PLDs, with 94.7% (36/38) of participants correctly selecting at least two of the emotions and 89.5% (34/38) selecting all three emotions. Identification rates for the neutral actions were similarly high, with 97.4% (37/38) of participants correctly selecting at least two of the three actions and 81.6% (31/38) selecting all three actions. A further question is whether viewing emotional movements influences subjective reports of emotional states. However, we could not examine this in the current design because different emotional movements were pseudo-randomly interleaved along with neutral movements, making it unclear what the cumulative effect would be.
To assess participants’ monitoring performance during the task, we computed d′ scores (d′ = ZFalse Alarm Rate – ZHit Rate) (Macmillan and Creelman, 2004) for the one-back task (mean = 3.25, s.d. = 0.65). A 2 × 2 repeated-measures ANOVA with factors of content (emotional/neutral) and action coherency (coherent/scrambled) found no significant main effects of content [F(1,37) = 0.06, P = 0.81, ηp2 = 0.002] or action coherency [F(1,37) = 0.94, P = 0.34, ηp2 = 0.03], though the interaction of content × action coherency approached significance [F(1,37) = 2.92, P = 0.10, ηp2 = 0.07], driven by a larger difference in accuracy for emotional content in the coherent vs scrambled conditions. However, the overall lack of significant differences between content and action coherency levels suggests that participants were deploying attention across all conditions.
Event-related potentials
To assess whether our experimental conditions evoked differential neural responses, as well as assessing the quality of the neural data, we examined the ERP response. Grand average data time-locked to the onset of the stimulus were entered into a 2 × 2 ANOVA with content (emotional/neutral) and action coherency (coherent/scrambled) as factors using a threshold-free cluster enhancement approach (Mensen and Khatami, 2013). Taking advantage of spatial and temporal relationships in ERP data, this analysis has been demonstrated to have high sensitivity to detect meaningful signals while maintaining statistical integrity (Mensen and Khatami, 2013; Pernet et al., 2015). Figure 2 displays the temporal distribution of significant main effects for each factor, along with representative scalp plots and grand average waveforms. The interaction effect failed to reach significance in any sensors or time windows (all P > 0.2).
Fig. 2.
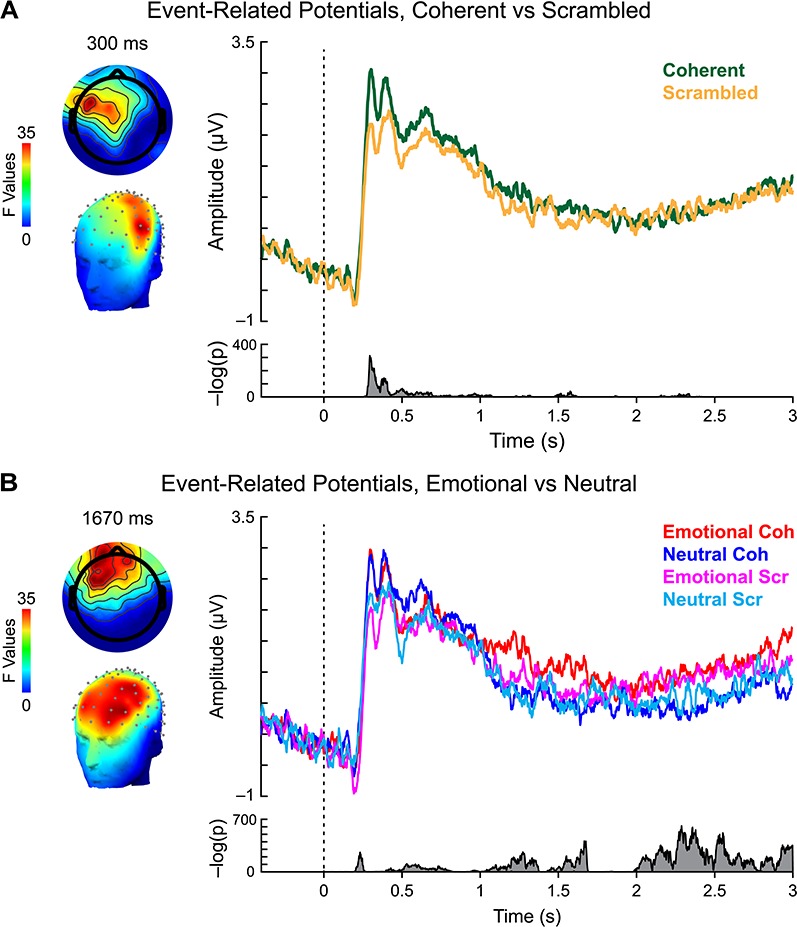
ERP analysis. (A) Main effect of action coherency. Left: scalp topography of significant activity for coherent vs scrambled movements, 300 ms after stimulus onset. Right: grand average waveforms for coherent (green) and scrambled (gold) PLDs. Bottom right: summed inverse P values across all sensors and time points revealed significant activity 288–330 ms after stimulus onset. (B) Main effect of content. Left: scalp topography of significant activity for emotional vs neutral movements, 1670 ms after stimulus onset. Right: grand average waveforms for emotional coherent (red), neutral coherent (blue), emotional scrambled (magenta) and neutral scrambled (cyan) PLDs. Bottom right: summed inverse P values across all sensors and time points revealed significant clusters of activity within our time window of interest, roughly 1288–1680 ms after stimulus onset.
A significant effect of action coherency (Figure 2A) emerged relatively early in the trial, from 288 to 330 ms after stimulus onset in left frontal and anterior temporal sensors (Figure 2A, left). Reflecting greater activity to coherent vs scrambled PLDs (Figure 2A, top right), the timing of this effect is largely consistent with latencies reported in prior ERP studies (for a review, see Thompson and Parasuraman, 2012). However, no significant effects were observed across the rest of the trial (Figure 2A, bottom right). Therefore, to the extent that coherent biological motion stimuli elicit additional perceptual and cognitive processing, these effects are unlikely to explain any observed differences in μ-suppression during the time window of interest (1–2 s post-stimulus onset).
In contrast, significant neural activity associated with content was observed across the time course of action perception (Figure 2B). Although the largest effects occurred ∼2 s after stimulus onset, significant clusters were visible within the time window of interest for μ-suppression effects, with a local peak significance of ∼1.67 s after stimulus onset (Figure 2B, bottom right). Examination of the evoked signals for each condition within this time window revealed this effect to be driven largely by the response to coherent emotional PLDs (Figure 2B, top right). Although the timing of this effect is similar to a previously reported slow-wave potential over centroparietal sensors that is associated with viewing of emotional pictures (Cuthbert et al., 2000), the response observed here differs notably both in terms of scalp topography (Figure 2B, left) and level of sustained activity, complicating the interpretation of this result. Nonetheless, the divergence of this ERP scalp topography from the canonical distribution of μ-suppression over central sensors suggests that cognitive processing indexed by this component may be separable from action simulation.
Time-frequency analysis: coherent vs scrambled action
Our first time-frequency analysis examined whether the comparison of coherent vs scrambled PLDs elicited significant μ-suppression over central sensors, as reported in previous studies. Averaging across power spectra for emotional and neutral stimuli, we then subtracted the 4–20 Hz power spectra for scrambled PLDs from the power spectra for coherent PLDs. If biologically plausible movements produce additional sensorimotor activity compared to non-biological motion, as posited by simulation theory, we should see significant reductions in power over central sensors within the alpha and/or beta frequency bands.
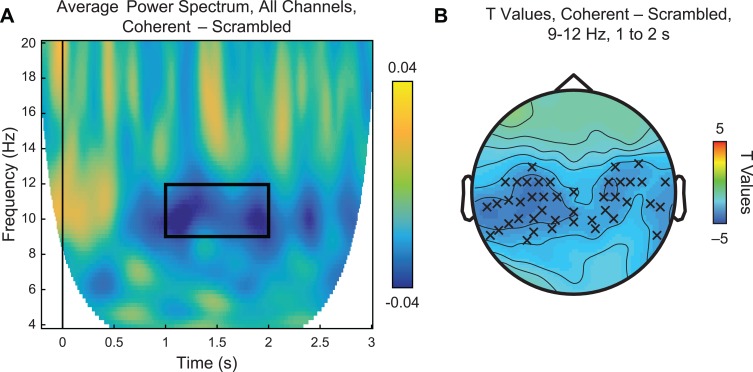
Figure 3 displays the difference in average power for coherent vs scrambled PLDs across all 128 channels. Consistent with previous reports of μ-suppression (e.g. Fox et al., 2016), we observed a clear band of spectral power suppression between ∼9 and 12 Hz from roughly 1 to 2 s after stimulus onset (Figure 3A, black box). Statistical analyses of this time and frequency window revealed significant reductions at central and temporal sensors roughly overlying sensorimotor cortex (Figure 3B), surviving cluster-based multiple-comparisons permutation correction at P = 0.03. Calculation of effect size using Cohen’s d with pooled standard deviation for within-subjects designs (Cohen, 1988; Morris and DeShon, 2002) likewise suggested moderate practical significance (d = –0.35). No significant effects were observed in the beta band for this contrast.
Fig. 3.
Main effect of action coherency. (A) Time-frequency plot of average power spectrum from 4 to 20 Hz for coherent–scrambled PLDs across all 128 channels. In the time window between 1 and 2 s, we observed reduced power within the alpha band of 9–12 Hz (black box). (B) Statistical analysis of the highlighted time and frequency window using a paired samples t-test revealed significant suppression (P < 0.05, permutation-corrected for multiple comparisons) in centroparietal and temporal sensors, as indicated by Xs.
Time-frequency analysis: emotional vs neutral movement
To assess the main experimental question of whether emotional movements elicit greater μ-suppression, we next compared spectral power for emotional and neutral content conditions after controlling for low-level motion information. Thus, this analysis used a difference of differences, or double subtraction, in which we first subtracted the response to the scrambled PLDs (‘s’) from the response to the equivalent coherent versions (‘c’): (EmotionalC–S = EmotionalCoherent – EmotionalScrambled; NeutralC–S = NeutralCoherent – NeutralScrambled) and then compared the two (EmotionalC–S – NeutralC–S).
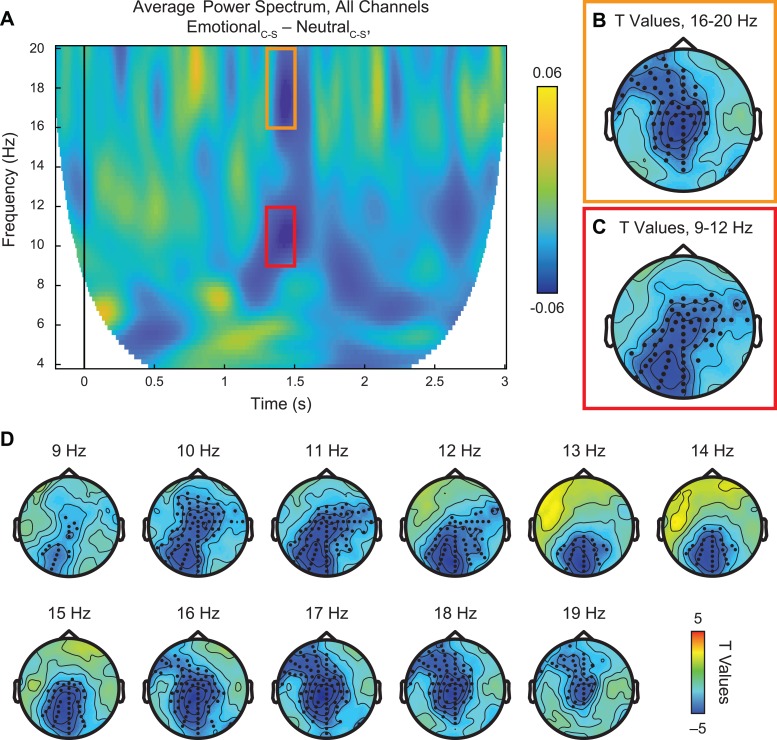
As shown in Figure 4A, this analysis identified a more focal time window of suppression in the alpha (Figure 4A, red box) and beta (Figure 4A, orange box) frequency bands, from ∼1.3 to 1.5 s after stimulus onset. Examining the specific ranges associated with spectral peaks for μ-alpha and beta, we found differential distribution of significant suppression effects (Figure 4B and C), surviving cluster-corrected multiple-comparisons correction at P < 0.01 (d = –0.6).
Fig. 4.
Neural response to emotional vs neutral PLDs, controlling for low-level visual properties. (A) Time-frequency plot of average power spectrum from 4 to 20 Hz across all 128 channels for the double subtraction of (EmotionalCoherent – EmotionalScrambled) – (NeutralCoherent – NeutralScrambled). Within the time window of ∼1.3–1.5 s after stimulus onset, we identified two major suppression effects in the alpha (9–12 Hz, red box) and central beta (16–20 Hz, orange box) frequency bands. (B–C) Statistical analysis using paired samples t-test revealed significant suppression effects (P < 0.01, permutation-corrected) in the beta (B) and alpha (C) frequency bands at frontocentral and centroparietal/occipital sites, respectively, as indicated by black dots. (D) Scalp plots of significant t values across all frequencies from 4 to 20 Hz, 1.3 to 1.5 s after stimulus onset. This less constrained statistical test across all frequencies found major effects between 9 and 20 Hz, in line with the targeted results from alpha and beta bands.
Whereas power reductions in the beta band (16–20 Hz) were found over central and frontal sensors and were most prominent at central locations (Figure 4B), μ-suppression in the canonical 9–12 Hz range was distributed over central and occipital sensors (Figure 4C). Examining the full range of frequencies from 4 to 20 Hz within this time window confirmed these findings, with significant reductions in power (cluster-corrected P < 0.01) emerging from ∼9 to 20 Hz and progressing from occipital and central sensors to more frontal electrodes with increasing frequency (Figure 4D).
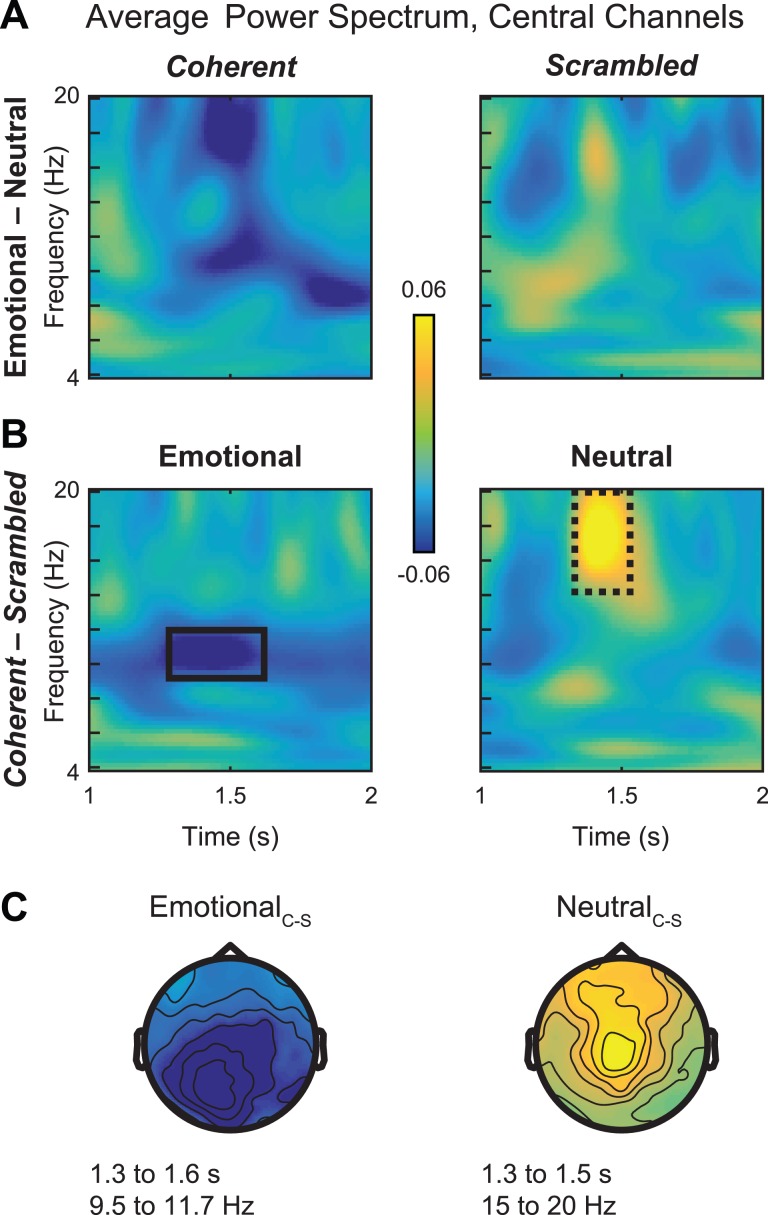
Given the complex nature of the double subtraction comparison, a further question is which conditions are driving the overall effect. Therefore, as a follow-up, we also plotted the simple subtractions of emotional – neutral in the coherent (Figure 5A, left) and scrambled (Figure 5A, right) conditions from 1 to 2 s. As expected, reductions in power were clearly visible within the time window of interest from 1.3 to 1.5 s for coherent stimuli but not for scrambled stimuli. This finding supports the idea that differential μ-suppression to emotional vs neutral stimuli reflects cognitive processes rather than low-level visual differences between the stimuli.
Fig. 5.
Simple comparisons of μ-suppression effects in alpha and beta bands. (A) Average power spectrum across central sensors for contrast of emotional–neutral in the coherent (left) and scrambled (right) conditions. Alpha and beta suppression effects are most strongly apparent in coherent PLDs, supporting the idea that this activity reflects action simulation rather than low-level visual properties. (B) Average power spectrum across central sensors for contrast of coherent–scrambled in the emotional (left) and neutral (right) PLD conditions. Whereas EmotionalCoherent stimuli are associated with alpha-band suppression between 9 and 12 Hz (solid black box, left), beta ‘suppression’ in the double subtraction reflects an enhancement of beta activity to NeutralCoherent PLDs (dashed black box, right). (C) Scalp topographies of these effects display alpha-band suppression for EmotionalC–S over occipital and centroparietal sites (left) and beta-band enhancement for NeutralC–S over frontocentral sites (right), matching the topography of the double subtraction in Figure 3.
However, this analysis does not specify the direction of these significant suppression effects with respect to the EmotionalC–Svs NeutralC–S comparisons. Therefore, in simple subtractions, we directly compared the response to emotional and neutral stimuli while controlling for low-level visual factors by subtracting EmotionalCoherent – EmotionalScrambled (Figure 5B, left). Consistent with previous reports of μ-alpha suppression, coherent vs scrambled emotional body movements were associated with decreased power between 9 and 12 Hz (Figure 5B, left, black box). These effects were strongest over central and occipital sensors (Figure 5C, left), in line with the results of the double subtraction; in contrast, no beta suppression was observed in this contrast. Surprisingly, however, comparison of NeutralCoherent – NeutralScrambled revealed enhanced power in the beta range between 1.3 and 1.5 s (Figure 5B, right, dashed black box) and this effect was strongest for central and frontal electrodes (Figure 5C, right). Thus, despite similar underlying sources, μ-suppression effects in the alpha and beta bands may reflect dissociable cognitive processes that are differentially influenced by emotional stimulus content.
Individual differences analysis: median-split by AQ
The above results are consistent with the idea that μ-suppression indexes action simulation, and this simulation is enhanced by emotional content. To help interpret this finding, we examined whether μ-suppression varies with autistic tendencies, given that ASD has previously been correlated with reduced μ-suppression. Although our sample may be underpowered for individual difference tests, we performed an exploratory analysis based on AQ scores (Baron-Cohen et al., 2001). Because of the relatively small sample size and restricted range of AQ scores within our sample, we expected that power would be an issue for a correlational analysis; therefore, we opted to perform a median-split analysis on the data. Across participants, the mean AQ score was 19.3 (median = 19.5, s.d. = 5.13), within the typical range of social functioning (i.e. 11–21). Dividing participants using a median-split, we obtained two groups with low AQ (mean = 15.4, s.d. = 2.99) and high AQ (mean = 23.3, s.d. = 3.51), associated respectively with neurotypical performance and above-average autistic tendencies.
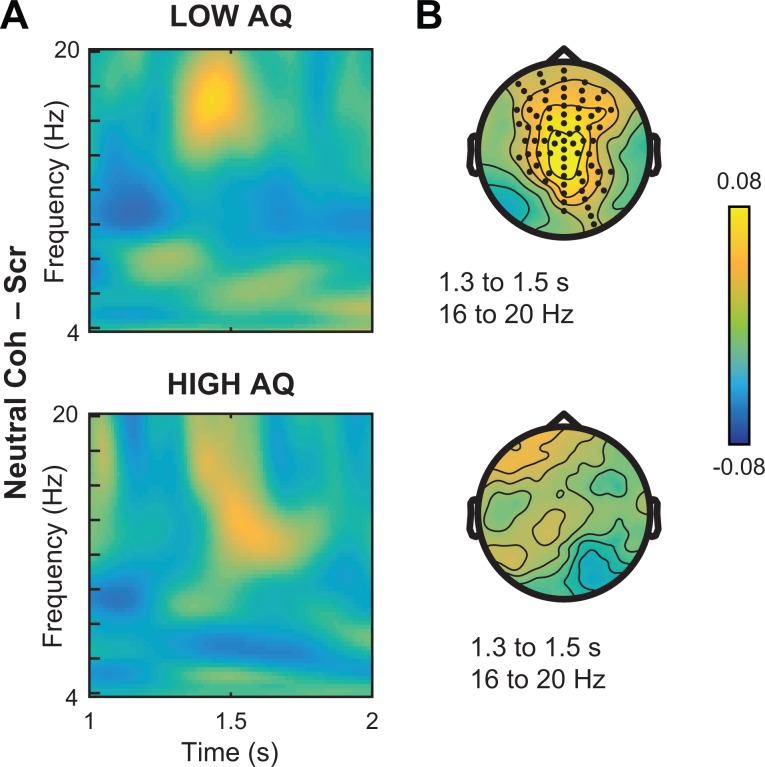
Comparing the neural responses to emotional actions in these two groups, we observed striking differences in the extent of alpha-band suppression associated with coherent vs scrambled stimuli (Figure 6A). Whereas the low AQ group showed extensive reductions in power across the window from 1 to 2 s (Figure 6A, top), μ-suppression effects were weaker in the high-AQ group (Figure 6A, bottom). Additionally, these effects varied in their scalp distribution, with the low-AQ group showing significant effects across central, parietal and temporal sensors (Figure 6B, top, white dots; P = 0.002, d = –0.86). In contrast, alpha-band suppression in the high-AQ group was concentrated over occipital sensors, though the within-group EmotionalC–S contrast did not reach significance at any sensor location (Figure 6B, bottom). To directly assess these group-level differences, we developed an index of μ-alpha suppression by defining sensors of interest (SOIs) using the significant sensors from the initial contrast of coherent – scrambled across both emotional and neutral stimuli, computing the average power between 9 and 12 Hz from 1 to 2 s after stimulus onset. Comparing power in these ‘μ-alpha SOIs’ to that over occipital electrodes within the same time range revealed higher suppression at μ-alpha SOIs in the low-AQ group, despite similar levels of occipital alpha suppression (Figure 6C). Confirming this effect, we found a significant interaction of group by location [t(36) = –2.18, P = 0.036, d = –0.71]. Again, these effects were dissociable from the enhancement of beta activity for neutral actions (Figure 7), which was strongest in the low-AQ group (P = 0.0001, d = 0.97), suggesting that oscillations in the alpha and beta bands contribute differentially to action simulation processes.
Fig. 6.
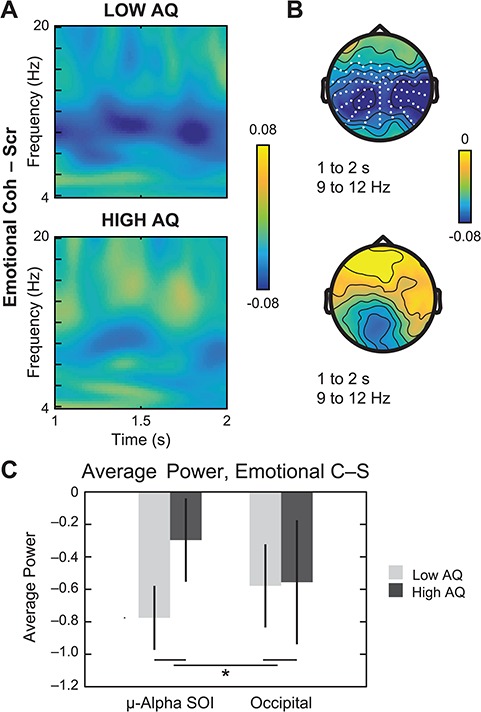
Neural response to emotional PLDs median-split by AQ. (A) Simple comparison of EmotionalCoherent – EmotionalScrambled in participants with population-typical scores (‘Low AQ’, top) vs autistic tendencies (‘High AQ’, bottom). Across the time window from 1 to 2 s, suppression effects in the alpha band (9 to 12 Hz) are more pronounced in the low-AQ subgroup. (B) Paired samples t-tests in the 9–12 Hz frequency range for the low-AQ group (top) revealed significant suppression effects across central, temporal and parietal sites (white dots, permutation-corrected P < 0.005), whereas suppression over occipital sensors was not significant in the high-AQ subgroup (bottom). (C) Direct comparison of average power from 9 to 12 Hz and 1 to 2 s at sensors associated with the main effect of coherent–scrambled (‘μ-Alpha SOI’) vs occipital sites for low vs high AQ. Error bars indicate standard error of the mean.
Fig. 7.
Neural response to neutral PLDs median-split by AQ. (A) Simple comparison of NeutralCoherent – NeutralScrambled in participants with population-typical scores (‘Low AQ’, top) vs autistic tendencies (‘High AQ’, bottom). As in the group data, we observed beta enhancement between ∼1.3 and 1.5 s after stimulus onset, with a stronger effect in the low-AQ subgroup. (B) Paired samples t-tests in the 16–20 Hz frequency range for the low-AQ group (top) found significant effects in frontocentral sensors (black dots, permutation-corrected P < 0.001). In contrast, no significant effects for this time or frequency range were observed for the high-AQ subgroup.
Discussion
Although μ-suppression is often cited in support of simulation theory, little work has focused on the selective effects of emotional actions on μ-power. Further, many studies of μ-suppression conflate perceptual, attentional and action simulation processes, making it difficult to disentangle which aspects of emotion might drive reductions in oscillatory power. Here, we more effectively isolated μ-rhythms specific to action simulation processes by implementing recent methodological recommendations, such as using high-density EEG and controlling for perceptual and attentional confounds (Fox et al., 2016; Hobson and Bishop, 2016; Bowman et al., 2017). With these controls, we successfully replicated previous reports of greater μ-alpha suppression for coherent vs scrambled biological motion from PLDs (Ulloa and Pineda, 2007). Further, we identified ERP evidence for early cognitive differences in processing coherent vs scrambled stimuli. However, they occur substantially prior to our window of interest (1–2 s) for μ-alpha suppression effects, suggesting that the sustained process of action-related simulation is likely separable from these more immediate perceptual responses to coherent biological stimuli.
Simultaneously mapping power changes over the whole head for emotional vs neutral stimuli, we found that μ-alpha suppression over central sites was associated with the perception of emotional content, supporting our hypothesis that action-related simulation processes are additionally engaged by emotional content in others’ movements. At the same time, we observed alpha suppression over occipital sensors even after controlling for low-level visual properties, supporting the idea that low-level visual features alone cannot explain the attentional salience of emotional content (Nummenmaa et al., 2006).
Given the inherent salience of emotional stimuli, effects of emotion and attentional processing are inevitably difficult to completely dissociate. However, the lack of performance differences in an attentionally demanding one-back task, along with the markedly different scalp distribution of the ERP waveform and μ-alpha suppression, suggest that the time-frequency effects cannot be wholly accounted for by differences in motivated attention associated with emotional stimuli.
Although many studies of μ-suppression focus on the alpha band, we also found significant effects in the beta band, from ∼16 to 20 Hz, distributed over frontocentral sensors. Simple subtractions of coherent vs scrambled stimuli revealed effects for emotional stimuli in the canonical μ-alpha (9–12 Hz) range, whereas these beta-band effects appeared to arise instead from enhancement of the response to coherent emotionally neutral actions. In contrast to the extensive literature on μ-suppression, beta enhancement is more difficult to explain. Beta enhancement has been previously reported for inhibition of motor responses in GO-NO GO tasks (Zaepffel et al., 2013). In studies of sensorimotor simulation, beta enhancement or event-related synchronization is often described as a ‘rebound’ effect, because it is seen as a brief increase in power that follows a period of suppression during which motion is executed (Hari et al., 1998; Babiloni et al., 2002). However, beta power does not only passively reflect the lack of movement; rather, it has been shown to be relevant to the active maintenance of the current motor set or cognitive state (Gilbertson et al., 2005; Engel and Fries, 2010). Notably, Brinkman et al. (2014) reported differential roles of alpha vs beta oscillations in suppressing task-irrelevant regions vs inhibiting movement parameter computations.
Consistent with this idea, our finding of differential effects of coherency for emotional and neutral stimuli suggests that beta and μ-alpha are not interchangeable measures of sensorimotor simulation, as often assumed in previous μ-suppression studies (Hobson and Bishop, 2017). Rather, μ-alpha and beta may reflect dissociable cognitive processes that are involved in the perception of different types of body movement. One clue regarding the nature of this distinction comes from recent evidence that μ-alpha reflects tactile rather than motor aspects of mirroring (Coll et al., 2015; Coll et al., 2017). Consistent with this idea, μ-alpha and beta power are inversely related to functional magnetic imaging (fMRI) signal in primary somatosensory and motor cortex, respectively (Ritter et al., 2009). Although the lack of a tactile component to our stimuli complicates any direct comparison between these results, it is interesting to note that our μ-suppression effects appear to be driven by perception of emotional content, which is known to engage somatosensory networks (e.g. Adolphs et al., 2000). In this light, we can hypothesize that μ-suppression in the alpha band represents somatosensory aspects of emotion simulation, whereas beta enhancement maintains action simulation processes while inhibiting motor output.
Yet, if beta enhancement is tied to motor inhibition, why did emotional body movements fail to elicit this processing? One possible answer arises from the evolutionary significance of emotional, as opposed to neutral, body movements. While it is appropriate to inhibit unwanted movement and maintain the status quo in response to neutral stimuli, inhibiting automatic motor responses may be less desirable when confronted with emotional stimuli. Because automatic facial and bodily responses to emotional stimuli can serve social goals (Reichmann-Decker et al., 2009; Moody et al., 2017), it may be disadvantageous for the sensorimotor system to inhibit potentially beneficial automatic approach/avoidance responses to the intensely affective bodily movement of others.
Furthermore, the emotional body movement of others may be more difficult to mimic because emotional expressions are idiosyncratic (Wilbarger et al., 2011). Therefore, there may be less need for an effortful suppression of an alternative motor state. Conversely, common actions such as touching one’s toes are performed in a similar manner by most individuals. As such, neutral actions may more easily activate automatic motor programs which would then have to be inhibited. The increase in beta power may reflect this inhibition of physical movement in order to maintain the current state of mental simulation.
If this mental simulation of others’ body movements allows us to better understand their emotional states, then it follows that individuals who are less sensitive to social cues may exhibit reduced neural correlates of action simulation. To address this question, we conducted an exploratory analysis examining alpha and beta power for two subgroups median-split by AQ, a behavioral measure of emotional intelligence and receptivity to social information. Looking at the neural response to emotional stimuli, we found that whereas the high- and low-AQ groups did not differ in terms of occipital alpha, the population-typical low-AQ group exhibited more pronounced μ-alpha suppression over centroparietal SOIs. Consistent with previous results from clinical populations with ASD (Oberman et al., 2005; Oberman and Ramachandran, 2007; Dumas et al., 2014), these results suggest that autistic tendencies are associated with selectively lower simulation activity for emotional content, even when occipitoparietal alpha-band activity suggests similar levels of attentional engagement.
At the same time, we observed strongest beta enhancement for neutral actions in the low-AQ group. Notably, this effect showed high statistical and practical significance, as indicated by a low P value and high effect size, despite having only half the sample size of the original experiment. Thus, rather than reflecting variability in simulation processes associated with autistic tendencies, these results confirm that, like μ-alpha, the beta effects are driven by ‘normal’ receptiveness to social information from the movements of others.
However, it is important to note that the AQ is not a clinical measure, but rather a preliminary tool for identifying traits associated with ASD in adults with normal intelligence (Baron-Cohen et al., 2001). Within our undergraduate sample, only one participant (AQ = 34) had an AQ score above the cut off indicating high likelihood of diagnosable ASD. Therefore, it is possible we would observe more pronounced differences in μ-alpha and beta effects in clinically diagnosed ASD populations.
Collectively, these results demonstrate that emotional content in body movements elicits higher levels of alpha-band μ-suppression, whereas increased power in the beta range reflects dissociable responses to neutral, rather than emotional, coherent actions. Consistent with theories of embodied emotion, these data support a link between simulation and social perception while more firmly connecting emotional processing to the activity of sensorimotor systems. In line with recent data identifying dissociations between sensorimotor processes in the alpha and beta bands, future research should further investigate the computational implications of separable alpha suppression and beta enhancement effects during action simulation.
References
- Adolphs R., Damasio H., Tranel D., Cooper G., Damasio A.R. (2000). A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. Journal of Neuroscience, 20(7), 2683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson A.P., Dittrich W.H., Gemmell A.J., Young A.W. (2004). Emotion perception from dynamic and static body expressions in point-light and full-light displays. Perception, 33(6), 717–46. [DOI] [PubMed] [Google Scholar]
- Atkinson A.P., Vuong Q.C., Smithson H.E. (2012). Modulation of the face- and body-selective visual regions by the motion and emotion of point-light face and body stimuli. Neuroimage, 59(2), 1700–12. [DOI] [PubMed] [Google Scholar]
- Babiloni C., Babiloni F., Carducci F., et al. (2002). Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. Neuroimage, 17(2), 559–72. [PubMed] [Google Scholar]
- Bannerman R.L., Milders M., Gelder B., Sahraie A. (2009). Orienting to threat: faster localization of fearful facial expressions and body postures revealed by saccadic eye movements. Proceedings. Biological Sciences, 276(1662), 1635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. (2001). The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Development Disorders, 31(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Barsalou L.W. (2008). Grounded cognition. Annual Review of Psychology, 59, 617–45. [DOI] [PubMed] [Google Scholar]
- Beall P.M., Moody E.J., McIntosh D.N., Hepburn S.L., Reed C.L. (2008). Rapid facial reactions to emotional facial expressions in typically developing children and children with autism spectrum disorder. Journal of Experimental Child Psychology, 101(3), 206–23. [DOI] [PubMed] [Google Scholar]
- Belouchrani A., Abed-Meraim K., Cardoso J.-F., Moulines E. (1997). A blind source separation technique using second-order statistics. IEEE Transactions on Signal Processing, 45(2), 434–44. [Google Scholar]
- Blake R., Shiffrar M. (2007). Perception of human motion. Annual Review of Psychology, 58, 47–73. [DOI] [PubMed] [Google Scholar]
- Bowman L.C., Bakermans-Kranenburg M.J., Yoo K.H., et al. (2017). The mu-rhythm can mirror: insights from experimental design, and looking past the controversy. Cortex, 96, 121–5. [DOI] [PubMed] [Google Scholar]
- Brinkman L., Stolk A., Dijkerman H.C., Lange F.P., Toni I. (2014). Distinct roles for alpha- and beta-band oscillations during mental simulation of goal-directed actions. Journal of Neuroscience, 34(44), 14783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical Power Analysis for the Behavioral Sciences, 2nd edn, Hillsdale: Erlbaum Associates. [Google Scholar]
- Coll M.P., Bird G., Catmur C., Press C. (2015). Cross-modal repetition effects in the mu rhythm indicate tactile mirroring during action observation. Cortex, 63, 121–31. [DOI] [PubMed] [Google Scholar]
- Coll M.P., Press C., Hobson H., Catmur C., Bird G. (2017). Crossmodal classification of mu rhythm activity during action observation and execution suggests specificity to somatosensory features of actions. Journal of Neuroscience, 37(24), 5936–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. [DOI] [PubMed] [Google Scholar]
- Gelder B. (2006). Towards the neurobiology of emotional body language. Nature Reviews. Neuroscience, 7(3), 242–9. [DOI] [PubMed] [Google Scholar]
- Decety J. (1996). Do imagined and executed actions share the same neural substrate? Brain Research. Cognitive Brain Research, 3(2), 87–93. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Dimberg U., Thunberg M. (1998). Rapid facial reactions to emotional facial expressions. Scandinavian Journal of Psychology, 39(1), 39–45. [DOI] [PubMed] [Google Scholar]
- Dittrich W.H., Troscianko T., Lea S.E., Morgan D. (1996). Perception of emotion from dynamic point-light displays represented in dance. Perception, 25(6), 727–38. [DOI] [PubMed] [Google Scholar]
- Dumas G., Soussignan R., Hugueville L., Martinerie J., Nadel J. (2014). Revisiting mu suppression in autism spectrum disorder. Brain Research, 1585, 108–19. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P. (2010). Beta-band oscillations—signalling the status quo? Current Opinion in Neurobiology, 20(2), 156–65. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Bakermans-Kranenburg M.J., Yoo K.H., et al. (2016). Assessing human mirror activity with EEG mu rhythm: a meta-analysis. Psychological Bulletin, 142(3), 291–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V., Goldman A. (1998). Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences, 2(12), 493–501. [DOI] [PubMed] [Google Scholar]
- Gilbertson T., Lalo E., Doyle L., Di Lazzaro V., Cioni B., Brown P. (2005). Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. Journal of Neuroscience, 25(34), 7771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman E.D., Blake R. (1999). Perception of coherent motion, biological motion and form-from-motion under dim-light conditions. Vision Research, 39(22), 3721–7. [DOI] [PubMed] [Google Scholar]
- Guntekin B., Basar E. (2014). A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia, 58, 33–51. [DOI] [PubMed] [Google Scholar]
- Hari R., Forss N., Avikainen S., Kirveskari E., Salenius S., Rizzolatti G. (1998). Activation of human primary motor cortex during action observation: a neuromagnetic study. Proceedings of the National Academy of Sciences of the United States of America, 95(25), 15061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield E., Cacioppo J.T., Rapson R.L. (1993). Emotional Contagion: Current Directions in Psychological Science, 2(3), 96–100. [Google Scholar]
- Hobson H.M., Bishop D.V. (2017). The interpretation of mu suppression as an index of mirror neuron activity: past, present and future. Royal Society Open Science, 4(3), 160662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson H.M., Bishop D.V.M. (2016). Mu suppression—a good measure of the human mirror neuron system? Cortex, 82, 290–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M. (2001). Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage, 14(1), S103–9. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. (2006). Motor Cognition: What Actions Tell the Self, Oxford: Oxford University Press. [Google Scholar]
- Jung T.P., Makeig S., Humphries C., et al. (2000). Removing electroencephalographic artifacts by blind source separation. Psychophysiology, 37(2), 163–78. [PubMed] [Google Scholar]
- Kilner J.M., Marchant J.L., Frith C.D. (2006). Modulation of the mirror system by social relevance. Social Cognitive and Affective Neuroscience, 1(2), 143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S.J. (2014). An Introduction to the Event-Related Potential Technique. MIT Press. [Google Scholar]
- Macmillan N.A., Creelman C.D. (2004). Detection Theory: A User's Guide, New York, NY: Psychology Press. [Google Scholar]
- McFarland D.J., Miner L.A., Vaughan T.M., Wolpaw J.R. (2000). Mu and beta rhythm topographies during motor imagery and actual movements. Brain Topography, 12(3), 177–86. [DOI] [PubMed] [Google Scholar]
- McIntosh D.N., Reichmann-Decker A., Winkielman P., Wilbarger J.L. (2006). When the social mirror breaks: deficits in automatic, but not voluntary, mimicry of emotional facial expressions in autism. Developmental Science, 9(3), 295–302. [DOI] [PubMed] [Google Scholar]
- Mensen A., Khatami R. (2013). Advanced EEG analysis using threshold-free cluster-enhancement and non-parametric statistics. Neuroimage, 67, 111–8. [DOI] [PubMed] [Google Scholar]
- Moody E.J., McIntosh D.N. (2006). Bases and consequences of rapid, automatic matching behavior. In: Imitation and the Social Mind: Autism and Typical Development, Vol. 71 New York, NY: Guilford Press. [Google Scholar]
- Moody E.J., McIntosh D.N., Mann L.J., Weisser K.R. (2007). More than mere mimicry? The influence of emotion on rapid facial reactions to faces. Emotion, 7(2), 447–57. [DOI] [PubMed] [Google Scholar]
- Moody E.J., Reed C.L., Bommel T., App B., McIntosh D.N. (2017). Emotional Mimicry Beyond the Face? Rapid Face and Body Responses to Facial Expressions. Social Psychological and Personality Science, 10.1177/1948550617726832. [DOI] [Google Scholar]
- Moore A., Gorodnitsky I., Pineda J. (2012). EEG mu component responses to viewing emotional faces. Behavioural Brain Research, 226(1), 309–16. [DOI] [PubMed] [Google Scholar]
- Moore M.R., Franz E.A. (2017). Mu rhythm suppression is associated with the classification of emotion in faces. Cognitive, Affective and Behavioral Neuroscience, 17(1), 224–34. [DOI] [PubMed] [Google Scholar]
- Morris S.B., DeShon R.P. (2002). Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods, 7(1), 105–25. [DOI] [PubMed] [Google Scholar]
- Niedenthal P.M. (2007). Embodying emotion. Science, 316(5827), 1002–5. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E., Silva F.L. (2005) Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Nummenmaa L., Hyönä J., Calvo M.G. (2006). Eye movement assessment of selective attentional capture by emotional pictures. Emotion, 6(2), 257–68. [DOI] [PubMed] [Google Scholar]
- Oberman L.M., Hubbard E.M., McCleery J.P., Altschuler E.L., Ramachandran V.S., Pineda J.A. (2005). EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Research. Cognitive Brain Research, 24(2), 190–8. [DOI] [PubMed] [Google Scholar]
- Oberman L.M., Ramachandran V.S. (2007). The simulating social mind: the role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychological Bulletin, 133(2), 310–27. [DOI] [PubMed] [Google Scholar]
- Oberman L.M., Winkielman P., Ramachandran V.S. (2009). Slow echo: facial EMG evidence for the delay of spontaneous, but not voluntary, emotional mimicry in children with autism spectrum disorders. Developmental Science, 12(4), 510–20. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. (2011). FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet C., Latinus M., Nichols T., Rousselet G. (2015). Cluster-based computational methods for mass univariate analyses of event-related brain potentials/fields: a simulation study. Journal of Neuroscience Methods, 250, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A., Bentin S., Bartal I.B., Lamm C., Decety J. (2010). ‘Feeling’ the pain of those who are different from us: modulation of EEG in the mu/alpha range. Cognitive, Affective and Behavioral Neuroscience, 10(4), 493–504. [DOI] [PubMed] [Google Scholar]
- Pineda J.A., Hecht E. (2009). Mirroring and mu rhythm involvement in social cognition: are there dissociable subcomponents of theory of mind? Biological Psychology, 80(3), 306–14. [DOI] [PubMed] [Google Scholar]
- Reichmann-Decker A., DePrince A.P., McIntosh D.N. (2009). Affective responsiveness, betrayal, and childhood abuse. Journal of Trauma and Dissociation, 10(3), 276–96. [DOI] [PubMed] [Google Scholar]
- Ritter P., Moosmann M., Villringer A. (2009). Rolandic alpha and beta EEG rhythms' strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Human Brain Mapping, 30(4), 1168–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Fogassi L., Gallese V. (2001). Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews. Neuroscience, 2(9), 661–70. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S., Kleinschmidt A. (2016). Brain networks and alpha-oscillations: structural and functional foundations of cognitive control. Trends in Cognitive Sciences, 20(11), 805–17. [DOI] [PubMed] [Google Scholar]
- Tang A.C., Liu J.Y., Sutherland M.T. (2005). Recovery of correlated neuronal sources from EEG: the good and bad ways of using SOBI. Neuroimage, 28(2), 507–19. [DOI] [PubMed] [Google Scholar]
- Thompson J., Parasuraman R. (2012). Attention, biological motion, and action recognition. Neuroimage, 59(1), 4–13. [DOI] [PubMed] [Google Scholar]
- Ulloa E.R., Pineda J.A. (2007). Recognition of point-light biological motion: mu rhythms and mirror neuron activity. Behavioural Brain Research, 183(2), 188–94. [DOI] [PubMed] [Google Scholar]
- Wilbarger J.L., Reed C.L., McIntosh D.N. (2011). Implicit influence of affective postures on the perception of others: you can't show me how I feel. Emotion, 11(3), 481–91. [DOI] [PubMed] [Google Scholar]
- Wood A., Rychlowska M., Korb S., Niedenthal P. (2016). Fashioning the face: sensorimotor simulation contributes to facial expression recognition. Trends in Cognitive Sciences, 20(3), 227–40. [DOI] [PubMed] [Google Scholar]
- Zaepffel M., Trachel R., Kilavik B.E., Brochier T. (2013). Modulations of EEG beta power during planning and execution of grasping movements. PLoS One, 8(3), e60060. [DOI] [PMC free article] [PubMed] [Google Scholar]