Abstract
Growing evidence of transcranial alternating current stimulation (tACS) modulating intrinsic neural oscillations has spawned interest in applying tACS to treat psychiatric disorders associated with aberrant neural oscillations. The alpha rhythmic activity is known to dominate neural oscillations at the awake, restful state, while attenuated resting-state alpha activity has been implicated in anxious mood. Administering repeated alpha-frequency tACS (α-tACS; at individual peak alpha frequency; 8–12 Hz) over four consecutive days (in the experiment group, sham stimulation in the control group), we demonstrated immediate and lasting (>24 h) increases in resting-state posterior ➔frontal connectivity in the alpha frequency, quantified by Granger causality. Critically, this connectivity enhancement was accompanied by sustained reductions in both anxious arousal and negative perception of sensory stimuli. Resting-state alpha power also increased, albeit only transiently, reversing to the baseline level within 24 h after tACS. Therefore, the lasting enhancement of long-range alpha connectivity due to α-tACS differs from local alpha activity that is nonetheless conserved, highlighting the adaptability of alpha oscillatory networks. In light of increasing recognition of large-scale network dysfunctions as a transdiagnostic pathophysiology of psychiatric disorders, this enduring connectivity plasticity, along with the behavioral improvements, paves the way for tACS applications in clinical interventions of psychiatric ‘oscillopathies’.
Keywords: alpha oscillations, resting state, Granger causality, neuromodulation, sensory affect
Introduction
Brain oscillations play important roles in various mental activities (Başar et al., 2001; Buzsaki et al., 2013). Aberrations in these oscillations have been observed in a host of neuropsychiatric disorders, potentially underpinning their pathophysiology (i.e. ‘oscillopathies’; Basar, 2013; Buzsaki et al., 2013). Recent advances in transcranial alternating current stimulation (tACS) demonstrate that by applying weak electric fields tuned to the rhythms of endogenous oscillations through the scalp, tACS can directly interact with and thereby modify brain oscillations (Frohlich and McCormick, 2010; Thut et al., 2011; Antal and Paulus, 2013; Herrmann et al., 2013). This non-invasive neuromodulation technology thus holds great promise for therapeutic interventions of oscillopathies (Frohlich, 2015; Abend et al., 2016). However, clinical efficacy of tACS hinges on whether its neuromodulatory effects would persist and induce lasting behavioral changes.
Alpha oscillations (8–12 Hz; posteriorly distributed) are the most prominent rhythmic activity in the awake restful human brain and are thought to be generated in the thalamo-cortical network (Hughes and Crunelli, 2005; Bollimunta et al., 2008; Buzsaki et al., 2013). Alpha oscillations exert largely inhibitory modulation on multiple cognitive processes, including attention and sensory perception (Palva and Palva, 2007; Bollimunta et al., 2008; Foxe and Snyder, 2011; Klimesch, 2012). For example, heightened pre-stimulus alpha power is associated with reduced perceptual sensitivity to the stimuli (Mathewson et al., 2009; Dugue et al., 2011), and attenuated alpha power is coupled with enhanced visual attention (Rajagovindan and Ding, 2011). Furthermore, via long-range, posterior ➔ frontal projections, alpha oscillations mediate inhibitory bottom-up information flow from the sensory cortex to frontal regions to influence global neural activity (Hillebrand et al., 2016; Sadaghiani et al., 2016; Johnson et al., 2017; Tang et al., 2017a). For instance, weak posterior ➔frontal alpha connectivity is associated with strong frontal gamma activity and impaired cognitive control (Clancy et al., 2017; Johnson et al., 2017).
Alpha oscillations are particularly important for inter-regional communication across large-scale resting-state networks (RSNs), especially the default mode network (DMN) and the salience network (SN; Laufs et al., 2003; Mantini et al., 2007; Sadaghiani et al., 2010; Mo et al., 2013; Tang et al., 2017a), which are strongly implicated in emotion- and arousal-related processes (Raichle et al., 2001; Sridharan et al., 2008) and affective disorders (Sripada et al., 2012; Peterson et al., 2014; Alvarez et al., 2015). Furthermore, akin to the reciprocal roles these two RSNs play in regulating arousal (Menon and Uddin, 2010; Young et al., 2017), alpha activity is positively correlated with DMN activity and negatively with SN activity (Sadaghiani et al., 2010; Ros et al., 2013), serving a key function in maintaining a state of restful alertness (Gath et al., 1983; Klimesch, 1999). Relatedly, neurofeedback training targeting alpha activity has been shown to modulate DMN and SN activities (Ros et al., 2013) and reduce anxiety (Hammond, 2005), while meditation training that leads to reduced anxious mood has been linked to augmentation of resting-state alpha activity and reorganization of these RSNs (Cahn and Polich, 2006; Fan et al., 2014; Tang et al., 2017b).
Consistent with growing evidence of DMN and SN dysfunctions in post-traumatic stress disorder (PTSD; Sripada et al., 2012), recent data from our laboratory revealed deficits in resting-state alpha power and posterior ➔frontal alpha connectivity in patients with PTSD (Clancy et al., 2017). Importantly, reduced alpha connectivity correlated with greater hypervigilance, a key hyperarousal symptom of PTSD. These findings thus implicate reduced alpha activity that translates to behavioral expressions of hypervigilance and anxious arousal, thereby presenting a concrete and spatiotemporally specific target for clinical intervention via alpha-frequency tACS (α-tACS). That is, provided alpha enhancement via α-tACS could persist, tACS could achieve stable anxiety reduction and clinically meaningful symptom alleviation.
Empirical and computational models suggest that while tACS induces short-term effects via entrainment of local neuronal firing synchrony, long-term plastic effects would arise from strengthened oscillatory circuits via spike-timing-dependent plasticity (STDP) and long-term potentiation (LTP) at the synapse (Thut et al., 2011; Herrmann et al., 2013; Reato et al., 2013). ‘Plasticity-like’ effects of tACS (primarily, local power enhancement outlasting the period of stimulation) have been consistently demonstrated (Neuling et al., 2013; Vossen et al., 2015; Kasten et al., 2016), but evidence for long-term effects remains limited. A recent α-tACS study tracked alpha power change over 90 min post-stimulation, but in contrast to enduring LTP that typically lasts for hours to days (Abraham, 2003), alpha power enhancement persisted only for 70 min post-stimulation (Kasten et al., 2016).
The acute but transient tACS effects could reflect the labile, early phase of LTP that is susceptible to other synaptic activities such as endogenous, corrective inputs from other neurons and changes in the extracellular environment (Abraham, 2003; Huang et al., 2017). Targeting primarily the cortex (Frohlich and McCormick, 2010; Thut et al., 2011; Herrmann et al., 2013), exogenous oscillatory inputs of most tACS applications rarely reach deep subcortical generators (Neuling et al., 2012). Since the thalamo-cortical circuit operates as a unified generator of alpha oscillations (Hughes and Crunelli, 2005; Bollimunta et al., 2008; Buzsaki et al., 2013), deep thalamic drives can thwart early-phase LTP at the synapses, resetting cortical alpha oscillations after α-tACS. That said, as repeated stimulation can facilitate the induction of late-phase LTP and long-term neural plasticity (Fregni et al., 2006; Reis et al., 2009; Huang et al.,2017), lasting tACS effects may emerge with multi-session (vs single-session reported thus far) tACS protocols.
Besides local cortical power increases, tACS, via the purported synaptic strengthening, is likely to induce inter-cortical connectivity plasticity. Indeed, tACS can intensify rhythmic reverberations across the abundant recurrent inter-cortical connections in the brain, thereby modifying large-scale network dynamics (Clopath et al., 2010; Alagapan et al., 2016; Moisa et al., 2016). This account would hold especially true for α-tACS given the instrumental role of alpha oscillations in long-range interactions (Palva and Palva, 2007; Cabral-Calderin et al., 2016). Importantly, while local cortical alpha oscillations can be restored by thalamic inputs, plasticity in inter-cortical connectivity, presumably relatively independent of thalamic drives, may persist over time and mediate lasting anxiety reduction.
Therefore, administering repeated sessions of high-density (HD) α-tACS targeting the occipitoparietal cortex implicated in PTSD-related alpha deficiency (Clancy et al., 2017; Figure 1), we set out to test two hypotheses. First, α-tACS could generate lasting enhancement of alpha activity, including alpha power and long-range connectivity, particularly posterior ➔frontal projections that dominate resting-state alpha connectivity (Engel et al., 2001; Tang et al., 2007; Sadaghiani et al., 2012; Hillebrand et al., 2016; Wang et al., 2016). Second, these neural changes would be accompanied by lasting anxiety reduction, measured here by anxious arousal and negative perception of sensory stimuli (in the auditory and olfactory modalities given their sensitivity to states of anxious arousal) (Adler et al., 1988; Grillon et al., 1991; Krusemark et al., 2013). Assessments were administered immediately and 30 min after α-tACS on the first and last (fourth) day, except for anxious arousal that was rated daily to track sustained and accumulating effects of α-tACS on this key outcome.
Fig. 1.
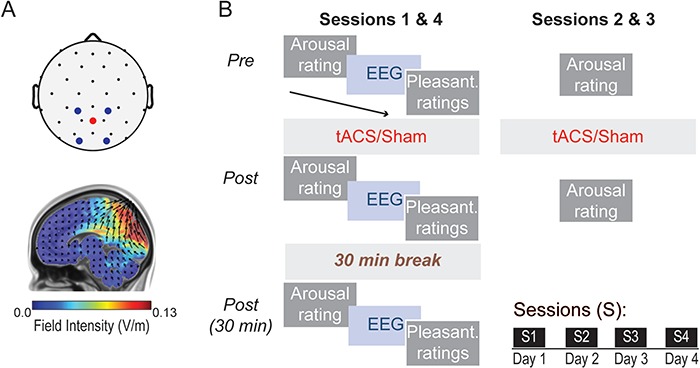
Stimulation setup and experimental protocol. (A) Top: tACS montage, with stimulation electrodes placed over occipitoparietal sites. Bottom: Modeled current flow of tACS, demonstrating maximal electrical field intensity over the occipitoparietal cortex. (B) Participants completed four sessions of tACS or sham stimulation, each separated by 24 h. tACS was administered for 30 min at each individual’s peak alpha frequency (PAF). Eyes-open resting state EEG data and ratings of anxious arousal and perceived pleasantness of auditory and olfactory stimuli were acquired before, immediately and 30 min after stimulation.
Methods
Participants
Thirty-eight healthy volunteers (18 female, 19.7 ± 2.0 years of age) participated in this study after providing written, informed consent approved by the Florida State University Institutional Review Board. No participants reported a history of neurological or psychiatric disorders and all were deemed tACS compatible (e.g. no metal implants, neurological surgery or pregnancy). Participants were randomly assigned to two groups, an Active (N = 21) and a Sham control group (N = 17). Four participants in the Active group did not provide behavioral ratings and were thus included in the electroencephalogram (EEG) analyses only. Groups did not differ in age or gender distribution (P > 0.14). Participants were blind to their group assignment (see tACS procedures below).
Ratings and questionnaires
Pleasantness ratings
Auditory and olfactory stimuli were rated for perceived pleasantness on a visual analog scale presented on a computer monitor (0–100 from most unpleasant to most pleasant; 50 = neutral). Auditory stimuli included a neutral (flat tone) and two negative (screaming and vomiting) sounds, delivered through headphones for one second each. Olfactory stimuli included a neutral (acetophenone; Fisher Scientific, NH, USA) and negative (burning rubber; ScentAir™, NC, USA) odors, delivered through a bottle. All stimuli were presented at three intensities: weak, medium and strong.
Subjective units of anxious arousal
Participants rated their current state of anxious arousal using a visual analog scale from 0 (not at all) to 100 (extremely), in accordance with standard ratings of subjective units of distress (Wolpe and Lazarus, 1966).
tACS
tACS was administered using a Soterix Medical 4 × 1 HD transcranial electrical stimulation system (New York, NY, USA).Stimulation electrodes were placed in a 4 × 1 montage over occipitoparietal sites, with the central electrode receiving electrical current from the four surrounding electrodes (Figure 1A). Stimulation sites were selected to maximally target the occipitoparietal area (cuneus/precuneus) where reduced alpha oscillations in patients with PTSD have been observed (Clancy et al., 2017). Current flow modeling (Soterix Medical HDExplore Software) indicated maximal electric field intensity (0.21 V/m; with a ±2 mA stimulation current) in the right dorsal occipital cortex (peak voxel: 29,−91, 18; Montreal Neurological Institute coordinates; Figure 1A). Electric field intensity did not exceed 0.02 V/m in frontal regions.
Stimulation was administered with eyes open for 30 min using a ±2 mA sinusoidal current oscillating at individual participants’ occipitoparietal PAF. The PAF was identified as the peak frequency within the alpha range (8–12 Hz) with a 0.5 Hz frequency resolution across occipitoparietal electrodes during the initial (session 1) baseline EEG recordings. Individual PAFs ranged from 8–11.5 Hz (M = 9.93, s.d. = 0.66 Hz) in the sample, equivalent between the two groups (P = 0.853). All participants were familiarized with tACS-induced skin sensations with a brief, 30 s pulse of random noise stimulation. During stimulation, the current ramped up to 2 mA over a span of 10 s. Stimulation was then discontinued in Sham participants and reintroduced during the last 10 s to mitigate awareness of experimental condition. All participants were told that they would receive stimulation, and their lack of awareness of sham stimulation was confirmed during the debriefing at the end of the study and by their responses to the Adverse Effects Questionnaire (Supplementary data).
Experimental design
The experiment consisted of four sessions, each separated by 24 h (Figure 1B). During the first and last sessions, participants started with a subjective rating of anxious arousal, followed by a 2 min resting, eyes-open EEG recording and then pleasantness ratings. Participants then received 30 min of α-tACS and then repeated a set of arousal ratings, resting EEG recordings and pleasantness ratings. After a 30 min break, participants again repeated a set of arousal ratings, resting EEG recordings and pleasantness ratings. The second and third sessions included α-tACS and pre-/post-stimulation ratings of anxious arousal.
EEG acquisition and analyses
EEG data were recorded from a 32-channel BrainProducts actiChamp system (Munich, Germany; 1000 Hz sampling rate, 0.05–200 Hz online bandpass filter, referenced to the FCz channel). Electro-oculogram (EOG) was recorded using four electrodes with vertical and horizontal bipolar derivations. EEG/EOG data were downsampled to 250 Hz, high-pass (1 Hz) and notch (60 Hz) filtered and re-referenced to the average of all EEG channels. We applied the ‘Fully Automated Statistical Thresholding for EEG artifact Rejection’ algorithm for artifact detection and rejection (Nolan et al., 2010).
Power analyses
EEG oscillation power was computed for individual channels for each epoch (1 s) using the multitaper spectral estimation technique (Mitra and Pesaran, 1999). Alpha (8–12 Hz) power was normalized by the mean power for the global spectrum (1–50 Hz) within each epoch. Alpha power was extracted from occipitoparietal (right, middle and left) electrodes, where they were maximally distributed (Palva and Palva, 2007; Foxeand Snyder, 2011; Klimesch, 2012).
Directed alpha-frequency connectivity (Granger causality) analyses
Alpha-frequency Granger causality (GC) analysis (Geweke, 1982; Ding et al., 2006) was performed to assess posterior ➔frontal causal connectivity in the alpha band. Following transformation into reference-free current source density data using the surface Laplacian algorithm (Perrin et al., 1989; Nunez et al., 1997; Wang et al., 2016), EEG data from ipsilateral frontal–posterior pairs were submitted to bivariate autoregressive (AR) modeling, from which GC spectra were derived (Ding et al., 2000, 2006). A model order of 20 (80 ms in time for a sampling rate of 250 Hz) was chosen in a two-step process: (i) Akaike Information Criterion (AIC) and (ii) comparing spectral estimates obtained by the AR model and by the Fourier-based method for data pooled across all subjects (Wang et al., 2016).
Statistical analyses
Three key effects were directly relevant to our hypotheses: (i) immediate and short-term (30 min) effects based on within-session changes (effect of Time); (ii) long-term effects based on differences between the initial and final sessions (i.e. effects lasting over 24 h from the preceding stimulation; effect of Session); and (iii) improved within-session tACS effectiveness, i.e. whether within-session effects increased from the initial to the final session (interaction of Time-by-Session). As such, we examined interactions of Group with Time and/or Session, in that these time/session effects differed between Active and Sham groups. Wherever appropriate, we started the analysis with an omnibus analysis of variance (ANOVA) and followed significant effects with post hoc tests, which were further controlled for Type I error using the false discovery rate (FDR) criterion.
Occipitoparietal alpha power and feedforward alpha GC at each time point (t) were decibel-normalized relative to the initial (i), first-session pre-tACS and baseline values [powerdb = 10*log10(powert/poweri); GCdb = 10*log10(GCt/GCi)] to control for variations in baseline activity (Cohen, 2014; Vossen et al., 2015; Kasten et al., 2016). Note that the groups did not differ in initial baseline alpha power or GC (P > 0.19). Decibel-normalized values were submitted to omnibus repeated-measures ANOVAs (rANOVAs) of Site (power: left, middle, right; GC: left, right hemisphere), Session (initial, final), Time (pre, post, post-30 minutes), and Group (active, sham). Site was included to consider previously reported effects of right-hemisphere laterality of alpha power on directed attention (Corbetta and Shulman, 2011; Rajagovindan and Ding, 2011) and connectivity deficits in PTSD (Clancy et al., 2017).
Pleasantness and anxious arousal ratings at each time point (t) were normalized using the difference-over-sum method to the initial (i) baseline [(ratingt − ratingi)/(ratingt + ratingi)] to account for variations in baseline ratings (Cohen and Maunsell, 2010). The groups did not differ in initial baseline ratings (P > 0.12). Pleasantness ratings were submitted to omnibus rANOVAs of Valence (neutral, threat), Intensity (strong, medium, weak), Session (initial, final), Time (pre, post, post-30 minutes), and Group. Ratings of anxious arousal were submitted to an omnibus rANOVA of Session (initial, second, third, final), Time (pre, post) and Group. As post-30 min anxious arousal ratings were acquired only in the first and last sessions and there was no difference between immediate and 30 min ratings in either sessions (P > 0.36), we averaged the immediate and 30 min ratings to reflect post-stimulation ratings for each session. Individual differences in trait anxiety are known to be associated with state anxiety and so can influence anxious arousal ratings (especially over repeated measurements across the 4 days). Therefore, we decided a priori to include scores of the Behavioral Inhibition Scale (BIS; Carver and White, 1994; a measure of trait anxiety) as a covariate in the ANOVA for anxiety ratings (not for other dependent variables).
Finally, a prospective examination of anxiety reduction on subsequent days (Days 2–4) as predicted by Day 1 alpha enhancement was conducted. This analysis was restricted to Day 1 alpha changes as the predictor because EEG was not acquired on Days 2 and 3 in order to ease the burden on participants. As the key outcome measure of the study, anxious arousal ratings were collected each day, which allowed for examination of long-term effects of Day 1 alpha and/or GC enhancement on subsequent anxiety levels. Specifically, we entered baseline shifts in anxious arousal on Days 2–4 (from Day 1) into an rANCOVA with Session (Sessions 2, 3 and 4) as an independent variable and Day 1 alpha activity change (power/GC) as a covariate. Additional correlational analyses on pleasantness ratings are reported in the Supplementary data.
To control for potential experimenter biases and to replicate the anxiety reduction effect, we conducted a double-blind, single-session replication study (on a smaller scale), explained in greater detail in the Supplementary data.
Results
tACS induced immediate and short-term increases in occipitoparietal alpha power
An omnibus rANOVA of Site (left, middle, right), Session (initial, final), Time (pre, post, post-30 min) and Group (active, sham) on posterior alpha power revealed a significant interaction of Time and Group (F1.99, 71.79 = 3.35, P = 0.041, ηp2 = 0.09), such that, across occipitoparietal sites and the two sessions, an effect of Time arose in the Active group (F1.55, 30.98 = 7.99, P = 0.003, ηp2 = 0.29) but not the Sham group (P = 0.485; Figure 2A). Follow-up contrasts within the Active group demonstrated power (collapsed across three posterior sites) increases both immediately (P = 0.0003) and 30 min (P = 0.006) after stimulation. A complementary bin-by-bin (0.25 Hz each) examination across the global frequency spectrum (1–50 Hz) revealed that this significant Time-by-Group interaction appeared selectively in the targeted alpha band (8–10.5 Hz; Figure 2A yellow line), confirming the specific modulation of alpha power. However, baselines for the two sessions did not differ in either group (P > 0.598), failing to indicate a long-term shift in alpha power (Figure 2B). Finally, there was no Session-by-Time-by-Group interaction (P = 0.268) such that the within-session effects did not change from the first to the last session. Overall, the results confirmed that α-tACS produced an immediate and specific increase in alpha power, lasting 30 min post-stimulation.
Fig. 2.
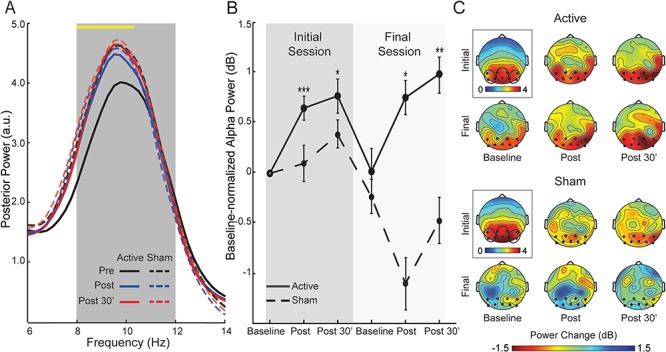
Changes in posterior alpha power. (A) Spectra of normalized power (collapsed across occipitoparietal electrodes) for each group at the three time points, averaged across the initial and final sessions. The yellow line at the top of the waveforms indicates frequency bins (0.25 Hz each) showing a significant Time-by-Group interaction. The interaction effect appeared selectively in the lower half of the targeted alpha range (8–10.5 Hz), confirming the specific modulation of alpha power. Note, the Active group demonstrated post-stimulation increases in the alpha band from the baseline while the Sham group demonstrated equivalent alpha power before and after stimulation. (B) Alpha power magnitudes, decibel-normalized to the initial baseline, for each group. (C) Scalp topographical maps of normalized alpha power at the initial baseline (surrounded by a black box and associated with its own color scale) and decibel-normalized power changes at each assessment thereafter. Electrodes included in occipitoparietal sites are bolded and encircled. Error bars = SEM. *P < 0.05; **P < 0.01; ***P < 0.005. All P-values are relative to the initial baseline. Note that the groups did not differ in the initial baseline values (P > 0.19).
tACS induced immediate, short-term and long-term increases in posterior ➔frontal alpha connectivity
A similar omnibus rANOVA of Site (right, left hemisphere), Session, Time, and Group on posterior ➔frontal alpha GC showed a significant four-way interaction of Site-by-Session-by-Time-by-Group (F1.98, 71.13 = 4.18, P = 0.020, ηp2 = 0.10). A follow-up ANOVA (Session-by-Time-by-Group) in the right hemisphere revealed a Session-by-Group interaction (F1, 36 = 7.71, P = 0.009, ηp2 = 0.18; Figure 3A), which was substantiated by a significant increase from the initial to the final session in the Active (F1, 20 = 12.11, P = 0.002, ηp2 = 0.38) but not Sham (P = 0.487) group. Furthermore, as illustrated in Figure 3B, relative to the initial baseline, GC at all subsequent assessments was augmented in the Active group (t’s < −2.28, P < 0.05 FDR corrected) but not in the Sham group (P > 0.190), reflecting immediate and sustained enhancement of connectivity via tACS. A bin-by-bin (0.25 Hz each) survey across the global frequency spectrum showed that this Session-by-Group interaction spanned almost the entire alpha band (8.25–12 Hz) but no other frequency band, supporting specific modulation of alpha-frequency GC. Importantly, the Active group (but not the Sham group, P = 0.225) demonstrated a significant baseline shift from the initial to the final session (F1, 20 = 18.46, P = 0.0004, ηp2 = 0.48), highlighting a long-term effect of tACS. Finally, there was no Session-by-Time-by-Group interaction (P = 0.147) such that the within-session effects did not change with repeated stimulation.
Fig. 3.
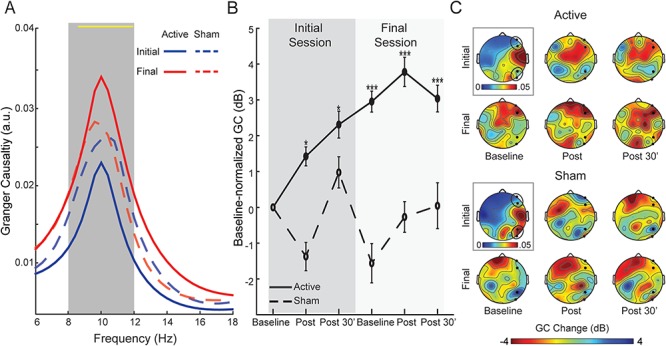
Changes in posterior ➔frontal GC. (A) Spectra of right-hemisphere posterior ➔frontal GC for each group (averaged across time for simplicity). The yellow line at the top of the waveforms indicates frequency bins (0.25 Hz each) showing a significant Session-by-Group interaction, which spans almost the entire alpha band (8.25–12 Hz) but no other frequency band, confirming the specific modulation of alpha-frequency GC. (B) Magnitudes of right posterior ➔frontal alpha GC, decibel-normalized to initial baseline, for each group. (C) Scalp topographical maps of alpha GC sent from right occipitoparietal electrodes at the initial baseline (surrounded by a black box and associated with its own color scale) and decibel-normalized changes at each assessment thereafter. Sending occipitoparietal and receiving frontal electrodes are bolded and encircled. Error bars = SEM. *P < 0.05; **P < 0.01; ***P < 0.005. All P-values are relative to the initial baseline. Note that the groups did not differ in the initial baseline values (P > 0.19).
A similar follow-up ANOVA in the left hemisphere showed an interaction of Session-by-Time-by-Group (F1.99, 71.58 = 2.84, P = 0.065, ηp2 = 0.07). However, this effect did not lend support to the notion of increased tACS potency over repeated stimulation. The three-way interaction was substantiated by an interaction of Session-by-Time in the Active (F1.99, 39.81 = 3.13, P = 0.055, ηp2 = 0.14) but not Sham (P = 0.363) group. Specifically, the Active group exhibited an effect of Time in the initial session (F1.96, 39.28 = 3.87, P = 0.030, ηp2 = 0.16) with a trend of immediate alpha GC increase (P = 0.065) and no change 30 min later (P = 0.504), in contrast to no effect of Time in the final session (P = 0.730). Finally, there was no baseline shift in the Active (vs Sham) group (P = 0.367), suggesting no lasting effect of stimulation in the left hemisphere.
tACS reduced anxious arousal
An omnibus rANOVA of Session, Time, and Group on anxious arousal ratings revealed a main effect of Group (F1, 32 = 4.64, P = 0.039, ηp2 = 0.13); following the initial baseline, the two groups diverged in anxious arousal ratings, with the Active group showing consistently lowered ratings relative to initial baseline (P < 0.05 FDR corrected), while no change was observed in the Sham group (P > 0.317; Figure 4A). Importantly, the Active group, but not Sham (P = 0.607) group, demonstrated sustained baseline shifts across sessions (F2.60, 41.62 = 4.76, P = 0.008, ηp2 = 0.23), such that, relative to the initial baseline on Day 1, the anxious arousal baseline was consistently lowered on Day 2 (P = 0.003), Day 3 (P = 0.021) and Day 4 (P = 0.005; Figure 4A). Therefore, tACS led to a consistent and lasting reduction in anxious arousal.
Fig. 4.
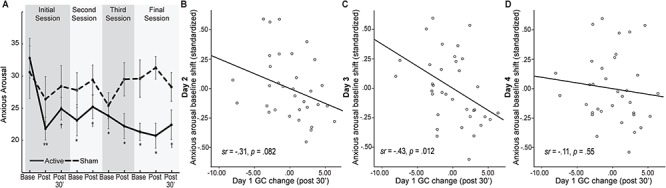
Anxious arousal outcome. (A) Anxious arousal ratings for each group at each assessment over the four sessions/days. (B–D) Day 1 (initial session) GC change at post-30 min predicted anxious arousal baseline shift (after controlling for individual differences in pre-study trait anxiety/BIS scores) on Day 2 (second session; B) and Day 3 (third session; C) but the association greatly declined on Day 4 (final session; D). Error bars = adjusted standard error (SEE). *P < 0.05; **P < 0.01; †P < 0.1. All P-values are relative to the initial baseline. The groups did not differ in the initial baseline (P = 0.718).
Next, to test whether alpha GC enhancement could underpin sustained anxiety reduction, we examined whether the right-hemisphere GC increase on Day 1 (post-30 min) could predict anxiety baseline shifts on the following days (relative to the Day 1 baseline). We observed a marginal effect of GC (F1,32 = 3.55, P = 0.069, ηp2 = 0.10), which was further qualified by a trending Session-by-GC interaction (F1.93, 62.89 = 2.80, P = 0.071, ηp2 = 0.08), suggesting a temporal progression in the association between GC and anxious arousal. Follow-up regression tests for each session revealed that Day 1 GC enhancement predicted reduction in baseline anxious arousal marginally on Day 2 (sr = −0.27, P = 0.082) and significantly on Day 3 (sr = −0.41, P = 0.012, P < 0.05 FDR corrected), but not on Day 4 (sr = −0.10, P = 0.539; Figure 4B–D).
tACS increased perceived pleasantness of sensory stimuli
Auditory stimuli
An omnibus rANOVA (Valence, Intensity, Session, Time, and Group) on pleasantness ratings of auditory stimuli revealed a main effect of Group (Active > Sham; F1, 32 = 4.19, P = 0.049, ηp2 = 0.12), which was further characterized by a Session-by-Time-by-Group interaction (F1.57, 50.17 = 3.84, P = 0.038, ηp2 = 0.11; Figure 5A). However, this effect did not clearly support increased potency of tACS on affective perception. Follow-up tests for each session showed an interaction of Time-by-Group in the initial (F1.70, 54.28 = 4.10, P = 0.028, ηp2 = 0.11) but not final (P = 0.707) session. That is, following the initial tACS (but not sham stimulation, P = 0.644), auditory stimuli were perceived as more pleasant (F1.35, 21.59 = 4.10, P = 0.045, ηp2 = 0.20), immediately (P = 0.073) and 30 min (P = 0.040) after stimulation. Although no additional increases were seen after the final stimulation, final baseline ratings were significantly higher than initial baseline ratings (t = −2.43, P = 0.027) and remained elevated post-stimulation (immediately, P = 0.053; 30 min, P = 0.083), suggesting sustained increases in pleasantness at the final session. No such between- or within-session changes were seen in the Sham group (P > 0.572). Therefore, sound pleasantness ratings paralleled anxious arousal ratings, increasing after the initial tACS and persisting through the last assessment on Day 4.
Fig. 5.
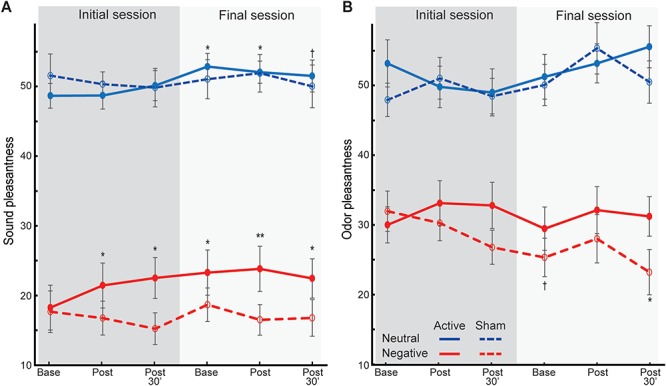
Pleasantness ratings of neutral and negative. (A) sounds and (B) odors. Neutral valence = 50. Error bars = SEM. *P < 0.05; **P < 0.01; †P < 0.1. All P-values are relative to the initial baseline. The groups did not differ in the initial baseline ratings (P > 0.215).
Olfactory stimuli
A similar omnibus rANOVA on pleasantness ratings of olfactory stimuli revealed an interaction of Valence-by-Group (F1, 32 = 8.51, P = 0.006, ηp2 = 0.21), which was further qualified by a Valence-by-Time-by-Group interaction (F1.75, 55.95 = 5.20, P = 0.011, ηp2 = 0.14; Figure 5B). Follow-up ANOVAs revealed a Time-by-Group interaction for negative (F1.82, 58.10 = 4.60, P = 0.017, ηp2 = 0.13) but not neutral (P = 0.230) odor ratings. That is, the Active group showed trending increases in pleasantness of negative odors (F1.88, 30.14 = 3.15, P = 0.060, ηp2 = 0.16), both immediately (P = 0.050) and, marginally, 30 min (P = 0.066) after stimulation, paralleled by opposing marginal decreases in pleasantness in the Sham group (F1.72, 27.56 = 3.32, P = 0.058, ηp2 = 0.17). There were no Session-by-Time-by-Group interactions or baseline shifts (P > 0.202) to indicate between-session or long-term effects.
Discussion
Combining multi-session α-tACS and multi-wave assessment in an experimental design, we demonstrated immediate and lasting neural and behavioral effects of tACS. α-tACS not only enhanced posterior ➔frontal alpha-frequency connectivity (in the right hemisphere) immediately and 30 min post-stimulation but also shifted the connectivity baseline 24 h after the preceding stimulation, indicating long-term plasticity. In parallel, anxious arousal decreased and perceived pleasantness of auditory stimuli increased both acutely (immediately and 30 min) and 24 h after α-tACS. By contrast, local posterior alpha power showed transient (immediately and 30 min post-stimulation) increases only, as did perceived pleasantness of negative olfactory stimuli. That repeated stimulation failed to induce lasting alpha power enhancement underscores the conservation of local alpha oscillations, contrasting the long-range alpha connectivity that is adaptive to long-term modulation by tACS. Our double-blind, single-session replication study with random noise stimulation as the control condition not only replicated the effect of anxiety reduction but also ruled-out potential confounds of experimenter biases and random electrical stimulation (Supplementary data).
The acute (immediate and 30 min) enhancement of local alpha power was evident in both initial and final sessions, echoing previous reports of reliable α-tACS after-effects (Herrmann et al., 2013; Neuling et al., 2013; Vossen et al., 2015). Furthermore, bin-by-bin examination across the entire frequency spectrum confirmed the specific modulation in the alpha band. In addition, the effect appeared largely in the lower alpha band (8–10.5 Hz), in line with prior findings of leftward shifting of alpha peaks by α-tACS (Kasten et al., 2016) and a general trend of higher alpha power being associated with lower alpha peaks (Smit et al., 2006). Transient alpha power enhancement following a single tACS session as previously reported (Kasten et al., 2016) could be attributed to the insufficient stimulation dosage to induce lasting, late LTP to support long-term plasticity. That our protocol of multiple stimulation sessions still failed to generate lasting power increases refuted the account of insufficient dosage, especially in light of lasting alpha connectivity enhancement indicating that the current dosage was sufficient for long-term plasticity. Instead, the transient alpha power increase likely reflected neural entrainment that temporally synchronized occipitoparietal cortical oscillations to exogenous stimulation (Reato et al., 2013; Helfrich et al., 2014). Therefore, as tACS ceased, the highly integrated thalamo-cortical alpha pacemaker (Hughes and Crunelli, 2005; Bazanova and Vernon, 2014) might allow thalamic inputs to restore cortical alpha oscillations to endogenous levels. Overall, the restoration of alpha power, despite repeated stimulation, accentuated the resistance of cortical alpha oscillators to long-term changes. This intrinsic regulation of resting-state alpha oscillations in healthy participants thus implicated a conserved, closed-loop (potentially encapsulated) system of alpha oscillators, critical for preserving neural homeostasis and mental stability (Sterling, 2014).
By contrast, alpha-frequency posterior ➔frontal connectivity exhibited both immediate and lasting changes, representing some of the first empirical evidence for long-term neural effects of tACS. The immediate effects replicated a recent report of immediate increases in resting-state connectivity between parietal and frontal cortices following α-tACS (Cabral-Calderin et al., 2016). The lasting enhancement in alpha connectivity, in contrast to alpha power, ruled out the possibility that augmented alpha connectivity merely resulted from a potentiated posterior alpha sender (via enhanced alpha synchrony in the occipitoparietal cortex) or a higher signal-to-noise ratio of scalp EEG at the assessment on Day 4. Rather, the evidence implicated mechanisms beyond local neuronal synchrony, in favor of the interpretation of improved efficiency in alpha projections across large-scale networks, such that with alpha power at the sender remaining constant, directed transmission of alpha oscillations to the distant receiver was intensified.
This improved efficiency in posterior ➔frontal alpha transmission was likely mediated by synaptic plasticity induced by tACS, via mechanisms such as STDP and LTP (Frohlich and McCormick, 2010; Reato et al., 2010; Zaehle et al., 2010; Polanía et al., 2012; Vossen et al., 2015), which is known to be conducive to enduring Hebbian synaptic plasticity and lasting increases in cortico-cortical oscillatory connectivity (Clopath et al., 2010; Siegel et al., 2012). Since such inter-cortical connectivity (driven by cortical alpha generators; Rockland and Virga, 1989; Bollimunta et al., 2008) operates outside the relatively encapsulated thalamo-cortical loop that constrains local cortical oscillations, its plasticity can outlast the effect of local alpha entrainment. This account concurs with the notion that tACS directly modifies interactions across proximal and distant neurons to the extent that the dynamics of macroscopic, global networks are altered (Frohlich and McCormick, 2010; Reato et al., 2010). Here, given the role of alpha oscillations in long-range interactions, α-tACS would generate pronounced connectivity plasticity in long-range pathways. In particular, the posterior ➔frontal alpha connectivity exhibited maximal gain, akin to its dominance in resting long-range interactions and, critically, tACS stimulation at the posterior site. Connectivity in the opposite direction (frontal ➔posterior) failed to show acute or delayed effects (P > 0.16). This highlights the importance of computing directional measures of connectivity (e.g. GC) such that directional effects would not be obscured by non-directional measures of connectivity. In addition, to the extent that GC can be influenced by signal power, the fact that GC increases persisted through Day 4 when power had reversed to the baseline largely mitigated this concern.
Paralleling alpha posterior ➔frontal connectivity increases, anxious arousal decreased both immediately and 24 h after tACS, which persisted across the 4 day period, highlighting reliable and robust effects on anxiety. These anxiety effects outlasted local alpha power enhancement and so were likely underpinned by strengthened posterior ➔frontal alpha connectivity, which presumably modulated large-scale RSNs, especially the DMN and SN that are closely linked to alpha oscillations (Laufs et al., 2003; Buzsáki and Draguhn, 2004; Mantini et al., 2007; Sadaghiani et al., 2010; Mo et al., 2013; Zhan et al., 2014; Tang et al., 2017a) and play critical roles in emotion and arousal (Raichle et al., 2001; Sridharan et al., 2008). The non-clinical nature, and relatively small size (for correlational effects), of the current sample may have occluded the full observation of previously demonstrated direct links between alpha connectivity and hyperarousal in a largely clinical sample (Clancy et al., 2017). However, the fact that neural effects preceded lasting anxiety changes underscores neural plasticity that develops and consolidates over time, giving rise to enduring amelioration of anxiety and related behavioral metrics. Nevertheless, this alpha connectivity enhancement failed to predict anxiety reduction on Day 4, suggesting that neural effects of a single tACS would not sustain prolonged behavioral changes and that multiple sessions would be needed for enduring behavioral effects. Future research with daily resting-state EEG recordings and follow-up assessments (e.g. at 1 week post-treatment) would delineate detailed temporal profiles of neural changes and related behavioral impacts.
Anxiety is often associated with negative stimulus perception (Krusemark and Li, 2011; Forscher and Li, 2012; Krusemark and Li, 2012). Auditory processing is particularly sensitive to states of anxious arousal (Grillon et al., 1991; Braff et al., 2001). In addition, alpha oscillations are known to modulate sensory processing and perception (Palva and Palva, 2007; Foxe and Snyder, 2011; Klimesch et al., 2011; Lou et al., 2014) such that altered alpha activity via α-tACS could further mitigate aversive responses to auditory stimuli by modifying auditory processing. Indeed, immediate and lasting increases in perceived pleasantness of sounds (especially negative sounds) coincided with anxious arousal reduction and alpha connectivity enhancement, providing additional support to our hypotheses. Interestingly, perceived pleasantness of negative odors increased in a transient manner while lasting increases emerged in the auditory domain. Unlike the auditory system that is neocortical and has direct and close connections with the targeted visual cortex (Falchier et al., 2002; Kayser et al., 2008; Novak et al., 2015), the olfactory system is largely subcortical or paleocortical without direct connections with visual cortex (Carmichael et al., 1994; Haberly, 2001; Krusemark et al., 2013). As such, olfactory perception may be relatively impervious to alpha purtabations with the transient changes (for negative odors) presumably reflecting brief adaptation to internal state changes because of tACS, as a phenomenon of olfactory alliesthesia (Krusemark et al., 2013).
Finally, we explored the possibility of intensified potency of tACS effects over repeated sessions. While there were hints of greater within-session effects on Day 4 relative to Day 1 (Figures 2 and 3), three-way interactions of Session, Time, and Group failed to support this possibility. We surmise that these null effects implicated a biological ceiling among healthy individuals, especially considering the already elevated baselines (for GC connectivity) on Day 4. Similarly, in contrast to clinical patients in a clinical trial, our participants in this proof-of-concept study had normal levels of baseline anxious arousal (mean scores 32/100), leaving only modest room for anxiety reduction. Nonetheless, anxiety reduced by 34% after tACS on Day 1 and maintained throughout the study, resisting the natural regression to the baseline (as seen in the Sham group). That said, the possibility of tACS potency intensification among patients with reduced alpha activity and greater anxious arousal warrants investigation through double-blind clinical trials, which could provide useful dosage guidance in clinical applications.
Increasing recognition of aberrant oscillatory dynamics in the pathology of various neuropsychiatric disorders has spawned strong interest in directly modulating endogenous oscillations (Schnitzler and Gross, 2005; Uhlhaas and Singer, 2012; Basar, 2013). Meanwhile, network views of psychopathology have gained rapid traction in recent years, advancing conceptualization of mental disorders from local neural aberrations to large-scale circuit anomalies (Kaiser et al., 2015; Clancy et al., 2017; McTeague et al., 2017). In fact, while our laboratory demonstrated both reduced posterior alpha power and posterior ➔frontal alpha connectivity in PTSD, we found that only the latter was directly coupled with symptom severity (Clancy et al., 2017). Therefore, the ability of tACS to induce lasting plasticity in long-range connectivity makes this neuromodulatory technique especially advantageous for treating disorders rooted in pathological oscillatory networks. While meditation and neurofeedback can modulate RSNs and reduce anxiety and arousal, along with alpha power enhancement (Hammond, 2005; Cahn and Polich, 2006; Ros et al., 2013; Fan et al., 2014; Tang et al., 2017b), their long-term impact on alpha-frequency connectivity remain unclear. In this regard, we call for attention to oscillatory connectivity modification and network adaptation in future investigation of all forms of neuromodulation.
Funding
This research was supported by the National Institute of Mental Health (R01MH093413 to W.L.) and (R21MH112206 to M.D.) and the FSU Chemical Senses Training Grant Award (T32DC000044 to K.C.) from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders.
Supplementary Material
References
- Abend R., Jalon I., Gurevitch G., et al. (2016). Modulation of fear extinction processes using transcranial electrical stimulation. Translational Psychiatry, 6(10), e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham W.C. (2003). How long will long-term potentiation last? Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1432), 735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler L.E., Pang K., Gerhardt G., Rose G.M. (1988). Modulation of the gating of auditory evoked potentials by norepinephrine: pharmacological evidence obtained using a selective neurotoxin. Biological Psychiatry, 24(2), 179–90. [DOI] [PubMed] [Google Scholar]
- Alagapan S., Schmidt S.L., Lefebvre J., Hadar E., Shin H.W., Fröhlich F. (2016). Modulation of cortical oscillations by low-frequency direct cortical stimulation is state-dependent. PLoS Biology, 14(3), e1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez R.P., Kirlic N., Misaki M., et al. (2015). Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Translational Psychiatry, 5, e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A., Paulus W. (2013). Transcranial alternating current stimulation (tACS). Frontiers in Human Neuroscience, 7, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basar E. (2013). Brain oscillations in neuropsychiatric disease. Dialogues in Clinical Neuroscience, 15(3), 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar E., Başar-Eroglu C., Karakaş S., Schürmann M. (2001). Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology, 39(2), 241–8. [DOI] [PubMed] [Google Scholar]
- Bazanova O.M., Vernon D. (2014). Interpreting EEG alpha activity. Neuroscience and Biobehavioral Reviews, 44, 94–110. [DOI] [PubMed] [Google Scholar]
- Bollimunta A., Chen Y., Schroeder C.E., Ding M. (2008). Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. The Journal of Neuroscience, 28(40), 9976–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff D.L., Geyer M.A., Swerdlow N.R. (2001). Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology, 156(2–3), 234–58. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Draguhn A. (2004). Neuronal oscillations in cortical networks. Science, 304(5679), 1926–9. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Logothetis N., Singer W. (2013). Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron, 80(3), 751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral-Calderin Y., Williams K.A., Opitz A., Dechent P., Wilke M. (2016). Transcranial alternating current stimulation modulates spontaneous low frequency fluctuations as measured with fMRI. NeuroImage, 141, 88–107. [DOI] [PubMed] [Google Scholar]
- Cahn B.R., Polich J. (2006). Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin, 132(2), 180–211. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T., Clugnet M.C., Price J.L. (1994). Central olfactory connections in the macaque monkey. The Journal of Comparative Neurology, 346(3), 403–34. [DOI] [PubMed] [Google Scholar]
- Carver C.S., White T.L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology, 67(2), 319–33. [Google Scholar]
- Clancy K., Ding M., Bernat E., Schmidt N.B., Li W. (2017). Restless ‘rest’: intrinsic sensory hyperactivity and disinhibition in post-traumatic stress disorder. Brain, 140(7), 2041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopath C., Büsing L., Vasilaki E., Gerstner W. (2010). Connectivity reflects coding: a model of voltage-based STDP with homeostasis. Nature Neuroscience, 13(3), 344–52. [DOI] [PubMed] [Google Scholar]
- Cohen M.R., Maunsell J.H. (2010). A neuronal population measure of attention predicts behavioral performance on individual trials. The Journal of Neuroscience, 30(45), 15241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.X. (2014). Analyzing Neural Time Series Data: Theory and Practice. London, England: MIT Press. [Google Scholar]
- Corbetta M., Shulman G.L. (2011). Spatial neglect and attention networks. Annual Review of Neuroscience, 34, 569–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M., Bressler S.L., Yang W., Liang H. (2000). Short-window spectral analysis of cortical event-related potentials by adaptive multivariate autoregressive modeling: data preprocessing, model validation, and variability assessment. Biological Cybernetics, 83(1), 35–45. [DOI] [PubMed] [Google Scholar]
- Ding M., Chen Y., Bressler S.L. (2006). Granger causality: basic theory and application to neuroscience In: Handbook of Time Series Analysis, Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA. [Google Scholar]
- Dugué L., Marque P., VanRullen R. (2011). The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. The Journal of Neuroscience, 31(33), 11889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A.K., Fries P., Singer W. (2001). Dynamic predictions: oscillations and synchrony in top-down processing. Nature Reviews. Neuroscience, 2(10), 704–16. [DOI] [PubMed] [Google Scholar]
- Falchier A., Clavagnier S., Barone P., Kennedy H. (2002). Anatomical evidence of multimodal integration in primate striate cortex. The Journal of Neuroscience, 22(13), 5749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Tang Y.Y., Tang R., Posner M.I. (2014). Short term integrative meditation improves resting alpha activity and stroop performance. Applied Psychophysiology and Biofeedback, 39(3–4), 213–7. [DOI] [PubMed] [Google Scholar]
- Forscher E.C., Li W. (2012). Hemispheric asymmetry and visuo-olfactory integration in perceiving subthreshold (micro) fearful expressions. The Journal of Neuroscience, 32(6), 2159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe J.J., Snyder A.C. (2011). The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Frontiers in Psychology, 2, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F., Otachi P.T., Do Valle A., et al. (2006). A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Annals of Neurology, 60(4), 447–55. [DOI] [PubMed] [Google Scholar]
- Fröhlich F. (2015). Experiments and models of cortical oscillations as a target for noninvasive brain stimulation. Progress in Brain Research, 222, 41–73. [DOI] [PubMed] [Google Scholar]
- Fröhlich F., McCormick D.A. (2010). Endogenous electric fields may guide neocortical network activity. Neuron, 67(1), 129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gath I., Lehmann D., Bar-On E. (1983). Fuzzy clustering of EEG signal and vigilance performance. The International Journal of Neuroscience, 20(3–4), 303–12. [DOI] [PubMed] [Google Scholar]
- Geweke J. (1982). Measurement of linear dependence and feedback between multiple time series. Journal of the American Statistical Association, 77(378), 304–13. [Google Scholar]
- Grillon C., Ameli R., Woods S.W., Merikangas K., Davis M. (1991). Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology, 28(5), 588–95. [DOI] [PubMed] [Google Scholar]
- Haberly L.B. (2001). Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chemical Senses, 26(5), 551–76. [DOI] [PubMed] [Google Scholar]
- Hammond D.C. (2005). Neurofeedback with anxiety and affective disorders. Child and Adolescent Psychiatric Clinics of North America, 14(1), 105–23vii. [DOI] [PubMed] [Google Scholar]
- Helfrich R.F., Schneider T.R., Rach S., Trautmann-Lengsfeld S.A., Engel A.K., Herrmann C.S. (2014). Entrainment of brain oscillations by transcranial alternating current stimulation. Current Biology, 24(3), 333–9. [DOI] [PubMed] [Google Scholar]
- Herrmann C.S., Rach S., Neuling T., Strüber D. (2013). Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Frontiers in Human Neuroscience, 7, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A., Tewarie P., Dellen E., et al. (2016). Direction of information flow in large-scale resting-state networks is frequency-dependent. Proceedings of the National Academy of Sciences of the United States of America, 113(14), 3867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.Z., Lu M.K., Antal A., et al. (2017). Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clinical Neurophysiology, 128(11), 2318–2329. [DOI] [PubMed] [Google Scholar]
- Hughes S.W., Crunelli V. (2005). Thalamic mechanisms of EEG alpha rhythms and their pathological implications. The Neuroscientist, 11(4), 357–72. [DOI] [PubMed] [Google Scholar]
- Johnson E.L., Dewar C.D., Solbakk A.K., Endestad T., Meling T.R., Knight R.T. (2017). Bidirectional frontoparietal oscillatory systems support working memory. Current Biology, 27(12), 1829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry, 72(6), 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten F.H., Dowsett J., Herrmann C.S. (2016). Sustained aftereffect of α-tACS lasts up to 70 min after stimulation. Frontiers in Human Neuroscience, 10, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C., Petkov C.I., Logothetis N.K. (2008). Visual modulation of neurons in auditory cortex. Cerebral Cortex, 18(7),1560–74. [DOI] [PubMed] [Google Scholar]
- Klimesch W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research. Brain Research Reviews, 29(2–3), 169–95. [DOI] [PubMed] [Google Scholar]
- Klimesch W. (2012). α-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16(12), 606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W., Fellinger R., Freunberger R. (2011). Alpha oscillations and early stages of visual encoding. Frontiers in Psychology, 2, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusemark E.A., Li W. (2011). Do all threats work the same way? Divergent effects of fear and disgust on sensory perception and attention. The Journal of Neuroscience, 31(9), 3429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusemark E.A., Li W. (2012). Enhanced olfactory sensory perception of threat in anxiety: an event-related fMRI study. Chemosensory Perception, 5(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusemark E.A., Novak L.R., Gitelman D.R., Li W. (2013). When the sense of smell meets emotion: anxiety-state-dependent olfactory processing and neural circuitry adaptation. The Journal of Neuroscience, 33(39), 15324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H., Krakow K., Sterzer P., et al. (2003). Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences of the United States of America, 100(19), 11053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou B., Li Y., Philiastides M.G., Sajda P. (2014). Prestimulus alpha power predicts fidelity of sensory encoding in perceptual decision making. NeuroImage, 87, 242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D., Perrucci M.G., Del Gratta C., Romani G.L., Corbetta M. (2007). Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 104(32), 13170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson K.E., Gratton G., Fabiani M., Beck D.M., Ro T. (2009). To see or not to see: prestimulus alpha phase predicts visual awareness. The Journal of Neuroscience, 29(9), 2725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Huemer J., Carreon D.M., Jiang Y., Eickhoff S.B., Etkin A. (2017). Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. The American Journal of Psychiatry, 174(7), 676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function, 214(5–6), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P.P., Pesaran B. (1999). Analysis of dynamic brain imaging data. Biophysical Journal, 76(2), 691–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J., Liu Y., Huang H., Ding M. (2013). Coupling between visual alpha oscillations and default mode activity. NeuroImage, 68, 112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisa M., Polania R., Grueschow M., Ruff C.C. (2016). Brain network mechanisms underlying motor enhancement by transcranial entrainment of gamma oscillations. The Journal of Neuroscience, 36(47), 12053–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuling T., Rach S., Herrmann C.S. (2013). Orchestrating neuronal networks: sustained after-effects of transcranial alternating current stimulation depend upon brain states. Frontiers in Human Neuroscience, 7, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuling T., Wagner S., Wolters C.H., Zaehle T., Herrmann C.S. (2012). Finite-element model predicts current density distribution for clinical applications of tDCS and tACS. Frontiers in Psychiatry, 3, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan H., Whelan R., Reilly R.B. (2010). FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. Journal of Neuroscience Methods, 192(1), 152–62. [DOI] [PubMed] [Google Scholar]
- Novak L.R., Gitelman D.R., Schuyler B., Li W. (2015). Olfactory-visual integration facilitates perception of subthreshold negative emotion. Neuropsychologia, 77, 288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P.L., Srinivasan R., Westdorp A.F., et al. (1997). EEG coherency. I: statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalography and Clinical Neurophysiology, 103(5), 499–515. [DOI] [PubMed] [Google Scholar]
- Palva S., Palva J.M. (2007). New vistas for alpha-frequency band oscillations. Trends in Neurosciences, 30(4), 150–8. [DOI] [PubMed] [Google Scholar]
- Perrin F., Pernier J., Bertrand O., Echallier J.F. (1989). Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology, 72(2), 184–7. [DOI] [PubMed] [Google Scholar]
- Peterson A., Thome J., Frewen P., Lanius R.A. (2014). Resting-state neuroimaging studies: a new way of identifying differences and similarities among the anxiety disorders? Canadian Journal of Psychiatry, 59(6), 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R., Nitsche M.A., Korman C., Batsikadze G., Paulus W. (2012). The importance of timing in segregated theta phase-coupling for cognitive performance. Current Biology, 22(14), 1314–8. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagovindan R., Ding M. (2011). From prestimulus alpha oscillation to visual-evoked response: an inverted-U function and its attentional modulation. Journal of Cognitive Neuroscience, 23(6), 1379–94. [DOI] [PubMed] [Google Scholar]
- Reato D., Rahman A., Bikson M., Parra L.C. (2010). Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. The Journal of Neuroscience, 30(45), 15067–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reato D., Rahman A., Bikson M., Parra L.C. (2013). Effects of weak transcranial alternating current stimulation on brain activity—a review of known mechanisms from animal studies. Frontiers in Human Neuroscience, 7, 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J., Schambra H.M., Cohen L.G., et al. (2009). Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the United States of America, 106(5), 1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland K.S., Virga A. (1989). Terminal arbors of individual ‘feedback’ axons projecting from area V2 to V1 in the macaque monkey: a study using immunohistochemistry of anterogradely transported Phaseolus vulgaris-leucoagglutinin. The Journal of Comparative Neurology, 285(1), 54–72. [DOI] [PubMed] [Google Scholar]
- Ros T., Théberge J., Frewen P.A., et al. (2013). Mind over chatter: plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. NeuroImage, 65, 324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani L., Gleeson H.B., Youde S., Waddington R.J., Lynch C.D., Sloan A.J. (2016). Growth factor liberation and DPSC response following dentine conditioning. Journal of Dental Research, 95(11), 1298–307. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S., Scheeringa R., Lehongre K., Morillon B., Giraud A.L., Kleinschmidt A. (2010). Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. The Journal of Neuroscience, 30(30), 10243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., Scheeringa R., Lehongre K., et al. (2012). Alpha-band phase synchrony is related to activity in the fronto-parietal adaptive control network. The Journal of Neuroscience, 32(41), 14305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A., Gross J. (2005). Normal and pathological oscillatory communication in the brain. Nature Reviews. Neuroscience, 6(4), 285–96. [DOI] [PubMed] [Google Scholar]
- Siegel M., Donner T.H., Engel A.K. (2012). Spectral fingerprints of large-scale neuronal interactions. Nature Reviews. Neuroscience, 13(2), 121–34. [DOI] [PubMed] [Google Scholar]
- Smit C.M., Wright M.J., Hansell N.K., Geffen G.M., Martin N.G. (2006). Genetic variation of individual alpha frequency (IAF) and alpha power in a large adolescent twin sample. International Journal of Psychophysiology, 61(2), 235–43. [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., et al. (2012). Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine, 74(9), 904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P. (2014). Homeostasis vs allostasis: implications for brain function and mental disorders. JAMA Psychiatry, 71(10), 1192–3. [DOI] [PubMed] [Google Scholar]
- Tang A. C., Sutherland M. T., Sun P., et al. (2007). Top-down versus bottom-up processing in the human brain: distinct directional influences revealed by integrating SOBI and Granger causality. In: Davies M.E., James C.J., Abdallah S.A., Plumbey M.D., editors. Independent Component Analysis and Signal Separation, 7th International Conference, ICA 2007, London, UK, 9 to 12 September 2007, Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Tang W., Liu H., Douw L., et al. (2017a). Dynamic connectivity modulates local activity in the core regions of the default-mode network. Proceedings of the National Academy of Sciences of the United States of America, 114(36), 9713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.Y., Tang Y., Tang R., Lewis-Peacock J.A. (2017b). Brief mental training reorganizes large-scale brain networks. Frontiers in Systems Neuroscience, 11, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G., Schyns P.G., Gross J. (2011). Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Frontiers in Psychology, 2, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. (2012). Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron, 75(6), 963–80. [DOI] [PubMed] [Google Scholar]
- Vossen A., Gross J., Thut G. (2015). Alpha power increase after transcranial alternating current stimulation at alpha frequency (alpha-tACS) reflects plastic changes rather than entrainment. Brain Stimulation, 8(3), 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Rajagovindan R., Han S.M., Ding M. (2016). Top-down control of visual alpha oscillations: sources of control signals and their mechanisms of action. Frontiers in Human Neuroscience, 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe J., Lazarus A.A. (1966). Behaviour Therapy Techniques; A Guide to the Treatment of Neuroses, New York: Pergamon. [Google Scholar]
- Young C.B., Raz G., Everaerd D., et al. (2017). Dynamic shifts in large-scale brain network balance as a function of arousal. The Journal of Neuroscience, 37(2), 281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle T., Rach S., Herrmann C.S. (2010). Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One, 5(11), e13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Z., Xu L., Zuo T., et al. (2014). The contribution of different frequency bands of fMRI data to the correlation with EEG alpha rhythm. Brain Research, 1543, 235–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


