Abstract
Gelastic seizures (GS) are a rare form of epilepsy characterized by inappropriate, uncontrolled laughter. They are highly associated with abnormal cognitive development and behavioral problems in patients. Research has shown that GS can originate from hypothalamic hamartomas (HH), non- neoplastic masses consisting of gray matter with large and small neurons interspersed with glial nuclei. GS have also been observed in patients with frontal and temporal lobe lesions.
The patient in this case report is a 40-year-old man with a past medical history significant for brain tumor, diabetes mellitus, and schizophrenia who presented with a long standing history of sudden, involuntary laughter occurring 2–3 times a week since 8 years old. Since the onset of these laughing spells the patient has displayed gradual cognitive impairment and increasing behavioral problems. Subsequent EEG (21-channel electroencephalogram) showed focal epileptiform activity in the right frontotemporal region and MRI studies revealed a mass arising from the hypothalamus suggestive of a HH.
Other conditions should be considered in the differential diagnosis for laughing spells and distinguishing different causes can be challenging. As demonstrated by this case report, in patients with behavioral issues, especially those with inappropriate uncontrolled laughter, gelastic seizures need to be included in the differential diagnosis. Thus, a thorough workup should include neuroimaging with attention to the suprasellar region and EEG. Accurate, early diagnosis and patient education are critical in avoiding excessive and unnecessary treatments. This condition may be pharmacoresistant and is often associated with progressive cognitive and behavioral issues. Studies have shown a surgical treatment approach may be effective.
Introduction
Gelastic seizures (GS) are a rare seizure form characterized by inappropriate laughter. They classically occur in infancy with high frequency and periodicity.1–3 There is currently limited knowledge of the epidemiology, particularly in the adult population, but one study conducted at a major epilepsy center in London found that GS were found in fewer than 0.8% of patients.4 They may also occur in association with other seizure semiologies and therefore may go unrecognized.
Laughing seizures were first described by Trousseau in 1877 as bursts of laughter lasting only a few seconds.5 In 1957, Daly and Mulder coined the term “gelastic seizure,” from the greek word “gelos” meaning laughter, to describe a seizure pattern with laughter as the predominant feature.6 GS have been classically associated with hypothalamic hamartomas (HH), rare lesions that often present in early infancy.3 However, they can also be caused by temporal and frontal lobe pathologies.4 Clinical features range in severity from a pressure to laugh to more debilitating seizures, precocious puberty, and cognitive impairment.2,7,8 Given the rarity of this condition, practice guidelines for treatment are currently not available. We present a case of a 40-year-old man with a history of inappropriate laughter in the context of worsening behavioral issues and MRI findings suggestive of a HH.
Case Report
A 40-year-old man was referred to a Hawai‘i comprehensive epilepsy center for management of medically refractory seizures. His seizures began at the age of 8 and occurred at a stable frequency of approximately 2–3 times per week. His episodes were not preceded by any noticeable aura and the ictal semiology comprised of sudden, involuntary laughter that was unrelated to external stimuli. The patient also described several episodes of breath-holding and one occasion in which he suffered a generalized tonic-clonic seizure. Medications that he had tried in the past included carbamazepine, sodium valproate, and levetiracetam; however, these agents were unsuccessful in controlling the episodes.
The patient's seizures had been attributed to an optic glioma that was diagnosed in childhood, for which he received radiation therapy. Subsequently, the patient's father noted the gradual development of cognitive impairment in his son. The patient's past medical history also included diabetes mellitus and schizophrenia. His medications at the time of consultation included metformin, quetiapine, aripiprazole, fluoxetine, low-dose aspirin, and B12 supplements. He was unemployed and received assistance with activities of daily living from his father. He denied any substance abuse. His family history included neoplastic disease in his grandparents, although the tissue origin and degree of invasiveness were unknown.
On physical examination, the patient appeared as an overweight man of stated age. He was afebrile and hemodynamically stable. He was alert and orientated to time, place, and person. He exhibited mild cognitive deficits. Cranial nerve examination found no deficits. His pupils were equal and reactive, he had full range of extra-ocular movements and he had no visual field defects. He had normal facial movements and sensation, and his tongue and palate moved symmetrically. Likewise, examination of the patient's extremities demonstrated normal tone, power, reflexes, coordination, and sensation.
In light of the patient's reported history of optic glioma and recurrent seizures, a standard 21-channel electroencephalogram (EEG) and magnetic resonance imaging (MRI) scan were performed. The patient's EEG was reviewed by an epileptologist and showed several bursts of diffuse spike and wave activity, more prominent in the right frontotemporal region and ranging from 1 to 6 seconds in duration (Figure 1). The activity increased in amplitude as time progressed and then stopped sharply, consistent with epileptiform activity. Although no clinical changes were noted by the recording technician, the patient appeared to blink abruptly afterwards suggesting a possible alteration in awareness. The blinking occurred several times following this type of event during the recording.
Figure 1.
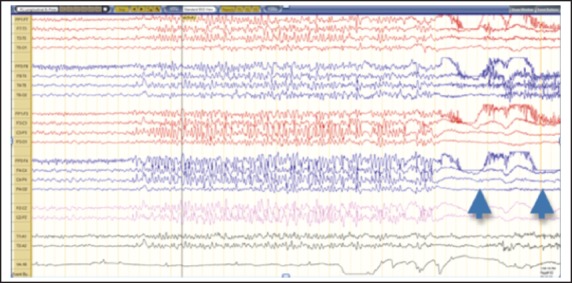
EEG activity showing 6 seconds of generalized epileptiform activity during monitoring.
The patient's MRI, reviewed by a neuroradiologist, demonstrated several abnormalities. Of these, the most notable was a mildly T1 hypointense, T2 hyperintense, non-enhancing mass arising from the hypothalamus that projected inferiorly into the prepontine cistern (Figures 2A, 2B). It exhibited no visible mass effect and conformed to the shape of adjacent structures including the clivus anteriorly and the brainstem posteriorly. Interestingly, the mass did not appear to arise from the optic nerves, chiasma, or tracts (Figure 3). Previous MRI's dating back to 2001 were obtained and demonstrated stability of the mass over time. Collectively, these findings were suggestive of a hypothalamic hamartoma.
Figure 2.
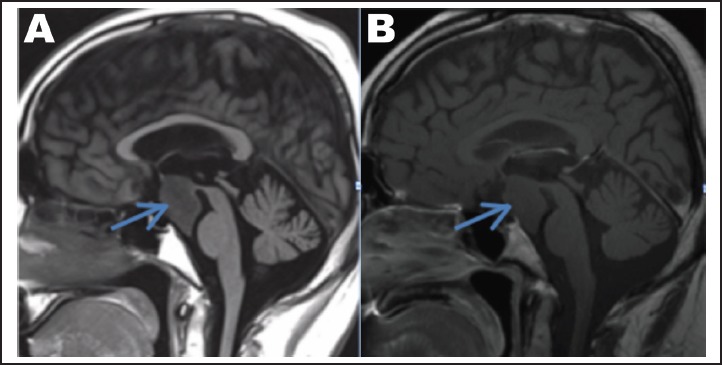
(A) Sagittal T1W image demonstrating a mildly hypointense mass arising from the hypothalamus and extending inferiorly into the prepontine cistern without any significant mass effect. (B) Sagittal T1 post contrast demonstrates no enhancement within the mass.
Figure 3.
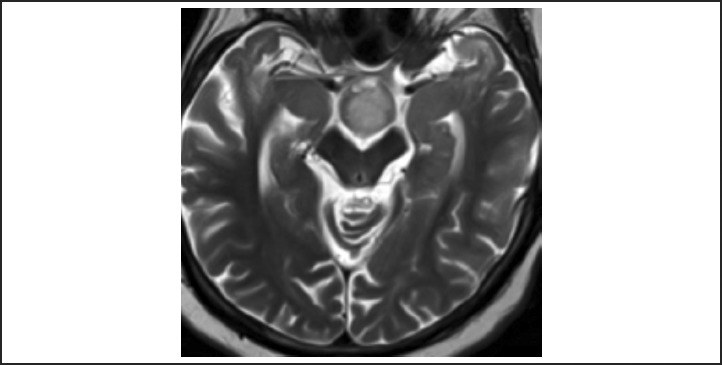
Axial T2W image demonstrates a mildly T2 hyperintense lesion posterior to the optic chiasm (arrow) without involvement of the chiasm or optic tracts.
Secondly, the appearance of the anterior temporal lobes was abnormal, with areas of T2 hyperintensity and T1 hypointensity (Figure 4A), and multiple scattered foci of susceptibility consistent with hemosiderin (Figure 4B). Given the patient's history and foci of hemosiderin, these findings were most consistent with post-radiation change, although trauma was also in the differential.9 Dysplasia was considered unlikely due to the presence of hemosiderin.
Figure 4.
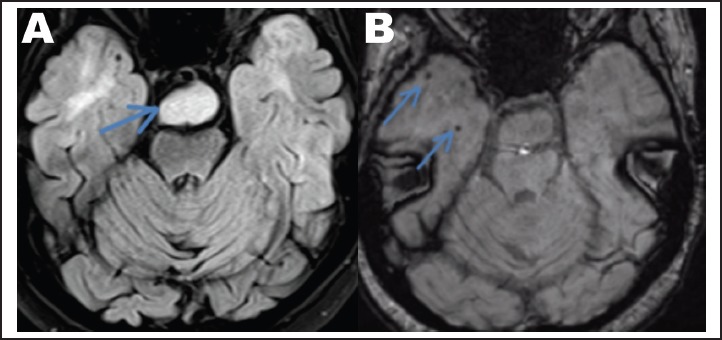
(A) Axial T2 FLAIR demonstrates abnormal T2 hyperintensity within the anterior temporal lobes. The hypothalamic hamartoma is visible within the prepontine cistern (arrow). (B) Axial susceptibility weighted imaging demonstrates foci of susceptibility consistent with hemosiderin (arrows).
Thirdly, there was an abnormality involving the superior aspects of the cerebellum with focal interdigitation of the two hemispheres, consistent with a developmental anomaly.
The patient was educated on the nature of his brain lesion. His management plan included continuing antiepileptic treatment until further clinical workup was performed, including plasma drug levels. An overnight, long-term video EEG was also scheduled to facilitate clinical EEG correlation.
Discussion
To understand the clinical manifestations of this patient's condition, it is helpful to understand the mechanisms involved in the production of laughter. Laughter has both an emotional and a motor component. Various parts of the brain are involved in producing laughter when it is emotionally provoked, including the amygdala, fusiform gyrus, parahippocampal gyrus, thalamus, hypothalamus, and dorsal tegmental brainstem.10 In contrast, non-emotional laughter involves the anterior cingulate premotor frontal and opercular areas. The emotional response is modified by the cerebral cortex and the physiological manifestations are controlled by the bulbar nuclei. Additionally, the hypothalamus plays an important role in integrating cortical and bulbar signals. Theoretically, a disruption anywhere along these pathways can generate laughter separate from emotional provocation.
Although there are many anatomical parts involved in the generation of laughter, researchers have shown through functional imaging studies and EEG recordings that gelastic seizures originate in hypothalamic hamartomas, especially those located at the mammillary level of the posterior hypothalamus.11–13 More specifically, hamartomas of the tuber cinereum have been known to cause GS. Hypothalamic hamartomas are masses consisting of gray matter with large and small neurons interspersed with glial nuclei.13 Both myelinated and unmyelinated fibers are present and can be haphazardly arranged, diffusely distributed, or clustered. Within the HH, two different types of neurons have been noted. The first are small, clustered GABAergic neurons that display spontaneous rhythmic firing. These “pacemaker” cells project to other HH neurons, creating synchronous activity that serves as the foundation of the intrinsic epileptogenicity of HH and the starting point for the generation of gelastic seizures.14 Supporting this hypothesis, ictal SPECT has shown hyperperfusion of HH during gelastic seizures.15 The second type of cell found in HH are large, quiescent pyramidal-like neurons. These neurons have more extensive dendritic and axonal arborization.13
Hypothalamic hamartomas can be divided into subtypes based on anatomical structures and clinical correlations: parahypothalamic and intrahypothalamic hamartomas. Parahypothalamic hamartomas are pedunculated and attached to the floor of the hypothalamus by a narrow base and seem to be more associated with precocious puberty with less frequent neuropsychological compromise.16 Intrahypothalamic hamartomas are sessile with a broad attachment to the hypothalamus and are more associated with GS, mental retardation and aggressive behavior.17 Both forms are associated with seizures that can evolve into tonic, tonic-clonic, and secondary generalized seizures.7,16 Our patient's MRI and clinical evaluation suggest he had an intrahypothalamic hamartoma associated with psychological and behavioral changes, which may have evolved into tonic clonic seizures.
One question that arises is: Can the epileptiform activity observed in patients with gelastic seizures arise from areas of the brain independent from the hypothalamus? A review of the literature shows cases of GS in patients with frontal and temporal lobe lesions, rather than hypothalamic lesions.18 A majority of these cases have been observed in adults, although large case studies are lacking. Patients with GS originating from the frontal lobe presented with motor signs but lacked laughter associated emotions (the laughter generated is described as unnatural and is not associated with feelings of mirth).13 In contrast, GS with temporal origin were more often associated with a sense of joy and happiness or pleasant sensations and feelings.19–20 It remains difficult to differentiate between HH induced seizure activity and secondary epileptogenesis—that is GS due to seizure activity progressing over time to involve connections of the hypothalamus, frontotemporal lobes, limbic circuitry, and thalamus in a patient with a HH.13
The clinical presentation of GS ranges in severity from a “pressure to laugh” to severe cases with the triad of early onset gelastic seizure, precocious puberty, and developmental delay that may progress to epileptic encephalopathy.1,7–8 Laughter may be combined with facial contortion suggestive of a smile.8,19 GS are unmotivated and mostly involuntary21—although rarely patients are able to suppress the urge to laugh.8 GS have been associated with altered consciousness and patients may be amnestic of the event.20–21 Autonomic features such as tachycardia, flushing, and changes in respiration are often present. In addition, many adults report an unpleasant epigastric sensation.22 Seizures are usually brief and stereotyped with a high frequency that usually occurs in clusters.13
GS in pediatric patients are highly associated with abnormal cognitive development and major behavioral problems. The extent of cognitive deficits largely depends on the severity and onset of the seizures as well as the size and location of HH.13 Late onset cases of patients with small HH might not cause cognitive deficits or behavioral disturbances.7,13 Our patient was diagnosed with schizophrenia when he was 7 years old—around the same time the hypothalamic mass was first discovered. His family also reported that he has had episodes of anger and rage. It is unclear whether his behaviors preceded the mass. However, it is possible that the patient's behavioral and cognitive changes are manifestations of the HH.
The diagnostic criteria for GS were first outlined in 1971 by Gascon and Lambroso. Criteria included stereotyped recurrence, absence of external precipitants, concomitance of other epileptic manifestations (tonic or clonic movements, loss of consciousness, automatisms), presence of interictal or ictal EEG epileptiform discharges, and absence of other causes of pathologic laughter.23
The diagnostic workup should include an EEG, video EEG (VEEG), and MRI. Previous studies indicate that a majority of GS fail to demonstrate EEG changes.24 However, present findings include slowing of background activity, interictal focal activity (mostly in patients with temporal/frontal region onset), generalized paroxysmal activity in the form of slow spike and wave complexes, and other various ictal patterns.13 Because EEG changes are variable, it is vital that gelastic epilepsy not be ruled out in the absence of EEG changes.
VEEG may play an important role in making the diagnosis in cases where neuro examination and MRI appear normal. In the case of a 2-year-old boy who presented with abrupt laughter without provocation, VEEG captured a GS with concurrent head deviation and twitching. Subsequent EEG revealed GS originating from frontal and temporal regions of brain.19
On MRI, HH appear as abnormalities in the region of the tuber cinereum and third ventricle.13 They are characteristically well-defined, pedunculated or sessile lesions that can be solid or cystic. They appear iso- or mildly hypointense with T1-weighted sequences, and iso- to hyperintense with T2-weighted sequences. Contrast enhancement and calcifications, as well as long-term changes in the size, shape, and signal intensity, are not characteristically found on MRI.25 The characteristic findings of HH were noted on our patient's MRI scans: the hypothalamic mass appeared mildly hypointense on T1-weighted sequences and mildly hyperintense on T2-weighted sequences. Furthermore, the hypothalamic mass observed in our patient remained stable in size when compared to previous studies, which, as described in the literature, is typical of hypothalamic hamartomas.
Management of GS should begin with patient education, especially as these seizures can be quite distressing for the patient and family. They tend to be refractory to antiepileptic drugs (AEDs) and therefore surgery is the predominant treatment option.
Neurosurgical techniques, particularly removal of the HH, tend to rapidly resolve symptoms. Rosenfeld first popularized surgical resection by utilizing a transcallosal approach to access the lesion from above.26 Less invasive surgical techniques, such as microsurgical resection and endoscopic disconnection through the foramen of Monro, are favored over open surgery due to decreased associated morbidity.16 Endoscopic treatment has yielded a 48.5% seizure free rate27 but may only be applicable in approximately 50% of cases.16
Newer procedures include radiofrequency ablation, disconnecting surgery, interstitial brachytherapy, stereotactic radiosurgery, and gamma knife radiation.13–28 The last has reported favorable outcomes (60% saw dramatic improvement and 37% are completely seizure free) according to a recent trial.28 This has the potential for being a safe and effective treatment option for sessile HH in small children.28 However, frequency of complications and adverse effects remain unknown.
Temporal lobe or frontal lobe resections are also performed in some cases. However, outcomes have not been studied in depth.
Differential Diagnosis
The differential diagnosis for inappropriate, uncontrollable laughter is broad. As demonstrated in a case report by Holmes and Goldman, recognizing the different conditions that cause incessant laughter is critical in the emergency setting as some diagnoses warrant acute treatment, while others necessitate further work-up.29 The differential should include gelastic seizures, pseudobulbar affect, intoxication, poisoning, psychogenic nonepileptic seizures, Angelman syndrome, psychiatric pathology, and normal laughter.29–31 Since gelastic seizures have been observed in patients with hypothalamic, frontal lobe, and temporal lobe lesions a thorough workup should include neuroimaging of the brain, as well as an EEG.
From a neurological perspective, another important consideration is whether the laughter represents pseudobulbar affect (PBA), also known as involuntary emotional expression disorder.30 PBA is characterized by uncontrolled crying or laughing which may be disproportionate or inappropriate to the social context and may be encountered in the setting of amyotrophic lateral sclerosis (ALS), as well as extrapyramidal and cerebellar disorders, such as Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. It has also been noted in the setting of multiple sclerosis, traumatic brain injury, Alzheimer's disease, various types of dementias, stroke, and brain tumors.30 The etiology is thought to originate from disruption of pathways involving serotonin and glutamate, particularly in the corticolimbic and cerebellar pathways, leading to disinhibition. PBA is commonly misdiagnosed as depression or bipolar disorder. It is effectively treated therapeutically with a combination of dextromethorphan and quinidine. Thus, early diagnosis can be beneficial. The use of DSM-5 criteria for the diagnosis of mood disorders, as well as the use of published criteria to help differentiate PBA from depression, can aid in distinguishing PBA from other psychiatric diagnoses in the setting of incessant laughter.30
Another important diagnosis to consider on the differential is intoxication especially with alcohol, LSD, and cannabis.29 Urine toxicology screening may be warranted if the history and clinical presentation raise suspicion of accidental or intentional ingestion of these substances. Laughter may also be a manifestation of psychogenic nonepileptic seizures (PNES), which are abrupt, involuntary seizure-like attacks characterized by changes in consciousness or behavior.31 PNES are not associated with electrophysiologic seizures, unlike with epilepsy. Video- EEG monitoring can be useful in differentiating these attacks from seizures. Other diagnoses to consider on the differential are genetic conditions, such as Angelman syndrome, other psychiatric conditions, and normal laughter. Genetic testing and psychological assessment can be performed to investigate genetic and psychiatric basis of the behavior.
In summary, the differential for incessant laughter is extensive and distinguishing different causes can be difficult. Neuroimaging techniques, video EEG, toxicology and psychiatric screening, and genetic testing can all aid in narrowing the differential, in addition to a thorough history and physical exam.
Conclusion
In patients with behavioral issues, especially those with inappropriate, uncontrolled laughter or giggling, gelastic seizure needs to be included in the differential diagnosis. Furthermore, a thorough workup should include neuroimaging to rule out a hypothalamic or temporal/frontal lobe lesion, and an EEG to investigate epileptiform activities. Because this condition is often pharmacoresistant and progressive with worsening cognitive and behavioral issues, early and accurate diagnosis, as well as patient and family education, is critical.7
Unfortunately, GS usually does not respond to AED and therefore minimally invasive surgical or radiosurgical therapies are often used to remove primary lesion with variable results for improvement of symptoms.13,28 Existing data shows early removal of HH can rapidly resolve symptoms. However, long term outcomes and complications have not been studied in depth.
Conflict of Interest
None of the authors report a conflict of interest.
References
- 1.Maixner W. Hypothalamic hamartomas—clinical, neuropathological and surgical aspects. Child' Nervous System. 2006;22(8):867–873. doi: 10.1007/s00381-006-0129-0. [DOI] [PubMed] [Google Scholar]
- 2.Berkovic Sf, Arzimanoglou A, Kuzniecky R, Harvey AS, Palmini A, Andermann F. Hypothalamic hamartoma and seizures: a treatable epileptic encephalopathy. Epilepsia. 2003;44(7):969–973. doi: 10.1046/j.1528-1157.2003.59102.x. [DOI] [PubMed] [Google Scholar]
- 3.Tellez-Zenteno J, Serrano-Almeida C, Moien-Afshari F. Gelastic seizures associated with hypothalamic hamartomas. An update in the clinical presentation, diagnosis and treatment. NDT Neuropsychiatric Disease and Treatment. 2008;4(6):1021–1031. doi: 10.2147/ndt.s2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovac S, Diehl B, Wehner T, Fois C, Toms N, Walker MC, Duncan JS. Gelastic Seizures: Incidence, clinical and EEG features in adult patients undergoing video-EEG telemetry. Epilepsia. 2015;56(1):e1–e5. doi: 10.1111/epi.12868. [DOI] [PubMed] [Google Scholar]
- 5.Trousseau A. De L'Epilepsie. 1877. Clinique Medicale de L'Hotel-Dieu de Paris; pp. 89–155. (Fre). [Google Scholar]
- 6.Daly DD, Mulder DW. Gelastic epilepsy. Neurology. 1957;7:189–192. doi: 10.1212/wnl.7.3.189. [DOI] [PubMed] [Google Scholar]
- 7.Striano S, Striano P, Sarappa C, et al. The clinical spectrum and natural history of gelastic epilepsy-hypothalamic hamartoma syndrome. Seizure. 2005;14:232–239. doi: 10.1016/j.seizure.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Sturm JW, Andermann F, Berkovic SF. Pressure to laugh”: an unusual epileptic symptom associated with small hypothalamic hamartomas. Neurology. 2000;54(4):971–973. doi: 10.1212/wnl.54.4.971. [DOI] [PubMed] [Google Scholar]
- 9.Chan YL, Leung SF, King AD, Choi PH, Metreweli C. Late radiation injury to the temporal lobes: morphologic evaluation at MR imaging. Radiology. 1999 Dec;213(3):800–807. doi: 10.1148/radiology.213.3.r99dc07800. [DOI] [PubMed] [Google Scholar]
- 10.Wild B, Rodden FA, Grodd W, Ruch W. Neural correlates of laughter and humour. Brain. 2003;126(Pt. 10):2121–2138. doi: 10.1093/brain/awg226. [DOI] [PubMed] [Google Scholar]
- 11.Munari C, Kahane P, Francione S, et al. Role of the hypothalamic hamartoma in the genesis of gelastic fits (a video-stereo-EEG study) Electroencephalogr Clin Neurophysiol. 1995;95:154–160. doi: 10.1016/0013-4694(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 12.Leal AJR, Monteiro JP, Secca MF, et al. Functional brain mapping of ictal activity in gelastic epilepsy associated with hypothalamic hamartoma: a case report. Epilepsia. 2009;50:1624–1631. doi: 10.1111/j.1528-1167.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 13.Striano S, Santulli L, Ianniciello M, Ferretti M, Romanelli P, Striano P. The gelastic seizures-hypothalamic hamartoma syndrome: Facts, hypotheses, and perspectives. Epilepsy & Behavior. 2012;24(1):7–13. doi: 10.1016/j.yebeh.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Xu L, Kim DY, Rho JM, St John PA, Lue LF. Electrophysiological properties of human hypothalamic hamartomas. Ann Neurol. 2005;58(3):371–382. doi: 10.1002/ana.20580. [DOI] [PubMed] [Google Scholar]
- 15.Kuzniecky RI, Guthrie B, Mountz J, et al. Intrinsic epileptogenesis of hypothalamic hamartomas in gelastic epilepsy. Ann Neurol. 1997;42(1):60–67. doi: 10.1002/ana.410420111. [DOI] [PubMed] [Google Scholar]
- 16.Schwarts T. Treatment options for hypothalamic hamartomas—no laughing matter. Epilepsy Currents. 2007;7(3):72–74. doi: 10.1111/j.1535-7511.2007.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arita K, Ikawa F, Kurisu K, et al. The relationship between magnetic resonance imaging findings and clinical manifestations of hypothalamic hamartoma. J Neurosurg. 1999;91:212–222. doi: 10.3171/jns.1999.91.2.0212. [DOI] [PubMed] [Google Scholar]
- 18.Kovac S, Diehl B, Wehner T, Fois C, Toms N, Walker MC, Duncan J. Gelastic seizures: Incidence, clinical and EEG features in adult patients undergoing video-EEG telemetry. Epilepsia. 2015;56(1):e1–e5. doi: 10.1111/epi.12868. [DOI] [PubMed] [Google Scholar]
- 19.Sivaswamy L, Ah Lee Y, Borgohain P. A 2-year-old boy with gelastic seizures. The Journal of Pediatrics. 2014;165:1067. doi: 10.1016/j.jpeds.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Uribe-San-Martin R, Ciampi E, Lawson-Peralta B, Acevedo-Gallinato K, Torrealba-Marchant G, Campos-Puebla M, Godoy-Fernandez J. Gelastic epilepsy: Beyond hypothalamic hamartomas. Epilepsy & Behavior Case Reports. 2015;4:70–73. doi: 10.1016/j.ebcr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maximov GK, Maximov KG, Maximova YK. Late-Onset Cryptogenic Gelastic Epilepsy. Pharmacologyonline. 2009;1:1–4. [Google Scholar]
- 22.Striano S, Meo R, Bilo L, Cirillo S, Nocerino C, Ruosi P, Striano P, Estraneo A. Gelastic epilepsy: symptomatic and cryptogenic cases. Epilepsia. 1999;40(3):294–302. doi: 10.1111/j.1528-1157.1999.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 23.Gascon GG, Lombroso CT. Epileptic laughter. Epilepsia. 1971;12:63–76. doi: 10.1111/j.1528-1157.1971.tb03916.x. [DOI] [PubMed] [Google Scholar]
- 24.Troester M, Haine-Schlagel R, Ng Y, Chapman K, Chung S, Drees C, Prenger E, Rekate H, Kerrigan JF. EEG and video-EEG seizure monitoring has limited utility in patients with hypothalamic hamartoma and epilepsy. Epilepsia. 2011;52(6):1137–1143. doi: 10.1111/j.1528-1167.2011.03095.x. [DOI] [PubMed] [Google Scholar]
- 25.Saleem S, Said AH, Lee D. Lesions of the hypothalamus: MR Imaging diagnostic features. Radiographics. 2007;27:1087–1108. doi: 10.1148/rg.274065123. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld JV, Harvey AS, Wrennall J, Zacharin M, Berkovic SF. Transcallosal resection of hypothalamic hamartomas, with control of seizures, in children with gelastic epilepsy. Neurosurgery. 2001;48:108–118. doi: 10.1097/00006123-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Procaccini E, Dorfmuller G, Fohlen M, Bulteau C, Delalande O. Surgical management of hypothalamic hamartomas with epilepsy: the stereoendoscopic approach. Operative Neurosurgery. 2006;59(4):ONS-336–ONS-346. doi: 10.1227/01.NEU.0000233900.06146.72. [DOI] [PubMed] [Google Scholar]
- 28.Regis J, Scavarda D, Tamura M, Nagayi M, Villeneuve N, Bartolomei F, Brue T, Dafonseca D, Chauvel P. Epilepsy related to hypothamalic hamartomas: surgical management with special reference to gamma knife surgery. Child's Nervous System. 2006;22:881–895. doi: 10.1007/s00381-006-0139-y. [DOI] [PubMed] [Google Scholar]
- 29.Holmes C, Goldman M. Seizures presenting as incessant laughter: A case of gelastic epilepsy. Journal of Emergency Medicine. 2012;43(6):e447–e449. doi: 10.1016/j.jemermed.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Therapeutics and Clinical Risk Management. 2013;9:483–489. doi: 10.2147/TCRM.S53906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mascia A, Quarato PP, D'Aniello A, Di Gennaro G. Psychogenic nonepileptic seizures mimicking gelastic seizures: A description of two cases. Epilepsy & Behavior Case Reports 4. 2015:67–69. doi: 10.1016/j.ebcr.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


