Abstract
Urban slums provide suitable conditions for infestation by rats, which harbour and shed a wide diversity of zoonotic pathogens including helminths. We aimed to identify risk factors associated with the probability and intensity of infection of helminths of the digestive tract in an urban slum population of Rattus norvegicus. Among 299 rats, eleven species/groups of helminths were identified, of which Strongyloides sp., Nippostrongylus brasiliensis and, the human pathogen, Angiostrongylus cantonensis were the most frequent (97, 41 and 39%, respectively). Sex interactions highlighted behavioural differences between males and females, as eg males were more likely to be infected with N. brasiliensis where rat signs were present, and males presented more intense infections of Strongyloides sp. Moreover, rats in poor body condition had higher intensities of N. brasiliensis. We describe a high global richness of parasites in R. norvegicus, including five species known to cause disease in humans. Among these, A. cantonensis was found in high prevalence and it was ubiquitous in the study area – knowledge which is of public health importance. A variety of environmental, demographic and body condition variables were associated with helminth species infection of rats, suggesting a comparable variety of risk factors for humans.
Keywords: Rattus norvegicus, helminth community, slum settlements, human pathogens, Angiostrongylus cantonensis, risk factors, spillover risk, urban
Introduction
Economic development has led to an increase in urbanization, as people migrate from rural areas in search of job and education opportunities (UN-Habitat, 2016). In many developing countries, this has, in turn, led to an increase in urban poverty, reflected in poor housing, infrastructural support, and social and health services – characteristics that define slum settlements (UN, 2003; Ooi and Phua, 2007). Although there have been initiatives to reduce poverty, such as the strategies undertaken in pursuit of the Millenium Development Goals, the number of slum residents increased by 28% from 1990 to 2014 (from 689 to 881 million) (UN, 2005; UN-Habitat, 2016).
The conditions imposed on urban slum residents, such as lack of adequate sanitation and garbage collection, provide resource-rich environments supporting large populations of rats (Riley et al. 2007; Costa et al. 2014a). The Norway rat, Rattus norvegicus Berkenhout (1769), has been the most successful invasive mammalian species, exhibiting tolerance to a wide range of temperatures, a high reproductive rate and adaptability to rural and urban environmental modification (Long, 2003; Vadell et al. 2014; Panti-May et al. 2016; Puckett et al. 2016). This species has been associated with economic losses due to its eclectic diet, contamination of food stores with feces and urine, and infrastructure damage inflicted through gnawing and burrowing (Childs et al. 1998; Singleton et al. 2003; Almeida et al. 2013). Additionally, R. norvegicus is a host for several agents that can infect humans (Webster and Macdonald, 1995; Ko et al. 1999; de Faria et al. 2008; Costa et al. 2014b) including the bacteria Leptospira (primarily of the Icterohaemorrhagiae serogroup). These pathogens impart a large burden of disease in human populations, with leptospirosisis being by far the most important with an estimated 1 000 000 annual human cases and 60 000 deaths (Costa et al. 2015a). Norway rats are also reservoir hosts of several helminths that cause disease in humans (Rafique et al. 2009; Hancke et al. 2011; Walker et al. 2017). For example, the cestode Hymenolepis nana is associated with diarrhoea cases, whereas the nematode Angiostrongylus cantonensis can cause eosinophilic meningitis (EoM) (Alicata, 1965; Schantz, 1996; Alruzug et al. 2016).
Notwithstanding the importance of the descriptive work in characterizing helminth communities of R. norvegicus (Gómez Villafañe et al. 2008; Kataranovski et al. 2010; Hancke et al. 2011; Nursyazana et al. 2013), it is additionally important to understand the potential risk factors structuring these communities (Mohd Zain et al. 2012; Simões et al. 2016), enabling understanding of the risk of spillover to humans. This is especially relevant to slum settlement since the peridomestic habits of Norway rats and high human densities lead to frequent human–rodent contact and human exposure to environments contaminated with the infectious stages of parasites shed in rat excreta (McGarry et al. 2015; Costa et al. 2015b). Despite the effort to characterize the risk factors aforementioned, these studies have been especially concentrated on environmental variables and demographic variables of the rats. However, individuals in natural populations will also vary in their ability to acquire necessary resources, which can affect their body condition, and fitness, by influencing vulnerability and resistance to infection (Beldomenico and Begon, 2010). In this sense, susceptible hosts are more likely to get an infection, further reducing their body condition, in a vicious circle. This, in turn, may influence the concentration of parasites excreted in the environment (e.g., Costa et al. 2015b), raising the chance for infection of other hosts, including humans. In this study, therefore, we combined environmental variables, variables of rat demography and body condition, and signs of rat infestation (as proxies for rat density), to identify potential risk factors that are associated with the probability and intensity of infection of helminths of the digestive tract in an urban population of R. norvegicus.
Materials and methods
Study area
The study area was located in the periphery neighbourhood of Pau da Lima (13°32′53·47″ S; 38°43′51·10″ W) in the city of Salvador (BA – Brazil), which has a high human population density, varying from 13 742 to 128 997 inhabitants km−2 (IBGE, 2010). The area, 0·17 km2 in extent, covers residential areas in three valleys characterized by poor sanitation conditions, with open sewers and lack of refuse collection (Reis et al. 2008). The valleys are occupied by people commonly living in an informal infrastructure of houses (determined by residents’ patterns of settlement and permeable to rats), also lacking access to officially-provided services (Riley et al. 2007). The area was selected for study as it presents high annual incidence of Leptospira asymptomatic infection (35·4 per 1000 person-years) and of severe leptospirosis cases (19·8 per 100 000 pop.) (Felzemburgh et al. 2014; Hagan et al. 2016), acquired from spirochetes shed in the contaminated urine of Norway rats, and residents are therefore at high risk of infection by intestinal parasites maintained by the same reservoir host.
The sampling design has been reported previously (Panti-May et al. 2016). Briefly, it included a randomized sample, from spatial maps, of 150 trapping points in the three valleys of Pau de Lima. However, in slum environments, such as in Pau da Lima, residents and visitors are subject to violence due to drug traffic and hence there is restricted access, so that the trapping locations were reduced to 101 (valley 1:25 points, 2 : 36 and 3 : 40) (see Supplementary Fig. S1). Each of the 101 trapping points included three different trapping sites within an area of 30 m2.
Trapping methods and tissue sampling
Rodents were trapped with Tomahawk traps (45 × 16 × 16 cm3) from the three valleys during two sampling campaigns in 2014: a rainy season (March to July 2014) and a dry season (October to December 2014). In each campaign, two trapping sessions were performed at each of the 101 points, at least one month apart. One of three sites randomly chosen within the area of 30 m2 was sampled; a sampling scheme previously described (Panti-May et al. 2016). In each site, two traps were set and baited at 9:00 am, and checked within 24 h. The expected number of trap-nights was of 2424 and 1616 (points × sessions × traps × nights) for the rainy and the dry seasons, respectively. The trapping efforts were shortened for the dry season sampling (from 6 to 4 nights) due to a complementary analysis performed with trapping information from previous campaigns, which showed that the mean abundance estimations of rats did not change significantly when reducing sampling effort for four nights. This was consistent with the previous expectation of capturing a higher number of rats during the first days of sampling (Hacker et al. 2016).
Previously developed environmental surveys were conducted at each point of capture once in each session (Costa et al. 2014a; Santos et al. 2017). Variables, subsequently included as covariates in our model development, were used to assess evidence of the presence of rats (presence of feces and trails, and number of burrows) and availability of food and harbourage sites for rats (pet food, human garbage, water sources and vegetation coverage by number of trees), in addition to assessing information relevant to helminth parasite survival and potential for human exposure (completely permeable or partially paved ground, and site proximity of at least 10 m to an open sewer). Daily rainfall data (mm) was obtained from the Environment and Water Resources Institute of the state of Bahia (INEMA) station, and the total rainfall (mm) per month was computed for the campaigns of active trapping and for lags of 30, 60 and 90 days. Traps containing individuals of R. norvegicus were placed in plastic bags and transported to a field laboratory, where individual rats were anesthetized and humanely killed. For each individual rat we obtained characteristics informative of population demography (mass, body length, sex and reproductive status) and of body condition (weight and length – for the estimate of an index later described – presence of wounds and internal fat – visceral or subcutaneous) (see Supplementary Table S1) (Glass et al. 1988; Costa et al. 2014b; Himsworth et al. 2014). These were recorded in an online database (REDCap). During the necropsies, performed for purposes of a larger project on leptospirosis (Costa et al. 2015b; Panti-May et al. 2016), fecal samples were collected directly from the intestines and placed in 10% formalin for subsequent analysis. Trapping and handling of rodents followed protocols previously validated and described (Mills et al. 1995; Costa et al. 2014b, 2015b) and approved by the Ethical Committee of the Animal Use (CEUA) protocol 003/ 2012 of the CPqGM – Oswaldo Cruz Foundation (Fiocruz).
Data collection
Formalin-fixed fecal samples were taken to a laboratory of parasitology for identification of helminth eggs by sedimentation (Hoffman et al. 1934), taking into account helminth lists previously described for R. norvegicus (Martins and Pessôa, 1977; Vicente et al. 1997). Subsequently, Gordon and Whitlock’s (1939) flotation technique with McMaster chambers was carried out for the quantification of helminth eggs in eggs per gram of feces (EPG). Because fecal samples had already been processed for the previous method, to perform the flotation technique we discarded the supernatant (10% formalin) and mixed the fecal content with a saline solution (Zinc Sulphate, gravity: 1·20), as recommended (Faust et al. 1938; Bartlett et al. 1978; Schnyder et al. 2011). The volume of saline solution added to each fecal sample varied with fecal weights since it was not always possible to collect 2 g of feces from the rats (we adapted the technique by adding a proportional volume of saline solution).
Statistics
The variable mass was used as a surrogate estimate of rat ‘age’ (Table 1) as used by other studies to allow for direct comparisons (Glass et al. 1988; Costa et al. 2014a). However, we also estimated age in days [age(d)] using a statistical model derived from published age–weight growth curve for wild rats (Calhoun, 1962), as described by Panti-May et al. (2016) (see Supplementary Fig. S2). We also improved the use of a weight/length ratio index of overall body condition variable, by using a scaled mass index (Smi), which accounts for the effect of age (Peig and Green, 2009). Then, to assess whether the demographic features of the populations sampled in the two seasons were similar, we compared age categories, sex and reproductive status through two-way contingency tables using chi-squared (χ2) test, and Smi using Wilcoxon rank sum test (since the assumption of normality was not met) (Table 1) (Crawley, 2007). Variables of reproductive status (e.g., presence of placental scars in females or presence of coiled seminal vesicle in males) were used to create a single three-level categorical variable of maturity for males and females – classified as immature, young-mature (first pregnancy; only females) or mature – to be assessed as a risk factor later (for details see Supplementary Table S1).
Table 1.
Demographic characterization of R. norvegicus populations in the two campaigns of capture
| Rainy season |
Dry season |
||||
|---|---|---|---|---|---|
| Total n of rats | n (%) or Median (IQR) | Total n of rats | n (%) or Median (IQR) | p | |
| n of trapped rats | 179 | 120 | |||
| Age categories | 179 | 120 | |||
| Young (<200 g) | 40 (22·4) | 25 (20·8) | 0·87 | ||
| Subadult (200–399 g) | 113 (63·1) | 72 (60·0) | 0·67 | ||
| Adult (≥400 g) | 26 (14·5) | 23 (19·2) | 0·37 | ||
| Scaled mass index (Smi)a | 179 | 262·3 (236·6–289·0) | 120 | 272·9 (237·6–304·8) | 0·14 |
| Sex | 179 | 120 | |||
| Male | 87 (48·6) | 65 (54·2) | 0·41 | ||
| Female | 92 (51·4) | 55 (45·8) | 0·41 | ||
| Female | 92 | 55 | |||
| Placental scars | 44 (47·8) | 32 (58·2) | 0·30 | ||
| Pregnant | 33 (35·9) | 18 (32·7) | 0·84 | ||
| Lactating | 23 (25·0) | 20 (36·4) | 0·20 | ||
| Male | 87 | 65 | |||
| Scrotal testes | 77 (88·5) | 57 (87·7) | 1·00 | ||
| Coiled seminal vesicles | 72 (82·8) | 51 (78·5) | 0·65 | ||
IQR, Interquartile range.
Helminth richness (the number of species present) was estimated for each individual rat and means were compared between sex and maturity, using Wilcoxon rank sum test and permutation ANOVA (Fisher LSD post-test), respectively, as richness distributions between sexes or maturity categories did not follow the normal distribution. Whether helminth richness varied with rats age (d) was also assessed by a generalized linear model (GLM) with Poisson family errors. The prevalence of each helminth species or group (see below) was estimated (% positive of feces sampled) and compared between sampling campaigns through two-way contingency tables using χ2 test. The laboratory technique used to estimate eggs in feces produces an estimate of EPG, rounded to the nearest 50, hence estimated zeros correspond to EPG between zero and 25. Samples in which a helminth egg infection was identified during sedimentation (presence/absence method), but found to be absent during McMaster counting are ‘false negatives’. By this definition, approximately 25% of the rats found positive to one helminth species were false negatives. For these, we replaced the zero by an imputed value as follows. Cumulative plots of the recorded EPG showed a variety of shapes. We assumed that the lower tail of the cumulative distribution for each species followed a power law, i.e. a linear relationship on a log-log scale. We fitted this relationship to each species ignoring the false negatives, then shifted the false negatives to the corresponding x-value on the fitted line, and back-transformed this value to the original scale to give an imputed value (see Supplementary Fig. S3).
To investigate the risk factors associated with the probability (dependent binary variable) and intensity (dependent continuous variable, including only positive individuals) of infection of each helminth species, GLMs with binomial or Gamma errors, respectively, were applied (Crawley, 2007). Because the EPG values are proxies for the intensity of helminth infestation in R. norvegicus, the EPG values were log2 transformed for ease of interpretation. Additionally, we included the time interval (in months) between fecal sampling and laboratory quantification analysis’ – Interval-sampling-analysis’ – as a covariate in the intensity models to control for any effect of formalin fixation in the egg quantification. Initially, univariate analyses were applied and only variables with values of P < 0·1 were included in subsequent models, which were developed in steps. First, a model was built with statistically significant environmental variables, collected during the application of the environmental surveys as previously described. Then, model simplifications were conducted using a threshold of 2 Akaike Information Criterion, corrected for small samples [corrected Akaike’s information criterion (AICc)] according to Hurvich and Tsai (1989), to generate a minimal adequate model of environmental predictors. Next, the rat demographic variables [sex, maturity and age(d)] were added and the model simplifications were re-run. Finally, to the minimal adequate model containing both environmental and rat demographic predictors, variables of rat body condition (Smi, the presence of wounds and internal fat) were added and model simplifications again re-conducted. The most parsimonious model was chosen with a ΔAICc < 2 compared with the final minimum model (indistinguishable explanatory power). We present the odds ratio (OR) and rates (Rate) of the significant variables in the binomial and gamma models, respectively. For all models, observations with missing values for any of the variables under evaluation were excluded. All the analyses were performed in R (R Development Core Team, 2011), considering a significance level of P < 0·05.
Results
Samples from 299 individuals of R. norvegicus were obtained: 179 in the rainy season, and 120 in the dry season, after an effort of 2318 and 1494 trap-nights, respectively. The demographic features of rats did not differ between campaigns of capture (Table 1).
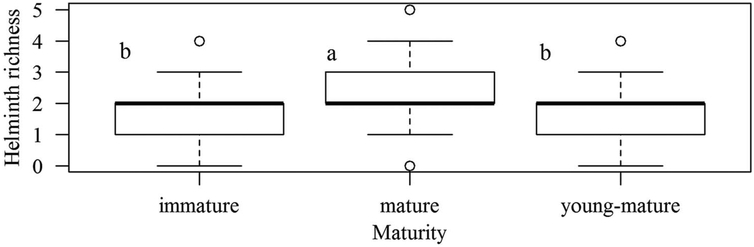
Eleven species/groups of helminths were identified in the feces of R. norvegicus (Table 2). Richness ranged from zero to five helminth species per individual rat, with a median of two (interquartile range of 1–2 species) (n = 299 rats). Mean richness did not differ between sex or age (d), however mature rats presented with higher richness than immature and young mature rats (5000 iterations; P < 0·0001) (Fig. 1). Overall, the most frequent species were Strongyloides sp., A. cantonensis and Nippostrongylus brasiliensis. For N. brasiliensis, the prevalence in the dry season was significantly higher than in the rainy season χ2 = 19·802, df = 1, P < 0·0001). The five potential pathogenic species for humans are shown in bold in Table 2. Of these, A. cantonensis, which in feces occurs in the larval stage (L1), was among the most prevalent species and Hymenolepis diminuta also moderately common. Eggs of Toxocara sp. and Ascaris sp. were also observed in feces samples, but not included in any analysis as rats do not serve as natural reservoirs.
Table 2.
Prevalence of helminth species of the digestive tract identified, by eggs or larvae, in an urban population of R. norvegicus
| Total (n = 299) |
Rainy season (n = 179) |
Dry season (n = 120) |
|
|---|---|---|---|
| Species | % (n) | % (n) | % (n) |
| Strongyloides sp.a | 96·6 (289) | 97·8 (175) | 95·0 (114) |
| Nippostrongylus brasiliensisb | 40·8 (122) | 30·2 (54) | 56·7 (68) |
| Angiostrongylus cantonensis | 39·1 (117) | 43·0 (77) | 33·3 (40) |
| Hymenolepis diminuta | 13·4 (40) | 13·4 (24) | 13·3 (16) |
| Trichuridaec | 9·0 (27) | 7·3 (13) | 11·7 (14) |
| Gongylonema neoplasticum | 6·4 (19) | 5·6 (10) | 7·5 (9) |
| Aspiculuris tetraptera | 0·3 (1) | 0·6 (1) | 0·0 (0) |
| Hymenolepis nano | 0·3 (1) | 0·0 (0) | 0·8 (1) |
| Syphacia muris | 0·3 (1) | 0·6 (1) | 0·0 (0) |
| Toxocara sp. | 0·7 (2) | 0·6 (1) | 0·8 (1) |
| Ascaris sp. | 1·7 (5) | 0·0 (0) | 4·2 (5) |
Species potentially pathogenic to humans are shown in bold.
S. rotti or S. venezuelensis.
Prevalence was significantly higher in the dry season (P < 0·0001).
Capillaria gastrica or Trichuris muris.
Fig. 1.
Boxplots of richness by the maturity of rats (n = 299). Different letters mean statistical significance.
Aspiculuris sp. infection was likely to have been incidental (Falcón-Ordaz et al. 2010) and, together with Syphacia muris was excluded from the statistical models later performed due to the low prevalence. Positive results for H. nana were pooled with those of H. diminuta, because of phylogenetic proximity and mode of transmission; this group will be referred to, hereafter, as Hymenolepis spp. The summaries of model selections for the probabilities and intensities of infection of each helminth species or families of helminth species are provided in Table 3. Details of each final model are available in Supplementary Table S2.
Table 3.
Summary of model performances in explaining the probability of infection (glm, family = binomial) and intensity [glm, family = Gamma(link = ‘identity’)] of each helminth species
| Models for infection |
Models for intensity of infection |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parasite | Models | AICc | ΔAICc | wi | Models | AICc | ΔAICc | wi |
| Strongyloides sp. | i. y~ Burrows + Sewer + Sex + Age(d) + Smi + Age2(d) + Sex:Sewer | 80·99 | 0·00 | 0·54 | i. log2(y) ~ Season + Cumulative rain + Ground + Burrows + Sex + Age (d) + Sex:Ground + Sex:Age(d) | 963·62 | 0·00 | 0·57 |
| ii. y ~ Burrows + Sewer + Sex + Age(d) + Age2(d) + Sex:Sewer | 82·27 | 1·28 | 0·28 | ii. log2(y) ~ Season + Cumulative rain + Ground + Sex + Age(d) + Sex:Ground + Sex:Age(d) | 964·56 | 0·94 | 0·36 | |
| iii. y ~ Burrows + Sewer + Sex + Age(d) + Smi + Sex:Sewer | 83·25 | 2·27 | 0·17 | iii. log2(y) ~ Season + Cumulative rain + Ground + Burrows + Sex + Sex: Ground | 967·88 | 4·26 | 0·07 | |
| iv. y ~ 1 | 89·63 | 8·64 | 0·01 | iv. log2(y) ~ 1 | 1021·37 | 57·75 | 0·00 | |
| Nippostrongylus brasiliensis | i. y ~ Season + Trails + Sex + Age(d) + Age2(d) + Sex:Trails + Sex:Age(d) | 368·59 | 0·00 | 0·79 | i. log2(y) ~ Season + Fat presence + Interval-sampling-analysis | 412·92 | 0·00 | 0·51 |
| ii. y ~ Season + Trails + Sex + Age(d) + Sex:Trails + Sex:Age(d) | 371·24 | 2·65 | 0·21 | ii. log2(y) ~ Fat presence + Interval-sampling-analysis | 413·42 | 0·50 | 0·40 | |
| iii. y ~ Season + Trails + Sex + Sex:Trails | 378·95 | 10·37 | 0·00 | iii. log2(y) ~ Season + Interval-sampling-analysis | 416·44 | 3·52 | 0·09 | |
| iv. y ~ 1 | 403·50 | 34·91 | 0·00 | iv. log2(y) ~ 1 | 434·99 | 22·08 | 0·00 | |
| Angiostrongylus cantonensis | i. y ~ Valley + Sex + Maturity + Smi + Sex:Valley | 361·09 | 0 | 0·69 | i. log2(y) ~ Cumulative rain + Tree + Burrows + Sex +Sex:Cumulative rain | 497·13 | 0·00 | 0·36 |
| ii. y ~ Valley + Sex + Maturity + Sex:Valley | 362·72 | 1·628 | 0–31 | ii. log2(y) ~ Cumulative rain + Tree + Burrows + Sex + Smi + Sex: Cumulative rain | 497·20 | 0·07 | 0·35 | |
| iii. y ~ Valley + Sex + Sex:Valley | 383·85 | 22·76 | 0 | iii. log2(y) ~ Cumulative rain + Tree (n) + Sex + Sex:Cumulative rain | 497·65 | 0·52 | 0·28 | |
| iv. y ~ 1 | 392·67 | 31·58 | 0 | iv. log2(y) ~ 1 | 502·96 | 5·84 | 0·02 | |
| Hymenolepis spp. | i. y ~ Valley | 226·20 | 0·00 | 0·99 | ||||
| ii. y ~ 1 | 240·74 | 14·54 | 0·00 | |||||
| Trichuridae | i. y ~ Valley + Sex + Maturity + Sex:Maturity | 172·19 | 0·00 | 0·55 | ||||
| ii. y ~ Valley | 172·73 | 0·54 | 0·42 | |||||
| iii. y ~ 1 | 178·32 | 6·13 | 0·03 | |||||
| Gongylonema neoplasticum | i.y ~1 | 143·51 | 0·00 | 0·62 | ||||
| ii. y ~ Smi + Sex + Sex:Smi | 144·46 | 0·95 | 0·38 | |||||
AICc, corrected Akaike’s information criterion; ΔAICc, difference between AICc score and lowest AICc score; wi, Akaike’s model weight.
The most parsimonious models are shown in bold.
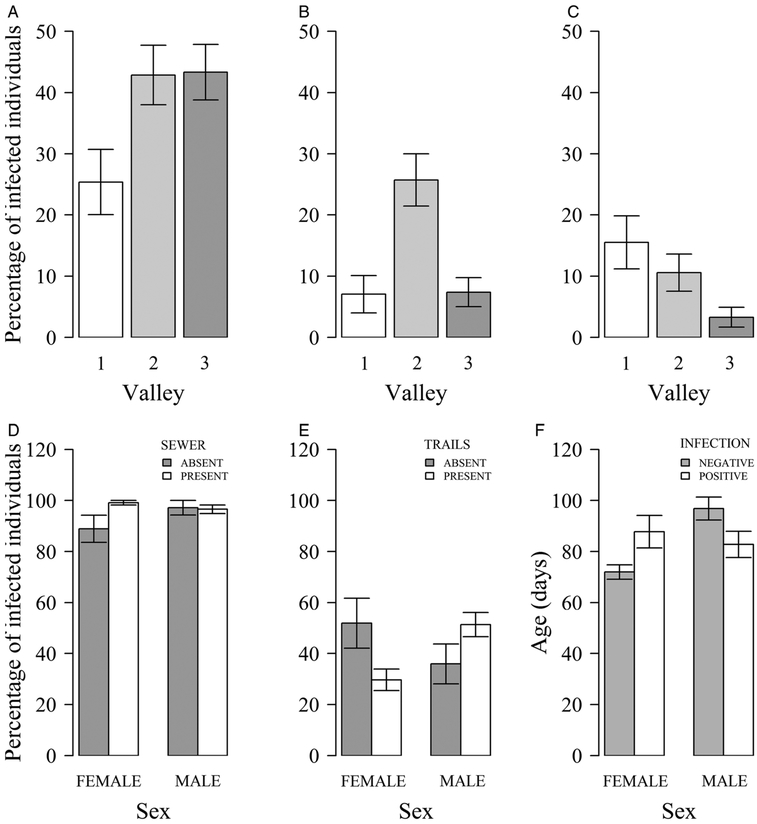
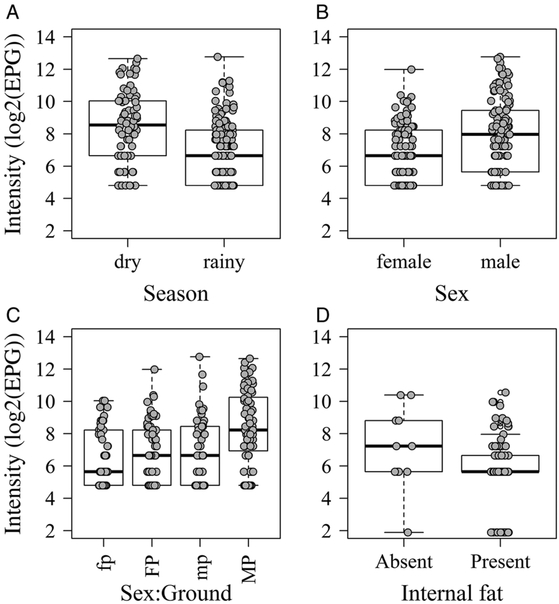
Variables of the environmental features and the demography and condition of the rats were significantly associated with the probabilities and intensities of infection of the helminth species, except for Gongylonema neoplasticum. Valleys 2 and 3 within the study area were significantly associated with higher probabilities of infection by A. cantonensis [Valley 3: OR, 3·67 (95% CI, 1·44–9·91)] and Hymenolepis spp. [Valley 2: 4·57 (1·80–14·07)] relative to valley 1 (Fig. 2A and B). On the other hand, compared with valley 1, valley 3 was significantly associated with lower probabilities of infection by Trichuridae [Valley 3: OR, 0·18 (95% CI, 0·05–0·57)] (Fig. 2C). The dry season was significantly associated with higher intensities of Strongyloides sp. [Rainy season: Rate, 0·24 (95% CI, 0·14–0·41)] (Fig. 3A).
Fig. 2.
Mean percentages of infected rats (A–E). Infections by A. cantonensis (A), Hymenolepis spp. (B) and Trichuridae (C) per valley; by Strongyloides sp. (D) per sex and presence of sewer; by N. brasiliensis (E) per sex and presence of trails. F – Mean ages (days) of N. brasiliensis infected and not infected individuals by sex. Whiskers represent standard errors.
Fig. 3.
Boxplots of helminths’ intensities (log2(EPG)). Strongyloides sp. by season (A); Strongyloides sp. by sex (B) and by sex and ground (C); N. brosiliensis by internal fat (D). In ‘C’, fp, female:partially-permeable-ground; FP, female:permeable-ground; mp, male:partially-permeable-ground; MP, male:permeable-ground.
For infections of both Strongyloides sp. and N. brasiliensis (females only) age(d) was only significant if combined with age2(d), indicating curvilinear associations. For Strongyloides sp., the fact that age(d) was positive and age2(d) negative indicates an increased probability of being infected that levelled off or even declined in older individuals (see Supplementary Table S2). For N. brasiliensis, on the other hand, age(d) in females, when considered alone, was positive and the inclusion of age2(d) also represented a positive association, indicating an increased probability of being infected that accelerated with age (see Supplementary Table S2). Maturity, a similar variable to age2(d), was also significantly associated with the infection by A. cantonensis [Mature: OR, 5·20 (95% CI, 2·65–10·89)], and therefore, after reaching maturity all individual rats shared a similar probability of being infected by this lungworm. Associations with sex alone only bordered significance. Nonetheless, with the probability of infection by Strongyloides sp. and N. brasiliensis, interactions terms with other variables (females used as reference in the analyses) indicated, first, that it was more likely to find Strongyloides sp. in females if there was an open sewer within 10 m of the trapping site [Male:Sewer: OR, 0·01 (95% CI, 0·00–0·41)] (Fig. 2D). Also, it was more likely to find male rats infected with N. brasiliensis if rat trails were present [Male: Trails: 5·02 (1·49–17·25)] or if males were younger [Male:Age (d): 0·97 (0·96–0·99)] (Fig. 2E and F). Once infected, male rats were significantly associated with high intensities of Strongyloides sp. [Rate, 4·324 (1·640–11·343)] compared with females, especially if the ground was permeable [2·501 (1·155–5·414)] (Fig. 3B and C). Among the variables used as a proxy for rat body condition, the only significant association was the presence of internal fat being significantly associated with lower intensities of N. brasiliensis [Rate, 0·39 (95% CI, 0·15–0·85)] (Fig. 3D).
Discussion
A high global richness of enzootic parasites was identified in urban populations of R. norvegicus, including five zoonotic species. Helminth species were differently associated with a variety of risk factors, including environmental features, and demographic and body condition variables of the rat population, together with sex interactions. The potential zoonotic transmission of some of these parasites to humans is significant in the environments studied, illustrated by the high prevalence, and ubiquity, of A. cantonensis (almost 40%) in the population of urban rats of the study area.
The fact that rats have been found infected by A. cantonensis indicates that the lungworm is able to complete its life cycle in the area. This may be due to the expansion of intermediate hosts distributions in Brazil (Thiengo et al. 2007; Carvalho et al. 2012; Moreira et al. 2013). Humans are accidental hosts of A. cantonensis, becoming infected after ingesting contaminated raw or undercooked intermediate hosts (snails or slugs), vegetables or paratenic hosts, such as crustaceans and other molluscs (Wang et al. 2008). The infective third stage larvae, then, migrate to the central nervous system, where they trigger a strong inflammatory response caused by the increased production of eosinophils, leading to EoM (>10% of eosinophils in the cerebrospinal fluid), alternatively they may move to the eye chamber leading to ocular angiostrongyliasis (Lo Re and Gluckman, 2003; Wang et al. 2012). This has been considered an emerging disease in Brazil – the first case was reported in 2006 (Garcia et al. 2008) – and it is probably under-recognized due to lack of proper diagnostics and the scarcity of information on the distribution of A. cantonensis in the country (Morassutti et al. 2014), and of its intermediate host species, which can vary at local scales. Apart from A. cantonensis, a moderate prevalence of H. diminuta and low prevalence of H. nana were identified in the rat population. While the first is considered to rarely infect humans with asymptomatic or mild symptoms (Tena et al. 1998; Kunwar et al. 2005; Patamia et al. 2010; Tiwari et al. 2014), the second can cause diarrhoea, especially in children (Mirdha and Samantray, 2002).
Of the 299 rat feces examined, all except for five were infected by at least one helminth species. Higher richness was observed among mature rats, which are likely to be more susceptible to infection due to reproductive expenditure (Zuk, 1990). The overall helminth richness found in our study, with a community mostly formed by nematodes, is consistent with helminth richness and community characteristics in R. norvegicus worldwide (richness range of four to eleven species) (Araújo, 1967; Waugh et al. 2006; Gómez Villafañe et al. 2008; Rafique et al. 2009; Kataranovski et al. 2010; Hancke et al. 2011; Mohd Zain et al. 2012). However, the distribution of parasites within an area can vary at small scales, since it depends on local environmental conditions, host densities and, in some cases, on the presence of intermediate hosts. In this study, we found that A. cantonensis, Hymenolepis spp. and Trichuridae infections were significantly associated with different geographic valleys, and this might be due to differences in the distribution of intermediate hosts within these valleys, which require further investigation.
Nippostrongylus brasiliensis was the only helminth species that presented with differing prevalence between campaigns of capture, being higher in the dry season. Moreover, the other high prevalent soil-transmitted helminth found in this study, Strongyloides sp., interestingly, presented with higher intensities in the dry season of capture compared with the rainy season. This finding is contrary to what might be expected, given Strongyloides species’ dependency on moist environments for larval viability (Gillespie and Chapman, 2006). Because this was a cross-sectional study, a potential explanation may be that in the rainy season, where there are more humid spots for infection, more rats were caught with recent acquired Strongyloides infections, which might have dragged down the intensity estimates in that season; whereas in the dry season, rats might have been caught mostly carrying old infections, which were therefore more intense.
Sex interactions associated with differing probabilities of infection or intensities of helminths in the rat population highlight behavioural differences in space use between males and females (Wolff, 2003). The fact that more females were found to be infected with Strongyloides sp. where an open sewer was close may indicate that they spend more time in burrows situated close to sewers which offer more access to humid environments. Males, in turn, have a larger home range than females, which might increase their rate of contact with contaminated environments. Moreover, males are more susceptible to infection, owing to more energy-costly reproductive behaviours and to hormones that can both reduce immunocompetence and affect genes which determine infection resistance (Zuk, 1990; Klein, 2000). Therefore, it is reasonable to have found more intense Strongyloides sp. infections in males, and more males infected with N. brasiliensis. This parasite was also found more in younger males. Adult rats are more capable of triggering an immune response for the expulsion of N. brasiliensis than younger rats, which, if exposed to N. brasiliensis, may have a persistent infection until adulthood (Jarret et al. 1966, 1968; Dineen and Kelly, 1973). Moreover, rats in poorer body condition (absence of fat) were found to be carrying higher intensities of N. brasiliensis. Despite this seeming an intuitive conclusion, body condition is often neglected when assessing the risk factors for the probability or intensity of infection of parasites in the field. This result suggests the importance of adding body condition (as a proxy of health) variables in the models, given that individuals vary within natural populations and, therefore, in their susceptibility to infection by a parasite. However, it is worth noting that rather than previous poor condition has caused the infection, N. brasiliensis may have reduced the body condition of infected rats, as the parasite can cause anorexia (Mercer et al. 2000).
Aside from these results, the observation of eggs of Toxocara sp. and Ascaris sp. in the feces of R. norvegicus indicates recent contact with these parasites’ eggs. While Ascaris sp. is likely to be a pseudoparasite for rats, presumably after ingesting human feces (Pinto et al. 2014), R. norvegicus are considered paratenic hosts for Toxocara sp., as after ingesting animal feces, mature eggs hatch and larvae migrate into rats’ tissues, where they do not mature (Lescano et al. 2004; Santos et al. 2009). These findings indicate the circulation of these pathogens in the study area – knowledge which is of public health importance, as both can infect humans.
The sample size was a limitation of this study. With one campaign per season, the confirmation of seasonal patterns of probability and intensity of infection of the helminth species may have been prevented. Apart from that, this study was limited by the method of assessing the helminth infection (eggs in feces), which, in terms of intensity, only allowed the use of proxies by EPG. However, fecal egg counts in EPG give reliable estimates of parasite burden for a diversity of host species and are an essential tool when other intensive methods are impractical (Cringoli et al. 2004; Adejinmi and Emikpe, 2011; Lynsdale et al. 2015). Moreover, the McMaster technique applied has been considered a robust method (accurate multiplication factor) and sensitive enough to allow comparisons between different laboratories (Levecke et al. 2011).
This study provides relevant information about the helminth community of rats in a tropical slum settlement. Among the helminths, it is important to highlight the presence of species pathogenic to humans, such as A. cantonensis found in high prevalence, a finding of notable public health system importance. Along with the prevalence of helminths, the main risk factors associated with the probabilities and intensities of infection of these parasites among rats included a variety of environmental features and the demography and body condition variables of the rat population. This potentially suggests different risk factors involved in the routes for human infection. Given the high richness of helminth species found within the rat population, further work should take co-infection into account in seeking to understand how potential associations between parasites may explain their probabilities and intensities of infection and the subsequent risk of transmission to humans.
Supplementary Material
Acknowledgements.
The authors are grateful to the Fundação de Amparo à Pesquisa do Estado da Bahia – Fapesb and the Brazilian Federal Agency for Support and Evaluation of Graduate Education – Capes, which granted Mrs. Carvalho-Pereira scholarships, making this work possible. We would like to thank the staff of the Center for the Control of Zoonoses (CCZ) from Salvador for their assistance in conducting the study. This work could not be accomplished without the joint collaborative effort of the resident associations, community leaders and residents, which constitute the Urban Health Council of Pau da Lima.
Financial support. This work was supported by the Oswaldo Cruz Foundation and Secretariat of Health Surveillance, Brazilian Ministry of Health, the National Institutes of Health of the United States (grant numbers F31 AI114245, R01 AI052473, U01 AI088752, R01 TW009504 and R25 TW009338) and by the Wellcome Trust (102330/Z/13/Z).
Footnotes
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017001755
References
- Adejinmi JO and Emikpe GE (2011) Helminth parasites of some wildlife in Asejire Game Reserve, Nigeria. South African Journal of Wildlife Research 41, 214–217. [Google Scholar]
- Alicata JE (1965) Biology and distribution of the rat lungworm, Angiostrongylus cantonensis, and its relationship to eosinophilic meningo-encephalitis and other neurological disorders of man and animals. Advances in Parasitology 3, 223–248. [DOI] [PubMed] [Google Scholar]
- Almeida A, Corrigan R and Sarno R (2013) The economic impact of commensal rodents on small businesses in Manhattan’s Chinatown: trends and possible causes. Suburban Sustainability 1, 1–12. [Google Scholar]
- Alruzug IM, Khormi MM and Alhanoo IK (2016) Hymenolepis nana human diagnosed through colonoscopy: a case report. Bacteriology & Parasitology 7, 265. [Google Scholar]
- Araújo P (1967) Helmintos de Rattus norvegicus (Berkenhout, 1769) da cidade de São Paulo. Revista da Faculdade de Farmácia e Bioquímica da Universidade de São Paulo 5, 141–159. [Google Scholar]
- Bartlett MS, Harper K, Smith N, Verbanac P and Smith JW (1978) Comparative evaluation of a modified zinc sulfate flotation technique. Journal of Clinical Microbiology 7, 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldomenico PM and Begon M (2010) Disease spread, susceptibility and infection intensity: vicious circles? Trends in Ecology & Evolution 25, 21–27. [DOI] [PubMed] [Google Scholar]
- Calhoun JB (1962) The Ecology and Sociology of the Norway Rat. US Department of Health Education and Welfare, Public Health Service Publication No. 1008, USA. [Google Scholar]
- Carvalho OS, Scholte RGC, Mendonça CLF, Passos LKJ and Caldeira RL (2012) Angiostrongylus cantonensis (Nematode: Metastrongyloidea) in molluscs from harbour areas in Brazil. Memórias do Instituto Oswaldo Cruz 107, 740–746. [DOI] [PubMed] [Google Scholar]
- Childs JE, McLafferty SL, Sadek R, Miller GL, Khan AS, DuPree ER, Advani R, Mills JN and Glass GE (1998) Epidemiology of rodent bites and prediction of rat infestation in New York city. American Journal of Epidemiology 148, 78–87. [DOI] [PubMed] [Google Scholar]
- Costa F, Ribeiro GS, Felzemburgh RDM, Santos N, Reis RB, Santos AC, Fragal DBM, Araujo WN, Santana C, Childs JE, Reis MG and Ko AI (2014a) Influence of household rat infestation on Leptospira transmission in the urban slum environment. PLoS Neglected Tropical Diseases 8, e3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Porter FH, Rodrigues G, Farias H, de Faria MT, Wunder EA, Osikowicz LM, Kosoy MY, Reis MG, Ko AI and Childs JE (2014b) Infections by Leptospira interrogans, seoul virus, and Bartonella spp. Among Norway rats (Rattus norvegicus) from the Urban Slum Environment in Brazil. Vector-Borne and Zoonotic Diseases 14, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B and Ko AI (2015a) Global morbidity and mortality of leptospirosis: a systematic review. PLoS Neglected Tropical Diseases 9, e0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Wunder EA Jr., De Oliveira D, Bisht V, Rodrigues G, Reis MG, Ko AI, Begon M and Childs JE (2015b) Patterns in Leptospira Shedding in Norway rats (Rattus norvegicus) from Brazilian Slum Communities at high risk of disease transmission. PLoS Neglected Tropical Diseases 9, e0003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley MJ (2007) The R Book. 1st edn. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- Cringoli G, Rinaldi L, Veneziano V, Capelli G and Scala A (2004) The influence of flotation solution, sample dilution and the choice of McMaster slide area (volume) on the reliability of the McMaster technique in estimating the faecal egg counts of gastrointestinal strongyles and Dicrocoelium dendriticum in sheep. Veterinary Parasitology 123, 121–131. [DOI] [PubMed] [Google Scholar]
- De Faria MT, Calderwood MS, Athanazio DA, McBride AJA, Hartskeerl RA, Pereira MM, Ko AI and Reis MG (2008) Carriage of Leptospira interrogans among domestic rats from an urban setting highly endemic for leptospirosis in Brazil. Acta Tropica 108, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineen JK and Kelly JD (1973) Immunological unresponsiveness of neonatal rats to infection with Nippostrongylus brasiliensis the competence of neonatal lymphoid cells in worm expulsion. Immunology 25, 141–150. [PMC free article] [PubMed] [Google Scholar]
- Falcón-Ordaz J, Pulido-Flores G and Monks S (2010) New species of Aspiculuris (Nematoda: Heteroxynematidae), parasite of Mus musculus (Rodentia: Muridae), from Hidalgo, Mexico. Revista Mexicana de Biodiversidad 81, 669–676. [Google Scholar]
- Faust EC, D’Antoni JS, Odom V, Miller MJ, Peres C, Sawitz W, Thomen LF, Tobie J and Walker JH (1938) A critical study of clinical laboratory technics for the diagnosis of protozoan cysts and helminth eggs in feces I. Preliminary communication. American Journal of Tropical Medicine 18, 169–183. [Google Scholar]
- Felzemburgh RDM, Ribeiro GS, Costa F, Reis RB, Hagan JE, Melendez AXTO, Fraga D, Santana FS, Mohr S, dos Santos BL, Silva AQ, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG and Ko AI (2014) Prospective study of leptospirosis transmission in an Urban Slum Community: role of poor environment in repeated exposures to the Leptospira agent. PLoS Neglected Tropical Diseases 8, e2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MHO, Moraes C, Almada GL and Araújo WN (2008) Investigação de casos de meningite eosinofílica causada pela infecção por angiostrongylus cantonensis no Espírito Santo, Brasil. Secretaria de Vigilância em Saúde – Boletim Eletrônico Epidemiológico, 8, 1–5. [Google Scholar]
- Gillespie TR and Chapman CA (2006) Prediction of parasite infection dynamics in primate metapopulations based on attributes of forest fragmentation. Conservation Biology 20, 441–448. [DOI] [PubMed] [Google Scholar]
- Glass GE, Childs JE, Korch GW and LeDuc JW (1988) Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvegicus). Epidemiology & Infection 101, 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Villafañe IE, Robles MR and Busch M (2008) Helminth communities and host-parasite relationships in argentine brown rat (Rattus norvegicus). Helminthologia 45, 126–129. [Google Scholar]
- Gordon H Mcl. and Whitlock HV (1939) A new technique four counting nematode eggs in sheep faeces. Journal of Scientific & Industrial Research 12, 50–52. [Google Scholar]
- Hacker KP, Minter A, Begon M, Diggle PJ, Serrano S, Reis MG, Childs JE, Ko AI and Costa F (2016) A comparative assessment of track plates to quantify fine scale variations in the relative abundance of Norway rats in urban slums. Urban Ecosystems 19, 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JE, Moraga P, Costa F, Capian N, Ribeiro GS, Wunder EA Jr., Felzemburgh RDM, Reis RB, Nery N, Santana FS, Fraga D, dos Santos BL, Santos AC, Queiroz A, Tassinari W, Carvalho MS, Reis MG, Diggle PJ and Ko AI (2016) Spatiotemporal determinants of urban leptospirosis transmission: four-year prospective Cohort study of slum residents in Brazil. PLoS Neglected Tropical Diseases 10, e0004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancke D, Navone GT and Suarez OV (2011) Endoparasite community of Rattus norvegicus captured in a shantytown of Buenos Aires City. Helminthologia 48, 167–173. [Google Scholar]
- Himsworth CG, Jardine CM, Parsons KL, Feng AYT and Patrick DM (2014) The characteristics of wild rat (Rattus spp.) populations from an inner-city neighborhood with a focus on factors critical to the understanding of rat-associated zoonoses. PLoS One 9, e91654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WA, Pons JA and Janer JL (1934) Sedimentation concentration method in schistosomiasis mansoni. Puerto Rico Journal of Public Health and Tropical Medicine 9, 283–298. [Google Scholar]
- Hurvich CM and Tsai C-L (1989) Regression and time series model selection in small samples. Biometrika 76, 297–307. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatistica (IBGE) (2010) http://www.censo2010.ibge.gov.br/sinopseporsetores/?nivel=st, last accessed in the 11th of February 2017.
- Jarret EEE, Jarret WFH and Urquhart GM (1966) Immunological unresponsiveness in adult rats to the nematode Nippostrongylus brasiliensis induced by infection in early life. Nature 211, 1310–1311. [DOI] [PubMed] [Google Scholar]
- Jarret EEE, Jarret WFH and Urquhart GM (1968) Immunological unresponsiveness to Helminth parasites: I. The pattern of Nippostrongylus brasiliensis infection in young rats. Experimental Parasitology 23, 151–160. [DOI] [PubMed] [Google Scholar]
- Kataranovski D, Kataranovski M and Deljanin I (2010) Helminth fauna of Rattus norvegicus Berkenhout, 1769 from the Belgrade area, Serbia. Archives of Biological Sciences 62, 1091–1099. [Google Scholar]
- Klein SL (2000) The effects of hormones on sex differences in infection: from genes to behavior. Neuroscience & Biobehavioral Reviews 24, 627–638. [DOI] [PubMed] [Google Scholar]
- Ko AI, Reis MG, Dourado CMR, Johnson WD Jr., Riley LW, Ferrer SR, Guerreiro H, Salgado K, Pereira MM, Velloso LF, Carvalho CC, de Codes LMG, Orrico GS, Tavares-Neto J, Pereira MM and Lee S (1999) Urban epidemic of severe leptospirosis in Brazil. The Lancet 354, 820–825. [DOI] [PubMed] [Google Scholar]
- Kunwar CB, Subba B, Shrestha M, Chapagain RH, Jha B, Subedi J, Blangero J, Williams Blangero S and Towne B (2005) A human case of Hymenolepis diminuta infection in Nepal. Journal of Institute of Medicine 27, 66–67. [Google Scholar]
- Lescano SZ, Queiroz ML and Chieffi PP (2004) Larval recovery of Toxocara canis in organs and tissues of experimentally infected Rattus norvegicus. Memórias do Instituto Oswaldo Cruz 99, 627–628. [DOI] [PubMed] [Google Scholar]
- Levecke B, Behnke JM, Ajjampur SSR, Albonico M, Ame SM, Charlier J, Geiger SM, Hoa NTV, Kamwa Ngassam RI, Kotze AC, McCarthy JS, Montresor A, Periago MV, Roy S, Tchuem Tchuenté L-A, Thach DTC and Vercruysse J (2011) A comparison of the sensitivity and fecal egg counts of the McMaster egg counting and Kato-Katz thick smear methods for soil-transmitted helminths. PLoS Neglected Tropical Diseases 5, el201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JL (2003) Introduced Mammals of the World. Collingwood, Victoria, AU: CSIRO Publishing; and Wallingford, UK: CABI Publishing. [Google Scholar]
- Lo Re V III and Gluckman SJ (2003) Eosinophilic meningitis. American Journal of Medicine 114, 217–223. [DOI] [PubMed] [Google Scholar]
- Lynsdale CL, Franco dos Santos DJ, Hayward AD, Mar KU, Htut W, Aung HH, Soe AT and Lummaa V (2015) A standardised faecal collection protocol for intestinal helminth egg counts in Asian elephants, Elephas maximus. International Journal for Parasitology: Parasites and Wildlife 4, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins AV and Pessôa SB (1977) Pessôa Parasitologia Médica, 10th edn. Rio de Janeiro, BR: Editora Guanabara-Koogan. [Google Scholar]
- McGarry JW, Higgins A, White NG, Pounder KC and Hetzel U (2015) Zoonotic helminths of urban brown rats (Rattus norvegicus) in the UK: neglected public health considerations? Zoonoses and Public Health 62, 44–52. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Mitchell PI, Moar KM, Bissett A, Geissler S, Bruce K and Chappell LH (2000) Anorexia in rats infected with the nematode, Nippostrongylus brasiliensis: experimental manipulations. Parasitology 120, 641–647. [DOI] [PubMed] [Google Scholar]
- Mills JN, Childs JE, Ksiazek TG, Peters CJ and Velleca WM (1995) Methods for trapping and sampling small mammals for virologic testing. U.S. Department of Health & Human Services, Edn. Atlanta, USA, 61p. [Google Scholar]
- Mirdha BR and Samantray JC (2002) Hymenolepis nana: a common cause of paediatric diarrhoea in Urban Slum Dwellers in India. Journal of Tropical Pediatrics 48, 331–334. [DOI] [PubMed] [Google Scholar]
- Mohd Zain SN, Behnke JM and Lewis JW (2012) Helminth communities from two urban rat populations in Kuala Lumpur, Malaysia. Parasites & Vectors 5, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morassutti AL, Thiengo SC, Fernandez M, Sawanyawisuth K and Graeff-Teixeira C (2014) Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Memórias do Instituto Oswaldo Cruz 109, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira VLC, Giese EG, Melo FTV, Simões RO, Thiengo SC, Maldonado A Jr. and Santos JN (2013) Endemic angiostrongyliasis in the Brazilian Amazon: Natural parasitism of Angiostrongylus cantonensis in Rattus rattus and R. norvegicus, and sympatric giant African land snails. Achatina fulica, Acta Tropica 125, 90–97. [DOI] [PubMed] [Google Scholar]
- Nursyazana MT, Mohdzain SN and Jeffery J (2013) Biodiversity and macro-parasitic distribution of the wild rat population of Carey Island, Klang. Tropical Biomedicine 30, 199–210. [PubMed] [Google Scholar]
- Ooi GL and Phua KH (2007) Urbanization and slum formation. Journal of Urban Health 84, i27–i34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panti-May JA, Carvalho-Pereira TSA, Serrano S, Pedra GG, Taylor J, Pertile AC, Minter A, Airam V, Carvalho M, Júnior NN, Rodrigues G, Reis MG, Ko AI, Childs JE, Begon M and Costa F (2016) A two-year ecological study of Norway rats (Rattus norvegicus) in a Brazilian Urban Slum. PLoS ONE 11, e0152511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patamia I, Cappello E, Castellano-Chiodo D, Greco F, Nigro L and Cacopardo B (2010) A human case of Hymenolepis diminuta in a child from Eastern Sicily. The Korean Journal of Parasitology 48, 167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peig J and Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. [Google Scholar]
- Pinto HA, Mati VLT and Melo AL (2014) Toxocara cati (Nematoda: Ascarididae) in Didelphis albiventris (Marsupialia: Didelphidae) from Brazil: a case of pseudoparasitism. Revista Brasileira de Parasitologia Veterinária 23, 522–525. [DOI] [PubMed] [Google Scholar]
- Puckett EE, Park J, Combs M, Blum MJ, Bryant JE, Caccone A, Costa F, Deinum EE, Esther A, Himsworth CG, Keightley PD, Ko A, Lundkvist A, McElhinney LM, Morand S, Robins J, Russell J, Strand TM, Suarez O, Yon L and Munshi-South J (2016) Global population divergence and admixture of the brown rat (Rattus norvegicus) Proceedings of the Royal Society of London B 283, 20161762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafique A, Rana SA, Khan HA and Sohail A (2009) Prevalence of some helminths in rodents captured from different city structures including poultry farms and human population of Faisalabad, Pakistan. Pakistan Veterinary Journal 29, 141–144. [Google Scholar]
- R Development Core Team (2011) R: A Language and Environment for Statistical Computing. Vienna, AT: R Foundation for Statistical Computing; http://www.R-project.org. ISBN 3-900051-07-0. Last accessed in the 20th of May 2013. [Google Scholar]
- Reis RB, Ribeiro GS, Felzemburgh RDM, Santana FS, Mohr S, Melendez AXTO, Queiroz A, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG and Ko AI (2008) Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Neglected Tropical Diseases 2, e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley LW, Ko AI, Unger A and Reis MG (2007) Slum health: diseases of neglected populations. BMC International Health and Human Rights 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos NJ, Sousa E, Reis MG, Ko AI and Costa F (2017) Rat infestation associated with environmental deficiencies in an urban slum community with high risk of leptospirosis transmission. Cadernos de Saúde Pública 33, e00132115. [DOI] [PubMed] [Google Scholar]
- Santos SV, Lescano SZ, Castro JM and Chieffi PP (2009) Larval recovery of Toxocara cati in experimentally infected Rattus norvegicus and analysis of the rat as potential reservoir for this ascarid. Memórias do Instituto Oswaldo Cruz 104, 933–934. [DOI] [PubMed] [Google Scholar]
- Schantz PM (1996) Tapeworms (Cestodiasis). Gastroenterology Clinics of North America 25, 637–653. [DOI] [PubMed] [Google Scholar]
- Schnyder M, Maurelli MP, Morgoglione ME, Kohler L, Deplazes P, Torgerson P, Cringoli G and Rinaldi L (2011) Comparison of faecal techniques including FLOTAC for copromicroscopic detection of first stage larvae of Angiostrongylus vasorum. Parasitology Research 109, 63–69. [DOI] [PubMed] [Google Scholar]
- Simões RO, Luque JL, Gentile R, Rosa MCS, Costa-Neto S and Maldonado A Jr. (2016) Biotic and abiotic effects on the intestinal helminth community of the brown rat Rattus norvegicus from Rio de Janeiro, Brazil. Journal of Helminthology 90, 21–27. [DOI] [PubMed] [Google Scholar]
- Singleton GR, Hinds LA, Krebs CJ and Spratt DM (2003) Rats, Mice and People: Rodent Biology and Management. Australian Centre for International Agricultural Research Monograph no. 96, Canberra, AU, 564p. [Google Scholar]
- Tena D, Pérez Simón M, Gimeno C, Pérez Pomata MT, Illescas S, Amondarain I, González A, Domínguez J and Bisquert J (1998) Human infection with Hymenolepis diminuta: case report from Spain. Journal of Clinical Microbiology 36, 2375–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiengo SC, Faraco FA, Salgado NC, Cowie RH and Fernandez MA (2007) Rapid spread of an invasive snail in South America: the giant African snail, Achatina fulica, in Brasil. Biological Invasions 9, 693–702. [Google Scholar]
- Tiwari S, Karuna T and Rautaraya B (2014) Hymenolepis diminuta infection in a child from a rural area: a rare case report. Journal of Laboratory Physicians 6, 58–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations (2003) The Challenge of Slums: Global Report on Human Settlements. London: UN: Human Settlements Programme; and London, UK: Sterling, Earthscan Publications Ltd., 310 p. [Google Scholar]
- United Nations (2005) Investing in Development: A Practical Plan to Achieve the Millennium Development Goals. New York, USA: UN Millennium Project. [Google Scholar]
- United Nations (2016) Urbanization and Development: Emerging Features. UN-Habitat World Cities Report 2016, UN Human Settlements Programme, Nairobi, Kenya, 262 p. [Google Scholar]
- Vadell MV, Gómez Villafañe IE and Cavia R (2014) Are life-history strategies of Norway rats (Rattus norvegicus) and house mice (Mus musculus) dependent on environmental characteristics? Wildlife Research 41, 172— 184. [Google Scholar]
- Vicente JJ, Rodrigues HO, Gomes DC and Pinto RM (1997) Nematóides do Brasil. Parte V: nematóides de mamíferos. Revista Brasileira de Zoologia 14, 1–452. [Google Scholar]
- Walker R, Carvalho-Pereira T, Serrano S, Pedra G, Hacker K, Taylor J, Minter A, Pertile A, Panti-May A, Carvalho M, Souza FN, Nery N Jr., Rodrigues G, Bahiense T, Reis MG, Ko AI, Childs JE, Begon M and Costa F (2017) Factors affecting carriage and intensity of infection of Calodium hepaticum within Norway rats (Rattus norvegicus) from an urban slum environment in Salvador, Brazil. Epidemiology & Infection 145, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q-P, Lai D-H, Zhu X-Q, Chen X-G and Lun Z-R (2008) Human angiostrongyliasis. The Lancet Infectious Diseases 8, 621–630. [DOI] [PubMed] [Google Scholar]
- Wang Q-P, Wu Z-D, Wei J, Owen RL and Lun Z-R (2012) Human Angiostrongylus cantonensis: an update. European Journal of Clinical Microbiology & Infectious Diseases 31, 389–395. [DOI] [PubMed] [Google Scholar]
- Waugh CA, Lindo JF, Foronda P, Ángeles-Santana M, Lorenzo-Morales J and Robinson RD (2006) Population distribution and zoonotic potential of gastrointestinal helminths of wild rats Rattus Rattus and R. Norvegicus from Jamaica. Journal of Parasitology 2, 1014–1018. [DOI] [PubMed] [Google Scholar]
- Webster JP and Macdonald DW (1995) Parasites of wild brown rats (Rattus norvegicus) on UK farms. Parasitology 111, 247–255. [DOI] [PubMed] [Google Scholar]
- Wolff JO (2003) Density-dependence and the socioecology of space use in rodents In Singleton GR, Hinds LA, Krebs CJ and Spratt DM (eds). Rats, Mice and People: Rodent Biology and Management, pp. 124–130. Australian Centre for International Agricultural Research Monograph No. 96; Canberra, AU. [Google Scholar]
- Zuk M (1990) Reproductive strategies and disease susceptibility: an evolutionary viewpoint. Parasitology Today 6, 231–233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.