Abstract
Idiopathic pulmonary arterial hypertension (IPAH) is characterized by pulmonary vascular remodeling. We have reported that high-dose prostaglandin I2 (PGI2) therapy markedly improved hemodynamics in IPAH patients and that PGI2 induced apoptosis of pulmonary artery smooth muscle cells obtained from IPAH patients. PGI2 is thought to have reverse remodeling effects, although it has not been histologically confirmed. In a case series, we examined the reverse pulmonary vascular remodeling effects of PGI2 in lung tissues obtained from an IPAH patient treated with high-dose PGI2 and an IPAH patient not treated with PGI2. Apoptotic cells were detected in small pulmonary arteries of the IPAH patient treated with high-dose PGI2 but not in those from the IPAH patient not treated with PGI2. Media of peripheral pulmonary arteries were thick in the IPAH patient not treated with PGI2. On the other hand, media of peripheral pulmonary arteries were thin in the IPAH patient treated with high-dose PGI2. The single case report suggested that high-dose PGI2 therapy has the potential for reverse pulmonary vascular remodeling by induction of apoptosis and reduction of medial hypertrophy. Accumulation of cases is needed for the application to generalized effect of high-dose PGI2.
<Learning objective: Reverse pulmonary vascular remodeling would provide further improvement in patients with IPAH. High-dose PGI2 therapy has the potential for reverse pulmonary vascular remodeling in patients with IPAH.>
Keywords: Remodeling, Medial hypertrophy, High-dose epoprostenol, Apoptosis, Idiopathic pulmonary arterial hypertension
Introduction
Idiopathic pulmonary arterial hypertension (IPAH) is characterized by vasoconstriction and vascular remodeling of pulmonary arteries [1]. Reverse remodeling as well as vasodilatation of pulmonary arteries would be desirable in patients with IPAH. We have reported that high-dose intravenous prostaglandin I2 (PGI2) therapy (>40 ng/kg/min) resulted in marked reduction in mean pulmonary artery pressure [2] and that PGI2 induced apoptosis of cultured pulmonary artery smooth muscle cells (PASMCs) obtained from patients with IPAH [3]. These results suggest that high-dose intravenous PGI2 therapy would reverse pulmonary vascular remodeling by induction of apoptosis in PAH-PASMCs, although it has not been histologically confirmed. We experienced a patient with IPAH who underwent cadaveric lung transplantation (CLT), although his clinical condition and hemodynamics were improved by high-dose intravenous PGI2 monotherapy. We examined the reverse pulmonary vascular remodeling effects of PGI2 in lung tissues obtained from patients with IPAH treated with high-dose PGI2 or not treated with PGI2.
Materials and methods
Lung tissues were obtained from two patients with IPAH at lung transplantation. As controls, lung tissues were obtained at lung lobectomy from a patient with lung cancer who had no pulmonary hypertension. All human subject protocols were approved by the Human Ethics Committee of Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, and written informed consent was obtained from all patients before the procedure. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays were performed on frozen sections with an ApopTag fluorescein in situ apoptosis detection kit (Chemicon International Inc., Temecula, CA, USA). Nuclear morphology was examined by labeling with 4′,6-diamidino-2-phenylindole (DAPI) (0.5 μL/mL). For identification of SMCs, α-smooth muscle actin mouse monoclonal antibody was used. The primary antibody was detected with rabbit anti-mouse immunoglobulin TRITC (DakoCytomation, Glostrup, Denmark). Tissues were analyzed using a fluorescence microscope (LSM5 EXCITER, Carl Zeiss Microimaging Inc., Oberkochen, Germany and Olympus IX71, Olympus Optical Co. Ltd., Tokyo, Japan). Morphometric analysis was performed on Elastica van Gieson-stained sections from paraffin-embedded blocks by standard techniques. Pulmonary arteries of 100–300 μm in external diameter were examined.
Results
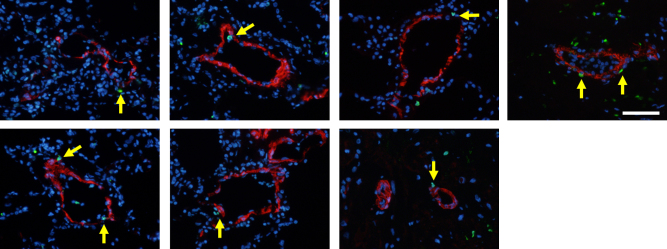
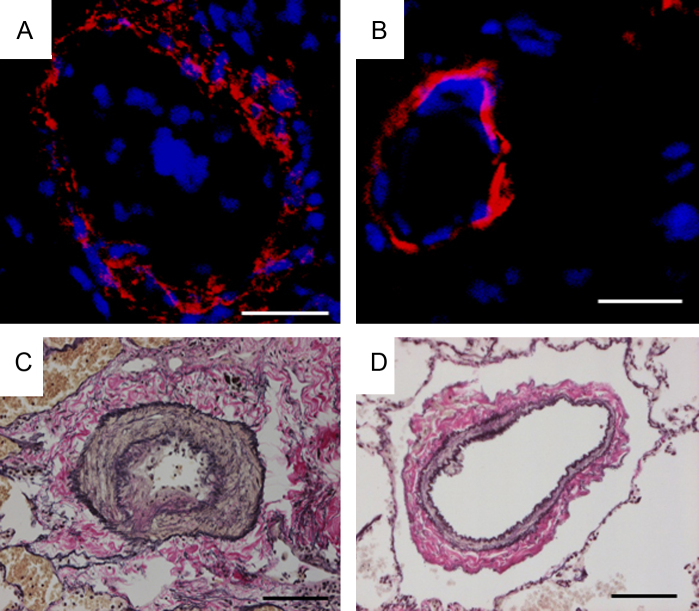
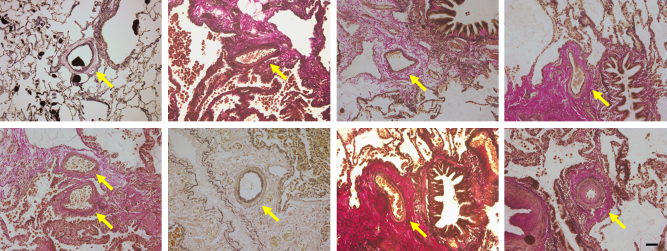
A 21-year-old man whose mother had died from pulmonary hypertension (PH) was diagnosed with familial PAH at the age of 9 years and had received intravenous PGI2 therapy from the age of 16 years. PGI2 dose had been gradually increased to 115 ng/kg/min over a period of about four years. Although mean pulmonary artery pressure decreased from 106 mmHg to 45 mmHg and symptoms were improved, he received CLT because of the appearance of a donor. In lung tissues obtained from the patient, apoptotic cells were detected in pulmonary arteries of his lung (Fig. 1). We also obtained lung tissues from a patient with IPAH who was not treated with PGI2 and from a patient with lung cancer. A 28-year-old woman was diagnosed with IPAH at the age of 22 years. Mean pulmonary artery pressure was 60 mmHg and she had received intravenous PGI2 therapy at the age of 25 years. However, the therapy was discontinued due to interstitial lung disease induced by PGI2 as previously described [4]. The patient was treated with an endothelin receptor antagonist and phosphodiesterase-5 inhibitor after the interstitial lung disease had been cured. However, mean pulmonary artery pressure was 59 mmHg at the age of 27 years and CLT was performed because of the appearance of a donor. No apoptotic cells were found in lung tissues obtained from the patient who had not received PGI2 therapy (Fig. 2A) or in lung tissues obtained from the non-PAH patient (Fig. 2B). Media of peripheral pulmonary arteries were thick in a patient with IPAH who had not received PGI2 therapy (Fig. 2C). On the other hand, media of peripheral pulmonary arteries were thin in a patient with IPAH treated with high-dose PGI2 (Fig. 3) and a non-PAH patient (Fig. 2D).
Fig. 1.
Pulmonary arteries stained by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay in a patient treated with high-dose prostaglandin I2. Green is TUNEL-positive. Red is α-smooth muscle actin. Blue is 4′,6-diamidino-2-phenylindole. Bar = 100 μm. Arrows indicated TUNEL-positive cells.
Fig. 2.
Pulmonary arteries stained by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and Elastica van Gieson staining in a patient not treated with prostaglandin I2 (PGI2) and in a non-pulmonary arterial hypertension (PAH) patient. Upper panel: TUNEL assay. Lower panel: Elastica van Gieson staining. (A and C) A 28-year-old woman who had not received PGI2 therapy. (B and D) A non-PAH patient.
Fig. 3.
Pulmonary arteries stained by Elastica van Gieson staining in a patient treated with high-dose prostaglandin I2. Arrows indicated the peripheral pulmonary arteries. Media of peripheral pulmonary arteries were thick.
Discussion
The pulmonary vasculature has been analyzed in almost all cases by autopsy in patients who died from progression of PH, and there have been few studies in which the pulmonary vasculature in patients whose clinical condition and hemodynamics improved was analyzed. In the present study, we analyzed the pulmonary vasculature in patients who received high-dose PGI2 therapy and whose clinical condition and hemodynamics improved. It was histologically confirmed that pulmonary vascular remodeling was reversible in patients with IPAH. Two case reports have shown that reduction in pulmonary flow caused a change in pulmonary vasculature in native lungs of patients with IPAH who had undergone single-lung transplantation 5, 6. Recently, pathological features of PAH patients treated with current medications were reported [7]. No significant differences were detected in medial thickness between patients with IPAH and controls. Thus, current medications for PAH have the potential for reversal of medial hypertrophy. In this study, improvement of hemodynamics, presence of apoptotic cells, and less medial hypertrophy were observed in a patient who received high-dose PGI2 therapy. These results suggest that high-dose PGI2 has the potential for reverse pulmonary vascular remodeling by reduction of medial hypertrophy. On the other hand, increased intimal thickness was observed in patients treated with currently recommended medications [7]. Reverse intimal hypertrophy will be a target for therapy of PAH in the future.
We previously reported that PGI2 at a high concentration (1.0 ng/mL) significantly induced apoptosis of cultured pulmonary artery SMCs obtained from patients with IPAH. However, PGI2 at a low concentration (0.5 ng/mL) did not significantly induce apoptosis [3]. The dose of PGI2 at a high concentration fitted to 100 ng/kg/min PGI2 in clinical settings by calculation from circulating blood volume of 50 kg body weight in humans. Thus, we consider that reverse remodeling of the pulmonary arteries would be a direct effect of high-dose PGI2.
In this case report, TUNEL-positive cells were stained with antibodies against α-SMA which are markers for SMC and media of peripheral pulmonary arteries were thin in a patient treated with high-dose PGI2. We previously reported that PGI2 at a high concentration induced apoptosis of cultured PASMCs obtained from patients with IPAH [3]. Therefore, TUNEL-positive cells have characteristics of PASMCs.
Limitation
Apoptotic cells were detected in only one IPAH patient with high-dose PGI2 therapy. Accumulation of cases is needed for the application to the generalized effect of high-dose PGI2.
In conclusion, the single case report suggested that high-dose PGI2 therapy has the potential for reverse pulmonary vascular remodeling by induction of apoptosis and reduction of medial hypertrophy.
Conflict of interest
Authors declare no conflict of interest.
Contributor Information
Satoshi Akagi, Email: akagi-s@cc.okayama-u.ac.jp.
Kazufumi Nakamura, Email: ichibun@cc.okayama-u.ac.jp.
References
- 1.Miura A., Nakamura K., Kusano K.F., Matsubara H., Ogawa A., Akagi S., Oto T., Murakami T., Ohtsuka A., Yutani C., Ohe T., Ito H. Three-dimensional structure of pulmonary capillary vessels in patients with pulmonary hypertension. Circulation. 2010;121:2151–2153. doi: 10.1161/CIR.0b013e3181e037c1. [DOI] [PubMed] [Google Scholar]
- 2.Akagi S., Nakamura K., Miyaji K., Ogawa A., Kusano K.F., Ito H., Matsubara H. Marked hemodynamic improvements by high-dose epoprostenol therapy in patients with idiopathic pulmonary arterial hypertension. Circ J. 2010;74:2200–2205. doi: 10.1253/circj.cj-10-0190. [DOI] [PubMed] [Google Scholar]
- 3.Akagi S., Nakamura K., Matsubara H., Kusano K.F., Kataoka N., Oto T., Miyaji K., Miura A., Ogawa A., Yoshida M., Ueda-Ishibashi H., Yutani C., Ito H. Prostaglandin I2 induces apoptosis via upregulation of Fas ligand in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2013;165:499–505. doi: 10.1016/j.ijcard.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Morimatsu H., Goto K., Matsusaki T., Katayama H., Matsubara H., Ohe T., Morita K. Rapid development of severe interstitial pneumonia caused by epoprostenol in a patient with primary pulmonary hypertension. Anesth Analg. 2004;99:1205–1207. doi: 10.1213/01.ANE.0000130615.28893.52. [DOI] [PubMed] [Google Scholar]
- 5.Levy N.T., Liapis H., Eisenberg P.R., Botney M.D., Trulock E.P. Pathologic regression of primary pulmonary hypertension in left native lung following right single-lung transplantation. J Heart Lung Transplant. 2001;20:381–384. doi: 10.1016/s1053-2498(00)00153-4. [DOI] [PubMed] [Google Scholar]
- 6.Deb S., Yun J., Burton N., Omron E., Thurber J., Nathan S.D. Reversal of idiopathic pulmonary arterial hypertension and allograft pneumonectomy after single lung transplantation. Chest. 2006;130:214–217. doi: 10.1378/chest.130.1.214. [DOI] [PubMed] [Google Scholar]
- 7.Stacher E., Graham B.B., Hunt J.M., Gandjeva A., Groshong S.D., McLaughlin V.V., Jessup M., Grizzle W.E., Aldred M.A., Cool C.D., Tuder R.M. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]