Abstract
Introduction.
Epidemiologic evaluations of Streptococcus pneumoniae nasopharyngeal (NP) colonization and pneumococcal disease suggest that newer serotypes in future formulations of pneumococcal conjugate vaccines (PCVs) are needed and there may need to be continued reformulations because there are many new emerging serotypes expressed by pneumococci.
Areas Covered:
Mechanisms of protection by next-generation whole-cell vaccine (WCV) and/or multi-component pneumococcal purified protein vaccines (PPVs) in development for prevention of pneumococcal infections.
Expert Commentary:
A long-term strategy for prevention of pneumococcal disease will likely include WCV and PPVs. However these vaccines will impact disease pathogenesis in a different manner than PCVs. Prevention of pneumococcal NP colonization should not be expected, nor is it desirable because risks for NP colonization by other replacement organisms into the ecological niche vacated by all pneumococci may have consequences. The expression biology of capsule and surface protein antigens are phase dependent. Therefore, the immune response will be different and the mechanism of protection divergent. WCVs and PPVs may be alternative strategies in low income developing countries to protect against invasive disease and reduce NP carriage load.
Keywords: Streptococcus pneumoniae, pneumococcal conjugate vaccines, pneumococcal whole-cell vaccine, pneumococcal protein vaccines, nasopharyngeal colonization, clinical trial, pathogenesis
1. Introduction
Pneumococcal conjugate vaccines (PCVs) provide clear and tangible public health benefits from direct effects on vaccinated persons and indirect effects from herd immunity, produced by elimination of nasopharyngeal (NP) colonization.[1] Early on, some experts envisioned that a PCV that included the 7 most common serotypes would have the potential to prevent up to 88% of invasive pneumococcal disease (IPD) in US children caused by the most virulent pneumococci with a concomitant herd effect.[2–4] The prediction was based on the notion that biologic fitness predominated among strains expressing the 7 most common serotypes and was lacking in the strains expressing other capsular types. The events that followed after PCV7 showed how rapidly bacteria adapted to vaccine-induced strain selection pressure with an increase of replacement strains expressing non-vaccine serotypes causing IPD.[5,6] As a consequence of emergence of new disease-causing strains of pneumococci expressing capsules not included in PCV7, vaccine companies chose to expand the number of serotypes included in PCVs to include additional strains expressing emergent capsular types and thus PCV10 and PCV13 became available. In a 2012 WHO position paper regarding IPD serotypes causing infection among children <5 years of age, PCV7 and PCV13 accounted for 49–82% and 74–88% geographic region coverage, respectively (http://www.who.int/wer/2012/wer8714.pdf). The Active Bacterial Core surveillance trends by serotype group, 1998–2015 clearly shows a decrease in IPD associated with use of PCV7 & PCV13 and a continued but constant level of IPD caused by non-PCV13 serotype groups in both children and adults (https://www.cdc.gov/abcs/reports-findings/surv-reports.html). WHO in their 2012 position paper state “for IPD caused by non-PCV serotypes, increases were evident among hospitalized cases”. However, it is now realized that predicting the overall impact of protection is more complex than knowing the number of pneumococcal serotypes within PCVs and the serotypes causing disease in a region.[7] Indeed, the pneumococcus has demonstrated remarkable biologic fitness along with an ability to exchange DNA to allow capsular switching and acquisition of antibiotic resistance.[8]
However, a few years after PCV13, we are once again observing emergence of additional strains expressing non-vaccine capsular serotypes.[9] The vaccine industry is responding by development of a PCV15 and PCVs with higher multivalent formulations.[10] Epidemiologic evaluations of pneumococcal NP colonization, IPD and acute otitis media (AOM), already suggest an insufficient solution with PCV’s because new types being added will not prevent infections by the many emerging serotypes expressed by pneumococci.[11–15] Moreover, differences between IPD and AOM strains are being observed more frequently and difference among countries are becoming more apparent. In addition, the current high cost of PCVs makes them less likely to be used extensively in developing countries where the need is the highest.[16] If one evaluates the report by Moore et al regarding emerging serotypes causing IPD in US children, a need to add 5 new non-PCV13 serotypes to the current PCV13 formulation to yield a PCV18 is already needed and only 2 of the 5 are in Merck’s PCV15.[17] Where and when do we end with newer PCVs?
NP colonization has been one of the key aspects in evaluating the PCVs because elimination of colonization provides a herd immunity effect. Most cost-effectiveness calculations take into account the impact of herd protection, and in a varying degree the replacement of disease. The bottom line from the public health perspective is the total disease burden has declined following introduction of PCVs and herd protection is really important, but if it comes with a cost of replacement disease, then it becomes less important.
A long-term strategy for prevention of pneumococcal disease is next-generation multi-component whole-cell vaccines (WCV) or purified protein vaccines (PPVs) as shown in Table 1. We have previously described key features of PPVs [18], the most important of which is selection of proteins shared by all or virtually all strains of the bacteria irrespective of capsular polysaccharide. Many multi-component PPVs in development that target the early stage of colonization include 1 or more surface-exposed, highly conserved proteins expressed by pneumococci that anchor the organism to NP epithelial cells.[18] However, the density of capsular polysaccharide on the surface of pneumococci during NP colonization is very different than what occurs with proteins.
Table 1.
Pneumococcal vaccine candidates in clinical trials.a
| Vaccine | Assignee | Indication | Identifier | Location | Age | Ph1 | Ph2 | Ph3 | Status |
|---|---|---|---|---|---|---|---|---|---|
| PCV | |||||||||
| PCV15b (V114) | Merck | Safety & Immuno | NCT01215175 | USA & Finland | 12–15mos & 18–45 | X | Completed[95,96] | ||
| PCV15 | Merck | Safety, Immuno | NCT01215188 | 6–12wks | X | Completed | |||
| PCV15 | Merck | Safety & Immuno | NCT02547649 NCT01513551 | 50yrs+ | X | Completed | |||
| PCV15 | Merck | Safety & Immuno | NCT02531373 NCT02037984 | 2mos-49yrs | X | X | Ongoing | ||
| PCV15 | Merck | Safety & Immuno | NCT02573181 | ≥65yrs | X | Ongoing | |||
| PCV12 (2830930A) | GlaxoSmithKline | Safety & Immuno | NCT01485406 | Germany | 12–23mos | X | Completed | ||
| PCV11, PCV12 (2830929A, 2830930A) | GlaxoSmithKline | Immuno | NCT01616459 | Czech Republic, Germany, Poland, Spain | 6–12wks | X | Completed | ||
| PCV12 | GlaxoSmithKline | The Gambia | X | Unknown[10] | |||||
| PCV10 | Panacea Biotech | Safety & Immuno | X | Unknown[10] | |||||
| PCV10 | Serum Institute of India | Immuno | CTRI/2015/12/006456 | India | 12–15mos | X | Not yet recruitting | ||
| Protein | |||||||||
| PhtD | GlaxoSmithKline | Safety & Immuno | NCT01767402 | Belgium | 18–45yrs ≥65yrs | X | Completed | ||
| dPly, PhtD-dPly +/− PCV10 | GlaxoSmithKline | Safety & Immuno | NCT00707798c | Belgium | 18–40yrs | X | Completed[64] | ||
| PhtD-dPly (GSK2189242A) | GlaxoSmithKline | Safety & Immuno | NCT00896064c | Belgium | 18–41yrs | X | Completed[64] | ||
| PhtD-dPly +/− PCV8 | GlaxoSmithKline | Safety & Immuno | NCT00756067 | Sweden | 65–85yrs | X | Completed[65] | ||
| PhtD-dPly + PCV10 | GlaxoSmithKline | Safety & Immuno | NCT01262872 | The Gambia | 2–4yrs | X | Completed[68] | ||
| PhtD-dPly + PCV10 | GlaxoSmithKline | Efficacy NP colonization | NCT01262872 | The Gambia | 8–10wks 2–4yrs | X | Completed | ||
| PhtD-dPly +/− PCV10 | GlaxoSmithKline | Immuno | NCT00985751 | Czech Republic | 12–23mos | X | Completed[66] | ||
| PhtD-dPly + PCV10 | GlaxoSmithKline | Immuno | NCT01204658 | Czech Republic Germany Poland | 6–14wks | X | Completed[67] | ||
| PhtD-dPly + PCV13 | GlaxoSmithKline | Ear & Lung | NCT01545375 | USA (Indian) | 6–12wks | X | Completed | ||
| PlyD1 | Sanofi Pasteur | Safety & Immuno | NCT01444352 | Switzerland | 18–50yrs | X | Completed[97] | ||
| PhtD | Sanofi Pasteur | Safety & Immuno | NCT01444001 | Switzerland | 18–50yrs | X | Completed[98] | ||
| PcpA, PcpA+PhtD | Sanofi Pasteur | Safety & Immuno | NCT01444339 | Switzerland | 18–50yrs | X | Completed[99] | ||
| PhtD+PcpA+PlyD1 (PPrV) | Sanofi Pasteur | Safety & Immuno | NCT01764126, NCT01446926, U1111–1117-7316 | Bangladesh | 6–7wks 12–13mos 18–50yrs | X | Completed[69] | ||
| SP2108+SP0148+SP1912 (GEN-004) | Genocea Biosciences | Safety & Immuno | NCT01995617 | US | 18–55yrs | X | Completed[59] | ||
| SP2108+SP0148+SP1912 (GEN-004) | Genocea Biosciences | Efficacy NP colonization | NCT02116998 | UK | 18–55yrs | X | Completed | ||
| PcsB, StkP, PsaA | Intercell AGd | Safety & Immuno | NCT00873431 | Germany | 18–65yrs | X | Completed[59] | ||
| Whole Cell | |||||||||
| WCV (Alum) | PATH | Safety & Immuno | NCT01537185 | US | 18–40yrs | X | Completed[59] | ||
| WCV +/− PCV10 (Alum) | PATH | Safety & Immuno | NCT02097472 | Kenya | 12–15mos 18–45yrs | X | X | Completed | |
| WCV (Aluminum hydroxide) | PATH | Safety & Immuno | NCT02543892 | Kenya | 12–19mos 18–40yrs | X | X | Not open yet | |
| S.typhi rPspA | Arizona State U. | Safety & Immuno | NCT01033409 | USA | 18–40yrs | X | Completed |
Data sources:
PCV15 reformulated and repeat of PhI-II trials
NCT00896064 is a continuation of NCT00707798 of a boost dose of PhtD-dPly given to the PhtD-dPly group only
Acquired by Valneva SE
2. Capsular Polysaccharide Versus Protein-Directed Antibody Effects on NP colonization
Pneumococci expresses various virulence factors making it a highly adaptable disease-causing pathogen in man.[19] In the NP, the expression biology of capsule and surface protein antigens are phase dependent (Figure 1). Therefore, it should be expected that raising an immune response would be different and the mode of protection against NP colonization and pathogenesis divergent. Specifically, while anti-capsular antibodies can prevent the acquisition of a subsequent carriage event of the same serotype, they fail to protect against already established NP colonization.[20] In contrast, antibodies to pneumococcal adhesins can partly block initiation of colonization and also reduce the load of pneumococci in the NP.[21] Several key points should be made:
Pneumococcal phase variation in the NP converts high capsule-expressing pneumococci (opaque phenotype) into less capsule-expressing pneumococci (transparent phenotype) and this process facilitates the establishment of commensal carriage.[16]
Most anti-polysaccharide capsule antibodies, which are naturally acquired by colonization or induced by PCVs, easily access their abundant target polysaccharide antigens resulting in a decreased incidence of vaccine serotype NP colonization (an exception is the lack or variable reduction of IPD caused by serotype 3 observed in Denmark, UK and USA [17,22,23]), and thereby, reduced rate of infection.[24 ] However, naturally acquired protection against IPD depends on antibody to protein antigens rather than capsule as shown in a murine model using human antibodies.[25]
While there is a significant difference in quantitative expression of capsule between variants [26], it is unclear as to differences in expression of membrane proteins between variants of the same strain.[27–29]
Transparent variants are selectively expanded during NP colonization [27,30] and demonstrate an increased ability to adhere to human epithelial cells.[29,31].
Due to phase variation, transparent variants are less likely to evade the immune system.[31] Therefore, antibodies to pneumococcal antigens are able to access the bacterial surface of transparent variants and prevent colonization or escalated growth of pneumococci in the NP resulting in IPD (Figure 1).[32–34]
As a commensal during health, pneumococci induce a greater rise in serum IgG responses to pneumococcal capsule than surface proteins.[35] and higher serum anticapsular polysaccharide antibodies are needed to prevent NP acquisition versus prevention of IPD after PCV vaccination.[36] Transudation of antibodies from serum into the nasal mucosa is minimal in times of health but increases during viral URI-induced NP inflammation.
The best physiological and immunological state to assess correlation of vaccine-induced anti-pneumococcal antibody levels and protection of pneumococcal colonization by WCVs and PPVs is during a viral URI- the clinical setting for development of pneumococcal infection.[18,37]
Figure 1.
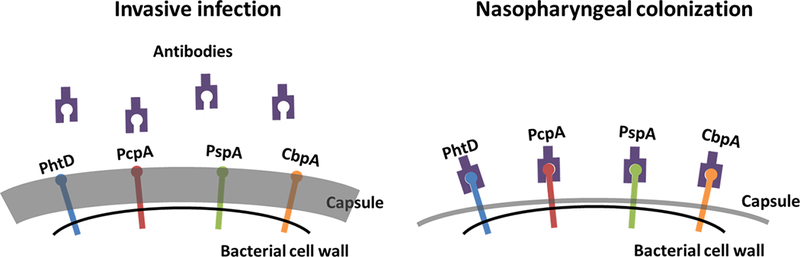
Spn capsule phase variants and antibody to surface antigens. During invasive infection, Spn produces a thick capsule, preventing host antibodies from accessing surface proteins. In the nasopharynx, Spn undergoes a phase shift, where capsule is much thinner, rendering surface proteins accessible.
3. Pneumococcal colonization is an essential precursor to disease pathogenesis and viral upper respiratory co-infection facilitates the transition of commensal pneumococcal colonization to infection.
Pneumococcal colonization is asymptomatic and commonly followed by horizontal dissemination.[38,39] In mouse studies, it elicits a mild NP inflammation that allows controlled immune surveillance, leading to the clearance of colonization without developing immunopathology.[40] It is an immunizing event that results in an antigen-specific immune response.[41] Over time, repeated colonization is therefore protective against subsequent colonization events.[42] Viral URI perturbs the host immune equilibrium in the NP and makes NP epithelium permissive for increased pneumococcal colonization.[37] Although, it remains to be understood how a sequentially protective immune response is mounted against colonization, it may be that vaccines that modulate NP mucosal immune responses and allow the persistence of low density NP colonization is a way forward. It is expected to mitigate the rapid evolution of new pneumococcal clones and serotypes and to promote antigen-specific immune priming to boost vaccine-induced immunity.
We recently studied healthy infants who had NP samples prospectively collected and whenever a child was diagnosed with AOM, tympanocentesis was performed and middle ear fluid samples collected to confirm the diagnosis by microbiologic culture. We found that NP mucosal antibody levels to PPV components pneumococcal histidine triad protein D (PhtD), pneumococcal choline-binding protein A (PcpA) and pneumolysin detoxified derivative (PlyD1) correlated with protection against pneumococcal acute otitis media (AOM) but not with protection against NP colonization.[43] Using a mouse model, we and others recently showed that a quantitative increase in density of pneumococci in the NP is associated with the transition of the organism from commensal to pathogen and that this occurs during a viral URI co-infection.[21,37] Therefore, viral URI converts asymptomatic pneumococcal colonization into a higher density pathogenic colonization. Moreover, in a mouse influenzae/pneumococcal co-infection model we recently showed that vaccination with a PPV adhesin, PhtD, can prevent pneumococcal density from reaching a pathogenic threshold during a viral URI without elimination of the organism from the NP.[21] The ability of WCVs and PCVs to maintain NP colonization below the pathogenic threshold during a viral co-infection might be considered an improved vaccination strategy to contain pneumococcal infections by harnessing the beneficial aspects of asymptomatic colonization (Figure 2).
Figure 2.
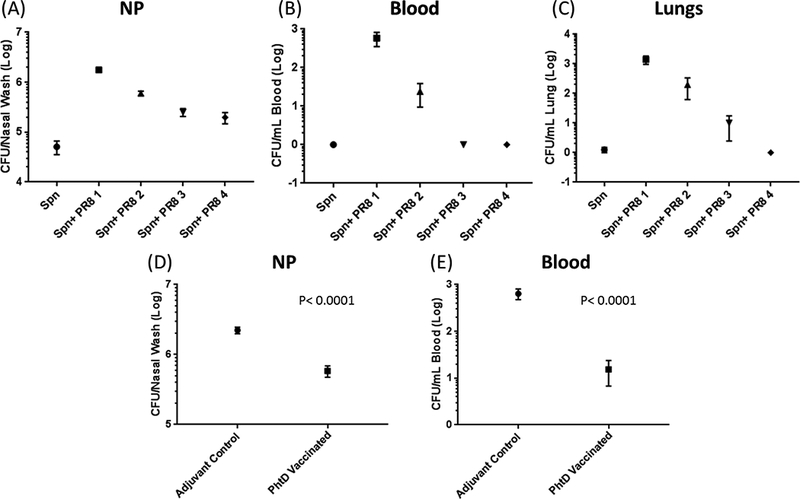
Spn NP bacterial threshold during a co-infection with influenza virus and after phtD vaccination: Six week old naive mice (C57BL/6) were i.n. inoculated (10 μl) with Spn serotype 6a (1 × 106 CFUs). 24 h later mice were inoculated i.n. (10 μl) with different infection doses of H1N1 PR8 influenza (PR8 1=20 × TCID50, PR8 2=25 × TCID50, PR8 3=10 × TCID50, PR8 4=5 × TCID50). Six days later, mice were euthanized and the Spn bacterial burden was ascertained in the NP (A), blood (B) and lings (c). PhtD vaccinated or adjuvant control mice (6 weeks old) were i.n. inoculated (10 μl) with 1106 s Spn. 24 h later, mice were inoculated i.n. with H1N1 PR8 (20 × TCID50. Six days later mice were euthanized and span bacterial burden was ascertained in Np (D) and blood (E).
4. A shift in expectation must be made as the evaluation of next generation WCVs and PPVs proceeds.
PCVs prevent pneumococcal NP colonization for the majority of vaccine serotypes but not for nonvaccine serotypes. However, for vaccines that are serotype independent such as WCVs and PPVs, complete pneumococcal NP elimination may have consequences.[38,44]
Although antibody induction is the principal mechanism of protection induced by PCVs, IL-17A has a role in protecting children from carriage[45] Likewise, in mice, WCVs and lipidated PPVs also protect against invasive disease and reducing carriage by inducing antibody and CD4+ Th17 cytokines[46,47]. Our work using a mouse influenzae/pneumococcal co infection model suggests CD4+ T cells are the principle modulators of protection against pneumococcal progression from colonization to invasive infection whereas infants require both CD4+ T cells and antibody.[21]
From a public health perspective the impact of PCVs on transmission and herd immunity is equally (or more) important as reduction of disease in the individual. Whether a similar effect can be achieved with WCVs or PCVs remains to be studied.
5. Pneumococcal whole cell vaccine
Historically vaccines were developed using methods that reduced or inactivated a pathogen’s virulence; such as attenuation or chemical treatment or preparations of whole-cell crude extracts. For example, whole-cell pertussis vaccines are suspensions of crude extracts of heat-killed B. pertussis.[48] An advantage of whole-cell vaccines is that they include molecules that act as pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS), which are recognized by host antigen presenting cells, and present antigens in their natural configuration.[49,50] Children immunized with the whole-cell pertussis vaccine mainly generate a Th1 response that may in part be due to the presence of LPS and/or peptidoglycan, TLR agonists.[49] Therefore, it is no surprise that whole-cell pertussis vaccines induce immune responses similar to what is observed during natural infection.[51] Although both whole-cell and acellular purified protein-based pertussis vaccines provide good protection, whole-cell pertussis vaccine are more efficacious and produce longer lasting immunity than acellular vaccines.[52,53] Importantly for this discussion, whole cell vaccines are more effective than protein-based acellular pertussis vaccines in developing countries and remain the choice for national childhood immunizations in many low-income countries.[54–56] The superiority of WCVs for pertussis prevention in developing countries over purified protein multicomponent vaccines is an important lesson in vaccinology that should be considered as relevant to clinical trial results using pneumococcal WCVs vs. PPVs.
Based on the observations regarding whole cell pertussis vaccines, a pneumococcal WCV may be the best strategy for developing countries. One such vaccine being tested is a non-capsulated pneumococcal strain. Protection against NP colonization and sepsis was demonstrated in mice immunized with the pneumococcal WCV.[57] and protection against NP colonization was shown to be CD4+ IL-17A dependent and non-capsular antibody mediated.[57,58]
The pneumococcal WCV was used in two clinical trials to study safety, dose tolerance and immunogenicity.[59] In healthy US adults (NCT01537185) WCV was shown to be safe and well tolerated; significant IgG responses to pneumococcal antigens were elicited, including PspA and pneumolysin, and functional antibody responses were detected in a pneumolysin toxin neutralizing assay.[60] Significant increases in T-cell cytokine responses, including IL-17, were measured among subjects receiving the highest dose of WCV. Passive transfer of human immune sera protected mice against fatal sepsis when challenged intravenously with a virulent strain of pneumococci.[61]
6. Pneumococcal Protein Vaccines
6.1. PPV designed to elicit Th17 responses
A PPV containing three pneumococcal Th17-stimulating antigens that protected mice from pneumococcal NP colonization via a CD4+ T-cell and IL-17A dependent manner has entered human clinical testing.[46,62] These proteins were identified as being recognized by Th17 cells from healthy humans.[63] Two of the proteins, SP_2180 and SP_0148, are lipoproteins and lipid moieties enhance the immunogenicity and protective efficacy through activation of TLR2.[46] This vaccine was tested in a Phase I clinical study of adults in the US (NCT01995617) and found to be safe and immunogenic although due to high pre-existing IL-17A levels, vaccine-induced increases were not observed.[59] A Phase II trial evaluated the efficacy of the vaccine on NP colonization of adults intranasally challenged with S. pneumoniae serotype 6B in the UK (NCT02116998). Consistent reductions versus placebo in the pre-specified endpoints of the rate and density of pneumococcal colonization were measured, but neither of the endpoints achieved statistical significance. Unfortunately, the study was powered for 50% or higher reduction in carriage and they only found a 25% reduction. How this would translate in young children is unknown.
6.2. Conserved proteins
PhtD + Pneumolysoid
Clinical studies of PhtD and chemically detoxified pneumolysin (dPly), were shown to be safe and immunogenic in young and old adults, toddlers and infants in Europe.[64–67] In Africa where PCV coverage is less than in the US or Europe, the PhtD-dPly vaccine was tested in 2–4 year old children not previously vaccinated with PCV in The Gambia. A single dose of PhtD-dPly combined with 10-valent polysaccharide conjugate was immunogenic.[68] A larger trial in Gambian infants (NCT01262872) assessed the impact of the PhtD-dPly vaccination on NP colonization While the vaccine raised serum antibodies to the vaccine components a reduction in NP colonization by pneumococci was not observed. (ISPPD 2016, NCT01262872)
PhtD + PcpA + Pneumolysoid
A pneumococcal protein recombinant vaccine (PPrV) containing PhtD, PcpA and genetically detoxified pneumolysin (PlyD1) was evaluated in Dhaka, Bangladesh (NCT01446926, NCT01764126).[69] Adults (18–50 yrs) and toddlers (12–13 mos.) received a single high dose injection of aluminum-adjuvanted PPrV. Infants (6–7wks) received 3 injections of high or middle or low doses of aluminum-adjuvanted PPrV; an additional infant cohort received 3 injections of middle dose unadjuvanted PPrV. The vaccine was safe and well tolerated and 75% or more of all PPrV + adjuvanted subjects had a ≥2-fold increase in serum antibody to all three antigens.[69]
6.3. Factors impacting pneumococcal vaccine trial results
There are many factors that may affect the immunogenicity and efficacy of pneumococcal vaccines
In many developing countries, general nutrition is poor and this adversely impacts both innate and adaptive immune responses. Differences in gut IgA levels are reported between healthy Bangladeshi versus UK adults [70] and microbiome diversity differences between healthy Bangladeshi versus US children[71].
Risk factors for high density pneumococcal NP colonization all prevail in The Gambia and Bangladesh such as high frequency of viral URI, high number of siblings in a family, smoke exposure and crowding.
High density NP pneumococcal colonization occurs in the first weeks of life among infants in developing countries[72] and early NP colonization may adversely impact immune responses to vaccines.[73,74]
Non-anticapsular antibodies and IL-17A production provide natural immunity to pneumococcus and these immunity mechanisms are different in children and adults in developing vs. developed countries.[75]
7. Pneumococcal Protein Vaccines to Prevent Acute Otitis Media
AOM is the best type of pneumococcal infection to be used in assessing efficacy of WCVs and PPVs because the etiology of AOM can be proven using tympanocentesis and because of higher costs and longer time to accumulate a sufficient number of subjects to prove efficacy of invasive pneumococcal disease with concurrent PCV administration. Prevention of AOM based on clinical diagnosis is an alternative study design. However, clinical diagnosis is challenging and over-diagnosis will reduce the ability to detect a true therapeutic benefit of vaccination. Diagnostic confirmation by tympanocentesis and specific otopathogen identification reduces the necessary sample size but involves the challenge of identifying clinical sites capable of performing tympanocentesis.
Study of populations that have a high incidence of AOM is appealing because total sample sizes needed to show differences induced by WCVs and PPVs are smaller. Such a clinical efficacy study is underway in a Native American population that is otitis-prone (NCT01545375). However, there are special risks in studying AOM in otitis-prone children. By microbiologically diagnosing every child with AOM by culture of middle ear fluid, collection of NP secretion samples and blood, our group found that susceptibility to recurrent AOM arises primarily from broad immunological defects. In fact, our published studies show that about 90% of otitis prone children have inadequate innate responses and diminished quantity and function of antibody generated to pneumococcal proteins, diminished memory B- and T-cell generation, and specific deficits in activation of B-cells and helper T-cells.[76] Many of these children also show an increased propensity to viral URI and fail to generate protective anti-viral antibody following infection[77] indicating broad immunologic defects. About 25% of otitis-prone children fail to produce immune responses when antigen is presented by injection of routine pediatric vaccines.[78] Therefore, while the sample size benefits are clear it should be realized that the response to PPVs in otitis-prone populations may not be of the same magnitude as the general population.
Indeed, our group studied 6 pneumococci antigens (PhtD, PhtE, LytB, PcpA, PspA and Ply) and showed an immune response to natural exposure through NP colonization and children with higher levels of mucosal antibodies are with reduced risk of AOM.[41,43,79] However, otitis-prone children produced lower levels of serum and mucosal antibody to most of these candidate protein vaccines compared to non-otitis-prone children.[80,81]
We also showed that otitis-prone children have dysregulated mucosal cytokine levels during viral URI, creating a permissive environment for bacterial colonization and invasion as well as increased expression of TLR2/4 on the surface of epithelial and innate immune cells present in the nasal mucosa at onset of AOM.[82] Higher TLR2 expression and signaling could significantly increase the production of IL-17A/IL-23 in the NP, which enhance pathogen clearance but may cause immunopathologic epithelial cell damage.[82] Based on our research, otitis-prone children display a complex of dysfunctional innate immune responses in the NP depending on the bacterium and virus, resulting in an altered cytokine milieu that contributes to the pathogenesis of recurrent AOM.[82]
8. Conclusion
PCVs are highly effective in reducing the incidence of IPD and AOM caused by vaccine serotypes in developing and developed countries. PCVs eliminate from the NP most strains expressing the included serotypes thereby producing highly cost-effective indirect herd immunity effects. However, risks for NP colonization by non-vaccine serotypes or other replacement organisms into the ecological niche vacated by the vaccine type pneumococci is high and raises concern. As discussed in this review, new pneumococcal WCVs are self-adjuvanting containing many antigens necessary for broad protection while PPVs are more likely to require an adjuvant and contain far less bacterial antigenic targets than WCVs. Effectiveness of both types of vaccines will depend on subject health issues such as nutrition, early and high NP colonization rates, gut and NP microbiome homeostasis, etc., all of which will impact immune response and consequent protection from pneumococcal diseases. The quest for developing a non-PCV vaccine is difficult but not insurmountable with already promising results.
9. Expert commentary
We are approaching a cross road regarding further testing of WCVs and PPVs. The results of phase I testing have shown the new vaccines are safe, well tolerated and immunogenic. However, comparisons with PCVs, with an expectation of eliminating NP carriage of pneumococci, have caused much discussion and concern about proceeding further with the development of WCVs and PPVs.[44] The vaccine industry has many targets and choices to consider including newly emerging pathogens such as Zika virus where no vaccine is available.[83] So a risk averse industry, concerned that the scientific community, regulators and agencies that endorse use of vaccines may not accept new pneumococcal vaccines that do not eliminate carriage, may cause a pause in further studies for now. In my opinion, a paradigm shift in expectations will be needed among scientists, regulators and industry if further evaluation of WCVs and PPVs is to proceed. These newer vaccines likely will not prevent NP colonization by pneumococci and the disadvantages of complete elimination of these organisms from their ecological niche should be viewed with a need for caution.[44] The vaccines may reduce the density of colonization and their mechanism of protection may come from maintaining the inocula of pneumococci in the NP below a pathogenic threshold, thereby preventing disease without preventing NP colonization. It is also possible that in more developed countries and in developing country populations where pneumococcal colonization occurs at a later time in childhood, with fewer organisms, less frequently and in settings where additional risk factors for high density colonization do not occur, WCVs and/or PPVs may be capable of preventing colonization similar to PCVs.
The key weaknesses in development of WCVs and PPVs so far has been the selection of the most challenging sites for phase I and II testing. Testing in The Gambia and Bangladesh where pneumococcal colonization and disease is highly prevalent and well above other developing countries [84–86] offered the vaccine industry an opportunity to study fewer subjects to provide efficacy results. However, the same health and epidemiology factors that cause such a high pneumococcal disease burden also create a very high bar to prove efficacy that may not be representative of other developing countries or developed countries.
Further research is needed to test the WCVs and PPVs in additional populations. Sensitive assays capable of detecting a one log drop in density of pneumococci in the nasopharynx during a viral URI will be needed to validate the hypothesis that protection against pneumococcal disease with WCVs and PPVs is associated with prevention of an inocula threshold that causes disease during a viral URI. The PPVs may need to include novel adjuvants with greater potency than aluminum salts. Particularly adjuvants that target stimulation of both antibody and cellular immunity would be desirable.
The ultimate goal in this field is to develop novel vaccines that are not serotype specific because the experience with PCVs has shown that the pneumococcus is a highly adaptable organism and emergence of replacement organisms expressing capsular polysaccharides not included in PCVs and/or nonencapsulated bacteria is almost a certainty. It is likely impossible to manufacture a 96 valent PCV.
The new knowledge needed to achieve this goal is defining correlates of protection with WCVs and PPVs. The biggest challenge for this goal to be achieved involves the acceptance by stake holders that the “rules” surrounding development and licensure of PCVs may not apply to WCVs and PPVs. Specifically, PCVs were licensed with a correlate of protection measured by an opsonophagocytic assay. WCVs and PPVs may not produce protection from disease by induction of antibody that promotes opsonophagocytic activity. The correlate of protection may be in prevention of sufficient adherence to nasopharyngeal epithelia cells so that a pathogenic inoculum is not achieved during a viral URI.[37] Or the correlate of protection may be the induction of sufficient Th17 cells to modulate neutrophil recruitment to the nasopharynx.[45]
The particular areas of the research our group is finding of interest at present includes defining the pneumococcal pathogenic inoculum in young children during viral URI, identifying a correlate of protection for PPVs, and understanding the immune defects in young children that permit pneumococcal infections to occur. Over the past 10 years, we have studied a large population of children during their first 3 years of life who experience repeated pneumococcal infections in the middle ear, sinuses and lungs.[76,87] Our research has identified deficiencies in T and B cell immunity in response to pneumococcal infections.[88,89] The deficiencies resemble a neonatal immunity response, giving our group reason to propose a new type of immunodeficiency termed “Prolonged Neonatal-Like Immune Profile” (PNIP).[76] We have shown that young children with PNIP do not respond with protective antibody titers to PCVs.[90] Although this population of young children is small (<10% of all young children), they experience the highest burden of pneumococcal disease. Our work suggests that specific pneumococcal proteins, such as PcpA, are more immunogenic than others in stimulating an immune response in PNIP young children when PCVs do not.[76,81,90] Currently, we are exploring novel adjuvants that facilitate immune responses in children especially prone to pneumococcal infections. Our early findings involving novel adjuvants have been encouraging.
10. Five-year view
Current pneumococcal conjugate vaccines have been highly successful in reducing the incidence of invasive pneumococcal diseases due to vaccine serotypes included in the product but the emergence of strains expressing other capsular polysaccharides to replace those eliminated by PCVs has been a consistent, recurring theme and there are 96 capsular types of pneumococci. The manufacturing challenges to produce a 13-valent PCV are well known so the expansion to 96 types is not likely possible in the next 5 years.. A PCV15, PCV16, PCV18 or even a PCV23 may be achievable but regulators may require each new product to be tested for efficacy for at least one disease state. Such studies are not feasible for IPD or pneumonia so that disease state will likely be AOM. AOM trials would be feasible because tympanocentesis derived cultures can document serotype-specific vaccine efficacy. However, to do such field testing of new PCVs the prevalence of the target serotype-specific strains in the new formulations will need to be known in order to calculate sample size of test children. About 2 years of data to justify sample size may be needed to secure investigational review board approvals to proceed with such clinical trials in young children. WCVs and PPVs will face the same challenge in phase III testing, i.e., clinical field testing of the vaccines with AOM the likely disease state to target. Yet there are very few sites globally that have expertise in AOM diagnosis, tympanocentesis and clinical trials. In the next 5 years investment is needed to prepare more investigators and sites to do such vaccine testing.
In the next 5 years it will likely become increasingly apparent that PCV efficacy erodes over time and that new vaccines based on alternative formulations will be necessary to prevent pneumococcal infections. WCVs and PPVs can confer serotype-independent protection and be cost-effective. Whole-cell vaccines may represent a path forward in developing countries but in developed countries regulators have become disinclined to approve whole cell vaccines and have shown a preference for component vaccines with demonstrated purity and consistency with manufacturing. PPVs have shown promise and may become the preferred pneumococcal vaccines in the next 5 years. At this writing the results of a phase III trial in American Indians of the GSK 2-componnet vaccine have not become publicly available. If the trial demonstrates efficacy in preventing AOM then further tests of that vaccine and other PPVs becomes more likely. If that trial fails to demonstrate efficacy then reformulations with novel adjuvants may become the primary activity for PPV development in the nearer term.
In the next 5 years additional clinical testing sites for pneumococcal vaccines will need to be identified and preliminary epidemiologic studies conducted. Further studies regarding determinants of protection from NP colonization vs. protection against pneumococcal disease should be done. Further work on cell-mediated vs. antibody mediated protection induced by WCVs and PPVs should occur. Development of in vitro assays to measure correlates of protection for WCVs and PPVs will be needed. We may also see newer technologies tested, such as reformulation of protein based pneumococcal vaccines into nanoparticles, another path forward. Nanoparticle presentation of antigens offers the ability to formulate vaccines containing multiple proteins and adjuvants targeting specific immune cells, and more effective uptake by antigen presenting cells.[91–94]
11. Key issues.
There are many risk factors such as health status, epidemiologic risk factors and NP pneumococcal colonization density that may influence the outcome of a pneumococcal vaccine trial
Newer vaccines are needed to target emerging serotypes of S. pneumoniae escaping from PCVs
Concern about eliminating all pneumococci rom the nasopharynx should be high
Adjuvants are needed for protein-based vaccines to elicit better and long lasting immunological memory
Newer assays to measure tvhe correlates of protection for WCVs and PPVs are needed
Acknowledgments
Funding
The manuscript was funded by a National Institute of Health grant (NIH R01 08671).
Abbreviations:
- AOM
(Acute Otitis Media)
- LVR
(Low Vaccine Responders)
- PCVs
(Pneumococcal Conjugate Vaccines)
- PPVs
(Purified Protein Vaccines)
- URI
(upper respiratory infection)
- WCV
(whole cell vaccines)
Footnotes
Declaration of interest
M.E Pichichero has served as a consultant to Sanofi Pasteur, GlaxoSmithKline, Merck and Pfizer regarding pneumococcal vaccine development. Author and his institution have received research funding from Sanofi Pasteur, Merck and Pfizer. Author is inventor and Sanofi Pasteur is owner of a patent regarding use of pneumococcal vaccines in otitis-prone children. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Stephens DS. Vaccines for the unvaccinated: protecting the herd. J Infect Dis, 197(5), 643–645 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Rennels MB, Edwards KM, Keyserling HL et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics, 101(4 Pt 1), 604–611 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 30(1), 100–121 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Butler JC, Breiman RF, Lipman HB, Hofmann J, Facklam RR. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978–1994: implications for development of a conjugate vaccine. J Infect Dis, 171(4), 885–889 (1995). [DOI] [PubMed] [Google Scholar]
- 5.Croucher NJ, Harris SR, Fraser C et al. Rapid pneumococcal evolution in response to clinical interventions. Science, 331(6016), 430–434 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinert R, Jacobs MR, Kaplan SL. Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development. Vaccine, 28(26), 4249–4259 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff WP, Hoet B, Adegbola RA. Predicting the impact of new pneumococcal conjugate vaccines: serotype composition is not enough. Expert review of vaccines, 14(3), 413–428 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Croucher NJ, Finkelstein JA, Pelton SI et al. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat Genet, 45(6), 656–663 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devine VT, Cleary DW, Jefferies JM et al. The rise and fall of pneumococcal serotypes carried in the PCV era. Vaccine, (2017). [DOI] [PubMed] [Google Scholar]
- 10.Alderson MR. Status of research and development of pediatric vaccines for Streptococcus pneumoniae. Vaccine, 34(26), 2959–2961 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Shimol S, Greenberg D, Givon-Lavi N et al. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: an active prospective nationwide surveillance. Vaccine, 32(27), 3452–3459 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Janoir C, Lepoutre A, Gutmann L, Varon E. Insight Into Resistance Phenotypes of Emergent Non 13-valent Pneumococcal Conjugate Vaccine Type Pneumococci Isolated From Invasive Disease After 13-valent Pneumococcal Conjugate Vaccine Implementation in France. Open forum infectious diseases, 3(1), ofw020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galanis I, Lindstrand A, Darenberg J et al. Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden. The European respiratory journal, 47(4), 1208–1218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. The Lancet infectious diseases, 15(5), 535–543 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Nakano S, Fujisawa T, Ito Y et al. Serotypes, antimicrobial susceptibility, and molecular epidemiology of invasive and non-invasive Streptococcus pneumoniae isolates in paediatric patients after the introduction of 13-valent conjugate vaccine in a nationwide surveillance study conducted in Japan in 2012–2014. Vaccine, 34(1), 67–76 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nature reviews. Microbiology, 6(4), 288–301 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Moore MR, Link-Gelles R, Schaffner W et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. The Lancet. Respiratory medicine, 4(5), 399–406 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Pichichero ME, Khan MN, Xu Q. Next generation protein based Streptococcus pneumoniae vaccines. Human vaccines & immunotherapeutics, 12(1), 194–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiser JN. The pneumococcus: why a commensal misbehaves. Journal of molecular medicine (Berlin, Germany), 88(2), 97–102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dagan R, Lipsitch M. Changing the Ecology of Pneumococci with Antibiotics and Vaccines In: The Pneumococcus. Tuomanen E (Ed. (ASM Press, 2004) 283–313. [Google Scholar]
- 21.Khan MN, Xu Q, Pichichero ME. Protection against Streptococcus pneumoniae Invasive Pathogenesis by a Protein-Based Vaccine Is Achieved by Suppression of Nasopharyngeal Bacterial Density during Influenza A Virus Coinfection. Infect Immun, 85(2) (2017).** This study demonstrated the increase in nasopharyngeal Spn bacterial load during a viral infection in mice and prevention of invasive pneumococcal disease in mice vaccinated with PhtD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slotved HC, Dalby T, Harboe ZB et al. The incidence of invasive pneumococcal serotype 3 disease in the Danish population is not reduced by PCV-13 vaccination. Heliyon, 2(11), e00198 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews NJ, Waight PA, Burbidge P et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. The Lancet infectious diseases, 14(9), 839–846 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Steens A, Caugant DA, Aaberge IS, Vestrheim DF. Decreased Carriage and Genetic Shifts in the Streptococcus pneumoniae Population After Changing the Seven-valent to the Thirteen-valent Pneumococcal Vaccine in Norway. The Pediatric infectious disease journal, 34(8), 875–883 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Wilson R, Cohen JM, Reglinski M et al. Naturally Acquired Human Immunity to Pneumococcus Is Dependent on Antibody to Protein Antigens. PLoS pathogens, 13(1), e1006137 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JO, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis, 177(2), 368–377 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Weiser JN, Austrian R, Sreenivasan PK, Masure HR. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun, 62(6), 2582–2589 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chai MH, Weiland F, Harvey RM, Hoffmann P, Ogunniyi AD, Paton JC. Proteomic comparisons of opaque and transparent variants of Streptococcus pneumoniae by two dimensional-differential gel electrophoresis. Scientific reports, 7(1), 2453 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overweg K, Pericone CD, Verhoef GG et al. Differential protein expression in phenotypic variants of Streptococcus pneumoniae. Infect Immun, 68(8), 4604–4610 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cundell DR, Weiser JN, Shen J, Young A, Tuomanen EI. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect Immun, 63(3), 757–761 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammerschmidt S, Wolff S, Hocke A, Rosseau S, Muller E, Rohde M. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun, 73(8), 4653–4667 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magee AD, Yother J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun, 69(6), 3755–3761 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen JM, Khandavilli S, Camberlein E, Hyams C, Baxendale HE, Brown JS. Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-cell responses to nasopharyngeal colonisation. PloS one, 6(10), e25558 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu HY, Virolainen A, Mathews B, King J, Russell MW, Briles DE. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microbial pathogenesis, 23(3), 127–137 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Turner P, Turner C, Green N et al. Serum antibody responses to pneumococcal colonization in the first 2 years of life: results from an SE Asian longitudinal cohort study. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 19(12), E551–558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dagan R, Juergens C, Trammel J et al. Modeling pneumococcal nasopharyngeal acquisition as a function of anticapsular serum antibody concentrations after pneumococcal conjugate vaccine administration. Vaccine, 34(36), 4313–4320 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Wolter N, Tempia S, Cohen C et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis, 210(10), 1649–1657 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. The Lancet infectious diseases, 4(3), 144–154 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Bogaert D, Engelen MN, Timmers-Reker AJ et al. Pneumococcal carriage in children in The Netherlands: a molecular epidemiological study. Journal of clinical microbiology, 39(9), 3316–3320 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. The Journal of clinical investigation, 119(7), 1899–1909 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichichero ME, Kaur R, Casey JR, Xu Q, Almudevar A, Ochs M. Antibody response to Streptococcus pneumoniae proteins PhtD, LytB, PcpA, PhtE and Ply after nasopharyngeal colonization and acute otitis media in children. Human vaccines & immunotherapeutics, 8(6), 799–805 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granat SM, Ollgren J, Herva E, Mia Z, Auranen K, Makela PH. Epidemiological evidence for serotype-independent acquired immunity to pneumococcal carriage. J Infect Dis, 200(1), 99–106 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Xu Q, Casey JR, Pichichero ME. Higher levels of mucosal antibody to pneumococcal vaccine candidate proteins are associated with reduced acute otitis media caused by Streptococcus pneumoniae in young children. Mucosal immunology, 8(5), 1110–1117 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDaniel LS, Swiatlo E. Should Pneumococcal Vaccines Eliminate Nasopharyngeal Colonization? mBio, 7(3) (2016).* Editorial comment on whether pneumococcal vaccines should target elimination of nasopharyngeal colonization [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoe E, Boelsen LK, Toh ZQ et al. Reduced IL-17A Secretion Is Associated with High Levels of Pneumococcal Nasopharyngeal Carriage in Fijian Children. PloS one, 10(6), e0129199 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moffitt K, Skoberne M, Howard A et al. Toll-like receptor 2-dependent protection against pneumococcal carriage by immunization with lipidated pneumococcal proteins. Infect Immun, 82(5), 2079–2086 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campos IB, Herd M, Moffitt KL et al. IL-17A and complement contribute to killing of pneumococci following immunization with a pneumococcal whole cell vaccine. Vaccine, 35(9), 1306–1315 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Edwards KM, Decker MD. Pertussis vaccines In: Vaccines. Plotkin, SA, Orenstein, WA, Offit PA (Eds.) (Elsevier Saunders, China, 2013) 447–492. [Google Scholar]
- 49.Gabutti G, Azzari C, Bonanni P et al. Pertussis. Human vaccines & immunotherapeutics, 11(1), 108–117 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moffitt KL, Yadav P, Weinberger DM, Anderson PW, Malley R. Broad antibody and T cell reactivity induced by a pneumococcal whole-cell vaccine. Vaccine, 30(29), 4316–4322 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higgs R, Higgins SC, Ross PJ, Mills KH. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal immunology, 5(5), 485–500 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Bolotin S, Harvill ET, Crowcroft NS. What to do about pertussis vaccines? Linking what we know about pertussis vaccine effectiveness, immunology and disease transmission to create a better vaccine. Pathogens and disease, 73(8), ftv057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Locht C Pertussis: acellular, whole-cell, new vaccines, what to choose? Expert review of vaccines, 1–3 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Prietsch SO, Axelsson I, Halperin SA. Acellular vaccines for preventing whooping cough in children. The Cochrane database of systematic reviews, 9, Cd001478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh M, Lingappan K. Whooping cough: the current scene. Chest, 130(5), 1547–1553 (2006). [DOI] [PubMed] [Google Scholar]
- 56.WHO. Pertussis vaccines: WHO position paper. Releve epidemiologique hebdomadaire, 85(40), 385–400 (2010). [PubMed] [Google Scholar]
- 57.Lu YJ, Leite L, Goncalves VM et al. GMP-grade pneumococcal whole-cell vaccine injected subcutaneously protects mice from nasopharyngeal colonization and fatal aspiration-sepsis. Vaccine, 28(47), 7468–7475 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu YJ, Gross J, Bogaert D et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS pathogens, 4(9), e1000159 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moffitt K, Malley R. Rationale and prospects for novel pneumococcal vaccines. Human vaccines & immunotherapeutics, 12(2), 383–392 (2016). ** Review on the rationale for development of pneumococcal vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alderson M, Malley R, Anderson P et al. A phase 1 study to assess the safety, tolerability and immunogenicity of inactivated nonencapsualted Streptococcus pneumoniae whole cell vaccine. In: [Abstract ISPPD - 0121]. pneumonia (Ed.^(Eds) (Hyderabad, India, 2014) 94. [Google Scholar]
- 61.Briles D, King J, Hale Y et al. IMMUNE SERA FROM ADULTS IMMUNIZED WITH KILLED WHOLE CELL NONENCAPSULATED VACCINE PROTECTS MICE FROM FATAL INFECTION WITH TYPE 3 PNEUMOCOCCI. In: ISPPD-9 / pneumonia. (Ed.^(Eds) (Hyderabad, India, 2014) Abstract 0122. [Google Scholar]
- 62.Moffitt KL, Gierahn TM, Lu YJ et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell host & microbe, 9(2), 158–165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Gierahn T, Thompson CM et al. Distinct effects on diversifying selection by two mechanisms of immunity against Streptococcus pneumoniae. PLoS pathogens, 8(11), e1002989 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leroux-Roels I, Devaster JM, Leroux-Roels G et al. Adjuvant system AS02V enhances humoral and cellular immune responses to pneumococcal protein PhtD vaccine in healthy young and older adults: randomised, controlled trials. Vaccine, 33(4), 577–584 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Pauksens K, Nilsson AC, Caubet M et al. Randomized Controlled Study of Pneumococcal Vaccine Formulations containing PhtD and dPly Proteins with Alum or Adjuvant System AS02V in Elderly Adults: Safety and Immunogenicity. Clinical and Vaccine Immunology, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prymula R, Pazdiora P, Traskine M, Ruggeberg JU, Borys D. Safety and immunogenicity of an investigational vaccine containing two common pneumococcal proteins in toddlers: A phase II randomized clinical trial. Vaccine, 32(25), 3025–3034 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Prymula R, Szenborn L, Silfverdal SA et al. IMMUNOGENICITY OF PRIMARY VACCINATION WITH AN INVESTIGATIONAL PROTEIN-BASED PNEUMOCOCCAL VACCINE IN INFANTS IN EUROPE: A PHASE II RANDOMIZED TRIAL. In: ISPPD-9 / pneumonia. (Ed.^(Eds) (Hyderabad, India, 2014) Abstract 0551. [Google Scholar]
- 68.Odutola A, Ota MO, Ogundare EO et al. Reactogenicity, safety and immunogenicity of a protein-based pneumococcal vaccine in Gambian children aged 2–4 years: A phase II randomized study. Human vaccines & immunotherapeutics, 12(2), 393–402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brooks WA, Chang LJ, Sheng X, Hopfer R, Team PPRS. Safety and immunogenicity of a trivalent recombinant PcpA, PhtD, and PlyD1 pneumococcal protein vaccine in adults, toddlers, and infants: A phase I randomized controlled study. Vaccine, 33(36), 4610–4617 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Hoque SS, Ghosh S, Poxton IR. Differences in intestinal humoral immunity between healthy volunteers from UK and Bangladesh. European journal of gastroenterology & hepatology, 12(11), 1185–1193 (2000). [DOI] [PubMed] [Google Scholar]
- 71.Lin A, Bik EM, Costello EK et al. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PloS one, 8(1), e53838 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Usuf E, Bojang A, Hill PC, Bottomley C, Greenwood B, Roca A. Nasopharyngeal colonization of Gambian infants by Staphylococcus aureus and Streptococcus pneumoniae before the introduction of pneumococcal conjugate vaccines. New Microbes New Infect, 10, 13–18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Licciardi PV, Russell FM, Balloch A et al. Impaired serotype-specific immune function following pneumococcal vaccination in infants with prior carriage. Vaccine, 32(20), 2321–2327 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madhi SA, Violari A, Klugman KP et al. Inferior quantitative and qualitative immune responses to pneumococcal conjugate vaccine in infants with nasopharyngeal colonization by Streptococcus pneumoniae during the primary series of immunization. Vaccine, 29(40), 6994–7001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lundgren A, Bhuiyan TR, Novak D et al. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine, 30(26), 3897–3907 (2012).** This study analyzed the PBMC responses to Spn whole cell antigen in children and adults from Sweden and Bangladesh. Different levels of Th17 responses between developed and developing countries are an important factor when evaluating pneumococcal vaccine candidates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pichichero ME. Ten-Year Study of the Stringently Defined Otitis-prone Child in Rochester, NY. The Pediatric infectious disease journal, 35(9), 1033–1039 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verhoeven D, Xu Q, Pichichero ME. Differential impact of respiratory syncytial virus and parainfluenza virus on the frequency of acute otitis media is explained by lower adaptive and innate immune responses in otitis-prone children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 59(3), 376–383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pichichero ME, Casey JR, Almudevar A et al. Functional Immune Cell Differences Associated With Low Vaccine Responses in Infants. J Infect Dis, 213(12), 2014–2019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren D, Almudevar AL, Pichichero ME. Synchrony in serum antibody response to conserved proteins of Streptococcus pneumoniae in young children. Human vaccines & immunotherapeutics, 11(2), 489–497 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu Q, Casey JR, Newman E, Pichichero ME. Otitis-prone Children Have Immunologic Deficiencies in Naturally Acquired Nasopharyngeal Mucosal Antibody Response after Streptococcus pneumoniae Colonization. The Pediatric infectious disease journal, 35(1), 54–60 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. The Pediatric infectious disease journal, 30(8), 645–650 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verhoeven D, Nesselbush M, Pichichero ME. Lower nasopharyngeal epithelial cell repair and diminished innate inflammation responses contribute to the onset of acute otitis media in otitis-prone children. Medical microbiology and immunology, 202(4), 295–302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gruber MF, Krause PR. Regulating vaccines at the FDA: Development and licensure of Zika vaccines. Expert review of vaccines, (2017). [DOI] [PubMed] [Google Scholar]
- 84.Hill PC, Cheung YB, Akisanya A et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian infants: a longitudinal study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 46(6), 807–814 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Granat SM, Mia Z, Ollgren J et al. Longitudinal study on pneumococcal carriage during the first year of life in Bangladesh. The Pediatric infectious disease journal, 26(4), 319–324 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Kwambana BA, Barer MR, Bottomley C, Adegbola RA, Antonio M. Early acquisition and high nasopharyngeal co-colonisation by Streptococcus pneumoniae and three respiratory pathogens amongst Gambian new-borns and infants. BMC infectious diseases, 11, 175 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pichichero ME. Ten-Year Study of Acute Otitis Media in Rochester, NY. The Pediatric infectious disease journal, 35(9), 1027–1032 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis, 204(4), 645–653 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J Infect Dis, 205(8), 1225–1229 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pichichero ME, Casey JR, Almudevar A. Nonprotective responses to pediatric vaccines occur in children who are otitis prone. The Pediatric infectious disease journal, 32(11), 1163–1168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aikins ME, Bazzill J, Moon JJ. Vaccine nanoparticles for protection against HIV infection. Nanomedicine (London, England), 12(6), 673–682 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dowling DJ, Scott EA, Scheid A et al. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. The Journal of allergy and clinical immunology, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zughaier SM. Analysis of novel meningococcal vaccine formulations. Human vaccines & immunotherapeutics, 1–5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richard K, Mann BJ, Qin A, Barry EM, Ernst RK, Vogel SN. Monophosphoryl Lipid A Enhances Efficacy of a Francisella tularensis LVS-Catanionic Nanoparticle Subunit Vaccine against F. tularensis Schu S4 Challenge by Augmenting both Humoral and Cellular Immunity. Clinical and vaccine immunology : CVI, 24(3) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sobanjo-ter Meulen A, Vesikari T, Malacaman EA et al. Safety, tolerability and immunogenicity of 15-valent pneumococcal conjugate vaccine in toddlers previously vaccinated with 7-valent pneumococcal conjugate vaccine. The Pediatric infectious disease journal, 34(2), 186–194 (2015). [DOI] [PubMed] [Google Scholar]
- 96.McFetridge R, Meulen AS, Folkerth SD et al. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine, 33(24), 2793–2799 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Kamtchoua T, Bologa M, Hopfer R et al. Safety and immunogenicity of the pneumococcal pneumolysin derivative PlyD1 in a single-antigen protein vaccine candidate in adults. Vaccine, 31(2), 327–333 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Seiberling M, Bologa M, Brookes R et al. Safety and immunogenicity of a pneumococcal histidine triad protein D vaccine candidate in adults. Vaccine, 30(52), 7455–7460 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Bologa M, Kamtchoua T, Hopfer R et al. Safety and immunogenicity of pneumococcal protein vaccine candidates: monovalent choline-binding protein A (PcpA) vaccine and bivalent PcpA-pneumococcal histidine triad protein D vaccine. Vaccine, 30(52), 7461–7468 (2012). [DOI] [PubMed] [Google Scholar]


