Abstract
Background
In the Netherlands, there are strong disparities in Chlamydia trachomatis (CT) prevalence between ethnic groups. The current study aims to identify whether socioeconomic status, sexual risk behavior and sexual healthcare seeking behavior may explain differences in CT seroprevalence between ethnic groups.
Methods
We used 2011–2014 baseline data of the HELIUS (HEalthy LIfe in an Urban Setting) study, a multi-ethnic population-based cohort study in Amsterdam, the Netherlands, including participants from Dutch, African Surinamese, South-Asian Surinamese, Ghanaian, Moroccan and Turkish origin. For this analysis, we selected sexually active, heterosexual participants aged 18–34 years old. CT seroprevalence was determined using a multiplex serology assay. The CT seroprevalence ratios between different ethnicities are calculated and adjusted for potential indicators of socioeconomic status, sexual risk behavior and sexual healthcare seeking behavior.
Results
The study population consisted of 2001 individuals (52.8% female) with a median age of 28 years (IQR 24–31). CT seropositivity differed by ethnicities and ranged from 71.6% (African Surinamese), and 67.9% (Ghanaian) to 31.1% (Turkish). The CT seroprevalence ratio of African Surinamese was 1.72 (95% CI 1.43–2.06) and 1.52 (95% CI 1.16–1.99) of Ghanaian as compared to the Dutch reference group, after adjustment for socioeconomic status, sexual risk behavior and sexual healthcare seeking behavior.
Conclusions
Indicators of socioeconomic status, sexual risk behavior, and sexual health seeking behavior could not explain the higher CT seroprevalence among African Surinamese and Ghanaian residents of Amsterdam.
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3533-7) contains supplementary material, which is available to authorized users.
Keywords: Ethnicity, Chlamydia trachomatis, Sexual healthcare seeking behavior, Socioeconomic status, Sexual risk behavior
Short summary
A multi-ethnic cohort in Amsterdam, the Netherlands, showed that the disparate Chlamydia trachomatis seroprevalence between ethnic groups was not explained by socioeconomic status, sexual healthcare seeking behavior and sexual behavior.
Background
Chlamydia trachomatis (CT) infections are a major public health concern. In many high income countries, including the Netherlands, CT prevalence, incidence and test positivity differs substantially across ethnic groups [1–5]. Urogenital CT infection is associated with sexual risk behavior (SRB) such as condomless sexual contact and higher number of sex partners [6, 7]. Some studies reported differences in these risk behaviors between ethnic groups [2, 8]. However, differences in individual risk behaviors fail to explain the differences in CT prevalence across ethnic groups [9–11]. Evidence from several population-based and STI clinic studies in the Netherlands suggested that sexual healthcare seeking behavior (sHSB) may play an important role in explaining the ethnic differences in CT infection rates [4]. Diagnosis and treatment of, especially asymptomatic, CT infections may be delayed by reduced sHSB, prolonging the time during which CT transmission is possible. This might lead to a higher CT prevalence in (sub)populations where members are characterized by low sHSB, high risk of CT infection, and have sex predominantly with members of the same group. Lower socioeconomic status (SES) is associated with lower sHSB [12]. In one study, CT infection was found to be associated with non-Dutch ethnicity and low SES [13]. In another study the uptake of CT screening tests was different between individuals with different ethnic backgrounds [4].
To better understand the contribution and interplay of SES, SRB and sHSB in their associations with CT and whether differences in these factors may explain differences in CT prevalence between ethnic groups, we performed a retrospective analysis of baseline data of a large multi-ethnic study: the HELIUS study. We examined whether SES, SRB and sHSB could explain the difference in heterosexual CT seroprevalence between ethnic groups in Amsterdam, the Netherlands.
Material and methods
Study population
The current study is based on baseline data from the HELIUS (HEalthy LIfe in an Urban Setting) study. The goals and design of the HELIUS study have been described before [14, 15]. In brief, HELIUS is a population-based study that includes individuals aged 18–70 years from the major ethnic groups living in Amsterdam (Surinamese, Turkish, Moroccan, Ghanaian and Dutch). Individuals were randomly selected, stratified by ethnicity, from the municipal registry of the city of Amsterdam and invited to participate in the study. The aim was to include similarly sized samples for each ethnic group, oversampling smaller groups to ensure this goal. Among each ethnic group, women were more likely to participate than men, and those who participated were slightly older than those who did not. Non-response analyses also indicated that, for each ethnic group, respondents and non-respondents did not differ regarding several SES indicators, suggesting representative samples for each ethnic group [15]. Baseline data collection took place from 2011 to 2015. Data were obtained by questionnaire and a physical examination, including collection of biological samples. Written informed consent was obtained from all participants. All HELIUS study protocols have been approved by the Ethics Review Board of the Academic Medical Center and are in accordance with the revised Declaration of Helsinki of 2000.
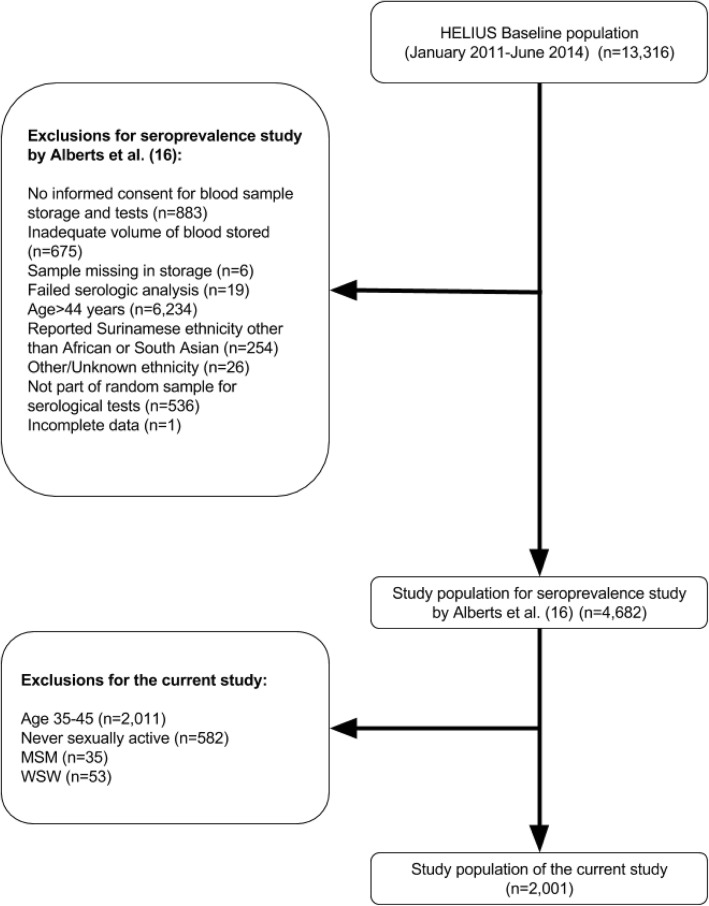
For the current study, a subset of the HELIUS baseline data and samples that were collected until June 2014 was used, on which CT serologic tests were performed. The selection procedure of the subsample has been described previously [16]. In short, among participants aged 18 to 44 years who gave informed consent for additional blood analyses and of whom an adequate volume of blood was available, a random sample per life year from each gender and ethnic group (Dutch, South-Asian Surinamese, African Surinamese, Ghanaian, Moroccan and Turkish ethnicity) was taken, resulting in a dataset of 4682 participants (Fig. 1) [16]. From this dataset we excluded men who have sex with men (MSM) and women who have sex with women (WSW) (based on self-reported behavior) and participants who had not yet had their sexual debut, because their Chlamydia infection risk differs from the general heterosexual population. All participants over 35 years old were excluded, as completion of questions on SRB was optional in this group and hence data were incomplete (Fig. 1).
Fig. 1.
Flowchart of the selection process of participants for the current study
Ethnicity and migration status
Ethnicity was defined according to the country of birth of the participant as well as that of his/her parents [17]. Specifically, the HELIUS study invited participants of non-Dutch ethnic origin if the following criteria were met: 1) the participant and at least one parent were born abroad (first generation), or 2) the participant was born in the Netherlands, but both parents were born abroad (second generation). For participants of Surinamese origin, ethnicity was further classified according to self-reported ethnic origin (e.g. South-Asian, African, Javanese, or other). For the Dutch sample, people were invited who were born in the Netherlands and whose parents were born in the Netherlands.
Chlamydia serological assay
To be able to take past CT infections into account, CT seroprevalence was determined as a proxy for lifetime CT exposure. Serum samples of fasting blood samples were tested for the presence of CT antibodies using a multiplex serology assay, developed at the German Cancer Research Center (DKFZ) in Heidelberg, Germany. This assay detects antibodies that bind to 7 CT antigens: the Major Outer Membrane Protein (MOMP) of serovars A, D, and L2, Translocated actin-recruiting phosphoprotein (Tarp, split into its N- and C-terminal fractions based on its size), and Porin B and Heat shock Protein-60 (Hsp60). The assay was validated against serum samples with known CT DNA status and a commercial ELISA (CT-ELISA, Medac, Wedel, Germany). The maximum of the 3 MOMP responses (MOMPmax) was used as a combined variable, and CT seropositivity was classified as an antibody response to 2 or more of the following antigens: Tarp-N, Tarp-C, PorB, and MOMPmax, or a median fluorescence intensity (MFI) > 1000 to MOMPmax alone, resulting in a sensitivity of 83% and a specificity of 87% for classification as CT seropositive (indicating CT exposure). Antibody cross-reactivity of Chlamydia trachomatis and Chlamydophila pneumonia was only minor, as indicated by correlation analyses (R2 < 0.05, data not shown) (Additional file 1: Appendix 1).
SES, SRB, sHSB and other measurements
Information on demographics, SES, SRB, and sHSB were obtained by questionnaire. SES was determined by occupational level and educational level. Occupational level was classified according to the Standard Classification of Occupations of 2010 by Statistics Netherlands (CBS) [18]. This document provides an extensive systematic list of all professions in the Dutch system. Based on this document, occupational level was classified into (1) elementary, or lower, (2) intermediate and (3) higher or (4) academic, based on job title and job description, including a question on fulfilling an executive function (directing personnel). Educational level was based on the highest qualification attained, either in the Netherlands or in the country of origin, and it was categorized into three groups, namely (1) no or elementary schooling, or lower vocational or lower secondary schooling, (2) intermediate vocational, or intermediate or higher secondary schooling and (3) higher vocational schooling or university.
sHSB was based on self-reports of having been tested for HIV or other STIs in the preceding 6 months. SRB characteristics were the lifetime number of sex partners, age of sexual debut and self-reported condom use in the preceding 6 months with casual and steady sex partners, categorized as (1) no sexual contact in the past 6 months, (2) sex exclusively with a steady partner in the past 6 months (regardless of condom use), (3) consistent condom use with casual sex partners (regardless of sex with a steady partner), and (4) inconsistent or no condom use with casual sex partners (regardless of sex with a steady partner). Self-reported consistent condom use is defined as having always used a condom with a sexual partner. All degrees of self-reported inconsistency with condom use were defined as inconsistent condom use.
Statistical analyses
The characteristics of the study population were described and differences between ethnic groups evaluated with χ2 tests for categorical variables or Kruskal-Wallis tests for continuous variables. The seroprevalence ratio (PR) of CT between the different ethnicities, and adjustments for potential explanatory indicators of SES, SRB, sHSB was calculated by Poisson regression analyses with robust variance [19].
For Poisson regression analyses, age was categorized into three strata: 18–24 years, 25–29 years and 30–34 years. The distribution of the number of lifetime sex partners was positively skewed and therefore transformed to its natural logarithm.
In univariable Poisson regression analyses with robust variance, we assessed the association of CT seropositivity with the variables selected in our theoretical model. These variables were age, sex and the indicators of SRB, SES and sHSB. Analyses were done overall, and stratified by ethnicity.
We assessed the CT seroprevalence ratios of the different ethnicities compared to the Dutch reference group, adjusting for potential explanatory indicators of SES, SRB, sHSB and the confounders age and gender in five steps. The first model included age and gender, the second age, gender and indicators of SRB, the third age, gender and indicators of SES, the fourth age, gender, and indicators of sHSB and the final model included age, gender and indicators of SRB, SES, and sHSB. In case of a PR > 1, a decrease of the CT seroprevalence ratio after adjustment with an indicator suggests an explanatory effect of that specific indicator for differences in CT seroprevalence between the compared groups. In case of a PR < 1, the same goes for an increase of CT seroprevalence ratio.
To assess the effect of migration generation on CT seroprevalence after adjustments for all indicators of SRB, SES and sHSB, we have performed a multivariable analysis stratified by ethnicity and migration generation.
Data were missing for some variables; as this was occurring in less than 5% of records per variable, we performed complete case analyses.
A statistical significance level of P < 0.05 was used. Statistical analyses were performed in STATA Intercooled 13.1 (College Station, Texas, USA).
Results
Study population selection and baseline characteristics including SES
Serological data were available for 4682 HELIUS participants. For the current analysis, 2011 participants aged 35 years or above, 582 participants who never had sexual intercourse, 35 MSM and 53 WSW were excluded. The resulting 2001 participants were included in the current study (Fig. 1).
In our study sample, the median age (28 years) of participants was similar across all ethnicities, and participants were mostly female (Table 1). Most participants of non-Dutch origin in the subsample were second generation migrants, except for Ghanaian participants who were mostly first generation migrants (78.2%). Overall, Dutch participants were higher educated and had higher occupational levels (Table 1).
Table 1.
Characteristics of the study population (n = 2001)a, stratified by ethnicity
| Dutch (n = 392) | South Asian Surinamese (n = 373) | African Surinamese (n = 317) | Ghanaian (n = 193) | Turkish (n = 389) | Moroccan (n = 337) | Total (n = 2001) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||||||
| Sex [n,%] (n = 2001) | ||||||||||||||
| Female | 214 | 54.6% | 185 | 49.6% | 193 | 60.9% | 121 | 62.7% | 167 | 43.4% | 174 | 51.6% | 1056 | 52.8% |
| Migration generation [n,%] (n = 1609) | ||||||||||||||
| First generation | N/A | N/A | 82 | 22.0% | 136 | 42.9% | 151 | 78.2% | 137 | 35.2% | 117 | 34.7% | 623 | 38.7% |
| Age in years [median, IQR] (n = 2001) | 28 | 25–31 | 28 | 23–31 | 27 | 23–31 | 27 | 23–32 | 29 | 25–32 | 29 | 25–32 | 28 | 24–31 |
| Socioeconomic status | ||||||||||||||
| Educational level [n,%] (n = 1995) | ||||||||||||||
| Low | 21 | 5.4% | 79 | 21.2% | 51 | 16.1% | 68 | 35.4% | 143 | 36.9% | 88 | 26.3% | 450 | 22.6% |
| Intermediate | 94 | 24.0% | 163 | 43.5% | 178 | 56.3% | 89 | 46.4% | 166 | 42.8% | 157 | 46.8% | 847 | 42.5% |
| High | 277 | 70.7% | 130 | 35.0% | 87 | 27.5% | 35 | 18.2% | 79 | 20.4% | 90 | 26.8% | 698 | 35.0% |
| Occupational level [n,%] (n = 1717) | ||||||||||||||
| Low | 53 | 15.2% | 125 | 38.1% | 112 | 44.1% | 94 | 64.0% | 172 | 49.3% | 112 | 38.6% | 668 | 38.9% |
| Intermediate | 64 | 18.3% | 104 | 31.7% | 90 | 35.4% | 37 | 25.2% | 110 | 31.5% | 114 | 39.3% | 519 | 30.2% |
| High | 232 | 66.5% | 99 | 30.2% | 52 | 20.5% | 16 | 10.9% | 67 | 19.2% | 64 | 22.1% | 530 | 30.9% |
| Sexual risk behavior | ||||||||||||||
| Lifetime number of sex partners [median, IQR] (n = 1901) | 7 | 3–13 | 3 | 1–6 | 5 | 3–10 | 4 | 2–6 | 1 | 1–7 | 2 | 1–7 | 4 | 1–10 |
| Age in years at sexual debut [median, IQR] (n = 1844) | 17 | 16–19 | 17 | 16–19 | 16 | 15–18 | 17 | 16–19 | 18 | 16–22 | 18 | 16–22 | 17 | 16–19 |
| Sexual contacts and condom use (preceding 6 months) [n,%] (n = 1836) | ||||||||||||||
| No sexual contact | 48 | 12.5% | 67 | 18.8% | 40 | 13.2% | 43 | 26.5% | 63 | 18.5% | 61 | 19.7% | 322 | 17.3% |
| Steady partner onlyb | 239 | 62.1% | 236 | 66.3% | 185 | 61.1% | 93 | 57.4% | 215 | 63.1% | 200 | 64.5% | 1168 | 62.9% |
| Consistent condom use with casual partnersc | 30 | 7.8% | 31 | 8.7% | 37 | 12.2% | 11 | 6.8% | 32 | 9.4% | 29 | 9.4% | 170 | 9.2% |
| Inconsistent condom use with casual partnersc | 68 | 17.7% | 22 | 6.2% | 41 | 13.5% | 15 | 9.3% | 31 | 9.1% | 20 | 6.5% | 197 | 10.6% |
| Sexual health seeking behavior | ||||||||||||||
| HIV testing (preceding 6 months) [n,%] (n = 1952) | ||||||||||||||
| Yes | 44 | 11.3% | 37 | 10.0% | 83 | 26.4% | 41 | 21.8% | 26 | 6.9% | 29 | 8.7% | 260 | 13.2% |
| STI testing (preceding 6 months) [n,%] (n = 1951) | ||||||||||||||
| Yes | 70 | 17.9% | 49 | 13.2% | 104 | 33.1% | 40 | 21.2% | 29 | 7.7% | 45 | 13.5% | 337 | 17.1% |
| Primary outcome | ||||||||||||||
| C. trachomatis seropositivity [n,%] (n = 2001) | ||||||||||||||
| Overall | 147 | 37.5% | 150 | 40.2% | 227 | 71.6% | 131 | 67.9% | 121 | 31.1% | 117 | 34.7% | 893 | 44.6% |
| Female | 79 | 36.9% | 77 | 41.6% | 147 | 76.2% | 81 | 66.9% | 46 | 27.2% | 66 | 37.9% | 496 | 47.0% |
| Male | 68 | 38.2% | 73 | 38.8% | 80 | 64.5% | 50 | 69.4% | 75 | 34.1% | 51 | 31.3% | 397 | 42.0% |
IQR Interquartile range, N/A Not available, HIV Human immunodeficiency virus, STI Sexually transmitted infection
Data are presented as n (%) or median (IQR)
aNumbers may not add up due to missing values
bSex exclusively with one partner, regardless of condom use
cHas had a casual sex partner, irrespective of having had a steady partner
Sexual risk behavior (SRB) and sexual healthcare seeking behavior (sHSB) between the different ethnicities
Several differences in SRB between the ethnic groups were observed. The median age at sexual debut was lowest among African Surinamese participants (16 years, IQR 15–18) and highest among Turkish and Moroccan participants (both 18 years, IQR 16–22).The median lifetime number of sex partners was highest among Dutch participants (7 sex partners, IQR 3–13), and lowest among Turkish participants (1 sex partner, IQR 1–7). Most individuals reported exclusive sexual contact with a steady partner (regardless of condom use). Compared to other participants, inconsistent condom use with casual sex partners was most frequent among Dutch and African Surinamese participants (Table 1).
sHSB was also dissimilar across the different ethnic groups. 13.2% of the study population reported having been tested for HIV and 17.1% for STI both in the preceding 6 months. The proportions that had been tested in the preceding 6 months for HIV (26.4%) and STI (33.1%) were highest among African Surinamese participants, and lowest among those of Turkish origin (Table 1).
Chlamydia seroprevalence
In the overall population, CT seroprevalence was 44.6%. CT seroprevalence was highest among African Surinamese (71.6%), followed by Ghanaian participants (67.9%). Overall CT seropositivity among male participants (42.0%) was slightly lower than among female participants (47.0%; P = 0.03).
CT seroprevalence ratios without adjustments
In the total study population the risk of CT seropositivity was increased by being female, a higher lifetime number of sex partners, a lower age at sexual debut, and in the preceding 6 months: inconsistent condom use with casual sex partners, STI testing and HIV testing. Second generation migrants were less likely to having been exposed to CT than first generation migrants. A high level of education and a high level of occupation were significantly associated with not having been exposed to CT (Table 2).
Table 2.
(Sero) prevalence ratios of CT for potential determinants, by ethnic group. Results of stratified unadjusted Poisson regression with robust variance
| Dutch (n = 392) | South Asian Surinamese (n = 373) | African Surinamese (n = 317) | Ghanaian (n = 193) | Turkish (n = 389) | Moroccan (n = 337) | Total (n = 2001) | |
|---|---|---|---|---|---|---|---|
| PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | |
| Demographics | |||||||
| Gender | |||||||
| Male | 1 | 1 | 1* | 1 | 1 | 1 | 1* |
| Female | 0.97 (0.75–1.25) | 1.08 (0.84–1.37) | 1.18 (1.01–1.38) | 0.96 (0.79–1.18) | 0.80 (0.59–1.09) | 1.21 (0.90–1.63) | 1.12 (1.01–1.23) |
| Migration generation | |||||||
| First | N/A | 1 | 1 | 1* | 1 | 1 | 1*** |
| Second | N/A | 1.00 (0.74–1.35) | 0.97 (0.85–1.12) | 0.73 (0.53–0.99) | 1.06 (0.77–1.45) | 0.95 (0.70–1.29) | 0.82 (0.74–0.91) |
| Age | |||||||
| 18–24 years | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 25–29 years | 0.98 (0.70–1.36) | 1.31 (0.93–1.83) | 1.03 (0.87–1.22) | 0.96 (0.73–1.27) | 1.11 (0.73–1.68) | 0.88 (0.58–1.32) | 0.98 (0.86–1.11) |
| 30–34 years | 0.99 (0.70–1.38) | 1.45 (1.05–2.00)* | 1.03 (0.87–1.23) | 1.17 (0.93–1.47) | 1.08 (0.72–1.63) | 0.96 (0.65–1.41) | 1.01 (0.89–1.14) |
| Socioeconomic status | |||||||
| Educational level | |||||||
| Low | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Intermediate | 1.24 (0.65–2.39) | 1.05 (0.76–1.44) | 0.97 (0.81–1.17) | 0.76 (0.62–0.94)* | 0.83 (0.60–1.14) | 1.01 (0.71–1.44) | 0.99 (0.87–1.11) |
| High | 1.09 (0.59–2.04) | 0.93 (0.66–1.32) | 0.91 (0.73–1.13) | 0.79 (0.60–1.05) | 0.66 (0.42–1.04) | 0.95 (0.63–1.42) | 0.84 (0.74–0.96)* |
| Occupational level | |||||||
| Low | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Intermediate | 0.69 (0.43–1.10) | 1.13 (0.83–1.53) | 1.09 (0.92–1.28) | 0.74 (0.53–1.02) | 0.86 (0.61–1.21) | 0.99 (0.68–1.36) | 0.91 (0.80–1.03) |
| High | 0.82 (0.58–1.15) | 1.11 (0.81–1.52) | 0.90 (0.71–1.14) | 0.77 (0.49–1.20) | 0.62 (0.38–1.01) | 0.81 (0.52–1.27) | 0.80 (0.70–0.91)** |
| Sexual risk behavior | |||||||
| Number of lifetime sex partnersa | 1.32 (1.17–1.50)*** | 1.19 (1.06–1.33)** | 1.05 (0.97–1.13) | 1.02 (0.90–1.17) | 1.12 (1.00–1.25) | 1.12 (1.00–1.25) | 1.16 (1.11–1.21)*** |
| Age at sexual debutb | 0.90 (0.84–0.98)* | 0.97 (0.93–1.01) | 0.99 (0.96–1.02) | 0.96 (0.93–1.00)* | 0.96 (0.92–1.00)* | 0.96 (0.93–1.00)* | 0.95 (0.93–0.96)*** |
| Sexual partners and condom use (preceding 6 months) | |||||||
| No sexual contact | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Steady partnerc | 0.97 (0.64–1.48) | 1.02 (0.72–1.43) | 1.16 (0.89–1.52) | 1.11 (0.86–1.43) | 1.16 (0.75–1.79) | 1.53 (0.95–2.45) | 1.11 (0.96–1.29) |
| Consistent condom used | 1.32 (0.77–2.26) | 1.16 (0.71–1.90) | 1.40 (1.04–1.87)* | 0.42 (0.16–1.13) | 0.88 (0.43–1.79) | 0.56 (0.20–1.54) | 1.10 (0.88–1.36) |
| Inconsistent condom use d | 1.37 (0.87–2.16) | 1.41 (0.86–2.29) | 1.34 (1.00–1.80) | 0.92 (0.58–1.47) | 1.35 (0.75–2.45) | 2.44 (1.38–4.30)** | 1.42 (1.18–1.70)*** |
| Sexual health seeking behavior | |||||||
| HIV testing (preceding 6 months) | |||||||
| No | 1 | 1 | 1* | 1 | 1* | 1* | 1*** |
| Yes | 1.24 (0.87–1.77) | 1.38 (1.00–1.92) | 1.16 (1.00–1.33) | 1.12 (0.90–1.40) | 1.68 (1.10–2.54) | 1.56 (1.06–2.30) | 1.51 (1.35–1.68) |
| STI testing (preceding 6 months) | |||||||
| No | 1* | 1*** | 1 | 1 | 1 | 1 | 1*** |
| Yes | 1.38 (1.04–1.83) | 1.64 (1.26–2.13) | 1.07 (0.93–1.24) | 1.15 (0.93–1.43) | 1.48 (0.96–2.29) | 1.18 (0.80–1.75) | 1.45 (1.31–1.61) |
OR Odds ratio, CI Confidence intervals, N/A not applicable, HIV Human immunodeficiency virus, STI Sexually transmitted infection, CT Chlamydia trachomatis
aPer increase of 1 natural log
bPer 1 year increase
cSex with exclusive sex partner, regardless of condom use
dHas had a casual partner, irrespective of having had a steady partner
*overall p < 0.05
**overall p < 0.01
***overall p < 0.001
Adjusted seroprevalence ratios of CT
The CT seroprevalence ratios (reference group: Dutch participants) were highest among African Surinamese (PR: 1.99; 95% CI 1.65–2.21) and Ghanaian participants (PR: 1.81; 95% CI 1.54–2.13) (Table 3). African Surinamese (adjusted PR (aPR): 1.72; 95% CI 1.43–2.06) and Ghanaians (aPR: 1.52; 95% CI 1.16–1.99) remained significantly more likely to be CT seropositive when adjusted for (1) age and gender, (2) age, gender and indicators of SRB, (3) age, gender and indicators of SES, (4) age, gender, and indicators of sHSB and (5) age, gender and indicators of SRB, SES, and sHSB .
Table 3.
Adjusted (sero)prevalence ratios (PR) of CT by ethnicity, as compared to the Dutch reference group
| Adjustments | South Asian Surinamese PR (95% CI) | African Surinamese PR (95% CI) | Ghanaian PR (95% CI) | Turkish PR (95% CI) | Moroccan PR (95% CI) |
|---|---|---|---|---|---|
| Unadjusted* | 1.07 (0.90–1.28) | 1.99 (1.65–2.21) | 1.81 (1.54–2.13) | 0.83 (0.68–1.01) | 0.93 (0.76–1.12) |
| Model 1: Adjusted for age and gender | 1.09 (0.91–1.30) | 1.90 (1.64–2.20) | 1.81 (1.54–2.13) | 0.82 (0.67–1.00) | 0.93 (0.76–1.13) |
| Model 2: Adjusted for age, gender and sexual risk behavior (SRB)a | 1.29 (1.07–1.56) | 1.87 (1.61–2.17) | 1.83 (1.51–2.21) | 1.01 (0.81–1.26) | 1.13 (0.91–1.40) |
| Model 3: Adjusted for age, gender, and socioeconomic status (SES)b | 1.11 (0.90–1.36) | 1.76 (1.47–2.12) | 1.58 (1.24–2.01) | 0.75 (0.59–0.97) | 0.89 (0.70–1.13) |
| Model 4: Adjusted for age, gender and sexual healthcare seeking behavior (sHSB)c | 1.11 (0.93–1.33) | 1.85 (1.59–2.14) | 1.78 (1.51–2.10) | 0.86 (0.70–1.05) | 0.94 (0.77–1.14) |
| Model 5: Adjusted for age, gender, SRB, SES and sHSBa, b, c | 1.27 (1.02–1.58) | 1.72 (1.43–2.06) | 1.52 (1.16–1.99) | 0.87 (0.66–1.13) | 1.09 (0.84–1.40) |
PR (Sero)prevalence ratio, CI Confidence intervals
*Results from unadjusted Poisson regression with robust variance
aSexual risk behavior includes sexual contacts and condom use in the preceding 6 months, the natural log of lifetime sex partners and age at sexual debut
bSocioeconomic status includes educational level and occupational level
cSexual healthcare seeking behavior includes HIV testing and STI testing in the preceding 6 months
Being of Turkish and Moroccan origin did not significantly increase the risk of CT seropositivity in univariable analysis, and the additional, stepwise, adjustments had little effect on the risk. In univariable analysis, South Asian Surinamese were as likely to be CT seropositive as the Dutch. When adjusted for age, gender, SRB, SES and sHSB (Step 5), the CT seroprevalence was significantly higher than in the Dutch (Table 3).
The multivariable analysis stratified by migration generation showed that among South Asian Surinamese second generation migration status, among Ghanaians first, among African Surinamese first and second was statistically associated with CT seropositivity, as compared to the Dutch. Of note, among South Surinamese first generation migration status and among Ghanaian second was not significantly associated with CT seropositivity compared to the Dutch (Table 4).
Table 4.
Adjusted CT seropositivity ratios of different ethnic groups (stratified by migration generation), as compared to Dutch. The analyses were adjusted for SRB, SES and sHSB
| Ethnicity | PR + 95% CI |
|---|---|
| South Asian Surinamese- first generation | 1.26 (0.89–1.79) |
| South Asian Surinamese- second generation | 1.27 (1.02–1.59)* |
| African Surinamese- first generation | 1.80 (1.47–2.20)* |
| African Surinamese- second generation | 1.66 (1.36–2.02)* |
| Ghanaian- first generation | 1.73 (1.31–2.29)* |
| Ghanaian- second generation | 1.04 (0.67–1.63) |
| Turkish- first generation | 0.91 (0.64–1.31) |
| Turkish- second generation | 0.85 (0.64–1.13) |
| Moroccan- first generation | 1.22 (0.86–1.71) |
| Moroccan- second generation | 1.04 (0.79–1.38) |
* Indicates a statistically significant result (p <0.05)
Discussion
In this study we assessed the disparate CT seroprevalence and aimed to explain the observed differences among various ethnic groups in the Netherlands. The current study showed a higher seroprevalence of CT among participants of African Surinamese and Ghanaian ethnic origin compared to the Dutch population. The seroprevalence of CT among three ethnicities (South-Asian Surinamese, Turkish and Moroccan participants) did not differ from that in the Dutch. Our study showed that SRB, SES and sHSB could not explain the disparate CT seroprevalence between the African Surinamese and Ghanaian groups and the Dutch. Being first or second generation migrant may play a role in explaining the ethnic differences in CT seroprevalence, especially among Ghanaians.
This is the first study to investigate the role of sHSB on disparate CT seroprevalence between ethnic groups. Other studies observed similar differences in CT between ethnic groups [3, 4, 9, 13], but were not based on diagnosis of CT by serology. Like in other studies [9–11] SRB failed to explain the discrepancies, but unlike a study performed by Matser et al. [9], SES was not explanatory for differences in CT between ethnicities.
The strength of this study, based on data from the large scale, population-based HELIUS study, lies in the sample sizes of the major ethnic groups of interest and data availability on demographics, SES, sHSB and SRB. These factors enabled us to assess whether these variables may explain in part the association between ethnicity and CT infection.
Another strength of this study is that similar ethnic group sizes were ensured during inclusion of HELIUS and only very small differences exist between participants and non-participants. Thus, the study sample is well suited for comparison of seroprevalence between the different ethnic groups. As no attempt is made to estimate the overall CT seroprevalence of Amsterdam oversampling of groups is of no concern [15].
A major limitation is the use of CT serology as a surrogate of a recent CT infection. Firstly, the specificity to detect acute infection is not optimal (87%), as it detects exposure to a (past) CT infection, rather than a recent infection. Moreover, CT seropositivity can also be induced by other CT infections like trachoma, which is a relatively common eye disease in West Africa [20] and in rare cases by pneumonia and inclusion conjunctivitis [21]. It may be that first generation migrants of Western African origin (i.e. Ghanaian participants) have been exposed to eye infections caused by CT, inflating the CT seroprevalence of the Ghanaian study group. Cross reactivity with C. pneumoniae, a well known issue with ELISA based CT tests, was only minor (details in Additional file 1: Appendix 1).
A specificity of less than 100% means that some participants are wrongly regarded as CT-exposed. There is no reason to believe that this misclassification differs by ethnicity or sHSB. Such random misclassification of CT status dilutes the strength of the effect of a risk factor with the outcome, but does not lead to the false identification of risk factors [22]. The use of Pgp3 based CT serological assays may improve the inherent properties of a serological CT test, as it has a higher specificity for CT [23, 24]. Lastly, the sensitivity of the CT serology may be affected by waning antibodies to CT overtime. For the current serological test the degree of waning is unknown. However, very recently Horner et al. [23] have shown that the waning of CT Pgp3 antibodies is less than 5% over 12 years, which suggests only a very modest effect of waning of CT antibodies. Use of NAAT based diagnosis of CT, might have alleviated some of these limitations caused by the suboptimal sensitivity and specificity of serological tests, but such results were not available [25].
Another limitation of the study is that no assessment could be performed on other areas of health care seeking behavior than sHSB. This might introduce a selection bias as low sHSB can either be appropriate or inappropriate depending on CT risk, but the difference between the two types of low sHSB cannot be discerned independently without an indication of healthcare seeking behavior in general. Likewise, if information on healthcare seeking behavior in general was available, but on sHSB was lacking, a similar selection would occur. In the multivariable models, adjustment for SRB was performed which minimizes the risk of selection bias due to this mechanism in the current study.
The cross-sectional design of the current study limits the possibilities to establish the causal role of SES, SRB, and sHSB in the association between ethnicity and CT. Follow-up data of CT infections and risk could establish temporal, etiologic causations of ethnicity with CT.
Another potential limitation concerns the representativeness of the HELIUS cohort with regards to health seeking behavior. It might be that HELIUS study participants differ from the general population in terms of health awareness and consequently health seeking behavior. Unfortunately, no information on these variables was available from the HELIUS (non)-response analysis, so no reasonable conclusion can be made [15].
Studies performed in the United States suggest that differences in sexual mixing patterns (e.g. assortative and disassortative mixing) between African-American and the Caucasian population as well as sexual concurrency play major roles in establishing and maintaining STI rate disparities [26, 27]. It may be that factors associated with the sexual network structure, such as assortative mixing patterns and concurrency, contribute to the increased CT risk among individuals of African Surinamese and Ghanaian ethnic origin in the Netherlands [8, 9, 26–29]. HELIUS does not collect sexual network data, so we were unable to evaluate the role of (dis)assortative mixing or concurrency, which limits the study in its potential to examine possible explanations of disparities in CT prevalence between different ethnicities in the Netherlands.
A last potential limitation is the lack of data on vaginal microbiota composition in the current study. The composition of vaginal microbiota differs between ethnicities [30–32], and might explain differences in CT prevalence as vaginal dysbiosis increases susceptibility to STIs [33–35]. Dysbiosis may partially or completely be responsible for the differences between ethnicities.
Conclusions
In conclusion, the current study shows that CT seroprevalence differs between ethnic groups in Amsterdam, the Netherlands. CT seropositivity is most common among people of African Surinamese and Ghanaian origin. The differences in CT seroprevalence could not be explained by differences in SRB, SES or sHSB between ethnic groups. To gain a better understanding of the factors that drive CT (sero)disparities in Amsterdam, additional research should be performed, directed at establishing accurate estimates of CT incidence and its explanatory factors, including sexual mixing networks and concurrency and possible biologic mechanisms.
Additional file
Appendix 1, Figure S1. Comparison of antibody detection in sera with defined cervical Ct-DNA status. Figure S2. Comparison of Ct multiplex serology and C. trachomatis p-Elisa (Medac) in 80 sera from Mongolian women. (DOCX 248 kb)
Acknowledgements
The HELIUS study is conducted by the Academic Medical Center Amsterdam and the Public Health Service of Amsterdam. Both organisations provided core support for HELIUS. The HELIUS study is also funded by the Dutch Heart Foundation, the Netherlands Organization for Health Research and Development (ZonMw), and the European Union (FP-7). We gratefully acknowledge the AMC Biobank for their support in biobank management and high-quality storage of collected samples. We are most grateful to the participants of the HELIUS study and the management team, research nurses, interviewers, research assistants and other staff who have taken part in gathering the data of this study. The study reported here was additionally supported by an additional grant from the Public Health Service of Amsterdam.
Funding
The Academic Medical Center (AMC) of Amsterdam and the Public Health Service of Amsterdam (GGD Amsterdam) provided core financial support for HELIUS. The HELIUS study is also funded by research grants of the Dutch Heart Foundation (Hartstichting; grant no. 2010T084), the Netherlands Organization for Health Research and Development (ZonMw; grant no. 200500003), the European Integration Fund (EIF; grant no. 2013EIF013) and the European Union (Seventh Framework Programme, FP-7; grant no. 278901).
Availability of data and materials
The HELIUS data are owned by the Academic Medical Center (AMC) in Amsterdam, The Netherlands. The HELIUS study has an open policy with regard to collaboration with other research groups and welcomes collaborations from a wide variety of disciplines. Information on procedures to acquire the available data and/or samples is described in the HELIUS Collaboration Policy, which can be found at www.heliusstudy.nl/nl/researchers/collaboration. In brief, to make use of the available data for research, we request a publication proposal describing background, aim, research questions, methods (analysis plan), and time table. All proposals should be submitted to HELIUS via m.b.snijder@amc.nl, or via info@heliusstudy.nl. The proposals are discussed in the HELIUS Executive Board regarding the study aims (compatibility with the general objectives of the HELIUS study/informed consent), the quality of the research proposal, and potential overlap with on going studies. After approval, the requested data will be provided after a Data Transfer Agreement has been signed. For more information, please visit the HELIUS website or contact the Scientific Coordinator of the HELIUS study.
Abbreviations
- (a)PR
(adjusted) Seroprevalence ratio
- CBS
Statistics Netherlands
- CI
Confidence interval
- CT
Chlamydia trachomatis
- DKFZ
German Cancer Research Center
- HELIUS
HEalthy Life In an Urban Setting
- Hsp60
Heat shock protein 60
- MOMP
Major outer membrane protein
- MSM
Men who have sex with men
- SES
Socioeconomic status
- sHSB
Sexual health seeking behavior
- SRB
Sexual risk behavior
- Tarp
Translocated actin-recruiting phosphoprotein
- WSW
Women who have sex with women
Authors’ contributions
SHH drafted the manuscript and performed the statistical analyses. AAM, CJA, MBS, HJCdV, MP and MFSvdL kindly contributed to the manuscript and statistical analyses. MWF, KH and TW developed the multiplex serological CT assay and are the authors of Additional file 1: Appendix 1. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All HELIUS study protocols have been approved by the Ethics Review Board of the Academic Medical Center (Reference number: NL32251.018.10) and are in accordance with the revised Declaration of Helsinki of 2000. Written informed consent was obtained from all HELIUS participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sebastiaan H. Hulstein, Email: bhulstein@ggd.amsterdam.nl, Email: s.h.hulstein@amc.nl
Amy Matser, Email: amatser@ggd.amsterdam.nl.
Catharina J. Alberts, Email: catharina.j.alberts@gmail.com
Marieke B. Snijder, Email: m.b.snijder@amc.nl
Martina Willhauck-Fleckenstein, Email: mwf-kg@willhauck.de.
Katrin Hufnagel, Email: k.hufnagel@Dkfz-Heidelberg.de.
Maria Prins, Email: mprins@ggd.amsterdam.nl.
Henry J. C. de Vries, Email: h.j.devries@amc.nl
Maarten F. Schim van der Loeff, Email: mschim@ggd.amsterdam.nl
Tim Waterboer, Email: T.Waterboer@dkfz-heidelberg.de.
References
- 1.Low N, Sterne JA, Barlow D. Inequalities in rates of gonorrhoea and chlamydia between black ethnic groups in south East London: cross sectional study. Sex Transm Infect. 2001;77(1):15–20. doi: 10.1136/sti.77.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenton KA, Mercer CH, McManus S, Erens B, Wellings K, Macdowall W, Byron CL, Copas AJ, Nanchahal K, Field J, et al. Ethnic variations in sexual behaviour in Great Britain and risk of sexually transmitted infections: a probability survey. Lancet. 2005;365(9466):1246–1255. doi: 10.1016/S0140-6736(05)74813-3. [DOI] [PubMed] [Google Scholar]
- 3.Gotz HM, van Bergen JE, Veldhuijzen IK, Broer J, Hoebe CJ, Steyerberg EW, Coenen AJ, de Groot F, Verhooren MJ, van Schaik DT, et al. A prediction rule for selective screening of chlamydia trachomatis infection. Sex Transm Infect. 2005;81(1):24–30. doi: 10.1136/sti.2004.010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Op de Coul EL, Gotz HM, van Bergen JE, Fennema JS, Hoebe CJ, Koekenbier RH, Pars LL, van Ravesteijn SM, van der Sande MA, van den Broek IV. Who participates in the Dutch chlamydia screening? A study on demographic and behavioral correlates of participation and positivity. Sex Transm Dis. 2012;39(2):97–103. doi: 10.1097/OLQ.0b013e3182383097. [DOI] [PubMed] [Google Scholar]
- 5.Van den Broek IVF, Van Aar F, Van Oeffelen A. Sexually transmitted infections in the Netherlands 2015. Bilthoven: National Institute of Health and the Enviroment (RIVM); 2016.
- 6.Navarro C, Jolly A, Nair R, Chen Y. Risk factors for genital chlamydial infection. Can J Infect Dis. 2002;13(3):195–207. doi: 10.1155/2002/954837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bebear C, de Barbeyrac B. Genital chlamydia trachomatis infections. Clin Microbiol Infect. 2009;15(1):4–10. doi: 10.1111/j.1469-0691.2008.02647.x. [DOI] [PubMed] [Google Scholar]
- 8.Gras MJ, Weide JF, Langendam MW, Coutinho RA, van den Hoek A. HIV prevalence, sexual risk behaviour and sexual mixing patterns among migrants in Amsterdam, The Netherlands. AIDS. 1999;13(14):1953–1962. doi: 10.1097/00002030-199910010-00019. [DOI] [PubMed] [Google Scholar]
- 9.Matser A, Luu N, Geskus R, Heijman T, Heiligenberg M, van Veen M, Schim van der Loeff M. Higher chlamydia trachomatis prevalence in ethnic minorities does not always reflect higher sexual risk behaviour. PLoS One. 2013;8(6):e67287. doi: 10.1371/journal.pone.0067287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallfors DD, Iritani BJ, Miller WC, Bauer DJ. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am J Public Health. 2007;97(1):125–132. doi: 10.2105/AJPH.2005.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellen JM, Aral SO, Madger LS. Do differences in sexual behaviors account for the racial/ethnic differences in adolescents’ self-reported history of a sexually transmitted disease? Sex Transm Dis. 1998;25(3):125–129. doi: 10.1097/00007435-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ward H, Mertens TE, Thomas C. Health seeking behaviour and the control of sexually transmitted disease. Health Policy Plan. 1997;12(1):19–28. doi: 10.1093/heapol/12.1.19. [DOI] [PubMed] [Google Scholar]
- 13.van den Broek IV, van Bergen JE, Brouwers EE, Fennema JS, Gotz HM, Hoebe CJ, Koekenbier RH, Kretzschmar M, Over EA, Schmid BV, et al. Effectiveness of yearly, register based screening for chlamydia in the Netherlands: controlled trial with randomised stepped wedge implementation. BMJ. 2012;345:e4316. doi: 10.1136/bmj.e4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stronks K, Snijder MB, Peters RJ, Prins M, Schene AH, Zwinderman AH. Unravelling the impact of ethnicity on health in Europe: the HELIUS study. BMC Public Health. 2013;13:402. doi: 10.1186/1471-2458-13-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snijder MB, Galenkamp H, Prins M, Derks EM, Peters RJ, Zwinderman AH, Stronks K. Cohort profile: the Healthy Life in an Urban Setting (HELIUS) study. BMJ Open. 2017. [DOI] [PMC free article] [PubMed]
- 16.Alberts CJ, Michel A, Bruisten S, Snijder MB, Prins M, Waterboer T, Schim van der Loeff MF. High-risk human papillomavirus seroprevalence in men and women of six different ethnicities in Amsterdam, the Netherlands: the HELIUS study. Papillomavirus Res. 2017;3:57–65. doi: 10.1016/j.pvr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stronks K, Kulu-Glasgow I, Agyemang C. The utility of ‘country of birth’ for the classification of ethnic groups in health research: the Dutch experience. Ethn Health. 2009;14(3):255–269. doi: 10.1080/13557850802509206. [DOI] [PubMed] [Google Scholar]
- 18.(CBS) SN: Standard Classification of Occupations. https://www.cbs.nl/nl-nl/onze-diensten/methoden/classificaties/onderwijs-en-beroepen/beroepenclassificatie%2D%2Disco-en-sbc--. Accessed 10 Sept 2017.
- 19.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West SK, Munoz B, Weaver J, Mrango Z, Dize L, Gaydos C, Quinn TC, Martin DL. Can we use antibodies to chlamydia trachomatis as a surveillance tool for National Trachoma Control Programs? Results from a District Survey. PLoS Negl Trop Dis. 2016;10(1):e0004352. doi: 10.1371/journal.pntd.0004352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schachter J, Grossman M, Azimi PH. Serology of chlamydia trachomatis in infants. J Infect Dis. 1982;146(4):530–535. doi: 10.1093/infdis/146.4.530. [DOI] [PubMed] [Google Scholar]
- 22.Gordis L. Epidemiology. 5. Philadelphia, PA: Elsevier; 2014. p. 265. [Google Scholar]
- 23.Horner PJ, Wills GS, Righarts A, Vieira S, Kounali D, Samuel D, Winston A, Muir D, Dickson NP, McClure MO. Chlamydia trachomatis Pgp3 antibody persists and correlates with self-reported infection and Behavioural risks in a blinded cohort study. PLoS One. 2016;11(3):e0151497. doi: 10.1371/journal.pone.0151497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodhall SC, Wills GS, Horner PJ, Craig R, Mindell JS, Murphy G, McClure MO, Soldan K, Nardone A, Johnson AM. Chlamydia trachomatis Pgp3 antibody population Seroprevalence before and during an era of widespread opportunistic chlamydia screening in England (1994-2012) PLoS One. 2017;12(1):e0152810. doi: 10.1371/journal.pone.0152810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geisler WM. Diagnosis and Management of Uncomplicated Chlamydia trachomatis infections in adolescents and adults: summary of evidence reviewed for the 2015 Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2015;61(Suppl 8):S774–S784. doi: 10.1093/cid/civ694. [DOI] [PubMed] [Google Scholar]
- 26.Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S. Concurrent partnerships and HIV prevalence disparities by race: linking science and public health practice. Am J Public Health. 2009;99(6):1023–1031. doi: 10.2105/AJPH.2008.147835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26(5):250–261. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Aral SO, Adimora AA, Fenton KA. Understanding and responding to disparities in HIV and other sexually transmitted infections in African Americans. Lancet. 2008;372(9635):337–340. doi: 10.1016/S0140-6736(08)61118-6. [DOI] [PubMed] [Google Scholar]
- 29.van Veen MG, Kramer MA, Op de Coul EL, van Leeuwen AP, de Zwart O, van de Laar MJ, Coutinho RA, Prins M. Disassortative sexual mixing among migrant populations in the Netherlands: a potential for HIV/STI transmission? AIDS Care. 2009;21(6):683–691. doi: 10.1080/09540120802511984. [DOI] [PubMed] [Google Scholar]
- 30.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Houdt R, Ma B, Bruisten SM, Speksnijder A, Ravel J, de Vries HJC. Lactobacillus iners-dominated vaginal microbiota is associated with increased susceptibility to chlamydia trachomatis infection in Dutch women: a case-control study. Sex Transm Infect. 2018;94(2):117–123. doi: 10.1136/sextrans-2017-053133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borgdorff H, van der Veer C, van Houdt R, Alberts CJ, de Vries HJ, Bruisten SM, Snijder MB, Prins M, Geerlings SE, Schim van der Loeff MF, et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS One. 2017;12(7):e0181135. doi: 10.1371/journal.pone.0181135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest. 2011;121(12):4610–4617. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwebke JR. Abnormal vaginal flora as a biological risk factor for acquisition of HIV infection and sexually transmitted diseases. J Infect Dis. 2005;192(8):1315–1317. doi: 10.1086/462430. [DOI] [PubMed] [Google Scholar]
- 35.van de Wijgert JH, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, Jespers V. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One. 2014;9(8):e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1, Figure S1. Comparison of antibody detection in sera with defined cervical Ct-DNA status. Figure S2. Comparison of Ct multiplex serology and C. trachomatis p-Elisa (Medac) in 80 sera from Mongolian women. (DOCX 248 kb)
Data Availability Statement
The HELIUS data are owned by the Academic Medical Center (AMC) in Amsterdam, The Netherlands. The HELIUS study has an open policy with regard to collaboration with other research groups and welcomes collaborations from a wide variety of disciplines. Information on procedures to acquire the available data and/or samples is described in the HELIUS Collaboration Policy, which can be found at www.heliusstudy.nl/nl/researchers/collaboration. In brief, to make use of the available data for research, we request a publication proposal describing background, aim, research questions, methods (analysis plan), and time table. All proposals should be submitted to HELIUS via m.b.snijder@amc.nl, or via info@heliusstudy.nl. The proposals are discussed in the HELIUS Executive Board regarding the study aims (compatibility with the general objectives of the HELIUS study/informed consent), the quality of the research proposal, and potential overlap with on going studies. After approval, the requested data will be provided after a Data Transfer Agreement has been signed. For more information, please visit the HELIUS website or contact the Scientific Coordinator of the HELIUS study.