Abstract
Background
Whether adiponectin (ADIPOQ) polymorphisms are associated with the risk of polycystic ovary syndrome (PCOS) remain controversial. Therefore, we performed this study to better explore correlations between ADIPOQ polymorphisms and PCOS risk.
Methods
Literature retrieve was conducted in PubMed, Medline and Embase. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated.
Results
Eighteen studies were enrolled for analyses. Pooled overall analyses showed that rs1501299 polymorphism was significantly associated with PCOS risk (recessive model: p = 0.02, OR = 0.77, 95%CI 0.62–0.95; allele model: p = 0.001, OR = 1.15, 95%CI 1.06–1.26). Further subgroup analyses according to ethnicity of participants revealed that rs1501299 and rs2241766 polymorphisms were both significantly correlated with PCOS risk in Caucasians. In addition, rs1501299 polymorphism was also significantly correlated with PCOS risk in East Asians.
Conclusions
Our findings indicated that rs1501299 and rs2241766 polymorphisms might serve as genetic biomarkers of PCOS in certain ethnicities.
Keywords: Adiponectin (ADIPOQ), Gene polymorphisms, Polycystic ovary syndrome (PCOS), Meta-analysis
Background
Polycystic ovary syndrome (PCOS), featured by oligomenorrhea, polycystic ovaries, anovulatory infertility, hyperandrogenism, insulin resistance or hyperinsulinemia, and an elevated risk of multiple metabolic diseases, is an extremely common reproductive endocrine disorder, with an estimated prevalence of approximately 5–10% in women of childbearing age [1–3]. Although the exact cause of PCOS remains unclear, mounting evidence supports that genetic factors play vital roles in its pathogenesis. First, family clustering of PCOS was not uncommon, and first-degree relatives of PCOS patients suffered an increased risk of developing PCOS and its associated disorders [4, 5]. Second, various genetic variants were found to be correlated with a higher PCOS risk [6]. However, PCOS is a highly heterogeneous disorder and genetic determinants underlying PCOS are still poorly understood [7, 8].
Adiponectin (ADIPOQ), a multifunctional adipocytokine that is primarily secreted by adipocytes, plays a pivotal role in regulating energy and material metabolism [9]. Previous studies showed that expression level of adiponectin was significantly reduced in patients with various metabolic disorders such as diabetes, obesity and insulin resistance, which suggested that adipoenctin might be involved in the pathogenesis of above-mentioned diseases [10, 11]. Considering the metabolic nature of PCOS and the fact that the expression levels of adiponectin and its receptors in female reproductive organs (ovary and uterus) vary in different phases of oestrous cycle [12], it is biologically plausible that adiponectin might also be implicated in the occurrence and development of PCOS.
Adiponectin is encoded by the ADIPOQ gene located on chromosome 3q27 [13]. It was evident that two common functional ADIPOQ polymorphisms, rs1501299 and rs2241766, were correlated with altered serum concentration of adiponectin [14, 15]. As a result, these two polymorphisms were thought to be ideal genetic biomarkers of multiple metabolic disorders including PCOS. So far, several studies already investigated associations between these ADIPOQ polymorphisms and PCOS risk, but the results of these studies were controversial [16–33]. Therefore, we performed the present meta-analysis to better explore potential roles of ADIPOQ polymorphisms in PCOS.
Methods
Literature search and inclusion criteria
This meta-analysis was adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline [34]. Potentially related literatures (published before September 2018) were retrieved from PubMed, Medline and Embase using the following searching strategy: (adiponectin OR ADIPOQ) AND (polymorphism OR variant OR mutation OR genotype OR allele) AND (polycystic ovary syndrome OR PCOS). Furthermore, the references of retrieved articles were also screened for other potentially relevant studies.
To test the research hypothesis of this meta-analysis, included studies must meet all the following criteria: (1) case-control study on correlations between ADIPOQ polymorphisms and PCOS risk; (2) provide genotypic and/or allelic frequency of investigated ADIPOQ polymorphisms in cases and controls; (3) full text in English or Chinese available. Studies were excluded if one of the following criteria was fulfilled: (1) not relevant to ADIPOQ polymorphisms and PCOS; (2) case reports or case series; (3) abstracts, reviews, comments, letters and conference presentations. For duplicate publications, we only included the study with the largest sample size for analyses.
Data extraction and quality assessment
The following data were extracted from included studies: (1) the name of the first author; (2) publication time; (3) country and ethnicity; (4) sample size; and (5) genotypic distributions of ADIPOQ polymorphisms in cases and controls. Additionally, the probability value (p value) of Hardy-Weinberg equilibrium (HWE) was also calculated. When necessary, we wrote to the corresponding authors for raw data. We used the Newcastle-Ottawa scale (NOS) to assess the quality of eligible studies [35]. This scale has a score range of zero to nine, and studies with a score of more than seven were thought to be of high quality. Two reviewers conducted data extraction and quality assessment independently. Any disagreement between two reviewers was solved by discussion until a consensus was reached.
Statistical analysis
All statistical analyses were conducted with Review Manager Version 5.3.3 (The Cochrane Collaboration, Software Update, Oxford, United Kingdom). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate strength of associations between ADIPOQ polymorphisms and PCOS risk in all possible genetic models, and p values ≤0.05 were considered to be statistically significant. Between-study heterogeneities were evaluated with I2 statistic. If I2 was greater than 50 %, random-effect models (REMs) would be used to pool the data. Otherwise, fixed-effect models (FEMs) would be employed for synthetic analyses. Subgroup analyses by ethnicity were subsequently performed. Sensitivity analyses were conducted to examine the stability of synthetic results. Funnel plots were used to evaluate possible publication bias.
Results
Characteristics of included studies
We found 331 potential relevant articles. Among these articles, a total of 18 eligible studies were finally included for synthetic analyses (see Fig. 1). The NOS score of eligible articles ranged from 7 to 8, which indicated that all included studies were of high quality. Baseline characteristics of included studies were shown in Table 1.
Fig. 1.
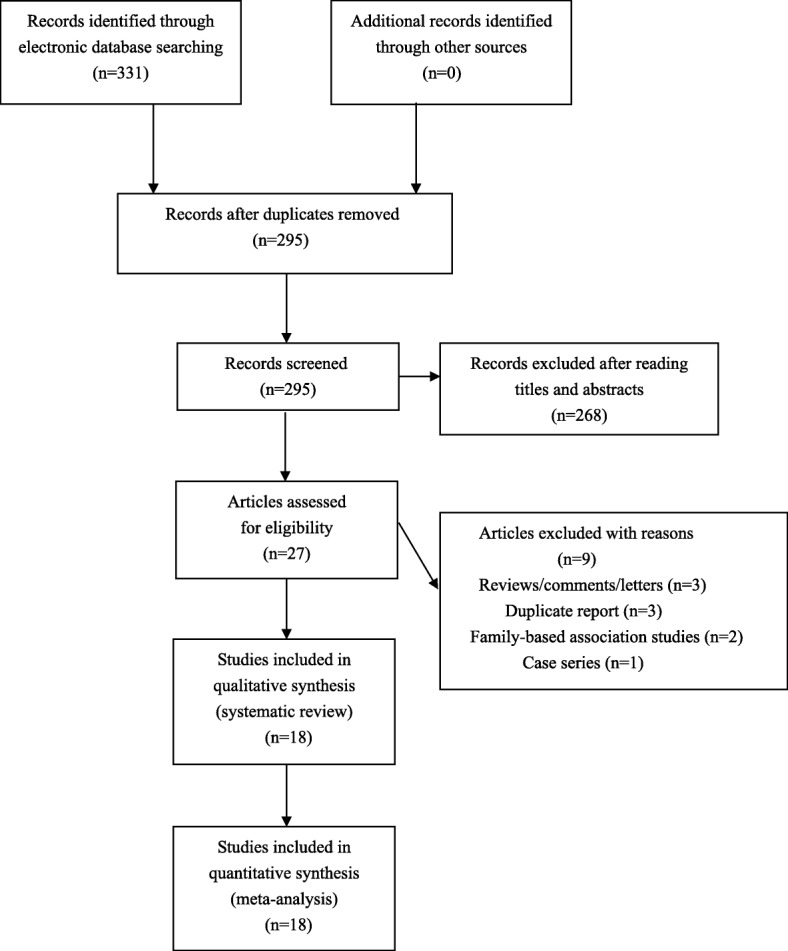
Flowchart of study selection for the present study
Table 1.
The characteristics of included studies
| First author, year | Country | Ethnicity | Age (years) Case/Control |
Sample size | Genotype distribution | P-value for HWE | NOS score | |
|---|---|---|---|---|---|---|---|---|
| Cases Controls | ||||||||
| rs1501299 G/T | GG/GT/TT | GG/GT/TT | ||||||
| Alfaqih 2018 [16] | Jordon | West Asian | 23.9/24.2 | 154/151 | 62/77/15 | 64/54/33 | 0.002 | 8 |
| Czeczuga-Semeniuk 2018 [17] | Poland | Caucasian | 24.6/23.2 | 294/78 | 156/117/21 | 25/49/4 | 0.002 | 8 |
| Escobar-Morreale 2006 [19] | Spain | Caucasian | NA | 76/40 | 30/39/7 | 15/21/4 | 0.390 | 7 |
| Heinonen 2005 [21] | Finland | Caucasian | NA | 143/245 | 77/58/8 | 110/110/25 | 0.744 | 7 |
| Li 2011 [22] | Korea | East Asian | NA | 144/159 | 61/73/10 | 48/87/24 | 0.131 | 7 |
| Nambiar 2016 [23] | India | West Asian | 28.6/31.1 | 282/200 | 94/165/23 | 86/99/15 | 0.060 | 8 |
| Pau 2013 [25] | USA | Mixed | NA | 525/472 | NA | NA | NA | 7 |
| Radavelli-Bagatini 2013 [26] | Brazil | Mixed | NA | 80/1500 | 42/27/11 | 671/672/157 | 0.556 | 7 |
| Ramezani Tehrani 2013 [27] | Iran | West Asian | 26.6/30.8 | 186/156 | 92/76/18 | 77/71/8 | 0.100 | 7 |
| Ranjzad 2012 [28] | Iran | West Asian | 27.1/31.1 | 181/181 | 92/77/12 | 91/79/11 | 0.254 | 8 |
| San Millán 2004 [29] | Spain | Caucasian | 24.6/31.1 | 72/42 | 28/34/10 | 18/20/4 | 0.643 | 7 |
| Xita 2005 [30] | Greece | Caucasian | 23.7/24.8 | 100/140 | 39/49/12 | 52/73/15 | 0.152 | 7 |
| Yoshihara 2009 [31] | Japan | East Asian | 29.1/29.8 | 38/97 | 19/15/4 | 58/24/15 | < 0.001 | 8 |
| Zhang 2008 [32] | China | East Asian | 28.7/29.6 | 120/120 | 56/46/18 | 41/50/29 | 0.083 | 8 |
| Zhang 2015 [33] | China | East Asian | 27.0/27.2 | 207/192 | 119/78/10 | 95/75/22 | 0.229 | 7 |
| rs2241766 T/G | TT/TG/GG | TT/TG/GG | ||||||
| Alfaqih 2018 [16] | Jordon | West Asian | 23.9/24.2 | 154/149 | 92/48/14 | 93/42/14 | 0.008 | 8 |
| Czeczuga-Semeniuk 2018 [17] | Poland | Caucasian | 24.6/23.2 | 294/78 | 255/39/0 | 62/16/0 | 0.313 | 8 |
| Demirci 2010 [18] | Turkey | Caucasian | 24.1/23.8 | 96/93 | 70/20/6 | 74/16/3 | 0.091 | 8 |
| Escobar-Morreale 2006 [19] | Spain | Caucasian | NA | 76/40 | 55/20/1 | 26/13/1 | 0.673 | 7 |
| Haap 2005 [20] | Germany | Caucasian | 27.4/38.9 | 53/542 | 38/8/7 | 414/112/16 | 0.016 | 8 |
| Heinonen 2005 [21] | Finland | Caucasian | NA | 143/245 | 125/17/1 | 222/22/1 | 0.572 | 7 |
| Li 2011 [22] | Korea | East Asian | NA | 144/159 | 79/59/6 | 72/84/3 | < 0.001 | 7 |
| Nambiar 2016 [23] | India | West Asian | 28.6/31.1 | 282/200 | 213/60/9 | 156/40/4 | 0.453 | 8 |
| Panidis 2004 [24] | Greece | Caucasian | 23.4/29.4 | 132/100 | 92/33/7 | 81/17/2 | 0.340 | 7 |
| Radavelli-Bagatini 2013 [26] | Brazil | Mixed | NA | 80/1500 | 64/14/2 | 1122/356/22 | 0.297 | 7 |
| Ramezani Tehrani 2013 [27] | Iran | West Asian | 26.6/30.8 | 186/156 | 142/42/2 | 106/46/4 | 0.707 | 7 |
| Ranjzad 2012 [28] | Iran | West Asian | 27.1/31.1 | 181/181 | 144/34/2 | 121/54/6 | 0.993 | 8 |
| San Millán 2004 [29] | Spain | Caucasian | 24.6/31.1 | 72/42 | 48/22/2 | 29/12/1 | 0.853 | 7 |
| Xita 2005 [30] | Greece | Caucasian | 23.7/24.8 | 100/140 | 77/23/0 | 106/30/4 | 0.306 | 7 |
| Yoshihara 2009 [31] | Japan | East Asian | 29.1/29.8 | 38/97 | 19/19/0 | 53/29/15 | 0.004 | 8 |
| Zhang 2008 [32] | China | East Asian | 28.7/29.6 | 120/120 | 57/54/9 | 74/42/4 | 0.504 | 8 |
| Zhang 2015 [33] | China | East Asian | 27.0/27.2 | 207/192 | 106/84/17 | 98/75/19 | 0.409 | 7 |
PCOS Polycystic ovary syndrome, HWE Hardy-Weinberg equilibrium, NOS Newcastle-Ottawa scale, NA Not available
Overall and subgroup analyses
To investigate potential correlations between ADIPOQ polymorphisms and PCOS risk, fifteen studies about rs1501299 polymorphism and seventeen studies about rs2241766 polymorphism were included for pooled analyses. A significant association with PCOS risk was detected for rs1501299 (recessive model: p = 0.02, OR = 0.77, 95%CI 0.62–0.95; allele model: p = 0.001, OR = 1.15, 95%CI 1.06–1.26) polymorphism in overall analyses. Further subgroup analyses according to ethnicity of participants revealed that rs1501299 and rs2241766 polymorphisms were both significantly correlated with PCOS risk in Caucasians. In addition, rs1501299 polymorphism was also significantly correlated with PCOS risk in East Asians (see Table 2).
Table 2.
Results of overall and subgroup analyses for ADIPOQ gene polymorphisms and PCOS
| Population | Sample size | Dominant comparison | Recessive comparison | Additive comparison | Allele comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P value | OR (95%CI) | I2 statistic | P value | OR (95%CI) | I2 statistic | P value | OR (95%CI) | I2 statistic | P value | OR (95%CI) | I2 statistic | ||
| rs1501299 G/T | GG vs. GT + TT | TT vs. GG + GT | GT vs. GG + TT | G vs. T | |||||||||
| Overall | 2602/3773 | 0.10 | 1.17 (0.97–1.42) | 52% | 0.02 | 0.77 (0.62–0.95) | 46% | 0.52 | 0.94 (0.76–1.15) | 58% | 0.001 | 1.15 (1.06–1.26) | 43% |
| Caucasian | 685/545 | 0.007 | 1.40 (1.09–1.79) | 42% | 0.79 | 0.94 (0.62–1.45) | 0% | 0.01 | 0.73 (0.58–0.93) | 48% | 0.03 | 1.22 (1.02–1.46) | 22% |
| East Asian | 509/568 | 0.006 | 1.41 (1.10–1.81) | 37% | 0.0003 | 0.48 (0.32–0.71) | 0% | 0.76 | 0.96 (0.75–1.23) | 17% | 0.0001 | 1.44 (1.20–1.73) | 2% |
| West Asian | 803/688 | 0.19 | 0.87 (0.71–1.07) | 4% | 0.88 | 0.95 (0.47–1.89) | 70% | 0.30 | 1.19 (0.85–1.68) | 62% | 0.52 | 0.95 (0.81–1.11) | 31% |
| rs2241766 T/G | TT vs. TG + GG | GG vs. TT + TG | TG vs. TT + GG | T vs. G | |||||||||
| Overall | 2358/4034 | 0.63 | 1.03 (0.91–1.18) | 46% | 0.54 | 1.10 (0.81–1.50) | 41% | 0.44 | 0.95 (0.83–1.09) | 44% | 1.00 | 1.00 (0.84–1.19) | 54% |
| Caucasian | 966/1280 | 0.32 | 0.89 (0.70–1.12) | 22% | 0.04 | 1.93 (1.05–3.56) | 26% | 0.84 | 1.03 (0.80–1.31) | 6% | 0.11 | 0.84 (0.68–1.04) | 46% |
| East Asian | 509/568 | 0.74 | 0.93 (0.62–1.40) | 61% | 0.86 | 1.10 (0.40–3.02) | 59% | 0.54 | 1.17 (0.71–1.93) | 73% | 0.95 | 0.99 (0.73–1.35) | 58% |
| West Asian | 803/686 | 0.31 | 1.22 (0.83–1.80) | 65% | 0.56 | 0.85 (0.49–1.47) | 8% | 0.31 | 0.83 (0.59–1.18) | 53% | 0.32 | 1.19 (0.84–1.69) | 67% |
OR Odds ratio, CI Confidence interval, NA Not available. PCOS Polycystic ovary syndrome
The values in bold represent there is statistically significant differences between cases and controls
Sensitivity analyses
We performed sensitivity analyses by excluding studies that deviated from HWE. No alterations of results were detected in sensitivity analyses, which suggested that our findings were statistically reliable.
Publication biases
Publication biases were evaluated with funnel plots. We did not find obvious asymmetry of funnel plots in any comparisons, which indicated that our findings were unlikely to be impacted by severe publication biases.
Discussion
To the best of our knowledge, this is so far the most comprehensive meta-analysis on correlations between ADIPOQ polymorphisms and PCOS risk. Our overall and subgroup analyses demonstrated that rs1501299 and rs2241766 polymorphisms were both significantly correlated with PCOS risk in Caucasians. Moreover, rs1501299 polymorphism was also significantly correlated with PCOS risk in East Asians.
There are several points that need to be addressed about this meta-analysis. Firstly, previous experimental studies showed that mutant alleles of investigated polymorphisms were correlated with decreased adiponectin generation, which may partially explain our positive findings [14, 15]. Secondly, the pathogenic mechanism of PCOS is highly complex, and hence it is unlikely that a single gene polymorphism could significantly contribute to its development. As a result, to better illustrate potential correlations of certain gene polymorphisms with PCOS, we strongly recommend further studies to perform haplotype analyses and explore potential gene-gene interactions.
As with all meta-analysis, this study certainly has some limitations. First, our results were derived from unadjusted analyses due to lack of raw data, and lack of further adjusted analyses for potential confounding factors may impact the reliability of our findings [36]. Second, obvious heterogeneities were found in several subgroups, which indicated that the controversial results of included studies could not be fully explained by differences in ethnic background, and other baseline characteristics of participants may also contribute to between-study heterogeneities [37]. Third, associations between ADIPOQ polymorphisms and PCOS risk may also be modified by gene-gene and gene-environmental interactions. However, most eligible studies ignore these potential interactions, which impeded us to perform relevant analyses accordingly [38]. To sum up, our findings should be cautiously interpreted on account of above mentioned limitations.
Conclusions
In conclusion, our meta-analysis suggested that rs1501299 and rs2241766 polymorphisms might serve as genetic biomarkers of PCOS in certain ethnicities. However, further well-designed studies are still warranted to confirm our findings.
Acknowledgments
None.
Funding
None.
Availability of data and material
The current study was based on results of relevant published studies.
Informed consent
For this type of study formal consent is not required.
Abbreviations
- ADIPOQ
Adiponectin
- HWE
Hardy-Weinberg equilibrium
- NOS
Newcastle-Ottawa scale
- PCOS
Polycystic ovary syndrome
Authors’ contributions
ZL and CH conceived of the study, participated in its design. ZL and ZW conducted the systematic literature review. YT and JF performed data analyses. ZL and CH drafted the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhengling Liu, Email: liuzhenglig@163.com.
Zengyan Wang, Email: wangzengyanga1@yeah.net.
Changhong Hao, Email: haochanghong99669@163.com.
Yonghui Tian, Email: tianyong1hui@yeah.net.
Jingjing Fu, Email: fujingjingj@163.com.
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Pasquali R, Gambineri A. Polycystic ovary syndrome: a multifaceted disease from adolescence to adult age. Ann N Y Acad Sci. 2006;1092:158–174. doi: 10.1196/annals.1365.014. [DOI] [PubMed] [Google Scholar]
- 3.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 4.Amato P, Simpson JL. The genetics of polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:707–718. doi: 10.1016/j.bpobgyn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Govind A, Obhrai MS, Clayton RN. Polycystic ovaries are inherited as an autosomal dominant trait: analysis of 29 polycystic ovary syndrome and 10 control families. J Clin Endocrinol Metab. 1999;84:38–43. doi: 10.1210/jcem.84.1.5382. [DOI] [PubMed] [Google Scholar]
- 6.Ioannidis A, Ikonomi E, Dimou NL, Douma L, Bagos PG. Polymorphisms of the insulin receptor and the insulin receptor substrates genes in polycystic ovary syndrome: a Mendelian randomization meta-analysis. Mol Genet Metable. 2010;99:174–183. doi: 10.1016/j.ymgme.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Urbanek M. The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3:103–111. doi: 10.1038/ncpendmet0400. [DOI] [PubMed] [Google Scholar]
- 8.Luque-Ramirez M, San Millan JL, Escobar-Morreale HF. Genomic variants in polycystic ovary syndrome. Clin Chim Acta. 2006;366:14–26. doi: 10.1016/j.cca.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Ghadge AA, Khaire AA, Kuvalekar AA. Adiponectin: a potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018;39:151–158. doi: 10.1016/j.cytogfr.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Dobrzyn K, Smolinska N, Kiezun M, Szeszko K, Rytelewska E, Kisielewska K, Gudelska M, Kaminski T. Adiponectin: a new regulator of female reproductive system. Int J Endocrinol. 2018:7965071. [DOI] [PMC free article] [PubMed]
- 11.Frankenberg ADV, Reis AF, Gerchman F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: a literature review. Arch Endocrinol Metab. 2017;61:614–622. doi: 10.1590/2359-3997000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18:E1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xian L, He W, Pang F, Hu Y. ADIPOQ gene polymorphisms and susceptibility to polycystic ovary syndrome: a HuGE survey and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2012;161:117–124. doi: 10.1016/j.ejogrb.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Dahlman I, Arner P. Genetics of adipose tissue biology. Prog Mol Biol Transl Sci. 2010;94:39–74. doi: 10.1016/B978-0-12-375003-7.00003-0. [DOI] [PubMed] [Google Scholar]
- 15.Al Hannan FA, O'Farrell PA, Morgan MP, Tighe O, Culligan KG. Associations between single-nucleotide polymorphisms of ADIPOQ, serum adiponectin and increased type 2 diabetes mellitus risk in Bahraini individuals. East Mediterr Health J. 2016;22:611–618. doi: 10.26719/2016.22.8.611. [DOI] [PubMed] [Google Scholar]
- 16.Alfaqih MA, Khader YS, Al-Dwairi A, Alzoubi A, Al-Shboul O, Hatim A. Lower Levels of Serum Adiponectin and the T Allele of rs1501299 of the ADIPOQ Gene Are Protective against Polycystic Ovarian Syndrome in Jordan. Korean J Fam Med. 2018;39:108–113. doi: 10.4082/kjfm.2018.39.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czeczuga-Semeniuk E, Galar M, Jarząbek K, Kozłowski P, Sarosiek NA, Wołczyński S. The preliminary association study of ADIPOQ, RBP4, and BCMO1 variants with polycystic ovary syndrome and with biochemical characteristics in a cohort of polish women. Adv Med Sci. 2018;63:242–248. doi: 10.1016/j.advms.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Demirci H, Yilmaz M, Ergun MA, Yurtcu E, Bukan N, Ayvaz G. Frequency of adiponectin gene polymorphisms in polycystic ovary syndrome and the association with serum adiponectin, androgen levels, insulin resistance and clinical parameters. Gynecol Endocrinol. 2010;26:348–355. doi: 10.3109/09513590903367051. [DOI] [PubMed] [Google Scholar]
- 19.Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Alvarez-Blasco F, Sanchón R, Luque-Ramírez M, San Millán JL. Adiponectin and resistin in PCOS: a clinical, biochemical and molecular geneticstudy. Hum Reprod. 2006;21:2257–2265. doi: 10.1093/humrep/del146. [DOI] [PubMed] [Google Scholar]
- 20.Haap M, Machicao F, Stefan N, Thamer C, Tschritter O, Schnuck F, Wallwiener D, Stumvoll M, Häring HU, Fritsche A. Genetic determinants of insulin action in polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2005;113:275–281. doi: 10.1055/s-2005-837665. [DOI] [PubMed] [Google Scholar]
- 21.Heinonen S, Korhonen S, Helisalmi S, Koivunen R, Tapanainen J, Hippeläinen M, Laakso M. Associations between two single nucleotide polymorphisms in the adiponectin geneand polycystic ovary syndrome. Gynecol Endocrinol. 2005;21:165–169. doi: 10.1080/09513590500238796. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Yun JH, Lee JH, Song S, Choi BC, Baek KH. Association study of +45G15G(T/G) and +276(G/T) polymorphisms in the adiponectin gene in patients with polycystic ovary syndrome. Int J Mol Med. 2011;27:283–287. doi: 10.3892/ijmm.2010.565. [DOI] [PubMed] [Google Scholar]
- 23.Nambiar V, Vijesh VV, Lakshmanan P, Sukumaran S, Suganthi R. Association of adiponectin and resistin gene polymorphisms in south Indian women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2016;200:82–88. doi: 10.1016/j.ejogrb.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Panidis D, Kourtis A, Kukuvitis A, Farmakiotis D, Xita N, Georgiou I, Tsatsoulis A. Association of the T45G polymorphism in exon 2 of the adiponectin gene with polycystic ovary syndrome: role of Delta4-androstenedione. Hum Reprod. 2004;19:1728–1733. doi: 10.1093/humrep/deh336. [DOI] [PubMed] [Google Scholar]
- 25.Pau C, Saxena R, Welt CK. Evaluating reported candidate gene associations with polycystic ovary syndrome. Fertil Steril. 2013;99:1774–1778. doi: 10.1016/j.fertnstert.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radavelli-Bagatini S, de Oliveira IO, Ramos RB, Santos BR, Wagner MS, Lecke SB, Gigante DP, Horta BL, Spritzer PM. Haplotype TGTG from SNP 45T/G and 276G/T of the adiponectin gene contributes to risk of polycystic ovary syndrome. J Endocrinol Investig. 2013;36:497–502. doi: 10.3275/8966. [DOI] [PubMed] [Google Scholar]
- 27.Ramezani Tehrani F, Daneshpour M, Hashemi S, Zarkesh M, Azizi F. Relationship between polymorphism of insulin receptor gene, and adiponectin gene with PCOS. Iran J Reprod Med. 2013;11:185–194. [PMC free article] [PubMed] [Google Scholar]
- 28.Ranjzad F, Mahmoudi T, Irani Shemirani A, Mahban A, Nikzamir A, Vahedi M, Ashrafi M, Gourabi H. A common variant in the adiponectin gene and polycystic ovary syndrome risk. Mol Biol Rep. 2012;39:2313–2319. doi: 10.1007/s11033-011-0981-1. [DOI] [PubMed] [Google Scholar]
- 29.San Millán JL, Cortón M, Villuendas G, Sancho J, Peral B, Escobar-Morreale HF. Association of the polycystic ovary syndrome with genomic variants related to insulin resistance, type 2 diabetes mellitus, and obesity. J Clin Endocrinol Metab. 2004;89:2640–2646. doi: 10.1210/jc.2003-031252. [DOI] [PubMed] [Google Scholar]
- 30.Xita N, Georgiou I, Chatzikyriakidou A, Vounatsou M, Papassotiriou GP, Papassotiriou I, Tsatsoulis A. Effect of adiponectin gene polymorphisms on circulating adiponectin and insulin resistance indexes in women with polycystic ovary syndrome. Clin Chem. 2005;51:416–423. doi: 10.1373/clinchem.2004.043109. [DOI] [PubMed] [Google Scholar]
- 31.Yoshihara K, Yahata T, Kashima K, Mikami T, Tanaka K. Association of single nucleotide polymorphisms in adiponectin and its receptor genes with polycystic ovary syndrome. J Reprod Med. 2009;54:669–674. [PubMed] [Google Scholar]
- 32.Zhang N, Shi YH, Hao CF, Gu HF, Li Y, Zhao YR, Wang LC, Chen ZJ. Association of +45G15G(T/G) and +276(G/T) polymorphisms in the ADIPOQ gene with polycystic ovary syndrome among Han Chinese women. Eur J Endocrinol. 2008;158:255–260. doi: 10.1530/EJE-07-0576. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Wu X, Ding M, Yu X, Liu G, Shi Y. Case-control based study between polymorphisms in the adiponectin gene and polycystic ovary syndrome. Zhonghua Fu Chan Ke Za Zhi. 2015;50:825–829. [PubMed] [Google Scholar]
- 34.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 35.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 36.Xie X, Shi X, Liu M. The roles of TLR gene polymorphisms in atherosclerosis: a systematic review and meta-analysis of 35,317 subjects. Scand J Immunol. 2017;86:50–58. doi: 10.1111/sji.12560. [DOI] [PubMed] [Google Scholar]
- 37.Shi X, Xie X, Jia Y, Li S. Associations of insulin receptor and insulin receptor substrates genetic polymorphisms with polycystic ovary syndrome: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2016;42:844–854. doi: 10.1111/jog.13002. [DOI] [PubMed] [Google Scholar]
- 38.Xie X, Shi X, Xun X, Rao L. Endothelial nitric oxide synthase gene single nucleotide polymorphisms and the risk of hypertension: a meta-analysis involving 63,258 subjects. Clin Exp Hypertens. 2017;39:175–182. doi: 10.1080/10641963.2016.1235177. [DOI] [PubMed] [Google Scholar]


