Abstract
Objective:
In previous decades, glaucoma has been primarily attributed to elevated intraocular pressure (IOP), but this has gradually been replaced by the development of optic neuropathy as the central concept of glaucoma in developed countries. However, there still remain strong controversies in the definition of glaucoma in China. In this current review, we are going to discuss these controversies and elaborate on the historical transitions of the definition of glaucoma both in China and developed countries. Furthermore, we will briefly describe the “ocular-cranial pressure gradient” theory and discuss the relationship between glaucoma and degenerative diseases of the central nervous system (CNS) in order to show the complex pathogenesis of glaucoma and the importance for the modification to the definition of glaucoma.
Data Sources:
We performed a comprehensive search in both PubMed and SinoMed using the following keywords: (a) “primary glaucoma” and “guideline,” (b) “ocular-cranial pressure gradient,” and (c) “glaucoma,” “Alzheimer's disease,” and “Parkinson's disease.” The literature search included the related articles with no restrictions on publication date.
Study Selection:
The primary references were Chinese and English articles including (a) original guidelines and expert consensuses of primary glaucoma, (b) reviews focusing on the differences between various versions of these guidelines and consensuses, and (c) papers about ocular-cranial pressure gradient theory and the relationship between glaucoma and CNS degenerative diseases.
Results:
The definitions and classifications of both primary open-angle glaucoma and primary angle-closure glaucoma differ between Chinese glaucoma consensuses and international primary glaucoma guidelines. Chinese definitions and classifications put more emphasis on the IOP, while international guidelines put more emphasis on the presence of optic neuropathy. The ocular-cranial pressure gradient theory and the research on the relationship between glaucoma and CNS degenerative diseases have provided new directions for exploring the pathogenesis of glaucoma.
Conclusions:
As regards the definition and classification of primary glaucoma, we find that there are still some discrepancies between Chinese expert consensuses and international guidelines. Glaucoma is a disease with complex etiologies, while its common characteristic is a specific optic neuropathy. The current definition and understanding of glaucoma is an ongoing and evolving process, reflecting our latest available evidence on its pathogenesis. Chinese ophthalmology community may need to update our guidelines, accommodating these latest developments.
Keywords: Central Nervous System Degenerative Disease, Intraocular Pressure, Ocular-Cranial Pressure Gradient, Optic Neuropathy, Primary Glaucoma
摘要
目的:
近年来,”特征性的视神经损害”逐渐取代了”病理性高眼压”成为发达国家青光眼临床定义的核心内涵。然而,上述青光眼内涵演变与定义变迁在我国尚存在广泛争议。本文阐述了国内外原发性青光眼内涵演变与定义变迁的历史,尝试剖析其主要脉络,并且探讨了当前存在的主要争论以及亟待解决的问题。同时本文简述了眼颅压力梯度理论和青光眼与中枢神经系统变性疾病之间关系的新进展,以体现青光眼病因的复杂性以及更新对青光眼的定义和理解的重要性。
数据来源:
在PubMed和SinoMed分别检索:(1)”原发性青光眼”和”指南”;(2)”眼颅压力梯度”;(3)”青光眼”,”Alzheimer’s 病”和”Parkinson’s 病”。检索年限不限。
研究选择:
语言为中文和英文,文献为各版原发性青光眼指南和专家共识以及重点阐述各版本指南和专家共识之间差异的综述性文章,还有关于眼颅压力梯度理论及青光眼与中枢神经系统变性疾病之间关系的论文。
结果:
国内原发性青光眼专家共识与国际上原发性青光眼指南在POAG和PACG的定义及分类上存在差异。在国内的定义和分类中,强调眼压的重要作用;国际指南中的定义和分类则强调青光眼的本质是一种视神经疾病。眼颅压力梯度理论的提出和青光眼与中枢神经系统变性疾病之间关系研究的新进展,为理解青光眼的病因提供了新的思路和研究方向。
结论:
对于原发性青光眼的定义和分类,国内专家共识与国际指南之间尚存一定的差异。青光眼是一种由多种复杂病因造成的、以特异性视神经损害为特征的疾病。随着对青光眼病因研究的不断深入,我们对青光眼的理解和定义也将不断发展和更新。
INTRODUCTION
Glaucoma is the leading cause of irreversible blindness worldwide. It is estimated that by the year 2020, 79.6 million people will develop primary glaucoma with 11.2 million having bilateral blindness throughout the world.[1] Early diagnosis and early treatment are the primary method to prevent blindness from glaucoma.
An accurate definition of glaucoma is important for guiding research in this field. Elevated intraocular pressure (IOP) and optic neuropathy are the two classic attributes of glaucoma. The evolution of the definition of primary glaucoma demonstrates the long-term efforts of the ophthalmology community to reach a unified hypothesis for the causality of the disease.
HISTORICAL EVOLUTION OF THE DEFINITION OF GLAUCOMA
The evolution of the definition of glaucoma can be classified into three historical periods.[2]
In 1745, Johann Zacharias Platner found that the eyes of glaucoma patients were firmer than those of healthy patients. In 1830, William Mackenzie highlighted the importance of elevated IOP in the identification of glaucoma, which marked the first instance that glaucoma was defined as “a disease in which IOP is elevated”.[3]
In 1857, von Graefe discovered the pitting atrophy of the optic nerve head (ONH) in glaucoma patients by ophthalmoscopy and named it “glaucomatous optic neuropathy (GON).” Glaucoma was then defined as “optic neuropathy caused by elevated IOP.”[2] To date, ophthalmologists have found that the characteristic signs of GON involved (a) a documented increase in cup size of the ONH, (b) atrophy surrounding the ONH in the peripapillary area, and (c) localized wedge-shaped defects or larger diffuse defects in the retinal nerve fiber layer (RNFL).[4]
In the late 20th century, the recognition of normal tension glaucoma (NTG) and ocular hypertension (OHT) suggested that elevation of IOP is not a necessary implication for glaucoma. Instead, research suggests that the primary implication of glaucoma is GON.[2]
TRANSITIONS OF THE DEFINITION OF PRIMARY GLAUCOMA IN RECENT GUIDELINES AND EXPERT CONSENSUSES
Transitions of the definition of primary open-angle glaucoma
The recognition of NTG and OHT revealed the complexity of the relationship between IOP and glaucoma, and thus, the definition of primary open-angle glaucoma (POAG) has been modified [Figure 1].
Figure 1.
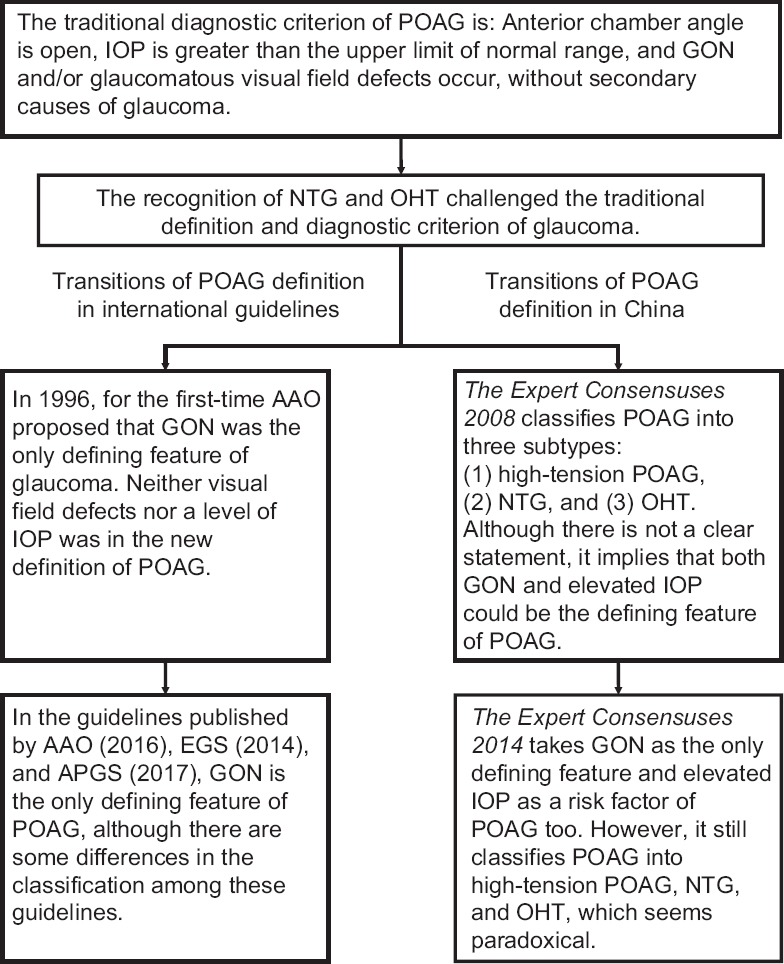
Transitions of the definition and classification of POAG. POAG: Primary open-angle glaucoma; IOP: Intraocular pressure; GON: Glaucomatous optic neuropathy; NTG: Normal tension glaucoma; OHT: Ocular hypertension; AAO: American Academy of Ophthalmology; APGS: Asia-Pacific Glaucoma Society.
Primary open-angle glaucoma definition among the expert consensuses in China
The diagnostic criteria of POAG were defined in the 1987 Chinese expert consensus The Preliminary Proposals for Early Diagnosis of Primary Glaucoma as follows: “POAG can only be diagnosed with the condition that the anterior chamber angle is open and IOP is >21 mmHg (measured with Goldmann applanation tonometer) together with GON and/or glaucomatous visual field defects.” Furthermore, The Preliminary Proposals mentions that “in the condition that GON and visual field defects occur without known reasons and IOP remains within normal limits, a diagnosis of NTG can be established. In the condition that IOP is greater than the normal limits through multiple measurements without the presence of GON, visual field defects, and secondary glaucoma, a diagnosis of OHT can be established.”[5]
The Preliminary Proposals emphasizes that elevated IOP is necessary for the diagnosis of POAG. In this proposal, the diagnostic criteria of NTG and OHT were related to POAG. However, the definitions of NTG and OHT did not meet the criteria for POAG described in this article. Therefore, The Preliminary Proposals did not clearly define the relationship between NTG, OHT, and POAG, which reflects the confusion of the glaucoma community at the time.
In 2008, The Expert Consensuses on Diagnosis and Treatments of Primary Glaucoma in China (2008) was published. In this article, POAG was classified into three subtypes: (1) high-tension POAG, in which the anterior chamber angle stays open and IOP >21 mmHg with GON and/or glaucomatous visual field defects without other known IOP-elevating factors; (2) NTG, in which there are characteristic glaucomatous damages (RNFL defects and/or changes of ONH) and/or glaucomatous defects of visual field, while IOP ≤21 mmHg (measured at least 6 times over a 24 h period) and the anterior chamber angle is open without the presence of other underlying diseases; and (3) OHT, which is defined similarly in The Preliminary Proposals.[6]
The latest edition of The Expert Consensuses on Diagnosis and Treatments of Primary Glaucoma in China was published in 2014. It defined POAG as a “chronic and progressive optic neuropathy for which pathologically elevated IOP is an important risk factor. The defining characteristics of POAG include the acquired atrophy of the optic nerve and the loss of retinal ganglion cells (RGCs) and their axons.” The classification of POAG in The Expert Consensuses 2014 was similar to that of The Expert Consensuses 2008 with the exception that the IOP measurement for NTG required a 24-h IOP curve.[7]
The Expert Consensuses 2008 did not provide a definition for POAG. Instead, it implied that both elevated IOP and GON could necessarily represent POAG since OHT and NTG were both regarded as subtypes of POAG. The Expert Consensuses 2014 defined POAG as “optic neuropathy” with IOP as a “risk factor.” This is paradoxical since OHT was still regarded as a subtype of POAG.
Primary open-angle glaucoma definition by American Academy of Ophthalmology
In 1996, the American Academy of Ophthalmology (AAO) published their 2nd edition of POAG preferred practice pattern (PPP). For the first time, both visual field defects and IOP were excluded from the definition of POAG. Instead, characteristic glaucomatous change in optic nerve or nerve fiber defects was regarded as sufficient for defining glaucoma. PPP 1996 then defined a list of defects of the optic nerve or nerve fiber layer including asymmetry, notching, thinning, progressive change, and nerve fiber layer defects.[8] Later editions of PPP follow the criteria that “GON is the only defining feature of glaucoma.”
In PPP 2016, POAG is defined as “a chronic and progressive optic neuropathy suffered by adults characterized by an acquired atrophy of the optic nerve and a loss of RGCs and their axons and is associated with an open angle by gonioscopy.”[9] PPP 2016 defines a POAG suspect as an individual with clinical findings and/or a constellation of risk factors that indicate an increased likelihood of developing POAG. Any of the following clinical findings in one or both eyes of an individual with an open angle can indicate a POAG suspect: (1) an appearance of the optic disc or RNFL that is suspicious for glaucomatous damage; (2) a visual field suspicious of glaucomatous damage in the absence of signs of other optic neuropathies; or (3) consistently elevated IOP associated with a normal appearance of the optic disc, RNFL, and visual field. This definition excludes angle-closure glaucoma and known secondary causes for open-angle glaucoma, such as pseudoexfoliation, pigment dispersion, and traumatic angle recession.[10]
Primary open-angle glaucoma definitions in other influential guidelines
In 2014, the European Glaucoma Society (EGS) published its 4th edition of Terminology and Guidelines for Glaucoma, in which the definition for POAG is nearly identical to that in PPP. Here, POAG is defined as a chronic progressive optic neuropathy with characteristic morphological changes at the ONH and RNFL in the absence of other ocular disease or congenital anomalies. Progressive RGC death and visual field loss are associated with these changes.[11]
However, there are some small differences between Terminology and Guidelines for Glaucoma and PPP 2016. In the guidelines published by EGS, POAG was classified into two subtypes: high-pressure glaucoma and NTG. The features of NTG were described as (1) an onset occurring at 35 years of age or older; (2) signs and symptoms including normal IOP without treatment (diurnal curve or 24-h phasing), asymptomatic until visual field loss, ONH damage typical of glaucoma, and disc hemorrhages; (3) visual field defects typical of glaucoma; (4) gonioscopy showing an open angle; and (5) no history or signs of other eye disease or steroid use. Furthermore, OHT was regarded as a separate diagnosis rather than a category of POAG suspect. The features of OHT were described as (1) signs and symptoms including IOP >21 mmHg without treatment, normal visual field, normal optic disc and RNFL, open angle (excluding intermittent angle closure) by gonioscopy, and no history or signs of other eye disease or steroid use and (2) no other risk factors. The features of POAG suspect were described as (1) a normal or suspicious visual field, optic disc, and/or RNFL with at least one being suspicious and (2) a normal or increased IOP.[11]
The definition and classification of POAG in another famous guideline, Asia Pacific Glaucoma Guidelines, published by Asia-Pacific Glaucoma Society are the same as those in the Terminology and Guidelines for Glaucoma.[12]
Contrast of the understanding of primary open-angle glaucoma between Chinese expert consensuses and international guidelines
According to the classification methods proposed by AAO, “high-tension POAG” and “NTG” should be collectively grouped to “POAG,” and “OHT” should be classified as “POAG suspect.” PPP 2016 did not mention the concepts of “high-tension POAG” and “NTG” but collectively named them “POAG.” This nomenclature revealed that in the definition and classification of POAG, PPP emphasized the essence of the disease and will no longer focus on the role of IOP on POAG classification.
Unlike international guidelines, Chinese expert consensuses did not mention “POAG suspect” and this may indicate that early screening for POAG in China is not as thorough as in other developed countries.
The definition and classification of POAG in China put more emphasis on the role of IOP. Not only do they highlight that “pathologically elevated IOP is an important risk factor” but also regard OHT as a subtype of POAG while influential international guidelines do not. The reasoning that OHT is considered a subtype of POAG in China is that OHT patients may develop GON and visual field defects over time. However, according to “The Ocular Hypertension Treatment Study,” 90–95% of patients with OHT will not develop glaucoma over 5 years,[13] and this suggests that OHT should be regarded as a separate diagnosis.
The definition of POAG in international guidelines puts more emphasis on the pathological essence of POAG; it indicates that POAG is not a single disease but may include multiple diseases whose common characteristic is the primary lesion of RGCs and their axons. Some researchers believe that the initiating point of POAG can be (1) the cytons of RGCs, (2) the axons of RGCs, or (3) both cytons and axons of RGCs. Pathogenesis for both lesions of cytons and axons can be (1) endogenous defects (genetic or epigenetic changes) or (2) exogenous harmful factors (such as inflammation).
Although a consensus on the definition and classification of POAG has not yet been reached, there is no controversy about the treatments of the disease. It is universally accepted that IOP reduction is the only evidence-based treatment strategy for both POAG and POAG suspects.[10,11] Even for OHT patients who may be at low risk of optical damage, reducing IOP can lower the risk of developing POAG from 9.5% to 4.5%.[13]
Enlightenment for the scientific community
Glaucoma is a neurodegenerative disease of the optic nerve. It presents itself at various stages of a continuum which is characterized by accelerated RGC death, subsequent axonal loss and optic nerve damage, and eventual visual field loss [Figure 2].[14]
Figure 2.
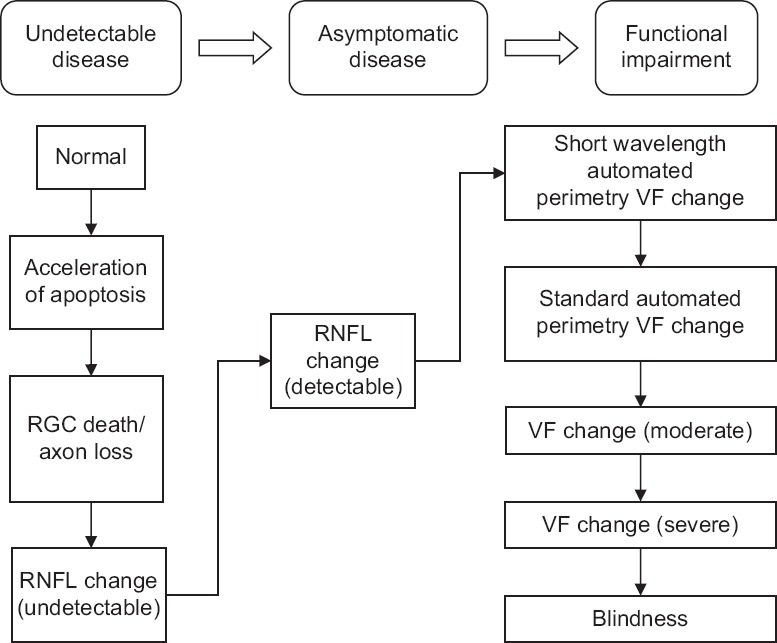
The glaucoma continuum. VF: Visual field; RGC: Retinal ganglion cells; RNFL: Retinal nerve fiber layer.
The essence of POAG is the death of RGCs and loss of their axons, and IOP is just an exogenous factor for POAG. The core indication of POAG has converted from elevated IOP to GON, and the key to diagnosis and treatment is to detect and monitor the lesions of RGCs and their axons.
Transitions of the definition of primary angle-closure glaucoma
The traditional definition of primary angle-closure glaucoma (PACG) is “the elevation of IOP caused by the primary closure of the anterior chamber angle.”[5] Inspired by the modification of the POAG definition, researchers proposed to also replace elevated IOP with GON as the defining characteristic of PACG [Figure 3].
Figure 3.
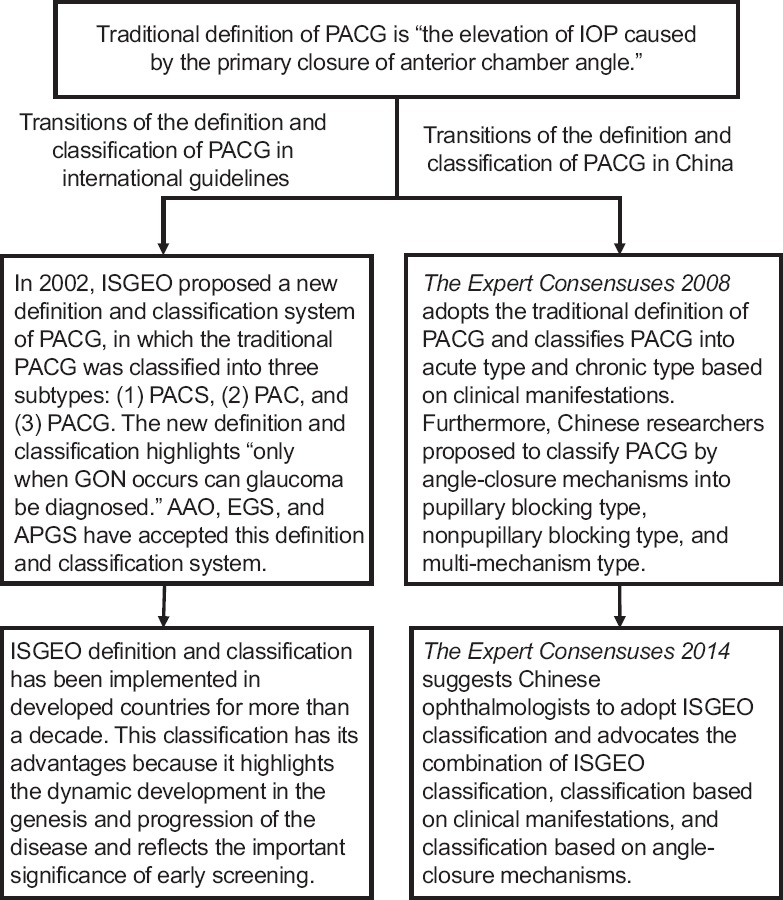
Transitions of the definition and classification of PACG. PACG: Primary angle-closure glaucoma; IOP: Intraocular pressure; ISGEO: International Society of Geographical and Epidemiological Ophthalmology; PACS: Primary angle-closure suspect; PAC: Primary angle closure; EGS: European Glaucoma Society; AAO: American Academy of Ophthalmology; APGS: Asia-Pacific Glaucoma Society; GON: Glaucomatous optic neuropathy.
The classification system by International Society of Geographical and Epidemiological Ophthalmology
In 2002, the International Society of Geographical and Epidemiological Ophthalmology (ISGEO) proposed a new definition and classification system of PACG, in which the traditional PACG was classified into three subtypes: (1) primary angle-closure suspect (PACS), (2) primary angle closure (PAC), and (3) PACG. The new definition and classification highlighted that “glaucoma can only be diagnosed when GON is present.”[15]
ISGEO defined PACS, PAC, and PACG as follows: (1) PACS shows appositional contact between the peripheral iris and posterior trabecular meshwork; (2) PAC shows an occluded drainage angle and shows features indicating that trabecular obstruction by the peripheral iris has occurred in the eye, such as peripheral anterior synechiae, elevated IOP, iris whorling (distortion of the radially orientated iris fibers), glaucomfleken lens opacities, or excessive pigment deposition on the trabecular surface. However, the optic disc does not have glaucomatous damage. (3) PACG is defined as PAC with evidence of GON.[15]
AAO accepted the ISGEO classification, and in PPP 2016, the diagnostic criteria of PACS, PAC, and PACG were listed in Table 1.[16]
Table 1.
Clinical findings that define patients with angle-closure diseases
| Clinical findings | PACS | PAC | PACG |
|---|---|---|---|
| ≥180° ITC | Present | Present | Present |
| Elevated IOP or PAS | Absent | Present | Present |
| Optic nerve damage | Absent | Absent | Present |
ITC: Iridotrabecular contact; PAS: Peripheral anterior synechiae; PACS: Primary angle-closure suspect; PAC: Primary angle closure; PACG: Primary angle-closure glaucoma; IOP: Intraocular pressure.
The definitions and diagnostic criteria of PACS, PAC, and PACG in Terminology and Guidelines for Glaucoma (2014) and Asia Pacific Glaucoma Guidelines (2016) are similar to those in PPP 2016.[11,12]
Definition of primary angle-closure glaucoma in China
The traditional definition and classification of PACG are still the most widely accepted in China despite the ISGEO definition having been proposed for more than a decade.
The Expert Consensuses 2008 defined PACG as “an acute or chronic elevation of IOP caused by primary closure of the anterior chamber angle with or without GON and visual field defects.” Based on clinical manifestations, it classified PACG into acute PACG and chronic PACG. Acute PACG was divided by the traditional approach into the preclinical, portent, acute episode, symptomatic relief, and chronic stages. Chronic PACG was divided into the early, progressing, and late stages. Complete blindness was the absolute stage.[6] Furthermore, Chinese researchers proposed to classify PACG into the pupillary blocking, nonpupillary blocking, and multi-mechanism types, based on the mechanisms in which angle closure happens.[17]
The Expert Consensuses 2014 kept the same definition and classification of PACG as that of The Expert Consensuses 2008; however, it suggested that Chinese ophthalmologists adopt the ISGEO classification and also advocated for the combination of the ISGEO classification, classification based on clinical manifestations, and classification based on angle-closure mechanisms.[7]
Contrast of primary angle-closure glaucoma definitions and classifications between Chinese expert consensuses and international guidelines
The basis of the ISGEO definition of PACG is that PAC is a unique disease of the anterior ocular chamber, which can increase the risk of developing PACG. PACG can only be diagnosed when GON is present concurrently with PAC.[18] This has been supported by substantial research. Surveys found that the acute symptomatic phase occurs in only a minority of those with PACG while a chronic asymptomatic form of PACG predominates.[19,20,21,22] Thus, ISGEO proposed that emphasis should be placed on optic nerve injury and visual loss rather than on symptomatic disease.[15] Other research has indicated that as many as 60–75% of people suffering from an acute symptomatic episode of angle closure recovered without optic disc or visual field damage in the short term,[23] which supports that PAC should be considered a separate diagnosis.
Some Chinese researchers believe that the ISGEO definition was disputable and argued that “there is a causal relationship between PAC, elevated IOP, and the occurrence of GON. Although PAC cannot immediately develop into GON, the eventual occurrence of GON is almost inevitable. Therefore, it is not appropriate to regard PAC as a separate diagnosis.”[24] In a cohort study, 28.5% of PAC patients developed GON in a 5-year period.[25] In another study, 17.8% of PAC patients developed blindness and almost half had glaucomatous optic nerve damage several years later after suffering from acute PAC.[26]
Ge[27] commented that “the radical divergence between these two definitions is whether PACS and PAC are separate diagnoses or the early stages in the course of PACG. It is difficult to reach a conclusion because the studies on the natural history of PACG are still scarce. Besides, the ISGEO definition does not reduce clinical interventions since both PACS and PAC may need to be treated as well.”
The ISGEO classification has its advantages because it stresses the dynamic development and progression of the disease and emphasizes the significance of early screening.[27] The fundamental aspect of the ISGEO classification is the early screening for glaucoma which has been conducted in many developed countries. However, early screening for glaucoma is not yet widely practiced in China, and researchers prefer the traditional classification of PACG which pays more consideration to the clinical manifestations after diagnosis of the disease. The classification based on angle-closure mechanisms is an important complement to the current classification of PACG because different treatments should be utilized based on these different angle-closure mechanisms.[17]
NEW HYPOTHESES AND THEORIES ABOUT THE RELATIONSHIP BETWEEN PRESSURE AND GLAUCOMA
From the transitions of the understanding and definition of primary glaucoma, we can learn that the relationship between IOP and glaucoma has been a longstanding issue in glaucoma research. In recent years, researchers have put forward some new hypotheses and theories trying to explain the pathogenesis of glaucoma, such as the “safe IOP hypothesis” and the “ocular-cranial pressure gradient theory,” which have provided a deeper understanding of the relationship between pressure and glaucoma.
Safe intraocular pressure hypothesis
The existence of NTG and OHT has demonstrated the problem in defining glaucoma with a specific IOP range. However, researchers have developed the “safe IOP hypothesis” which attempts to keep “pathologically elevated IOP” as the pathogenesis and defining feature of glaucoma. “Safe IOP hypothesis” states that “safe IOP” is a range of IOP that will not cause optic neuropathy in individuals, and “safe IOP” is individualized and can be different from statistically normal IOP. The occurrence of NTG and OHT can be explained with this hypothesis. “Safe IOP” is lower for NTG patients compared to OHT patients. GON can develop in NTG patients with statistically normal IOP, but GON might not develop in OHT patients with the same IOP. However, relevant studies of this hypothesis are still scarce.[28]
Ocular-cranial pressure gradient theory
The Consensuses and Suggestions on POAG Ocular-Cranial Pressure Gradient in China (2017) states that “the optic nerve is located in both the intraocular cavity and intracranial cavity. The difference between IOP and intracranial pressure (ICP) exists at the lamina cribrosa and forms a pressure gradient along the optic nerve, which is called ocular-cranial pressure gradient or the “trans-lamina cribrosa pressure difference (TLPD).”[29]
Based on conventional opinions, the compression of the ONH and the degeneration of the lamina cribrosa due to elevated IOP are the causes of GON. However, in the theory of TLPD, elevated TLPD causes impingement of the ONH and the degeneration of lamina cribrosa rather than elevated IOP. Elevated TLPD can be caused not only by elevated IOP but also by decreased ICP, which can induce GON as well.[30] The occurrence of NTG and OHT can also be explained by this new theory. When ICP is below normal limits, TLPD increases under the normal IOP and can cause NTG. When ICP is above normal limits, TLPD can remain within normal limits even if IOP is elevated, and thus, OHT occurs.
A series of researches have collected supportive evidence for this theory. In case–control studies, ICP was lower in POAG and NTG patients and elevated in OHT patients.[31,32] A prospective study found that lumbar cerebrospinal fluid pressure (CSFP) was significantly lower in the NTG group than in the high-tension POAG group and the control group, and also, the TLPD (IOP minus CSFP) was significantly higher in the NTG group and high-tension POAG group than in the control group.[33] Many studies indicated that TLPD was more significantly correlated with the amount of glaucomatous optic nerve damage compared to IOP and ICP alone.[34,35] Another study showed that lowering CSFP could induce glaucoma-like optic neuropathy in monkeys.[36] Furthermore, Zhang et al.[37] found that increasing TLPD by lowering CSFP could cause axonal transport failure of the optic nerve and could disrupt optic blood supply and lead to optic neuropathy.
Hou et al.[30] further proposed that there exists a pressure gradient along the entire length of the optic nerve determined by the surrounding tissues and fluid. Therefore, optic nerve damage is not only occurring at the site of the lamina cribrosa but also along the entirety of the optic nerve. Thus, they suggested that the concept of TLPD should be expanded to the “optic nerve pressure gradient.” There has been promising research on this theory.[30,38]
THE RELATIONSHIP BETWEEN GLAUCOMA AND CENTRAL NERVOUS SYSTEM NEURODEGENERATIVE DISEASES
The retina is the outer span of the central nervous system (CNS) with a similar histologic origin to the brain.[39] Glaucoma, a neurodegenerative disease of the optic nerve, has been found to have some association with CNS neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD).
Glaucoma and AD have several common characteristics. Both are chronic and age-related neurodegenerative disorders. Structural studies have shown that the optic nerves of both POAG and AD patients exhibit degeneration and loss of RGCs. On a molecular level, caspase activation induces abnormal amyloid precursor protein formation, which is the key event in the pathogenesis of AD, was observed in a rat model of chronic OHT.[40] Some studies have demonstrated that POAG prevalence in AD patients is significantly higher than that of the control groups. A study in Germany showed that the POAG prevalence of AD patients was 25.9% while that of the control was 5.2%.[41] Bayer et al.[42] reported that in their study involving 49 AD patients, 12 (24.5%) had glaucomatous visual defects or a cup-to-disc ratio of ≥0.8. Japanese researchers found that POAG prevalence of AD patients (23.8%) was much higher than that of the control (9.9%), and among AD patients, there was no significant difference between the IOP of the POAG group and the non-POAG group.[43] Furthermore, some studies found that the RNFL of AD patients was significantly thinner than that of the control group.[44,45,46] Some retrospective studies have reported that PD patients are likely to exhibit glaucomatous-like visual field defects.[42,47] Studies using optic coherence tomography have shown that peripapillary RNFL thinning occurs in PD patients.[48,49,50] Another retrospective study revealed that in elderly patients, POAG is a significant predictor of AD but not a significant predictor of PD.[40]
The studies above showed that there is an association between glaucoma and CNS neurodegenerative diseases, but the clinical and genetic relationship between them requires more elucidating. Liu et al.[51,52] proposed that glaucoma is not only an ocular neurodegenerative disease but also a CNS disease, and with this understanding, we may better research the pathogenesis of glaucoma and better establish comprehensive treatment strategies for this disease.
OTHER RISK FACTORS WHICH MAY PARTICIPATE IN THE PATHOGENESIS OF GLAUCOMA
Besides the conventional risk factors of primary glaucoma which include elevated IOP, family history, old age, ethnicity, type 2 diabetes, myopia, and thin central corneal thickness, there has recently been some new factors found to be associated with primary glaucoma such as obesity, obstructive sleep apnea-hypopnea syndrome (OSAHS), mental stress, anxiety, and depression. Many studies reported that body mass index is positively correlated with IOP.[53,54] Furthermore, studies have shown that an association exists between sleep pattern and primary glaucoma.[55] It has been shown that OSAHS increases the risk for glaucoma.[56,57] Psychological factors, such as mental stress, anxiety, and depression, have been shown to be associated with primary glaucoma, and primary glaucoma has been slowly accepted as a psychosomatic disease.[55,58,59] Further studies are needed to completely explain how these factors participate in the pathogenesis of primary glaucoma.
SUMMARY
As the ophthalmology community is gaining a deeper understanding of glaucoma, the definition of primary glaucoma has undergone a series of transitions. We have achieved much progress from defining glaucoma solely upon IOP to realizing that the essence of glaucoma is based on the atrophy of the optic nerve and the loss of RGCs and their axons. The complete definition of the disease should reflect the nature of its pathogenesis. As we continue to explore the pathogenesis of glaucoma, it can be anticipated that we will adopt further modifications and refinements to the definition of glaucoma. China has abundant clinical resources and a great magnitude of biological diversity and therefore has great potential for contributing to original findings in medicine, including the intriguing disease of glaucoma. We may need to update our guidelines on glaucoma, integrating its latest understandings. In this way, we can unify clinical guidelines among various hierarchies of practice, thus making significant contributions that are universally accepted within international ophthalmology community.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou H, Shen Z. The definition of glaucoma. In: Zhou H, Shen Z, editors. Intraocular Pressure and Glaucoma. 1st ed. Wuhan: Hubei Science & Technology Press; 2010. pp. 8–11. [Google Scholar]
- 3.Ge J, Fan Z. Current situation and development trend of glaucoma research in China (in Chinese) Chin J Ophthalmol Otorhinol. 2004;2:69–71. [Google Scholar]
- 4.Kanski JJ, Bowling B. Kanski's Clinical Ophthalmology: A Systematic Approach. Sydney: Elsevier; 2015. [Google Scholar]
- 5.Glaucoma Group of Chinese Ophthalmological Society. The preliminary proposals for early diagnosis of primary glaucoma (in Chinese) Chin J Ophthalmol. 1987;23:127. [Google Scholar]
- 6.Glaucoma Group of Chinese Ophthalmological Society. The expert consensuses on diagnosis and treatments of primary glaucoma in China (in Chinese) Chin J Ophthalmol. 2008;44:862–3. doi: 10.3321/j.issn:0412-4081.2008.09.022. [Google Scholar]
- 7.Glaucoma Group of Chinese Ophthalmological Society. The expert consensuses on diagnosis and treatments of primary glaucoma in China (in Chinese) Chin J Ophthalmol. 2014;5:382–3. doi: 10.3760/cma.j.issn.0412-4081.2014.05.022. [Google Scholar]
- 8.Lee PP. Understanding the new primary open-angle glaucoma preferred practice pattern. Int Ophthalmol Clin. 1998;38:93–9. doi: 10.1097/00004397-199803830-00011. doi: 10.1097/00004397-199803830-00011. [DOI] [PubMed] [Google Scholar]
- 9.Prum BE, Jr, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, et al. Primary open-angle glaucoma preferred practice pattern(®) guidelines. Ophthalmology. 2016;123:P41–111. doi: 10.1016/j.ophtha.2015.10.053. doi: 10.1016/j.ophtha.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 10.Prum BE, Jr, Lim MC, Mansberger SL, Stein JD, Moroi SE, Gedde SJ, et al. Primary open-angle glaucoma suspect preferred practice pattern(®) guidelines. Ophthalmology. 2016;123:P112–51. doi: 10.1016/j.ophtha.2015.10.055. doi: 10.1016/j.ophtha.2015.10.055. [DOI] [PubMed] [Google Scholar]
- 11.European glaucoma society terminology and guidelines for glaucoma, 4th edition-chapter 2: Classification and terminology Supported by the EGS foundation: Part 1: Foreword; introduction; glossary; chapter 2 classification and terminology. Br J Ophthalmol. 2017;101:73–127. doi: 10.1136/bjophthalmol-2016-EGSguideline.002. doi: 10.1136/bjophthalmol-2016-EGSguideline.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aung T, Crowston J, Chen HS, Covar RV, George R, Kim SH, et al. Asian Pacific Glaucoma Guidelines. 3rd ed. Amsterdam, The Netherlands: Kugler Publications; 2016. [Google Scholar]
- 13.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The ocular hypertension treatment study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 14.Weinreb RN, Friedman DS, Fechtner RD, Cioffi GA, Coleman AL, Girkin CA, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. 2004;138:458–67. doi: 10.1016/j.ajo.2004.04.054. doi: 10.1016/j.ajo.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 15.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prum BE, Jr, Herndon LW, Jr, Moroi SE, Mansberger SL, Stein JD, Lim MC, et al. Primary angle closure preferred practice pattern(R) guidelines. Ophthalmology. 2016;123:P1–40. doi: 10.1016/j.ophtha.2015.10.049. doi: 10.1016/j.ophtha.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Wu H, Fan Z. Primary angle closure glaucoma in Chinese and Western populations. Chin Med J. 2002;115:1706–15. doi: 10.3760/j.issn:0366-6999.2002.11.024. [PubMed] [Google Scholar]
- 18.He M, Ge J. Primary angle closure-a new term in the definition of primary angle closure glaucoma (in Chinese)? Chin J Ophthalmol. 2005;12:1061–4. doi: 10.3760/j:issn:0412-4081.2005.12.002. [PubMed] [Google Scholar]
- 19.Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Glaucoma in Mongolia. A population-based survey in Hövsgöl province, Northern Mongolia. Arch Ophthalmol. 1996;114:1235–41. doi: 10.1001/archopht.1996.01100140435011. doi: 10.1001/archopht.1996.01100140435011. [DOI] [PubMed] [Google Scholar]
- 20.Foster PJ, Oen FT, Machin D, Ng TP, Devereux JG, Johnson GJ, et al. The prevalence of glaucoma in Chinese residents of Singapore: A cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol. 2000;118:1105–11. doi: 10.1001/archopht.118.8.1105. doi: 10.1001/archopht.118.8.1105. [DOI] [PubMed] [Google Scholar]
- 21.Salmon JF, Mermoud A, Ivey A, Swanevelder SA, Hoffman M. The prevalence of primary angle closure glaucoma and open angle glaucoma in Mamre, Western Cape, South Africa. Arch Ophthalmol. 1993;111:1263–9. doi: 10.1001/archopht.1993.01090090115029. doi: 10.1001/archopht.1993.01090090115029. [DOI] [PubMed] [Google Scholar]
- 22.Congdon NG, Quigley HA, Hung PT, Wang TH, Ho TC. Screening techniques for angle-closure glaucoma in rural Taiwan. Acta Ophthalmol Scand. 1996;74:113–9. doi: 10.1111/j.1600-0420.1996.tb00053.x. doi: 10.1111/j.1600-0420.1996.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 23.Douglas GR, Drance SM, Schulzer M. The visual field and nerve head in angle-closure glaucoma. A comparison of the effects of acute and chronic angle closure. Arch Ophthalmol. 1975;93:409–11. doi: 10.1001/archopht.1975.01010020423004. doi: 10.1001/archopht.1975.01010020423004. [DOI] [PubMed] [Google Scholar]
- 24.Ren Z. Devoting much attention to further understanding the definition of glaucoma (in Chinese) Chin J Ophthalmol. 2006;3:193–5. doi: 10.3760/j:issn:0412-4081.2006.03.001. [PubMed] [Google Scholar]
- 25.Thomas R, Parikh R, Muliyil J, Kumar RS. Five-year risk of progression of primary angle closure to primary angle closure glaucoma: A population-based study. Acta Ophthalmol Scand. 2003;81:480–5. doi: 10.1034/j.1600-0420.2003.00135.x. doi: 10.1034/j.1600-0420.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 26.Aung T, Friedman DS, Chew PT, Ang LP, Gazzard G, Lai YF, et al. Long-term outcomes in Asians after acute primary angle closure. Ophthalmology. 2004;111:1464–9. doi: 10.1016/j.ophtha.2003.12.061. doi: 10.1016/j.ophtha.2003.12.061. [DOI] [PubMed] [Google Scholar]
- 27.Ge J. Scientific controversy to push the progress of glaucoma practice-inspiration by the debate on the classification of primary angle closure glaucoma (in Chinese) Chin J Ophthalmol. 2006;11:964–6. doi: 10.3760/j: issn:0412-4081.2006.11.002. [PubMed] [Google Scholar]
- 28.Lu D, Liu X, Wang C. Analysis of intraocular pressure, cup disc ration and systemic blood pressure for the prediction of prognosis of glaucoma patients (in Chinese) Eye Sci. 1985;1:77–80. [PubMed] [Google Scholar]
- 29.Glaucoma Group of Chinese Ophthalmological Society. The Consensuses and Suggestions on POAG Ocular-Cranial Pressure Gradient in China (in Chinese) Chin J Ophthalmol. 2017;53:89–91. doi: 10.3760/cma.j.issn. 0412-4081.2017.02.004. [Google Scholar]
- 30.Hou R, Zhang Z, Yang D, Wang H, Chen W. Pressure balance and imbalance in the optic nerve chamber: The Beijing intracranial and intraocular pressure (iCOP) study. Sci China Life Sci. 2016;46:1413–22. doi: 10.1007/s11427-016-5022-9. doi: 10.1360/N052016-00279. [DOI] [PubMed] [Google Scholar]
- 31.Berdahl JP, Allingham RR, Johnson DH. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology. 2008;115:763–8. doi: 10.1016/j.ophtha.2008.01.013. doi: 10.1016/j.ophtha.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: A case-control study. Invest Ophthalmol Vis Sci. 2008;49:5412–8. doi: 10.1167/iovs.08-2228. doi: 10.1167/iovs.08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren R, Jonas JB, Tian G, Zhen Y, Ma K, Li S, et al. Cerebrospinal fluid pressure in glaucoma: A prospective study. Ophthalmology. 2010;117:259–66. doi: 10.1016/j.ophtha.2009.06.058. doi: 10.1016/j.ophtha.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 34.Ren R, Wang N, Zhang X, Cui T, Jonas JB. Trans-lamina cribrosa pressure difference correlated with neuroretinal rim area in glaucoma. Graefes Arch Clin Exp Ophthalmol. 2011;249:1057–63. doi: 10.1007/s00417-011-1657-1. doi: 10.1007/s00417-011-1657-1. [DOI] [PubMed] [Google Scholar]
- 35.Jonas JB, Wang NL, Wang YX, You QS, Xie XB, Yang DY, et al. Estimated trans-lamina cribrosa pressure difference versus intraocular pressure as biomarker for open-angle glaucoma. The Beijing Eye Study 2011. Acta Ophthalmol. 2015;93:e7–13. doi: 10.1111/aos.12480. doi: 10.1111/aos.12480. [DOI] [PubMed] [Google Scholar]
- 36.Yang D, Fu J, Hou R, Liu K, Jonas JB, Wang H, et al. Optic neuropathy induced by experimentally reduced cerebrospinal fluid pressure in monkeys. Invest Ophthalmol Vis Sci. 2014;55:3067–73. doi: 10.1167/iovs.13-13657. doi: 10.1167/iovs.13-13657. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Liu D, Jonas JB, Wu S, Kwong JM, Zhang J, et al. Axonal transport in the rat optic nerve following short-term reduction in cerebrospinal fluid pressure or elevation in intraocular pressure. Invest Ophthalmol Vis Sci. 2015;56:4257–66. doi: 10.1167/iovs.14-16045. doi: 10.1167/iovs. 14-16045. [DOI] [PubMed] [Google Scholar]
- 38.Hou R, Zhang Z, Yang D, Wang H, Chen W, Li Z, et al. Intracranial pressure (ICP) and optic nerve subarachnoid space pressure (ONSP) correlation in the optic nerve chamber: The Beijing intracranial and intraocular pressure (iCOP) study. Brain Res. 2016;1635:201–8. doi: 10.1016/j.brainres.2016.01.011. doi: 10.1016/j.brainres.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Li Z, Han Y, Mou D, Wang N, Lu Y. Association between Alzheimer's disease and glaucoma (in Chinese) Int Eye Sci. 2017;17:964–7. [Google Scholar]
- 40.Lin IC, Wang YH, Wang TJ, Wang IJ, Shen YD, Chi NF, et al. Correction: Glaucoma, Alzheimer's disease, and Parkinson's disease: An 8-year population-based follow-up study. PLoS One. 2016;11:e0150789. doi: 10.1371/journal.pone.0150789. doi: 10.1371/journal.pone.0150789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayer AU, Ferrari F, Erb C. High occurrence rate of glaucoma among patients with Alzheimer's disease. Eur Neurol. 2002;47:165–8. doi: 10.1159/000047976. doi: 10.1159/000047976. [DOI] [PubMed] [Google Scholar]
- 42.Bayer AU, Keller ON, Ferrari F, Maag KP. Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer's disease and Parkinson's disease. Am J Ophthalmol. 2002;133:135–7. doi: 10.1016/s0002-9394(01)01196-5. doi: 10.1016/S0002-9394(01)01196-5. [DOI] [PubMed] [Google Scholar]
- 43.Tamura H, Kawakami H, Kanamoto T, Kato T, Yokoyama T, Sasaki K, et al. High frequency of open-angle glaucoma in Japanese patients with Alzheimer's disease. J Neurol Sci. 2006;246:79–83. doi: 10.1016/j.jns.2006.02.009. doi: 10.1016/j.jns.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Morphological and functional retinal impairment in Alzheimer's disease patients. Clin Neurophysiol. 2001;112:1860–7. doi: 10.1016/s1388-2457(01)00620-4. doi: 10.1016/S1388-2457(01)00620-4. [DOI] [PubMed] [Google Scholar]
- 45.Marziani E, Pomati S, Ramolfo P, Cigada M, Giani A, Mariani C, et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer's disease using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:5953–8. doi: 10.1167/iovs.13-12046. doi: 10.1167/iovs.13-12046. [DOI] [PubMed] [Google Scholar]
- 46.Kirbas S, Turkyilmaz K, Anlar O, Tufekci A, Durmus M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol. 2013;33:58–61. doi: 10.1097/WNO.0b013e318267fd5f. doi: 10.1097/WNO.0b013e318267fd5f. [DOI] [PubMed] [Google Scholar]
- 47.Yenice O, Onal S, Midi I, Ozcan E, Temel A, I-Gunal D. Visual field analysis in patients with Parkinson's disease. Parkinsonism Relat Disord. 2008;14:193–8. doi: 10.1016/j.parkreldis.2007.07.018. doi: 10.1016/j.parkreldis.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 48.Altintaş O, Işeri P, Ozkan B, Cağlar Y. Correlation between retinal morphological and functional findings and clinical severity in Parkinson's disease. Doc Ophthalmol. 2008;116:137–46. doi: 10.1007/s10633-007-9091-8. doi: 10.1007/s10633-007-9091-8. [DOI] [PubMed] [Google Scholar]
- 49.Moschos MM, Tagaris G, Markopoulos I, Margetis I, Tsapakis S, Kanakis M, et al. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol. 2011;21:24–9. doi: 10.5301/ejo.2010.1318. doi: 10.5301/EJO.2010.1318. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Martin E, Satue M, Fuertes I, Otin S, Alarcia R, Herrero R, et al. Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson's disease. Ophthalmology. 2012;119:2161–7. doi: 10.1016/j.ophtha.2012.05.003. doi: 10.1016/j.ophtha. 2012.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Chen X, Wang N. Is glaucoma a central nervous system disease.(in Chinese) Ophthalmol CHN. 2010;19:4–7. [PubMed] [Google Scholar]
- 52.Liu X, Chen X, Wang N. Is glaucoma a central nervous system disease: re-evaluation (in Chinese) Chin J Ophthalmol. 2010;46:1062–5. doi: 10.3760/cma.j.issn.0412-4081.2010.12.003. [PubMed] [Google Scholar]
- 53.Fukuoka S, Aihara M, Iwase A, Araie M. Intraocular pressure in an ophthalmologically normal Japanese population. Acta Ophthalmol. 2008;86:434–9. doi: 10.1111/j.1600-0420.2007.01068.x. doi: 10.1111/j.1600-0420.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 54.Memarzadeh F, Ying-Lai M, Azen SP, Varma R Los Angeles Latino Eye Study Group. Associations with intraocular pressure in Latinos: The Los Angeles Latino eye study. Am J Ophthalmol. 2008;146:69–76. doi: 10.1016/j.ajo.2008.03.015. doi: 10.1016/j.ajo.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma X, Shen G, Qi Q, Sun X. Disturbed sleep and psychosocial factors associated with primary open angle glaucoma patients. Fudan Univ J Med Sci. 2015;42:628–33. doi: 10.3969/j.issn.1672-8467.2015.05.012. [Google Scholar]
- 56.Wu X, Liu H. Obstructive sleep apnea/hypopnea syndrome increases glaucoma risk: Evidence from a meta-analysis. Int J Clin Exp Med. 2015;8:297–303. [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Y, Liu P, Guan J, Lu Y, Su K. Association between glaucoma and obstructive sleep apnea syndrome: A meta-analysis and systematic review. PLoS One. 2015;10:e0115625. doi: 10.1371/journal.pone.0115625. doi: 10.1371/journal.pone. 0115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erb C, Batra A, Lietz A, Bayer AU, Flammer J, Thiel HJ. Psychological characteristics of patients with normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 1999;237:753–7. doi: 10.1007/s004170050308. doi: 10.1007/s004170050308. [DOI] [PubMed] [Google Scholar]
- 59.Shily BG. Psychophysiological stress, elevated intraocular pressure, and acute closed-angle glaucoma. Am J Optom Physiol Opt. 1987;64:866–70. doi: 10.1097/00006324-198711000-00011. doi: 10.1097/00006324-198711000-00011. [DOI] [PubMed] [Google Scholar]


