Abstract
Background:
Several studies have shown that detection of minimal residual disease (MRD) in acute myeloid leukemia (AML) is an independent prognostic factor. This study aimed to evaluate the significance of dynamic MRD pretransplantation on outcome of AML patients receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Methods:
We retrospectively analyzed 145 consecutive AML patients undergoing allo-HSCT in complete remission status between June 2013 and June 2016. MRD was determined with multiparameter flow cytometry after the first and second courses of chemotherapy and pre-HSCT.
Results:
In matched sibling donor transplantation (MSDT) settings, patients with positive MRD had higher cumulative incidence of relapse (CIR) than those without MRD after the first (32.3 ± 9.7% vs. 7.7 ± 3.1%, χ2 = 3.661, P = 0.055) or second course of chemotherapy (57.1 ± 3.6% vs. 12.5 ± 2.7%, χ2 = 8.759, P = 0.003) or pre-HSCT (50.0 ± 9.7% vs. 23.0 ± 3.2%, χ2 = 5.547, P = 0.019). In haploidentical SCT (haplo-SCT) settings, the MRD status at those timepoints had no significant impact on clinical outcomes. However, patients with persistent positive MRD from chemotherapy to pre-HSCT had higher CIR than those without persistent positive MRD both in MSDT and haplo-SCT settings. Patients with persistent positive MRD underwent MSDT had the highest relapse incidence, followed by those with persistent positive MRD underwent haplo-SCT, those without persistent MRD underwent haplo-SCT, and those without persistent MRD underwent MSDT (66.7 ± 9.2% vs. 38.5 ± 6.0% vs. 18.8 ± 8.7% vs. 12.0 ± 1.0%, χ2 = 20.763, P < 0.001). Multivariate analysis showed that persistent positive MRD before transplantation was associated with higher CIR (hazard ratio [HR] = 1.69, 95% confidence interval [CI]: 1.200–2.382, P = 0.003), worse leukemia-free survival (HR = 1.812, 95% CI: 1.168–2.812, P = 0.008), and overall survival (HR = 2.354, 95% CI: 1.528–3.627, P < 0.001).
Conclusion:
Our results suggest that persistent positive MRD before transplantation, rather than positive MRD at single timepoint, could predict poor outcome both in MSDT and haplo-SCT settings.
Keywords: Allogeneic Stem Cell Transplantation, Flow Cytometry, Haploidentical Allograft, Human Leukocyte Antigen-Matched Sibling Donor Transplantation, Minimal Residual Disease
摘要
背景:
有研究显示流式检测的微小残留病变(Minimal residual disease,MRD)与急性髓系白血病(Acute myeloid leukemia,AML)患者预后相关。本研究旨在探讨异基因造血干细胞移植前MRD动态变化与AML患者预后的关系。
方法:
回顾性分析145例于我院接受异基因造血干细胞移植的AML患者。患者移植前均为形态学完全缓解状态。用多色流式细胞术在第一和第二程化疗后、移植前检测患者骨髓MRD。
结果:
接受同胞全合移植的AML患者,第一程化疗后MRD阳性组复发率较MRD阴性组高[(32.3±9.7)% vs. (7.7±3.1)%, P=0.055, χ2=3.661],第二程化疗后MRD阳性组较MRD阴性组复发率高[(57.1±3.6)% vs. (12.5±2.7)%, P = 0.003, χ2=8.759],移植前MRD阳性组较MRD阴性组复发率高[(50.0±9.7)% vs. (23.0±3.2)%, P = 0.019, χ2=5.547]。在单倍体移植患者,上述时间点MRD阳性组和阴性组预后无显著差异。然而,无论是接受同胞全合还是单倍体移植的患者,移植前MRD持续阳性组均较移植前MRD非持续阳性组复发率高。移植前持续MRD阳性且接受同胞全合移植组复发率最高,其次为移植前MRD持续阳性接受单倍体移植组、移植前MRD非持续阳性接受单倍体移植组,和移植前MRD非持续阳性接受同胞全合移植组[(66.7±9.2) % vs. (38.5±6.0) % vs. (18.8±8.7)% vs. (12.0±1.0)%, p < 0.001, χ2 = 20.763]。多因素分析显示,移植前MRD持续阳性预示复发风险增高(HR=1.69, 95%CI 1.200–2.382, P=0.003),无病生存率(HR=1.812, 95%CI 1.168–2.812,P=0.008)和总体生存率降低(HR=2.354, 95%CI 1.528–3.627, P<0.001)。
结论:
无论接受同胞全合还是单倍体移植,移植前持续MRD阳性,而非单一时间点MRD阳性能够预示AML患者预后较差。
INTRODUCTION
Minimal residual disease (MRD) determined by multicolor flow cytometry (MFC) has been successfully used to predict outcomes in acute myeloid leukemia (AML) patients treated with chemotherapy or received allogeneic stem cell transplantation (allo-SCT).[1,2,3,4,5,6,7,8] Several researchers have demonstrated the association of MRD after induction therapy and consolidation therapy with poor outcomes in AML patients.[4,9,10,11] However, the therapies of cases enrolled in these studies are heterogeneous, including chemotherapy alone or chemotherapy plus allo-SCT.[4,9,10,11,12,13] Furthermore, the negative effects of MRD pre- and/or post-transplantation on outcomes were also demonstrated in a number of studies.[8,14,15,16,17] In a cohort of 279 patients with AML, Zhou et al.[14] found that all patients with increased MRD levels over the peri-SCT period (n = 7) died of relapse with a median time of 125 (range: 43–836) days following transplantation. However, the effects of induction therapy or consolidation therapy on transplant outcomes were not demonstrated in these studies. Another limitation of above-mentioned studies is that they all focus on human leukocyte antigen (HLA)-matched sibling donor transplantation (MSDT) and unrelated donor transplantation.
More recently, the association of pre- and post-transplantation MRD with outcomes in haploidentical SCT (haplo-SCT) was confirmed by our groups.[8,18] We found that pretransplantation MRD was associated with cumulative incidence of relapse (CIR) in patients who underwent MSDT but not those receiving haploidentical allografts.[8,18] Unfortunately, the effects of MRD after the first and second courses of chemotherapy on haplo-SCT outcomes remain unclear.[19] Therefore, in this retrospective study including 145 cases in complete remission (CR) pretransplantation who treated with either haplo-SCT or MSDT, we investigated the dynamics of MRD detected by MFC from the first course of chemotherapy to pretransplantation to evaluate the correlation of MRD with clinical outcomes. Subgroup analysis was also performed to explore whether there were any differences in the association of MRD with outcomes of patients receiving either haplo-SCT or MSDT at different timepoints before transplantation.
METHODS
Ethical approval
The Institutional Review Board (IRB) of Peking University People's Hospital approved the protocol, and all patients or their guardians signed consent forms approved by the IRB.
Patients enrollment
Between June 2013 and June 2016, 145 consecutive patients diagnosed with AML undergoing allogeneic hematopoietic SCT (allo-HSCT) at the Peking University People's Hospital, Institute of Hematology, were enrolled. All patients were at CR status pre-HSCT. The patients' data were updated until January 31, 2018.
Transplant protocols
Transplant were performed following previously reported protocols.[20] For donor selection, a matched sibling donor was the first option, a suitable, closely HLA-matched unrelated donor, specifically with more than 8 of 10 matching HLA-A, HLA-B, HLA-C, HLA-DR, HLA-DQ loci and 5 of 6 or 6 of 6 matching HLA-A, HLA-B, HLA-DR loci, was the second choice. Haplo-SCT was performed if a matched sibling donor or a suitable HLA-matched unrelated donor was unavailable or if there was insufficient time for an unrelated donor search due to disease status.[20,21] All patients in this study received myeloablative conditioning regimens. The conditioning therapy for patients undergoing haploidentical or unrelated HSCT was as previously reported.[20,22,23]
Definitions and assessments
Engraftment was defined as the absolute neutrophil count (ANC) exceeded 0.5 × 109/L on three consecutive posttransplantation days, or the absolute platelet count exceeded 20,000/ml on 7 consecutive posttransplantation days without platelet transfusion. The criteria for grading acute graft-versus-host disease (GVHD) and chronic GVHD (cGVHD) were as previously published.[24,25] CR was defined as hematological CR, that is, <5% bone marrow blasts, the absence of blasts in peripheral blood, the absence of extramedullary disease, an ANC >1.0 × 109/L, and a platelet count >100 × 109/L with no red cell transfusions. Relapse was defined by morphologic evidence of disease in the peripheral blood, marrow, or extramedullary sites. Chimerism was evaluated in recipient peripheral blood cells by fluorescence in situ hybridization. When the patient and donor were of the same sex, chimerism was assessed using polymerase chain reaction-based analyses of polymorphic minisatellite or microsatellite regions (short tandem repeats).
Sample preparation
Bone marrow (BM) samples from patients were obtained at diagnosis, after the first course of chemotherapy, after the second course of chemotherapy, and before allo-HSCT. Follow-up samples were obtained at 1, 2, 3, 4.5, 6, 9, 12, 18, and 24 months post-allo-HSCT and once a year thereafter. All samples were analyzed for MFC within 4 h of sampling in the MFC laboratory of Peking University People's Hospital, Institute of Hematology.
Multicolor flow cytometry detection of minimal residual disease
Eight-color MFC was performed in all patients as a routine clinical test on BM samples that were obtained as part of baseline assessment at the time of diagnosis, the end of the first and second courses of chemotherapy, as well as pre-HSCT.[8,18] A panel of eight antibody combinations that recognize CD7, CD11b, CD13, CD14, CD16, CD19, CD33, CD34, CD38, CD41, CD45, CD56, CD61, CD64, CD71, CD117, CD123, and HLA-DR was used for MRD detection, and 0.2–1 million events per tube were acquired on a Fluorescence activated cell sorter (FACS Canto II) (BD Co., USA). The isotype control monoclonal antibodies were used. Positive MRD was considered when a cluster of more than 25 cells with leukemia-associated immunophenotype (LAIP) and side scatter (SSC) characteristics identified in all plots of interest and carrying at least two LAIP markers identified at diagnosis was observed. For those without LAIP markers at diagnosis, MRD was identified as a cell population showing deviation from the normal patterns of antigen expression seen on specific cell lineages at specific stages of maturation compared with either normal or regenerating marrow. A lower limit of detection (LOD) of 0.01% was targeted. Any measurable level of MRD was considered positive. The standardized assays and quality controls were performed according to previous reports. The significant level of MRD was set up by choosing a logarithmic scale that correlates with survival estimates and CIR as described previously[8,18] as a percentage of the total CD45+ white cell events.
Donor lymphocyte infusion
The modified donor lymphocyte infusion (mDLI) regimen consisted of granulocyte colony-stimulating factor-primed peripheral blood stem cells, instead of harvested nonprimed donor lymphocytes, and short-term immunosuppressive agents.[26] mDLI was administered to 31 patients post-allo-HSCT. Prophylactic DLI was administered to 2 patients between day 28 and day 60 posttransplant.
Statistical analysis
The primary endpoint studied was relapse rate, and the secondary endpoint was survival. The event for overall survival (OS) was death (regardless of the cause). The events for leukemia-free survival (LFS) included death in CR or relapse.
Differences in categorical variables between two groups were evaluated by the Chi-square test or Fisher's exact test. Continuous variables were compared using a nonparametric test. The associations between MRD status and clinical outcomes were analyzed by the Kaplan–Meier method or calculated using cumulative incidence curves to accommodate competing risks. Differences in relapse, LFS, and OS between groups were calculated using the log-rank test. A two-sided P ≤ 0.05 was regarded as significant. Multivariate Cox regression analysis was applied to test the independence of relapse-predicting factors. Statistical analyses were performed using IBM SPSS 22.0 statistical software (IBM SPSS Statistics, IBM Co., USA). R software (http://www.R-project.org) was used to calculate the cumulative incidence considering the presence of competing risk.
RESULTS
Patient characteristics and transplant outcomes
One hundred and forty-five patients were included in this study. All patients had <5% bone marrow blasts and met the morphological criteria for a leukemia-free state and CR before transplantation. Table 1 summarizes the characteristics of these patients. There were 17 pediatric patients (age ≤16 years) and 128 adults (age ≥17 years). A total of 31 patients received DLI, which was given for relapse prophylaxis (n = 2), intervention (n = 15), or treatment (n = 14). The median dose of infused mononuclear cells was 7.65 (2.7.00–10.46) ×108/kg. All patients achieved sustained, full-donor chimerism posttransplantation. The cumulative, 100-day incidence of acute GVHD Grades II–IV was 34.3 ± 4.2%. The cumulative incidence of acute GVHD Grades III–IV was 10.9 ± 3.5%. The cumulative incidence of severe cGVHD was 12.5 ± 3.4%. After a median follow-up of 907 days (range, 257–1442 days) for live cases, the 4-year cumulative incidences of nonrelapse mortality (NRM) and relapse were 8.2 ± 2.8% and 21.3 ± 5.6%, respectively. The 4-year probabilities of LFS and OS were 70.5 ± 8.2% and 78.6 ± 9.0%, respectively [Table 2].
Table 1.
Characteristics of consecutive AML patients undergoing allogeneic hematopoietic SCT
| Characteristics | Results |
|---|---|
| n | 145 |
| Age, median years (range) | 32 (5–61) |
| Gender, n (%) | |
| Male | 68 (46.9) |
| Female | 77 (53.1) |
| Diagnosis, n (%) | |
| De novo AML | 139 (95.9) |
| Secondary AML | 6 (4.1) |
| Pretransplantation disease status | |
| CR1 | 141 (97.2) |
| CR2 | 4 (2.8) |
| Donor type, n (%) | |
| MSD | 31 (21.4) |
| Haploid | 114 (78.6) |
| Cytogenetics, n (%) | |
| Favorable | 18 (12.4) |
| Intermediate | 80 (55.2) |
| Adverse | 47 (32.4) |
| Conditioning regimen, n (%) | |
| Myeloablative | 145 (100.0) |
| HLA-A-, HLA-B-, HLA-DR-mismatched grafts, n (%) | |
| 0 | 31 (21.4) |
| 1 | 2 (1.4) |
| 2 | 19 (13.1) |
| 3 | 93 (64.1) |
| ABO-matched grafts, n (%) | |
| Matched | 71 (49) |
| Major mismatch | 30 (20.7) |
| Minor mismatch | 32 (22.1) |
| Bidirectional mismatch | 11 (7.6) |
| Donor-recipient sex-matched grafts, n (%) | |
| Male-male | 43 (29.7) |
| Male-female | 54 (37.2) |
| Female-female | 23 (15.9) |
| Female-male | 25 (17.2) |
| Infused nuclear cells, median (range) (×108/kg) | 7.65 (2.70–10.46) |
| Infused CD34 + cells, median (range) (×106/kg) | 2.43 (1.20–5.73) |
| DLI for transplant, n (%) | 31 (20.7) |
| For relapse prophylaxis or intervention | 17 (54.8) |
| For relapse treatment | 14 (45.2) |
| Follow-up time of survivors, median (range) (days) | 907 (257–1442) |
AML: Acute myeloid leukemia; CR1: First complete remission; CR2: Second complete remission; SCT: Stem cell transplant; HLA: Human leukocyte antigen; DLI: Donor lymphocyte infusion.
Table 2.
Transplant outcomes of all patients (n = 145)
| Outcome | Results |
|---|---|
| Neutrophil engraftment time (days), median (range) | 13 (10–21) |
| Platelet engraftment time (days), median (range) | 17 (9–108) |
| Cumulative incidence of acute GVHD (%), mean ± SD | |
| None | 41.1 ± 7.2 |
| Grade I | 24.6 ± 4.9 |
| Grade II | 23.4 ± 4.5 |
| Grade III | 6.8 ± 3.1 |
| Grade IV | 4.1 ± 1.0 |
| Cumulative incidence of chronic GVHD (%), mean ± SD | |
| None | 53.0 ± 4.6 |
| Mild | 24.5 ± 2.8 |
| Moderate | 10.0 ± 3.7 |
| Severe | 12.5 ± 3.4 |
| Cumulative incidence of TRM (%), mean ± SD | 8.2 ± 2.8 |
| 4-year CIR (%), mean ± SD | 21.3 ± 5.6 |
| 4-year LFS (%), mean ± SD | 70.5 ± 8.2 |
| 4-year OS (%), mean ± SD | 78.6 ± 9.0 |
GVHD: Graft-versus-host disease; TRM: Transplant-related mortality; CIR: Cumulative incidence of relapse; LFS: Leukemia-free survival; OS: Overall survival; SD: Standard deviation.
Impact of minimal residual disease status after the first course of chemotherapy on outcomes in patients receiving hematopoietic stem cell transplantation versus matched sibling donor transplantation
As described in the MFC detection of MRD part, a lower LOD of 0.01% was targeted, and any measurable level of MRD was considered positive. Patients were classified into four groups according to their MRD status after the first course of chemotherapy and donor type: (1) patients undergoing MSDT with positive MRD after the first course of chemotherapy (n = 14), (2) patients undergoing MSDT without positive MRD after the first course of chemotherapy (n = 17), (3) patients undergoing haplo-SCT with positive MRD after the first course of chemotherapy (n = 48), and (4) patients undergoing haplo-SCT without positive MRD after the first course of chemotherapy (n = 66). The impact of MRD status on clinical outcomes was evaluated among the four groups.
The NRM, CIR, LFS, and OS of the four groups had no significant differences. However, analysis in the subgroups according to HLA compatibility showed contrasting results.
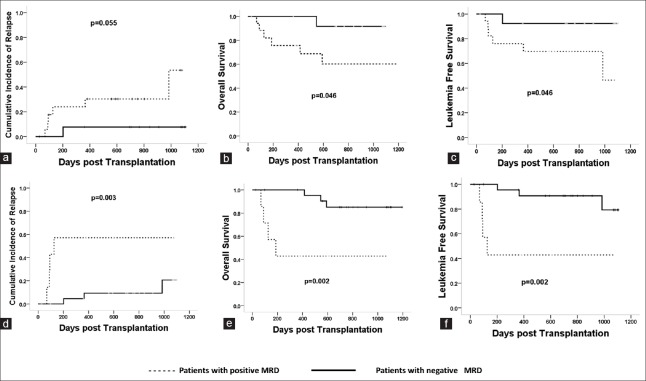
In MSDT settings, patients with positive MRD after the first course of chemotherapy had higher incidences of relapse (32.3 ± 9.7% vs. 7.7 ± 3.1%, P = 0.055, χ2 = 3.661) and lower probabilities of survival (LFS: 67.7 ± 6.1% vs. 92.3 ± 9.8%, P = 0.046, χ2 = 3.680; OS: 67.7 ± 6.1% vs. 92.3 ± 9.8%, P = 0.046, χ2 = 3.680) than those without MRD [Figure 1a–1c].
Figure 1.
The impact of MRD postchemotherapy on clinical outcomes in MSDT. Patients with positive MRD after the first chemotherapy had higher CIR (32.3 ± 9.7% vs. 7.7 ± 3.1%, P = 0.055, χ2 = 3.661) (a), lower LFS (67.7 ± 6.1% vs. 92.3 ± 9.8%, P = 0.046, χ2 = 3.680) (b), and OS (67.7 ± 6.1% vs. 92.3 ± 9.8%, P = 0.046, χ2 = 3.680) (c) than those without MRD. Patients with positive MRD after the second chemotherapy had higher CIR (57.1 ± 3.6% vs. 12.5 ± 2.7%, P = 0.003, χ2 = 8.759) (d), lower LFS (42.9 ± 6.3% vs. 87.5 ± 8.7%, P = 0.002, χ2 = 9.803) (e), and OS (42.9 ± 6.3% vs. 87.5 ± 8.7%, P = 0.002, χ2 = 9.803) (f) than those without MRD. MRD: Minimal residual disease; MSDT: Matched sibling donor transplantation; CIR: Cumulative incidence of relapse; LFS: Leukemia-free survival; OS: Overall survival.
This MRD classification showed no prognostic significance in patients undergoing haplo-SCT. Patients with or without positive MRD after the first course of chemotherapy had comparable incidence of relapse (10.8 ± 5.7% vs. 11.5 ± 6.4%, P = 0.947, χ2 = 0.001), NRM (16.3 ± 10.3% vs. 12.7 ± 9.7%, P = 0.948, χ2 = 0.003), and similar probabilities of survival (LFS: 72.9 ± 6.6% vs. 75.8 ± 7.1%, P = 0.761, χ2 = 0.092; OS: 81.3 ± 5.4% vs. 77.3 ± 5.5%, P = 0.641, χ2 = 0.217) after receiving haplo-SCT.
Impact of minimal residual disease status after the second course of chemotherapy on outcomes in patients receiving haploidentical stem cell transplantation versus matched sibling donor transplantation
The impact of MRD status at the end of the second chemotherapy on outcomes was evaluated in all patients. Patients were classified into four groups according to their MRD status after the second course of chemotherapy and donor type: (1) patients undergoing MSDT with positive MRD after the second course of chemotherapy (n = 7), (2) patients undergoing MSDT without positive MRD after the second course of chemotherapy (n = 24), (3) patients undergoing haplo-SCT with positive MRD after the second course of chemotherapy (n = 34), and (4) patients undergoing haplo-SCT without positive MRD after the second course of chemotherapy (n = 80).
In MSDT settings, patients with positive MRD after the second course of chemotherapy had higher incidences of relapse (57.1 ± 3.6% vs. 12.5 ± 2.7%, P = 0.003, χ2 = 8.759) and lower probabilities of LFS (42.9 ± 6.3% vs. 87.5 ± 8.7%, P = 0.002, χ2 = 9.803) and OS (42.9 ± 6.3% vs. 87.5 ± 8.7%, P = 0.002, χ2 = 9.803) than those without MRD [Figure 1d–1f].
However, in patients undergoing haplo-SCT, patients with or without positive MRD after the second course of chemotherapy had comparable incidences of relapse (29.4 ± 4.4% vs. 18.7 ± 4.6%, P = 0.241, χ2 = 1.486) and NRM (11.8 ± 5.5% vs. 10.0 ± 3.7%, P = 0.801) and similar probabilities of LFS (58.8 ± 4.9% vs. 71.3 ± 7.8%, P = 0.611, χ2 = 0.259) and OS (70.6 ± 4.5% vs. 82.5 ± 8.4%, P = 0.215, χ2 = 1.541).
Impact of pretransplant minimal residual disease on outcomes in patients receiving haploidentical stem cell transplantation versus matched sibling donor transplantation
To explore the impact of pretransplant MRD on outcomes in patients receiving haplo-SCT and MSDT, patients were classified into four groups according to their MRD status pretransplantation and donor type: (1) patients undergoing MSDT with positive MRD pretransplantation (n = 8), (2) patients undergoing MSDT without positive MRD pretransplantation (n = 23), (3) patients undergoing haplo-SCT with positive MRD pretransplantation (n = 33), and (4) patients undergoing haplo-SCT without positive MRD pretransplantation (n = 81).
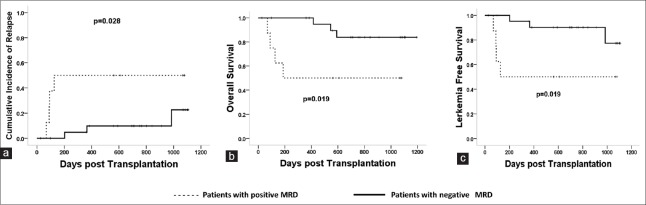
Data showed that in MSDT settings, patients with positive MRD pretransplant had higher CIR (50.0 ± 9.7% vs. 23.0 ± 3.2%, P = 0.028, χ2 = 4.809), lower probabilities of LFS (50.0 ± 9.7 vs. 77.0 ± 4.7%, P = 0.019, χ2 = 5.547), and OS (50.0 ± 9.7% vs. 77.0 ± 8.5%, P = 0.019, χ2 = 5.805) than those without positive MRD pretransplantation [Figure 2].
Figure 2.
The impact of MRD pretransplant on clinical outcomes in MSDT settings. Patients with positive MRD pretransplant had higher CIR (50.0 ± 9.7% vs. 23.0 ± 3.2%, P = 0.028, χ2 = 4.809) (a), lower LFS (50.0 ± 9.7% vs. 77.0 ± 4.7%, P = 0.019, χ2 = 5.547) (b), and OS (50.0 ± 9.7% vs. 77.0 ± 8.5%, P = 0.019, χ2 = 5.805) (c) than those without MRD. MRD: Minimal residual disease; MSDT: Matched sibling donor transplantation; CIR: Cumulative incidence of relapse; LFS: Leukemia-free survival; OS: Overall survival.
However, in patients undergoing haplo-HSCT, patients with and without positive MRD pretransplant had comparable incidence of relapse (27.9 ± 8.3% vs. 22.4 ± 4.9%, P = 0.362, χ2 = 1.016), LFS (60.0 ± 5.7% vs. 67.1 ± 8.2%, P = 0.512, χ2 = 0.429), OS (69.7 ± 8.9% vs. 81.7 ± 4.5%, P = 0.168, χ2 = 1.903), and NRM (12.1 ± 5.7% vs. 10.5 ± 3.6%, P = 0.761, χ2 = 0.104).
Impact of postinduction and pretransplant minimal residual disease dynamics on outcomes in patients receiving haploidentical stem cell transplantation versus matched sibling donor transplantation
Nineteen patients had persistent positive MRD from postinduction to pretransplant, while the remaining 126 patients had negative MRD after the first or second course of chemotherapy or pretransplantation.
Patients were classified into four groups: patients with (Group I, n = 6) or without (Group II, n = 25) persistent positive MRD from postinduction to pretransplant in MSDT settings, patients with (Group III, n = 13) or without (Group IV, n = 101) persistent positive MRD from postinduction to pretransplant in haplo-SCT settings.
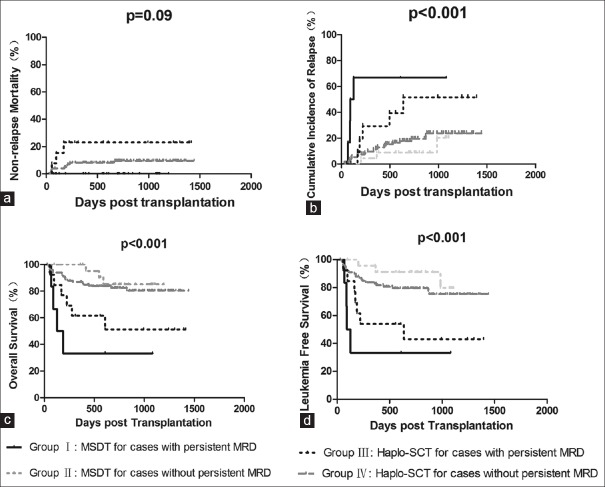
Patients with persistent positive MRD undergoing MSDT (Group I, n = 6) had the highest incidence of relapse, followed by patients with persistent positive MRD undergoing haplo-SCT (Group III, n = 13), patients without persistent positive MRD undergoing haplo-SCT (Group IV, n = 101), and patients without persistent MRD undergoing MSDT (Group II, n = 25) (66.7 ± 9.2% vs. 38.5 ± 6.0% vs. 18.8 ± 8.7% vs. 12.0 ± 1.0%, P < 0.001, χ2 = 20.763). Group I had the lowest probabilities of LFS and OS, followed by Groups III, IV, and II (LFS: 33.3 ± 9.2% vs. 46.2 ± 9.7% vs. 78.2 ± 6.0% vs. 88.0 ± 4.1%, P < 0.001, χ2 = 19.380; OS: 33.3 ± 9.2% vs. 82.2 ± 7.9% vs. 53.8 ± 10.6% vs. 88.0 ± 4.3%, P < 0.001, χ2 = 20.111). The NRM of four groups was comparable (0 vs. 8.9 ± 3.1% vs. 0 vs. 23.1 ± 10.0%, P = 0.09, χ2 = 6.513) [Figure 3].
Figure 3.
The impact of MRD dynamics before transplant on clinical outcomes. Patients were classified into four groups: patients with (Group I, n = 6) or without (Group II, n = 25) persistent positive MRD in MSDT settings and patients with (Group III, n = 13) or without (Group IV, n = 101) persistent positive MRD in haplo-SCT settings. The NRM was comparable (a). Group I had the highest CIR (66.7 ± 9.2% vs. 38.5 ± 6.0% vs. 18.8 ± 8.7% vs. 12.0 ± 1.0%, P < 0.001, χ2 = 20.763) (b), lowest LFS (33.3 ± 9.2% vs. 46.2 ± 9.7% vs. 78.2 ± 6.0% vs. 88.0 ± 4.1%, P < 0.001, χ2 = 19.380) (c), and OS (33.3 ± 9.2% vs. 82.2 ± 7.9% vs. 53.8 ± 10.6% vs. 88.0 ± 4.3%, P < 0.001, χ2 = 20.111) (d), followed by Groups III, IV, and II. MRD: Minimal residual disease; MSDT: Matched sibling donor transplantation; CIR: Cumulative incidence of relapse; LFS: Leukemia-free survival; OS: Overall survival.
In conclusion, patients with persistent positive MRD postinduction and pretransplant had higher CIR, lower LFS, and OS than those without persistent positive MRD both in MSDT and haplo-HSCT settings.
Multivariate analysis of factors associated with outcomes of acute myeloid leukemia patients who underwent allogeneic hematopoietic stem cell transplantation
Factors that might affect the transplant outcome including patient age, sex, donor type, cytogenetic abnormalities at diagnosis (including FLT3-ITD mutation), infused nuclear cells and CD34+ cells, prophylactic or intervention DLI, MRD at the first and second chemotherapy, MRD pretransplant, and MRD dynamics and so on. Multivariate analysis showed that persistence of MRD pretransplantation was an independent risk factors of relapse (hazard ratio [HR] = 1.69, 95% confidence interval [CI]: 1.2–2.382, P = 0.003), LFS (HR = 1.812, 95% CI: 1.168–2.812, P = 0.008), and OS (HR = 2.354, 95% CI: 1.528–3.627, P < 0.001). Cytogenetics and cGVHD were also independent risk factors of relapse, LFS, and OS. Acute GVHD (Grade III–IV) was associated with NRM [Table 3].
Table 3.
Multivariate analysis of factor associated with outcomes of patients who underwent allo-SCT (n = 145)
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Relapse | ||||||
| Persistent positive MRD | 1.517 | 1.103–2.087 | 0.010 | 1.690 | 1.200–2.382 | 0.003 |
| Cytogenetics | 2.484 | 1.346–4.585 | 0.002 | 2.258 | 1.226–4.159 | 0.009 |
| Chronic GVHD | 0.419 | 0.192–0.914 | 0.071 | 0.322 | 0.118–0.877 | 0.006 |
| Nonrelapse mortality | ||||||
| Acute GVHD (Grade III–IV) | 6.528 | 2.062–20.699 | <0.001 | 6.528 | 2.062–20.699 | <0.001 |
| Leukemia-free survival | ||||||
| Persistent positive MRD | 1.386 | 1.130–1.865 | 0.031 | 1.812 | 1.168–2.812 | 0.008 |
| Cytogenetics | 1.854 | 1.024–19.378 | 0.050 | 1.217 | 1.096–14.862 | 0.025 |
| Chronic GVHD | 0.371 | 0.143–0.961 | 0.023 | 0.297 | 0.114–0.779 | 0.014 |
| Acute GVHD (Grade III–IV) | 3.844 | 1.848–7.997 | 0.001 | 2.875 | 1.258–6.572 | 0.012 |
| Overall survival | ||||||
| Persistent positive MRD | 1.965 | 1.298–2.976 | 0.001 | 2.354 | 1.528–3.627 | <0.001 |
| Cytogenetics | 2.081 | 1.147–3.775 | 0.016 | 1.920 | 1.057–3.487 | 0.032 |
| Chronic GVHD | 0.347 | 0.120–0.992 | 0.040 | 0.248 | 0.084–0.732 | 0.012 |
| Acute GVHD (Grade III–IV) | 3.448 | 1.539–7.728 | 0.007 | 2.647 | 1.107–6.930 | 0.025 |
MRD: Minimal residual disease; GVHD: Graft-versus-host disease; SCT: Stem cell transplantation; CI: Confidence interval; HR: Hazard ratio.
DISCUSSION
In this study, we showed the negative effects of MRD after the first and second courses of chemotherapy on transplant outcomes in patients who underwent MSDT but not those receiving haplo-SCT. In addition, we found that positive MRD pretransplant was associated with poor prognosis in patients receiving MSDT but not those who underwent haplo-SCT, which is in agreement with previous studies.[8,18] However, the association of persistent positive MRD pretransplantation with poor transplant outcomes was observed both in the MSDT and haplo-SCT subgroups in the present study. Our results add new information for the association of MRD determined by MFC with transplant outcomes,[5,15,27,28,29] suggesting (i) some differences exist in the effects of MRD at single timepoint before transplant on outcomes between haplo-SCT and MSDT and (ii) persistent MRD is better than positive MRD at single timepoint before transplant in predicting outcomes.
In contrast to the studies performed by other researchers,[9,12,13,28] there was no association of MRD after the first and second courses of chemotherapy with transplant outcomes in total patients. Further analysis showed negative effects of MRD after the first and second chemotherapy on transplant outcomes in the MSDT subgroup. The results in our homogeneous patient cohort further confirmed the negative effects of MRD after induction and/or consolidation therapy on clinical outcomes after MSDT, which have demonstrated by others in cases with heterogeneous therapy.[9,13] Together with the results that there were no association of MRD after the first and second courses of chemotherapy with transplant outcomes in patients who underwent haplo-SCT, our data suggest there might be a difference in the antileukemia activity between haplo-SCT and MSDT.
In this study, we also demonstrated a negative association of positive pre-MRD with poor prognosis in patients receiving MSDT but not those who underwent haplo-SCT. Either in total patients or in pediatrics,[8,18] our previous studies have showed the negative effects of positive pre-MRD with poor outcomes after MSDT but not following haploidentical allografts. Given the fact that MRD allows better estimates of the leukemia burden and associated with higher CIR after transplantation,[30,31] our results indicate that haploidentical allograft could overcome the negative effects of positive pre-MRD on outcomes and suggest a stronger antileukemia effect of haploidentical allografts compared with HLA-identical sibling donor allografts.
Impressively, we found that persistent MRD was associated with higher CIR and poor survival both in haploidentical transplant settings and in MSDT modalities. This is contrast to single timepoint MRD, which had negative effects on outcomes after MSDT but not following haplo-SCT. Several studies have demonstrated that acute leukemia patients with persistent MRD had a very poor prognosis due to resistance to chemotherapy, resulting in a high relapse rate and inferior survival.[32,33] The results observed in the present study indicate that in both transplant modalities, persistent MRD is better than single timepoint MRD in predicting transplant outcomes, suggesting that kinetics of MRD determined by MFC before transplantation is more reliable in prognosis prediction.
Most MRD studies in AML have focused on adults, and relatively little is known about the prognostic significance of MRD in childhood AML.[9,10] There were 17 pediatric patients in this cohort. All of them received haplo-SCT. Only one child had persistent MRD before HSCT and he relapsed at 219 days post-HSCT. Limited by the deficient number of cases, we cannot demonstrate if there was any difference between the pediatric and adult patients in this study. Future studies might focus on this issue. Our study had some limitations. First, this is a retrospective, single-center study. Second, there are also other haplo-SCT modalities, such as haplo-SCT with post-cyclophosphamide and haploidentical allografts with T-cell depletion, our results should be confirmed in these transplant settings.
In summary, we demonstrated that persistent MRD before transplantation is better than positive MRD at single timepoint in prognosis prediction either in MSDT modalities or haplo-SCT settings. Our results provide a considerable way in accurately predicting transplant outcomes by monitoring the kinetics of MRD pretransplantation, although a prospective, multicenter study is warranted to confirm our findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: Results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–52. doi: 10.1016/S1470-2045(10)70090-5. doi: 10.1016/s1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29:1190–7. doi: 10.1200/JCO.2010.31.8121. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122:1813–21. doi: 10.1182/blood-2013-06-506725. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Xie H, Wood BL, Walter RB, Pagel JM, Becker PS, et al. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol. 2015;33:1258–64. doi: 10.1200/JCO.2014.58.3518. doi: 10.1200/JCO.2014.58.3518. [DOI] [PubMed] [Google Scholar]
- 5.Walter RB, Gyurkocza B, Storer BE, Godwin CD, Pagel JM, Buckley SA, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29:137–44. doi: 10.1038/leu.2014.173. doi: 10.1038/leu.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: Time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol. 2016;34:329–36. doi: 10.1200/JCO.2015.63.3826. doi: 10.1200/JCO.2015.63.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, et al. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med. 2016;375:944–53. doi: 10.1056/NEJMoa1602074. doi: 10.1056/NEJMoa1602074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang YJ, Zhao XS, Wang Y, Liu YR, Xu LP, Zhang XH, et al. Effects of pre – And post-transplantation minimal residual disease on outcomes in pediatric patients with acute myeloid leukemia receiving human leukocyte antigen-matched or mismatched related donor allografts. Am J Hematol. 2017;92:E659–61. doi: 10.1002/ajh.24910. doi: 10.1002/ajh.24910. [DOI] [PubMed] [Google Scholar]
- 9.van der Velden VH, van der Sluijs-Geling A, Gibson BE, te Marvelde JG, Hoogeveen PG, Hop WC, et al. Clinical significance of flowcytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia. 2010;24:1599–606. doi: 10.1038/leu.2010.153. doi: 10.1038/leu.2010.153. [DOI] [PubMed] [Google Scholar]
- 10.Sievers EL, Lange BJ, Alonzo TA, Gerbing RB, Bernstein ID, Smith FO, et al. Immunophenotypic evidence of leukemia after induction therapy predicts relapse: Results from a prospective Children's Cancer Group study of 252 patients with acute myeloid leukemia. Blood. 2003;101:3398–406. doi: 10.1182/blood-2002-10-3064. doi: 10.1182/blood-2002-10-3064. [DOI] [PubMed] [Google Scholar]
- 11.Venditti A, Buccisano F, Del Poeta G, Maurillo L, Tamburini A, Cox C, et al. Level of minimal residual disease after consolidation therapy predicts outcome in acute myeloid leukemia. Blood. 2000;96:3948–52. [PubMed] [Google Scholar]
- 12.Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013;121:2213–23. doi: 10.1182/blood-2012-10-462879. doi: 10.1182/blood-2012-10-462879. [DOI] [PubMed] [Google Scholar]
- 13.Buldini B, Rizzati F, Masetti R, Fagioli F, Menna G, Micalizzi C, et al. Prognostic significance of flow-cytometry evaluation of minimal residual disease in children with acute myeloid leukaemia treated according to the AIEOP-AML 2002/01 study protocol. Br J Haematol. 2017;177:116–26. doi: 10.1111/bjh.14523. doi: 10.1111/bjh.14523. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB, et al. Pre- and post-transplant quantification of measurable ('minimal') residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia. 2016;30:1456–64. doi: 10.1038/leu.2016.46. doi: 10.1038/leu.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oran B, Jorgensen JL, Marin D, Wang S, Ahmed S, Alousi AM, et al. Pre-transplantation minimal residual disease with cytogenetic and molecular diagnostic features improves risk stratification in acute myeloid leukemia. Haematologica. 2017;102:110–7. doi: 10.3324/haematol.2016.144253. doi: 10.3324/haematol.2016.144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah MV, Jorgensen JL, Saliba RM, Wang SA, Alousi AM, Andersson BS, et al. Early post-transplant minimal residual disease assessment improves risk stratification in acute myeloid leukemia. Biol Blood Marrow Transplant. 2018;24:1514–20. doi: 10.1016/j.bbmt.2018.02.003. doi: 10.1016/j.bbmt.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, Wang Z, Ruan G, Liu Y, Wang Y, Zhang X, et al. Impact of pre-transplantation minimal residual disease determined by multiparameter flow cytometry on the outcome of AML patients with FLT3-ITD after allogeneic stem cell transplantation. Ann Hematol. 2018;97:967–75. doi: 10.1007/s00277-018-3265-1. doi: 10.1007/s00277-018-3265-1. [DOI] [PubMed] [Google Scholar]
- 18.Chang YJ, Wang Y, Liu YR, Xu LP, Zhang XH, Chen H, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: A retrospective and prospective analysis. J Hematol Oncol. 2017;10:134. doi: 10.1186/s13045-017-0502-3. doi: 10.1186/s13045-017-0502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13:10–24. doi: 10.1038/nrclinonc.2015.128. doi: 10.1038/nrclinonc.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in china-recommendations from the Chinese Society of Hematology. J Hematol Oncol. 2018;11:33. doi: 10.1186/s13045-018-0564-x. doi: 10.1186/s13045-018-0564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124:843–50. doi: 10.1182/blood-2014-03-563130. doi: 10.1182/blood-2014-03-563130. [DOI] [PubMed] [Google Scholar]
- 22.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15:257–65. doi: 10.1016/j.bbmt.2008.11.025. doi: 10.1016/j.bbmt.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Yu W, Qi-Fa L, Ya-Zhen Q, Dai-Hong L, Lan-Ping X, Bin J, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of patients with mixed-lineage-leukemia (MLL)-rearranged acute leukemia: Results from a prospective, multi-center study. Am J Hematol. 2014;89:130–6. doi: 10.1002/ajh.23595. doi: 10.1002/ajh.23595. [DOI] [PubMed] [Google Scholar]
- 24.Martin P, Nash R, Sanders J, Leisenring W, Anasetti C, Deeg HJ, et al. Reproducibility in retrospective grading of acute graft-versus-host disease after allogeneic marrow transplantation. Bone Marrow Transplant. 1998;21:273–9. doi: 10.1038/sj.bmt.1701083. doi: 10.1038/sj.bmt.1701083. [DOI] [PubMed] [Google Scholar]
- 25.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 seattle patients. Am J Med. 1980;69:204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 26.Huang XJ, Wang Y, Liu DH, Xu LP, Liu KY, Chen H, et al. Administration of short-term immunosuppressive agents after DLI reduces the incidence of DLI-associated acute GVHD without influencing the GVL effect. Bone Marrow Transplant. 2009;44:309–16. doi: 10.1038/bmt.2009.26. doi: 10.1038/bmt.2009.26. [DOI] [PubMed] [Google Scholar]
- 27.Buckley SA, Appelbaum FR, Walter RB. Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia. Bone Marrow Transplant. 2013;48:630–41. doi: 10.1038/bmt.2012.139. doi: 10.1038/bmt.2012.139. [DOI] [PubMed] [Google Scholar]
- 28.Campana D, Leung W. Clinical significance of minimal residual disease in patients with acute leukaemia undergoing haematopoietic stem cell transplantation. Br J Haematol. 2013;162:147. doi: 10.1111/bjh.12358. doi: 10.1111/bjh.12358. [DOI] [PubMed] [Google Scholar]
- 29.Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nat Rev Clin Oncol. 2013;10:460–71. doi: 10.1038/nrclinonc.2013.100. doi: 10.1038/nrclinonc.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlenk RF, Döhner K, Mack S, Stoppel M, Király F, Götze K, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-austrian trial AMLHD98A. J Clin Oncol. 2010;28:4642–8. doi: 10.1200/JCO.2010.28.6856. doi: 10.1200/JCO.2010.28.6856. [DOI] [PubMed] [Google Scholar]
- 31.Sierra J, Storer B, Hansen JA, Bjerke JW, Martin PJ, Petersdorf EW, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: The effect of leukemic burden, donor HLA-matching, and marrow cell dose. Blood. 1997;89:4226–35. [PubMed] [Google Scholar]
- 32.Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: Data from the german AML cooperative group (AMLCG) 1992 trial. Blood. 2003;101:64–70. doi: 10.1182/blood-2002-02-0532. doi: 10.1182/blood-2002-02-0532. [DOI] [PubMed] [Google Scholar]
- 33.Manara E, Basso G, Zampini M, Buldini B, Tregnago C, Rondelli R, et al. Characterization of children with FLT3-ITD acute myeloid leukemia: A report from the AIEOP AML-2002 study group. Leukemia. 2017;31:18–25. doi: 10.1038/leu.2016.177. doi: 10.1038/leu.2016.177. [DOI] [PubMed] [Google Scholar]