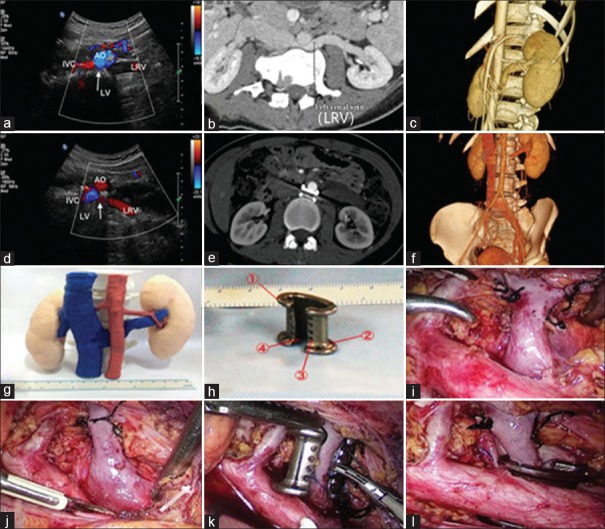
To the Editor: Posterior nutcracker syndrome (PNS) is a condition caused by compression of the left renal vein (LRV) between the vertebral column and the aorta (AO). Open surgery with LRV transposition has usually been recommended for patients with PNS.[1,2,3,4] However, existing surgical means of treatment are not sufficiently safe, effective, or minimally invasive. Here, we present one case of PNS treated with three-dimensional (3D) printed extravascular stent placement using laparoscopy.
A 29-year-old woman with a 9-month history of severe and persistent gross hematuria was admitted to the Department of Urology in Tangdu Hospital. On admission, urinalysis was positive for microscopic hematuria (+++) and proteinuria (+). Anemia was demonstrated by the reduced hemoglobin (89 g/L). A preoperative Doppler ultrasound (DUS) examination confirmed a retroaortic LRV with flow acceleration in the stenotic area (peak velocity 8 m/s measured in the hilum portion and 220 cm/s in the stenotic area; Figure 1a). The preoperative computed tomography (CT) and 3D imaging showed compression and stricture of the retroaortic LRV (2-mm diameter measured in the stenotic area and 9 mm in the hilum portion; Figure 1b and 1c). In addition, the 3D printed anatomical model gave us a perceptual intuition of the compressed retroaortic LRV [Figure 1g].
Figure 1.
Representative image of the patient. Preoperative DUS and CT examinations and 3D imaging showed compression and stricture of the retroaortic LRV with high blood flow acceleration in the stenotic area (a-c). Postoperative DUS and CT examinations and 3D imaging revealed patent blood flow of the LRV and confirmed the stability of the extravascular stent (d-f). PNS model was 3D printed (g). Patient-specific extravascular stent was designed properly (h): ① the raised edges, ② the small holes on both ends of the raised edge, ③ the porous design, and ④ the C-shaped design. The stent was placed and fixed step by step (i-l). DUS: Doppler ultrasound; CT: Computed tomography; PNS: Posterior nutcracker syndrome; AO: Aorta; LV: Lumbar vertebra; IVC: Inferior vena cava; LRV: Left renal vein; 3D: Three-dimensional.
We designed the C-shaped personalized stent with the 3D modeling software Auto Computer Aided Design (Autodesk, San Rafael, CA, US) and printed it using a 3D printer (SLM-250S; EOS, Krailling, Bayern, Germany). The extravascular stent weighed 3.1 g. It had a length of 17 mm and a vertical and a transverse diameter within the stent of 9 mm and 6 mm, respectively [Figure 1h]. The following designs were targeted to prevent stent migration: (1) the raised edges on two ends of the extravascular stent, (2) the small holes on both ends of the raised edge to fix the stent on the AO with silk thread, and (3) the porosity of the extravascular stent.
A standard, four-port, transperitoneal, laparoscopic approach was performed under general anesthesia with the patient placed in the lateral oblique position. The left adrenal central vein, ovarian vein, and lumbar vein were ligated and transected to release the LRV [Figure 1i]. The LRV and AO were successively exposed [Figure 1j]. The AO and the proximal end of the lumbar artery were gently pulled, while the C-shaped extravascular stent was placed around the compressed LRV through the gap [Figure 1k]. The stent was pushed into the space around the compressed LRV [Figure 1l]. Finally, through the small holes on the raised edge, the extravascular stent was fixed on the AO.
The duration of the operation was 140 min and the blood loss was 50 ml. The gross hematuria disappeared on the 23rd postoperative day. Repeat DUS and CT examinations were performed at 6, 12, and 24 months postoperatively, and the results revealed patent blood flow of the LRV (peak velocity 16 cm/s, 18 cm/s, and 18.5 cm/s, respectively, measured in the hilum portion, peak velocity within the stent was not measured as the effect of stent on ultrasound; Figure 1d) and stable position of the extravascular stent with no migration or erosion [Figure 1e and 1f]. The reduced hemoglobin rose to 127 g/L at 12 months and 127.3 g/L at 24 months postoperatively.
There have been reports of patients with PNS who underwent open surgery, including LRV transposition,[1,2] left renal autotransplantation,[2] left ovarian vein transposition,[3] and abdominal aortic transposition.[4] These vascular transposition methods are associated with technically difficult procedures and severe trauma. In addition, endovascular stents (EVS) have been proven to be an alternative treatment for PNS recently.[5] However, risks including incorrect stent placement, stent migration (e.g., to the right atrium), partial stent dislodgement to the inferior vena cava, and stent migration into the hilar region of the LRV cannot be avoided effectively.[6,7] Beyond this, patients treated with EVS have to take anticoagulants, which can increase the risk of postoperative complications.
The use of 3D printing technology and medicine in combination has solved a number of traditional medical problems. We built a vascular stent of appropriate size, designed with a series of anti-migration component design using 3D printing technology. Titanium alloy, the raw material used in the stent, was selected for its lightweight, pronounced compression strength, low cost, and biocompatibility. The extravascular stent was shown to be safe postoperatively without migration, collapse, or erosion. Since extravascular stent implantation was here relatively simple, the operation was easily completed using laparoscopic technology, making it a less invasive alternative to LRV transposition and open surgery.
In conclusion, a patient-specific 3D printed extravascular stent was made for the treatment of PNS, and the laparoscopic implantation of the stent was conducted successfully. Hence, 3D printed extravascular stents with titanium alloy might be a minimally invasive, safe, and effective technique for PNS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initial will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Marone EM, Psacharopulo D, Kahlberg A, Coppi G, Chiesa R. Surgical treatment of posterior nutcracker syndrome. J Vasc Surg. 2011;54:844–7. doi: 10.1016/j.jvs.2011.01.038. doi: 10.1016/j.jvs.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 2.Ali-El-Dein B, Osman Y, Shehab El-Din AB, El-Diasty T, Mansour O, Ghoneim MA, et al. Anterior and posterior nutcracker syndrome: A report on 11 cases. Transplant Proc. 2003;35:851–3. doi: 10.1016/s0041-1345(02)04026-5. doi: 10.1016/S1569-9056(03)80038-6. [DOI] [PubMed] [Google Scholar]
- 3.Hartung O, Barthelemy P, Berdah SV, Alimi YS. Laparoscopy-assisted left ovarian vein transposition to treat one case of posterior nutcracker syndrome. Ann Vasc Surg. 2009;23:413.e13–6. doi: 10.1016/j.avsg.2008.08.026. doi: 10.1016/j.avsg.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Ji C, Guo H. Abdominal aortic transposition as a treatment alternative for posterior nutcracker syndrome. Int J Urol. 2012;19:1043–4. doi: 10.1111/j.1442-2042.2012.03092.x. doi: 10.1111/j.1442-2042.2012.03092.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Zhang H, Shi H, Tian L, Jin W, Li M, et al. Endovascular stenting for treatment of nutcracker syndrome: Report of 61 cases with long-term followup. J Urol. 2011;186:570–5. doi: 10.1016/j.juro.2011.03.135. doi: 10.1016/j.juro.2011.03.135. [DOI] [PubMed] [Google Scholar]
- 6.Sebastian T, Erdoes G, Bratu VA, Baumgartner I, Kucher N. Endovascular extraction of a migrated large self-expanding laser-cut renal venous stent from the right ventricle. J Vasc Surg Cases Innov Tech. 2017;3:79–82. doi: 10.1016/j.jvscit.2017.03.001. doi: 10.1016/j.jvscit.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, Zheng X, He Y, Fang X, Li D, Tian L, et al. Stent migration after endovascular stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4:193–9. doi: 10.1016/j.jvsv.2015.10.005. doi: 10.1016/j.jvsv.2015.10.005. [DOI] [PubMed] [Google Scholar]