Abstract
Background:
There is growing evidence suggesting that both genetic and environmental factors modulate treatment outcome in, a highly heterogeneous, major depressive disorder (MDD). 5-HTTLPR variant of the serotonin transporter gene (SLC6A4) and MTHFR 677C>T polymorphisms have been linked to the pathogenesis of MDD, and antidepressant treatment response. The evidence is lacking on the clinical utility of yoga in patients with MDD who have 5-HTTLPR and MTHFR 677C>T polymorphisms and less likely to respond to medications (SSRIs).
Aims:
We aimed to examine the impact of YBLI in those who have susceptible 5-HTTLPR and MTHFR 677C>T polymorphisms and are less likely to drug therapy with SSRIs.
Settings and Design:
In a 12 week randomized active-controlled trial, MDD patients (n = 178) were randomized to receive YBLI or drug therapy.
Methods:
Genotyping was conducted using PCR-based methods. The clinical remission was defined as BDI-II score ≤ 9.
Statistical Analysis Used:
An intent-to-treat analysis was performed, and the association of genotype with treatment remission consisted of the logistic regression model. A P value of <0.05 was considered statistically significant.
Results:
Multivariate logistic regression models for remission including either 5-HTTLPR or MTHFR 677C>T genotypes showed statistically significant odds of remission in YOGA arm vs. DRUG arm. Neither 5-HTTLPR nor MTHFR 677C>T genotype showed any influence on remission to YBLI (P = 0.73 and P = 0.64, respectively). Further analysis showed childhood adversity interact with 5-HTTLPR and MTHFR 677C>T polymorphisms to decrease treatment response in DRUG treatment arm, but not in YOGA arm.
Conclusions:
YBLI provides MDD remission in those who have susceptible 5-HTTLPR and MTHFR 677C>T polymorphisms and are resistant to SSRIs treatment. YBLI may be therapeutic for MDD independent of heterogeneity in its etiopathogenesis.
Keywords: 5-HTTLPR, depression, gene-environment, meditation, MTHFR 677C>T, yoga
INTRODUCTION
Major depressive disorder (MDD), a chronic and devastating neuropsychiatric condition, is currently the leading cause of global burden of disease.[1] At any given time, over 4% of the global population suffers from MDD, with females being 1.7 times more likely than males to experience an MDD episode. The characteristic presentation of MDD is that of persistent sadness and loss of interest in almost all activities. MDD has ~37% heritability, and both hypothesis-driven and hypothesis-free molecular studies have suggested an association of several genes with MDD. It is complicated by accelerated biological aging[2] and increased mortality from chronic lifestyle diseases.[3,4] The cure for MDD is still elusive since the etiopathology of MDD is incompletely understood.[5] Current first-line treatment of MDD includes either, pharmacotherapy based on monoamine hypothesis, or psychotherapy. The vast spectrum of available treatments used alone or in combination, fail to provide recovery from MDD in ~50% of patients and are associated with suboptimal remissions, frequent relapses, and complications.[5,6]
Beyond monoamine hypothesis, neuroplasticity-based theories are increasingly being explored in emerging interventions for MDD.[7,8] In addition, accelerated biological aging is suggested to be the fundamental process in the pathobiology of MDD,[2] and a potential target for interventions in MDD. Accelerated aging is associated with dysregulated serotonin biology.[9,10,11] In this context, it is important to note that serotonin biology may have an important role in optimizing neuroplasticity,[12] and the latter is linked to optimized cellular health. Yoga is used for the management of MDD as shown by the limited but consistent studies.[13] The previous research has confirmed that yoga, an active mind-body intervention (MBI), improve neuroplasticity in both healthy and MDD[14,15,16,17] by correcting the accelerated biological aging in cells and organ systems throughout the body.[16,18,19,20] The effects of yoga are far beyond on anyone biological process.[21] Such modifications at the whole body level may ameliorate the fundamental pathological processes associated with MDD and provide an in-depth understanding of the pathobiology of this complex disorder. Previous studies have suggested that yoga may optimize serotonin biology[22,23,24] in novice practitioners. In this context, yoga-induced improvements in cellular health and neuroplasticity in MDD may be accompanied by optimization of serotonin biology.
Polymorphisms of the genes related to serotonin biology are the most widely studied biomarkers in MDD.[7] Increasing serotonin levels in the synapses by blocking SERT, encoded by the SLC6A4 gene, is the basis for the current first-line pharmacotherapy using selective serotonin reuptake inhibitors (SSRI's). Previous studies have highlighted, a priori, two candidate genetic markers: 5-HTTLPR in SLC6A4 and MTHFR 677C>T, which significantly impact serotonin biology and linked to MDD pathogenesis and treatment response in MDD.[25,26] It is interesting and clinically relevant to make further analysis and clarify if they have an impact on yoga-based interventions that are based on (neuroplasticity and cellular health based) theories beyond monoamine hypothesis.[7]
The 5-HTTLPR (serotonin transporter-linked polymorphic region) polymorphism primarily has either a long or short repeat length variations in the promoter region of the serotonin transporter gene (SLC6A4). Serotonin transporter protein recycles serotonin after its release, thereby determining the magnitude and duration of serotonergic responses.[27] This widely studied polymorphism is linked to both psychiatric and somatic disorders, including depression.[28,29,30,31] In particular, S allele is associated with increased risk[32] and LL genotype is associated with protection against MDD,[33] although some studies report contradictory findings.[34,35] Patients with S allele exhibit lower remission rates, increased side effects, and intolerance to drug therapy.[35,36,37,38] Serotonin can modify neuroplasticity both during development[39] and adult life, and alterations in serotonin homeostasis can modify the fine wiring of brain connections and cause behavior changes. 5-HTTLPR genotypes may affect neuroplasticity.[40] Epigenetic modifications including methylation of 5-HTTLPR mediate the differential effects of the genotypes like gray matter volumes in the brain of MDD patients.[41] Methylenetetrahydrofolate reductase (MTHFR), is an enzyme involved in catalyzing the reduction of 5,10-methylenetetrahydrofolate to 5-MTHF, primarily in the metabolism of folic acid. This reaction provides a methyl donor for methylation reactions. These pathways are critical to the synthesis of monoamines including serotonin, and methylation of genes including SLC6A4.[42] A study analyzing the impact of mother's depressed mood on infants provides further evidence that MTHFR 677C>T genotypes may affect SLC6A4 methylations.[43] Low-activity MTHFR 677C>T allele is shown to be associated with MDD,[44,45] but few studies refute this correlation.[46,47] MTHFR 677C>T may affect neuroplasticity and cellular health through epigenetic modifications of regulatory genes.
The evidence is lacking on the clinical utility of yoga in patients with MDD who have 5-HTTLPR and MTHFR 677C>T polymorphisms and less likely to respond to medications (SSRIs). Therefore, we aimed to analyze the impact of yoga-based lifestyle intervention (YBLI) in these MDD patients, and with a focus beyond monoamine theories, we hypothesized in this trial that YBLI, an active MBI, would be therapeutic in MDD in those who have 5-HTTLPR and MTHFR 677C>T polymorphisms and are poor responders to SSRIs.
METHODS
Design
This article reports the therapy genetic findings from a short-term randomized active-controlled trial. The 12-week trial was performed at a national apex health institute of India. MDD patients who attended outpatient clinics of the Psychiatry Department of institute hospital were referred to participate in the study. Participants were assigned to receive either YBLI (YOGA arm) or routine drug therapy with SSRIs (DRUG arm) for 12 weeks. The trial was prospectively registered on the Clinical Trials Registry of India (CTRI REF/2014/09/007532). It was approved by the Institutional Review Board, and all participants provided written informed consent before any study procedures.
Participants
Participants were adults aged 20–60 years with MDD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). The exclusion criteria included participants having (a) very severe depression (Beck Depression Inventory-II [BDI-II] scale score ≥50); (b) other comorbid neuropsychiatric conditions (except for anxious distress that is commonly present with MDD); and (c) unstable chronic medical conditions. For females, additional exclusion criterion was pregnancy or breastfeeding.
Sample size
For the outcome of MDD remission, assuming a 30% remission rate to drug treatment and a 30% therapeutic gain over drug treatment to be clinically significant, the number per group required was 64, to achieve approximately 80% power at a 2-sided α level of 0.05. We assumed a 25% dropout rate in each arm. The planned recruitment was for 80 per arm (160 total).
For a prior calculation of the study power to detect differences in remission between genotypes, we assumed 75 participants in each arm. Assuming common homozygous genotypes LL to be 35% and CC to be 45%, the required sample sizes were: 26 (LL) and 49 (LS/SS) for 5-HTT LPR, and 34 (CC) and 41 (CT/TT) for MTHFR 677C>T. Assuming a two-sided alpha level of 0.05, the power to detect difference of 60% versus 20% in remission for 5-HTTLPR (n = 26 vs. 49) was 83%, and the power to detect differences of 70% versus 25% in remission for MTHFR 677C>T (n = 34 vs. 41) was ~87%.
Randomization and blinding
To minimize referral bias, referring physicians were blind to both inclusion and randomization of the participants. Dynamic allocation randomization was used for randomization and blinding. The distribution across balancing factors for previous assignments was the basis for treatment assignment. A research assistant not otherwise involved in the study created the randomization allocation schedule. Smallest group with specific combinations of balancing factors received treatment assignment, with age, sex, body mass index, suicidal ideation, and childhood adversity as balancing factors. The concealed allocation was assured by use of a computer system. Participants were not blind to allocation. Single rater recorded the clinical parameters, who remained blind to the allocated group of each participant until after the data analysis was completed. Participants were instructed not to mention participation in YBLI.
Interventions
Eligible participants were allocated to one of the two groups: YOGA group, practicing YBLI 5 days a week, or DRUG group, receiving routine drug therapy with suitable SSRI.
Yoga-based lifestyle intervention
YBLI is an active MBI administered as per the pretested design.[16] YBLI is designed to be an integrative health strategy incorporating the classic components of Yoga including Āsana (physical postures), Prāṇāyāma (breathing exercises), and Dhyāna (Meditation) which are derived from a mix of Hatha Yoga and Raja Yoga. YBLI program included 2 h sessions 5 days per week for 12 weeks. For the first 2 weeks, the sessions were held at the Institute hospital, and taught by registered, specialized yoga instructors. For the first 2 weeks, YBLI included supervised group sessions (having 12–15 participants). Sessions in the first 2 weeks of YBLI included interactive lectures on yoga, lifestyle, lifestyle diseases including MDD, and the importance of their prevention and management. Remaining 10 weeks were home based and included one-one and unsupervised sessions with duration and frequency similar to the first 2 weeks. Monitoring of adherence to YBLI was through maintenance of a dairy and telephonic contact. Patients in the yoga group were asked to visit institute hospital every 2 weeks for follow-up and counseling. The details of the activities in a day during YBLI program are given in Table 1. The Hawthorne effect was insignificant because of YBLI's emphasis on environmental enrichment and self-awareness. Participants in the yoga group were asked to stop previous antidepressant medications if any, and they did not receive SSRIs or other antidepressant medications during trial. Any other medication for minor ailments was allowed after consultation with the investigators.
Table 1.
Details of activities in the yoga-and meditation-based lifestyle intervention program

Drug therapy
Drug therapy for the control group was provided at the psychiatry OPD of the institute. SSRIs were used as per the prescription of treating psychiatrists, who were not necessarily part of the research team. Escitalopram (5–20 mg/day), fluoxetine (20–40 mg/day), and paroxetine (20–40 mg/day) were used in 36, 24, and 29, patients, respectively. Adjunctive medications were prescribed for sleep (diphenhydramine, 25–50 mg; or zolpidem, 5–10 mg), and for anxiety (clonazepam, 0.25 mg twice daily; or lorazepam, 0.5 mg 3 times/day). Since the study did not include MDD patients with significant psychotic features, no antipsychotics were prescribed. For those subjects who were previously on antidepressant medications, they were asked to stop them. Only the drug group received SSRI antidepressant medications as per our study protocol. Antidepressant medications other than the SSRIs were not continued in the drug group. Patients in the drug group were also asked to visit institute hospital every 2 weeks for follow-up and counseling. The visits to hospital were of similar frequency (every two weeks) for both groups so that the influence of the confounding factor of number of visits by the patients is minimized.
Outcome measures
The a priori primary endpoint of the trial was MDD remission defined as BDI-II score ≤9 at 12 weeks.[48,49,50] The remission as defined by BDI-II scale is considered a clinically useful criterion and is widely accepted in MDD trials as the primary outcome.[48,49,50] BDI-II is chosen for assessment of depression since its stated purpose is not to diagnose major depressive episode, but as an appropriate instrument for detecting depressive symptoms and monitoring treatment efficacy.[51] BDI-II is specifically designed to address DSM-5 criteria for depression, and in both BDI-II and DSM-5, patients are asked to answer the questions based on happenings in the last 2 weeks. It has reliable comparability with observer-rated scales, such as the Hamilton Rating Scale for Depression (HAM-D) or the Montgomery-Åsberg Depression Rating Scale.[51] In the healthcare context, the perceived burden of scale completion by the clinician is the major obstacle to using standardized scales, such as the HAM-D, which is unlikely to meet with success. As a self-report questionnaire to measure depression, the BDI-II holds the advantages of releasing the overburdened clinician from the paperwork of scale administration and of improving the efficiency of the clinical encounter by providing mental status assessment that correlates well with clinician-rated tools.[51] In a recent study by Zhao et al.[52] that compared five depression measures in depressed adult patients using item response theory (IRT), BDI-II was found informative on a wider range of depression levels and had greater measurement precision than the other three measures, the Patient Health Questionnaire (PHQ-9), the depression subscale of the Depression, Anxiety and Stress Scale (DASS-Depression), and the depression subscale of the Hospital Anxiety and Depression Scale (HADS-Depression). The benefits that can be reaped from using the BDI-II come from its ease of administration and understandable questions that allow the user to maneuver through the 21 items of the questionnaire. It was evaluated at baseline and the end of 12-week therapy.
There is always a concern of the response bias among participants while rating with self-report measure BDI-II, especially in this study having a nonblinded design and a lifestyle-based treatment. Underreporting of symptoms by individuals may be common in epidemiological study settings, and one previous study has reported significantly more core depressive symptoms in the covert condition, suggesting that surveys of community samples may underestimate the prevalence of depression.[53] However, BDI-II rating by participants in this study is in the hospital settings in the psychiatry OPD, and response bias among participants may be less compared to epidemiological settings. Treating psychiatrists, who were not necessarily part of the research team, were involved in asking the patients to rate BDI-II. Persons involved in yoga training were not involved in asking the participants to rate the BDI-II to minimize response bias among participants.
This article reports the therapy genetic findings of the trial, and the secondary endpoint was to analyze 5-HTTLPR and MTHFR 677C>T polymorphisms in MDD treatment with either YBLI or routine drug therapy and analyze the clinical utility of yoga in those who have susceptible polymorphisms and are poor responders to medications (SSRIs).
Study procedure
The study participants were all asked to undergo clinical evaluation and provide a blood sample at baseline. DNA was extracted from blood samples, and genotyping was completed at baseline. During the 12-week intervention period, participants were asked to follow the procedures designed for their group. Evaluation of clinical parameters was repeated after completion of the intervention at 12 weeks.
Assessment of clinical parameters
Depressive symptoms
The BDI-II[54] is a 21-item, self-report inventory of the severity of current depressive symptoms. The BDI-II was used to assess the severity of depressive symptoms at baseline and at postintervention. Higher total scores reflect greater subjectively perceived depressive symptomatology. Suicidal ideation was assessed using BDI-II suicide item (item 9). A recent study by Green et al.[55] has recommended the use of the BDI-II suicide item as a brief, efficient screen for suicide risk in routine clinical care. It gives a score from 0 to 3, and scores 1–3 indicated the presence of suicidal ideation.
Comorbid psychiatric conditions
MDD participants were interviewed using the Mini Neuropsychiatric Interview, version 7.0 for DSM 5 (MINI 7.0) (Harm Research Institute, Tampa, Florida, USA)[56,57] to assess comorbid psychiatric conditions. MINI 7.0 is a short, structured diagnostic interview with an administration time of approximately 15 min, and it assesses the 17 most common disorders in mental health.
Childhood adversity
We defined childhood adversity as having experienced at least one traumatic event before age 18 including the following: Parental loss, sustained alcohol and/or drug abuse by caregiver, victim of a serious crime, victim of a serious accident, and victim of sexual and/or physical abuse.
Laboratory parameters: Genotyping
DNA was extracted from 5 ml of peripheral blood samples provided by all randomized patients in the trial (n = 178) and were diluted to a standard concentration of 50 ng/uL. There were a total of 178 DNA samples collected and received; 24 samples had an insufficient quantity and quality. The samples were processed, and the genotyping was conducted using polymerase chain reaction (PCR)-based genotyping. The genotyping personnel was blinded to treatment allocation and performed automated data entry. Lymphocyte DNA was extracted using a phenol-chloroform method. DNA was quantified using a spectrophotometer (SPECTRAmax PLUS 384, Nanodrop Technologies, USA). All samples were assessed in the same assays, and DNA specimens were randomly plated. Genotyping of the 5-HTTLPR and MTHFR 677C>T polymorphisms was performed using the S1000 Thermal Cycler (Bio-Rad).
5-HTTLPR
The primers for the 5-HTTLPR polymorphism were: the forward primer-5’-GTT TCT TGA GGG ACT GAG CTG GAC AAC CAC-3’, and the reverse primer-5’-GGC GTT GCC GCT CTG AAT GC-3’. PCR was performed in a 20 μL reaction containing 2 μL of the genomic DNA (100 ng), 2 μL dNTP (2.5 mM), 2 μL Taq buffer, 0.4 μL of the Taq polymerase, 2 μL of the primer mix, and 11.6 μL H2O. The PCR products were amplified for 10 min denaturation at 95°C, 45 cycles of 30 esc at 95°C, 45 s annealing at 65°C, and 1 min extension at 72°C, followed by a final extension of 5 min at 72°C. The PCR products were resolved on a 2% agarose gel prepared with ethidium bromide. Two allele variants of the gene polymorphism were identified based on the PCR fragment sizes: (short S; 486 bp and long L; 529 bp).
MTHFR 677C>T
The MTHFR 677C>T polymorphism is the critical variant that determines the enzyme activity level. For MTHFR 677C>T polymorphism,
The forward primer used was
5’-AGGCTGTGCTGTGCTGTTG-3’;
The reverse primer used was
5’-CGCTGTGCAAGTTCTGGAC-3’.
PCR substrates and conditions included 11.4 μl H2O, 1.6 μl MgCl2, 4 μl 5× buffer, 0.4 μl each primer (25 μmol l − 1), 0.2 μl Taq 90°C, 10 min; 40 cycles of: 95°C, 30 s; 63°C, 30 s; and 72°C, 30 s; followed by 72°C, 10 min.
MTHFR 677 C > T PCR amplified amplicon of 477 bp was subjected to Hinf1 digestion and resolved in 3.5% agarose gel. The C allele is cut by the enzyme and gives 425 bp and 52 bp products, whereas the T allele yields 260 bp, 165 bp, and 52 bp products.
Compliance
Study compliance including adherence to study treatments was ensured by monitoring completion of questionnaires, diaries, and pharmacy logs.
Statistical analysis
An intent-to-treat analysis included all randomized participants (89 drugs, 89 yoga). Association of genotype with treatment remission (BDI-II score ≤9 at the end of 12-week intervention) consisted of logistic regression adjusting for baseline characteristics. Assessment of genotype associated differential treatment effects was by including treatment by genotype interaction terms in the logistic regression model. Initial analyses of genotype and allele frequency were done in SHEsisPlus software. Remaining analyses were carried out using the SPSS Statistics v22.0 (Armonk, IBM Corp, NY, USA). The data points were identified as outliers if they do not fall within three standard deviation (SD) of the mean. If they have large effects on the estimates, they were dealt by transforming the observations. Outliers in the data were dealt by eliminating the observations if they are genuine outliers. P < 0.05 was considered statistically significant.
RESULTS
Participant characteristics
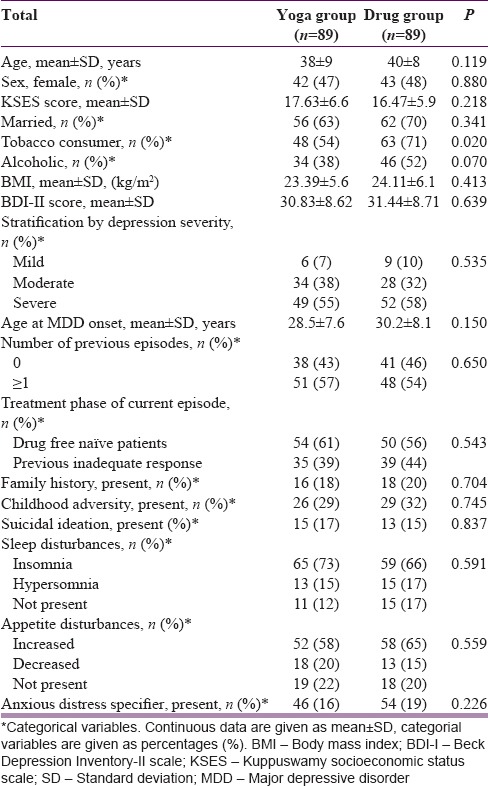
A total of 178 MDD patients (89 YOGA, and 89 DRUG) were randomized. Figure 1 shows the trial profile as per CONSORT guidelines. Table 2 shows the baseline characteristics of the participants in each group. The two groups showed no significant differences. Of the 178 participants, 144 (81%) participants completed the 12-week treatment trial. Among those completing treatment in the YOGA group, the median yoga sessions practiced was 54 (range 43–73) of 60 maximum suggested sessions, and median minutes per session was 110 min (range 93–168) of 120 maximum suggested minutes per session.
Figure 1.
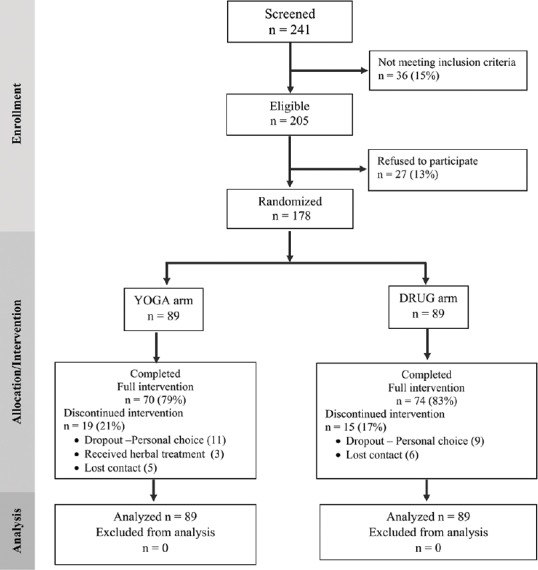
CONSORT flow diagram
Table 2.
Demographics and baseline characteristics
Among those completing treatment in the DRUG group, the number of pills taken was similar among subgroups receiving one of the three drugs in the study: Median (range) of 68 (40–84) escitalopram, 71 (42–84) fluoxetine, and 69 (38–84) paroxetine of 84 maximum.
Genotyping
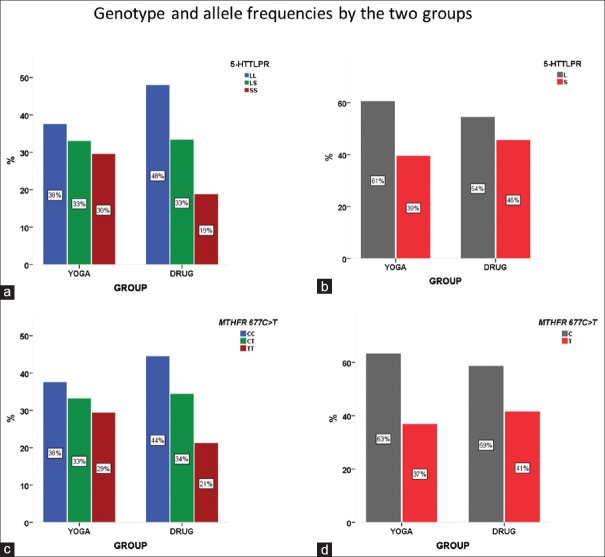
A total of 154 (87%) and 148 (83%) participants completed 5-HTTLPR and MTHFR 677C>T genotyping, respectively. The genotype distributions and allele frequencies for both polymorphisms are shown in Table 3. Both of these distributions were in Hardy–Weinberg equilibrium. The genotype and allele frequencies did not differ statistically between the two treatment arms for both 5-HTTLPR and MTHFR 677C>T [Figure 2].
Table 3.
Genotype and allele frequencies, n (%)
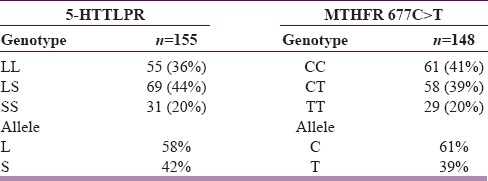
Figure 2.
5-HTTLPR and MTHFR 677C>T genotype and allele frequencies. (a) 5-HTTLPR genotype distributions by the two treatment arms. (b) 5-HTTLPR allele frequencies by the two treatment arms. (c) MTHFR 677C>T genotype distributions by the two treatment arms. (d) MTHFR 677C>T allele frequencies by the two treatment arms
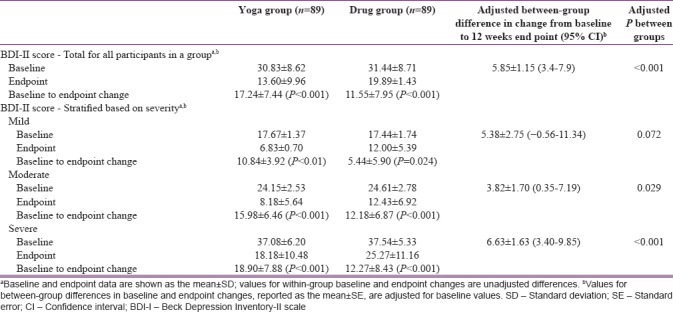
Treatment efficacy between groups
Mean change in BDI-II (depression severity) scores from baseline at the 12-week endpoint are shown in Table 4, along with the scores in MDD stratified by severity (mild, moderate, and severe). Within-group changes for all participants were significant for both the groups (both P < 0.001). Within-group changes analyzed for mild, moderate, and severe MDD were also significant for both the groups (all P < 0.05). In the between-group analysis including all participants, YOGA group showed significantly higher “post-intervention change in BDI-II score” than the DRUG group (P < 0.001). In the between-group analysis based on stratification by severity, while the “post-intervention change in BDI-II score” was significantly higher in YOGA group in comparison to DRUG group in both moderate (P = 0.029) and severe (P < 0.01) MDD, it was not significant for mild MDD (P < 0.072) [Table 4].
Table 4.
Changes in depression severity (Beck Depression Inventory-II scale) scores from baseline to 12 weeks in total score and scores in groups stratified into mild, moderate and severe major depressive disorder
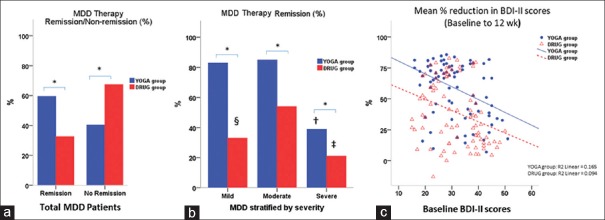
The rates for remission were 59% (n = 52) for YOGA group and 33% (n = 29) for DRUG group [Figure 3a], indicating a difference (P = 0.01) between the two treatment arms. Remission rates in MDD stratified into mild, moderate, and severe showed, significantly decreased remission in severe MDD compared to mild and moderate in YOGA group, and significantly higher remission in the moderate MDD compared to mild and severe in the DRUG group [Figure 3b] (all P < 0.001).
Figure 3.
Treatment efficacy by groups. (a) Rate of major depressive disorder therapy remissions and non-remissions by treatment arms (b) Remission rates in major depressive disorder stratified into mild, moderate, and severe in both YOGA and DRUG groups. (c) The “mean percent reduction in Beck Depression Inventory-II scores from baseline at the 12-week endpoint” in both YOGA and DRUG groups. *Between group difference, P < 0.001; †Severe major depressive disorder versus mild and moderate major depressive disorder in YOGA group, all P < 0.001; ‡Severe major depressive disorder versus moderate major depressive disorder in DRUG group, P < 0.001; §Mild major depressive disorder versus moderate major depressive disorder in DRUG group, P = 0.029
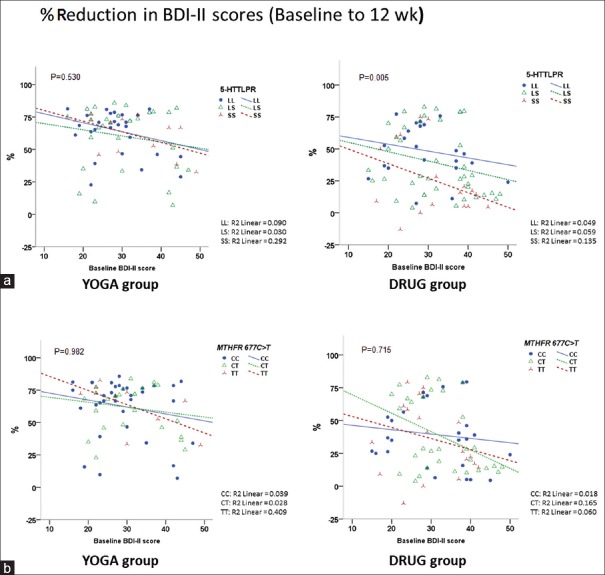
The “mean percent reduction in BDI-II scores from baseline at the 12-week endpoint” of both YOGA and DRUG groups were decreased with increasing baseline symptom severity, and YOGA group showed a significantly higher reduction (P < 0.001) [Figure 3c]. No outliers were found beyond the the limits of three standard deviations in both the groups. However; there was one outlier in DRUG group that worsened with treatment [Figures 3c, 4a, and b]. Analysis excluding the outlier did not have any significant influence on the findings.
Figure 4.
Reduction in Beck Depression Inventory-II scores from baseline to 12-week endpoint by genotypes. (a) Association of percent reduction in Beck Depression Inventory-II scores from baseline by 5-HTTLPR genotypes in both treatment arms. (b) Association of percent reduction in Beck Depression Inventory-II scores from baseline by MTHFR 677C>T genotypes in both treatment arms
The interaction between polymorphisms and treatment response
The “mean percent reduction in BDI-II scores from baseline at the 12-week endpoint” was significantly lower in SS genotype compared to LL genotype in DRUG group only (P = 0.025) [Figure 4a], and MTHFR 677C>T showed no significant differences in both groups [Figure 4b].
Analysis excluding one outlier in DRUG group that worsened with treatment [Figures 3c, 4a, and b] did not have any significant influence on the findings.
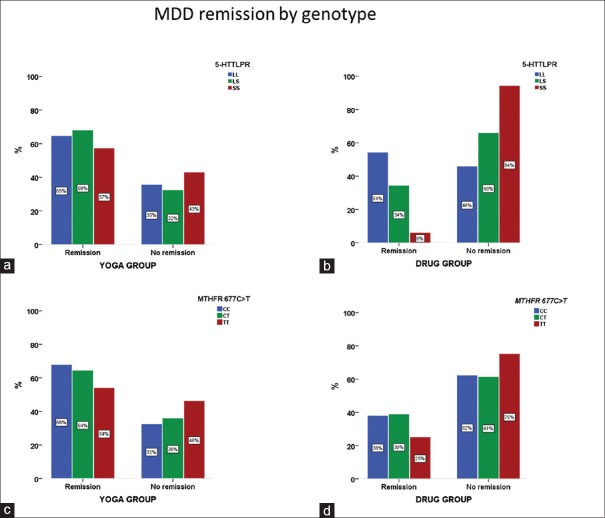
Analysis for the association of 5-HTTLPR and MTHFR 677C>T polymorphisms and treatments for MDD showed no significant differential treatment remission in YOGA group. Neither 5-HTTLPR (P = 0.73) nor MTHFR 677C>T (P = 0.64) genotypes predicted remission to YBLI [Figure 5a and b]. While, 5-HTTLPR genotype predicted remission to SSRI drug treatment (P = 0.02), MTHFR 677C>T genotype failed to predict remission in drug arm (P = 0.45) [Figure 5a and b]. Specifically, only the SS/LS genotype groups had a lower likelihood of remission to drug treatment (6% SS vs. 32% LS vs. 56% for LL). The difference in YBLI therapy remission in the LS and SS genotype arms in comparison to LL genotype was not significant (57% SS vs. 69% LS vs. 63% for LL). Similarly, the difference in YBLI therapy remission in the CT and TT genotype arms in comparison to CC genotype was not significant (54% TT vs. 64% CT vs. 68% for CC) [Figure 5c and d].
Figure 5.
Remission by genotype. (a and b) MDD remissions by 5-HTTLPR genotype and treatment arm. The treatment by genotype interaction P value is not significant in yoga arm compared to drug arm (yoga P = 0.73; drug P = 0.02 for LL vs. LS vs. SS by treatment). (c and d) MDD remissions by MTHFR 677C>T genotype and treatment arm. The treatment by genotype interaction P value is not significant in both yoga and drug arms (yoga P = 0.64; drug P = 0.45 for CC vs. CT versus TT by treatment). MDD – Major depressive disorder
Separate analysis for YOGA and DRUG groups were performed to explore specific genotype effects on postintervention depression severity. Reduction in BDI-II score was more significant for the LL versus SS genotype in DRUG group only. In YOGA group, the interaction of both 5-HTTLPR and MTHFR 677C>T polymorphisms with postintervention depression severity were not significant [Figure 4].
Findings of logistic regression models in our study (remission as the dependent variable) showed that 5-HTTLPR and MTHFR 677C>T polymorphisms were not associated with remission after adjusting for treatment, age, gender, basal BDI-II score, Kuppuswamy SES scale scores, age at MDD onset, anxious distress specifier, family history, and BMI [Figure 6a and b]. Interestingly, YOGA group versus DRUG group and childhood adversity present versus childhood adversity absent were significantly associated with remission.
Figure 6.
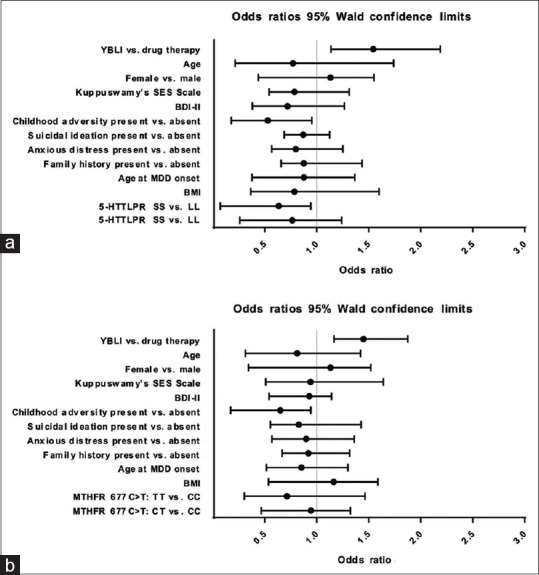
Results of multivariate logistic regression model for major depressive disorder remission. (a) Odds ratios and 95% confidence intervals for independent variables in the multivariate logistic regression model including 5-HTTLPR genotype. (b) Odds ratios and 95% confidence intervals for independent variables in the multivariate logistic regression model including MTHFR 677C>T genotype. BDI-II – Beck Depression Inventory-II scale
We, therefore, performed additional binary logistic regression analysis with the sample divided into those with or without childhood adversity and belonging to YOGA or DRUG group. There was a significant difference observed between the 5-HTTLPR and MTHFR 677C>T genotypes and remission only in childhood adversity present participants of drug arm (P = 0.02). Remission in childhood adversity present participants in the yoga arm was not significant (P = 0.78). Furthermore, we did not find any significant interaction between 5-HTT LPR and MTHFR 677C>T genotypes and suicidal ideation, SES, BMI, and other baseline variables in predicting remission.
Safety
No serious adverse events were reported in both treatment arms. Minor adverse events were reported by 14 individuals in the DRUG group. Dizziness, gastrointestinal disturbances, and insomnia were the most common side effects.
DISCUSSION
In this study, the widely studied 5-HTTLPR and MTHFR 677C>T polymorphisms showed no significant association on therapy response to 12-week YBLI in MDD. Patients who had susceptible polymorphisms and were poor responders to SSRI treatment showed significant improvement of depressive symptoms with yoga therapy. To the best of our knowledge, this is the first study aiming to investigate the influence of these polymorphisms in response to YBLI in MDD. Our study supports earlier studies for the association of 5-HTTLPR polymorphism with the DRUG group, where SS/LS genotypes, in comparison to LL genotype, showed significantly lower remission rates. Similar to YOGA group, DRUG group also showed no significant association on therapy response with MTHFR 677C>T polymorphism. In addition, a significant interaction of 5-HTTLPR and MTHFR 677C>T polymorphisms and childhood adversity for MDD remission was found in DRUG group only. No specific 5-HTTLPR effects could be observed in males or females.
Our findings show that MDD remission and response with YBLI is independent of 5-HTTLPR and MTHFR 677C>T genotypes and that higher response in YOGA group is due to MDD remission even in those who have susceptible gene polymorphisms and are resistant to SSRI treatment. In a recent systematic review, Cramer et al.[58] have investigated the previous RCTs for the efficacy and safety of yoga interventions in treating patients with MDD, where seven RCTs on yoga for participants with MDD were finally selected.[59,60,61,62,63,64,65,66] Another recent pilot RCT on yoga for participants with MDD not included in the review,[13] included only mild-to-moderate cases (scores of 14–28 on BDI-II scale). Similar to our study, yoga group in two of these previous studies[13,59] did not receive add-on antidepressant medications and used BDI-II scale to assess depression severity. In contrast to 12-week duration in our study, in both of these studies, yoga was practiced for 8 weeks. In the study by Prathikanti et al.,[13] yoga group practiced hatha yoga twice weekly, in contrast to five times per week in our study. Although in the study by Janakiramaiah et al.,[59] yoga was practiced six times per week, yoga sessions lasted 45 min and included Sudarshan Kriya Yog (SKY), which has emphasis mainly on Prāṇāyāma. Āsana limb was particularly missing in SKY. Some of the studies mentioned in the review by Cramer et al.[58] have employed physician administered instrument HAM-D[59,60,63,66] for assessing depression severity, and yoga group practiced was either SKY,[59,60,66] or hatha yoga as add-on treatment.[63] In some studies, participants were Women only,[62,64] or participants had MDD specifier like antenatal depression.[65] Our study also provides post-intervention change in these parameters for mild, moderate, and severe MDD. These differences in the components and duration of yoga, the instrument used to assess depression severity, and the severity levels of MDD patients in the studies must be taken into consideration while comparing the response rate of 59% achieving remission in our study with other studies. For example, yoga therapy MDD remission was 67% in the study by Janakiramaiah et al.[59] These differences must also be considered while comparing decrease in mean BDI-II scores of −17.24 or mean percent reduction in BDI-II scores of 56% after MDD treatment with YBLI in our study. For example, decrease in mean BDI-II scores was −9.47 in the study by Prathikanti et al.[13] and −8.92 in the study by Schuver and Lewis.[64] Mean reduction in HDRS-17 (HAM-D 17) score was −9.77 in the study by Sharma et al.[66]
YBLI optimizes serotonin homeostasis and increases neuroplasticity by mechanisms not restricted to modifying serotonin transporter function, the principal mechanism of action of SSRI drug therapy. Decreased serotonin transporter function is suggested to be present in those having susceptible 5-HTTLPR polymorphisms and is resistant to SSRI treatment. Unlike, passive interventions such as monoamine drug therapy, ketamine, electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS), an active MBI like YBLI may reduce the accelerated biological aging associated with MDD to improve serotonin biology and neuroplasticity, including in those who have susceptible gene polymorphisms and are resistant to SSRI treatment. There is rapid progress in severity of MDD in these patients. Since our findings indicate significant clinical improvement after yoga therapy in moderate to severe MDD, yoga therapy by YBLI may be clinically beneficial in these patients. Plausible mechanisms include: (1) Increasing neuroplasticity independent of SERT function. It has been argued that even in SERT deficient conditions, neurogenesis in hippocampus is unaffected.[67] Previous studies have suggested that neural stem cells have their own abundant regulatory control over serotonin function and no urge for SERT-driven serotonergic fine tuning.[68] In addition, a recent imaging study did not find any significant relationship between the 5-HTTLPR and alterations in white matter in the uncinate fasciculus (UF)–tract that is commonly associated with top-down regulation of mood and cognition. Neural networks including UF-tract are modified in regular practitioners of yoga and meditation.[69] YBLI may provide top-down signals from pre-frontal cortex, and increase neurogenesis and neuroplasticity by activating serotonin self-regulatory systems in the limbic regions. (2) Modifying activity of other neurotransmitters and transporters to optimize serotonin biology. The existence of additional 5-HT reuptake mechanisms on the cell membrane is strongly indicated, since 5-HT can be taken up by the dopamine transporter (DAT) and by the norepinephrine transporter (NET), and they may contribute to optimize serotonin homeostasis. Yoga and meditation practices have shown to increase the levels of dopamine and norepinephrine.[24,70] Both DAT and NET may receive top-down and bottom-up regulatory feedback related to eight limbs of yoga. (3) Improving circadian rhythms to optimize serotonin biology and neuroplasticity. MDD patients receiving YBLI in our study showed significantly improved sleep and vegetative symptoms. Previous studies suggest that yoga can optimize melatonin (5-methoxy-N-acetyltryptamine) levels in the brain and improve sleep.[71] Animal studies have suggested that melatonin has serotonergic functions.[72] Regularization of melatonin and circadian rhythms may contribute to increased neuroplasticity.[73] (4) Increase in the expression and efficiency of serotonin transporters. A recent study suggests that the significance of risk polymorphisms for depression, including SLC6A4, depends on stress exposure, and SERT expression and function in S allele is decreased only in the event of moderate-to-severe adversity.[74] The activities related to YBLI that cause moderate-to-severe physiological stress, may correct epigenetic modifications in SLC6A4 by different cellular mechanisms and pathways, and normalize SERT expressions even in the sensitive S allele. Therefore, top-down and bottom-up regulation of mood and cognition by yoga and meditation may benefit MDD remission irrespective of 5-HTTLPR genotype.
5-HTTLPR polymorphism is shown to be a therapy response biomarker for SSRI drug therapy in MDD.[35,75] Several studies have reported poor response with SS/SL genotype.[35,36,37,38,76,77] A meta-analysis by Porcelli et al.[37] show that L allele/LL genotype of 5-HTTLPR may be a predictor of better antidepressant response (odds ratio [OR] = 1.58) and remission (OR = 1.53) in the SSRI group. Findings in the DRUG group of our study treated with SSRIs (escitalopram, fluoxetine, and paroxetine) complement these findings. The plausible mechanisms for decreased efficacy in the DRUG group treated with SSRIs, particularly in those who have susceptible 5-HTTLPR polymorphism and are resistant to treatment with SSSRIs include (1) Selective action on neurotransmitter transporters and receptors. SSRIs are preferred to non-SSRI drugs because of the presumed selective rise of serotonin levels. This although may decrease complications, being selective may also decrease their efficacy. Factors responsible for the specificity of individual SSRIs include their actions at allosteric sites of SERT and their binding to other transporters and receptors. Escitalopram is shown to increase its efficacy by decreasing its own dissociation rate from the orthosteric site on the SERT through allosteric binding.[78] The evidence is limited for allosteric binding of paroxetine and fluoxetine. It is unknown if 5-HTTLPR polymorphism affect the drug action at allosteric sites. YBLI is shown to optimize several mediators of cellular aging and neuroplasticity[16] that may interact with 5-HTTLPR genotype. It is not known if their actions involve binding to different allosteric sites of SERT. (2) Impact of pharmacogenetics on drug metabolism. Recently, in a comprehensive systematic review and network meta-analysis, Cipriani et al. have reported comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with MDD.[79] Contrary to the findings in the review, paroxetine was more efficacious than escitalopram in the DRUG group of our study. A recent study with Asian population has also reported superior efficacy of paroxetine over escitalopram.[80] This discrepancy could be attributed to inter-ethnic differences in pharmacogenetics. Cytochrome P450 (CYP) enzymes account for over 90% of all drug metabolism. CYP2D6 and CYP2C19 that metabolize paroxetine and escitalopram respectively would require at least a doubling of the dose of extensive metabolizers (EMs) in comparison to poor metabolizers (PMs). While treatment with YBLI is not affected by these dose-related issues, the details of duration and frequency of yoga and meditation practice have not yet been standardized. However, more regular and 5 times a week practice for 1–2 h daily provide optimum benefits in MDD. (3) Decreased tolerability due to adverse events of treatment. No major adverse events were reported with YBLI in YOGA group. While no serious adverse events were reported, mild complications were noted in the drug group. Most common complications seen with MDD patients in the DRUG group of our study included dizziness, mild gastrointestinal disturbances, and insomnia. Similar to the review by Cipriani et al.,[79] fluoxetine was more acceptable in our study compared to escitalopram and paroxetine. A recent study suggested an association between fluoxetine response and SLC6A4 genotype and identified S allele carriers as being at risk for developing insomnia and agitation with treatment.[81] The same dose of SSRI may inhibit a higher proportion of SERT in individuals carrying the S allele, causing a rapid accumulation of synaptic serotonin and increasing the risk of adverse effects.[82] Higher acceptability of YBLI may be related to the absence of complications. While, SSRIs efficacy and tolerability is related to their action specifically at SERT, and at related receptors and transporters, benefits of YBLI are because of multi-way communications between humoral and neural regulatory systems and optimizing the crosstalk and biology of these regulatory mediators maintaining them within the homeostatic physiological range.[16,20] In fact, YBLI may improve homeostatic reserves of the organ systems and decrease allostatic load. Complications due to exaggerated increase or decrease of regulatory molecules are not observed.
Although numerous studies have identified a modest association with depression symptomology and disease, data linking polymorphisms in the MTHFR with depression have been inconclusive.[26,83] The MTHFR gene has not been directly linked to antidepressant response.[84,85,86,87,88] Our study supports these findings in MDD with MTHFR 677C>T polymorphism in both YOGA and DRUG groups. Considering that both DRUG and YOGA groups have effect on MTHFR function, possibility that the observed treatment effects are due to downstream effects cannot be ruled out. Modification of MTHFR function may, therefore, be possible in both DRUG and YOGA groups. Our findings must be interpreted cautiously by considering the impact of following contexts of MTHFR function in both YOGA and DRUG group, particularly in those who have susceptible gene polymorphisms and are resistant to SSRIs treatment: (1) Low activity MTHFR 677T allele, especially in homozygous state, is associated with a significant decrease in enzyme activity, leading to decrease in serotonin levels and DNA methylation capacity,[89] and it could deplete the levels of serotonin in the brain.[90] However, the presence of other polymorphisms that affect MTHFR function, including MTHFR 1298A>C,[91] also play an important role in this regard. For example, compound heterozygotes having one allele each of MTHFR 677C>T and MTHFR 1298A>C may also decrease MTHFR function. Therefore, information on other polymorphisms may be necessary for the precise interpretation of polymorphism effects. (2) In those who have these susceptible polymorphisms, when folic acid is given artificially as a one carbon source in the form of fortified diet or dietary supplements, MTHFR fails to provide methyl groups necessary for methylation reactions. Healthy diet comprised green leafy vegetables, legumes, and other natural food items, provide abundant 5-MTHF (also called L-methylfolate) that can provide methyl groups even in those who have MTHFR 677C>T MTHFR 1298A>C polymorphisms. 5-MTHF is active, circulating form of folate and the predominant form found naturally in food. Healthy diet by Indians, including those who regularly practice yoga, is rich in these natural food ingredients. The previous studies have reported improved nutritional behavior by yoga.[92] Therefore, YBLI may enable compensation of the deficient MTHFR function even in those who have susceptible MTHFR polymorphisms. (3) A recent study[93] suggests that exercise has beneficial impact on gut microbiota diversity, and optimized microbial diversity may contribute to improved MTHFR function. YBLI has physical exercise components such as Āsana and Prāṇāyāma, and therefore, optimized gut microbiota diversity in YOGA group may contribute to improved MTHFR function. Optimized vagus nerve function contributes to improve microbiota-gut-brain axis,[94] and there is significant evidence that yoga can optimize vagus nerve and gut functions. (4) A recent study[95] reports that the interaction between Helicobacter pylori seropositivity and reduced folate-cycle factor 5-MTHF might impair aspects of cognitive function. Previous research provides evidence for improved gut function by yoga and is mediated both by improved autonomic functions and anti-inflammatory effects.[96,97,98] YBLI mediated improvement in gut function may decrease H. pylori seropositivity. This can lead to improved gut function and nutrition behavior, and thereby increase 5-MTHF levels and methylation reactions related to gene function and neurotransmitter biology.(5) Previously, a research by Terruzzi et al.[99] studied genetic polymorphisms of the enzymes involved in DNA methylation and synthesis using elite athletes and in vitro C2C12 myoblasts cell-line model of DNA hypomethylation. Their findings suggest that the presence of DNA polymorphisms of MTHFR and other enzymes that cause reduced DNA methylation may provide an advantage to populations since their presence is significantly more common in elite athletes than control general population. DNA synthesis is significantly increased in the presence of these polymorphisms. In vitro findings in their study suggest that DNA hypomethylation due to lesser efficiency of polymorphic MTHFR and other related enzymes induces the activation of factors determining proliferation and differentiation of myoblasts promoting muscle growth and increase of muscle mass in athletes. In relation to YBLI treatment of MDD patients, particularly in those who have susceptible polymorphisms and are resistant to SSRIs treatment, these findings can be extrapolated in two ways. First, that exercise provided by yoga may induce the activation of factors from muscle cells that improve function of not only muscles but also cells of other organ systems including brain due to an optimized muscle cell secretory phenotype. Second, similar to muscle cells, other cells may also secrete activation factors during treatment with YBLI since it is comprised of diverse and active engagement of cells in the body through practice of the various limbs of yoga. Further research is warranted in this regard. Given the contexts mentioned above, any increase in MTHFR function or its compensation may be beneficial in depression, particularly in those who receive YBLI and have susceptible gene polymorphisms and resistance to SSRI treatment. Thus, these mechanisms may also contribute to clinical benefits of YBLI in moderate to severe MDD, who are more likely to have susceptible gene polymorphisms and are resistant to SSRI treatment. Increased MTHFR function may contribute to neurotransmitter homeostasis, maintaining serotonin, and melatonin levels in the central nervous system[100] and optimized neuroplasticity. To the best of our knowledge, there is no evidence to suggest SSRI antidepressants improve nutrition behavior, gut function, and gut microbiota diversity. Therefore, unlike yoga therapy, MDD treatment with SSRIs may not contribute to increase MTHFR function. This has particular relevance to decreased remission with drug therapy in those who have susceptible gene polymorphisms and are resistance to SSRI treatment.
5-HTTLPR S allele is associated with an increased risk for suicidal ideations.[101,102] Studies are limited for the association of MTHFR 677C>T with suicidal ideation. Due to a small sample size of patients with a history of suicidal ideation, the association of these therapygenetic markers with suicidal ideation was inconclusive in our study. Previous studies on childhood adversity associated with depression have reported lower antidepressant efficacy in SS genotype of the 5-HTTLPR with both SSRI and non-SSRI drugs.[103] Studies are limited for the association of MTHFR genetic variants with childhood adversity. In our current study, we found that 5-HTTLPR and MTHFR 677C>T polymorphisms were not associated with childhood adversity in yoga arm. However, childhood adversity significantly affected treatment response in 5-HTTLPR and MTHFR 677C>T variants in the drug arm. Since studies on the impact of the interactions of clinical variables and polymorphisms on therapy MDD remission have suggested a wide variation,[104,105] further research with larger samples is warranted.
Moreover, the multivariate logistic regression model in our study also did not show any significant association of 5-HTTLPR and MTHFR 677C>T genotypes with YBLI therapy response, while controlling for other factors. Our study did not show any significant gender differences to both YBLI and drug therapy. In our study, the S and T alleles were more common compared to the western population. This may be due to, increased prevalence of these genotypes in the Asian population, presumably a chance association during subject selection, or increased susceptibility of these alleles to MDD. These differences must be taken into consideration while findings from our analyses are interpreted. Although we have not assessed the attitudes and beliefs of study participants on yoga and drug therapy, the impact of polymorphisms in our study on altered remission based on the belief that a particular therapy will benefit cannot be ruled out.
Yoga, as practiced in YBLI, is based on the yoga sutras (principles) of Patanjali having eight limbs (Yama, Niyama, Āsana, Prāṇāyāma, Pratyāhāra, Dhāraṇā, Dhyāna, and Samādhi). The bottom-up and top-down regulation of brain and body[106] by YBLI, mediated by normative feedback loops,[107,108,109,110] may contribute to benefits of yoga in MDD patients who have susceptible gene polymorphisms and are resistant to treatment by SSRIs. Top-down mechanisms can inhibit the depression eliciting loops in the limbic system[111] by direct effects of meditation that are processed in the higher functional neural networks. Bottom-up regulation involving neurotransmitters include feedback, through sensory nerves, spinal cord, and brainstem, of neural activation by postures of asanas and breathing patterns of pranayama.[112] Several studies highlight the connections between the brain and periphery in promoting neurotransmitter biology and neuroplasticity by physical exercise and rhythmic breathing,[113,114,115,116,117,118] that are provided by yoga limbs such as Āsana and Prāṇāyāma. YBLI may lead to decrease in the stress-and inflammation-mediated[16] production of indoleamine 2,3-dioxygenase in microglia, and thereby shunting tryptophan from the production of a disruptive excitatory mediator quinolinic acid to the production of serotonin, melatonin, and a regulatory molecule kynurenic acid.[119] A recent study using a mouse model[120,121] has reported that physical exercise training-induced PGC-1α1 by skeletal muscle can modulate kynurenine metabolism and mediate resilience to stress-induced depression. Their findings suggest that activation of the PGC-1α1-PPARα/δ pathway increases skeletal muscle expression of kynurenine aminotransferases, thus enhancing the conversion of kynurenine into kynurenic acid, a metabolite unable to cross the blood-brain barrier. Reducing plasma kynurenine protects the brain from stress-induced changes associated with depression. Since Āsana and Prāṇāyāma provide good physical exercise to the body, yoga may be therapeutic in depression by targeting the PGC-1α1-PPAR axis in skeletal muscle, both by decreasing the adverse effects of kynurenine and by diverting tryptophan metabolism to increase the formation of serotonin and melatonin in the brain. In YBLI, there are repeating cycles of a metabolic challenge that induces ketosis. Intermittent metabolic switching (IMS) to ketosis is accompanied by cellular and molecular adaptations of neural networks in the brain that enhance their functionality and bolster resistance to stress, injury, and disease[122] by optimizing cellular health and neuroplasticity. The monoamine neurotransmitters serotonin, noradrenaline, and dopamine, also play important role in the effects of IMS on neuronal network activity.[123,124] Optimized neural and humoral regulatory feedback loops may also contribute to improved functions of organ systems.
Study is limited by not having an active control group for yoga. Active controls receiving treatments such as routine physical exercise, accupuncture, and taekwondo may provide more insight into the impact of yoga in depression. Use of BDI-II, a self-rated instrument, to assess the severity of depression is another limitation in the study. While, using BDI-II has several benefits, including physician-rated instruments, either alone or incombination with self-rated instruments, may be included in future studies for getting a different perspective. While measurement of adherence to treatments was performed using patient logs and diaries, more precise methods may need to be adopted in future studies. Our study is also limited by the exclusion of patients with very severe depression. Currently, neurological interventions such as rTMS and DBS are preferred for very severe depression. Future research is needed for exploring YBLI in these patients. Although MINI 7.0 was used to assess comorbid psychiatric conditions, it has limitations in translation research since it is based on DSM-5, which is in essence purely descriptive and emphasizes clinical pragmatism. Structured instruments based on the Research Domain Criteria proposed by National Institute of Mental Health or other criteria, which provide a causal framework for classification of behavioral disturbance may need to be developed and used for future research. The current study had several strengths that include precision in genotyping with study population in Hardy–Weinberg equilibrium. Findings from our study are very critical in highlighting the impact of YBLI in MDD independent of 5-HTTLPR and MTHFR 677C>T polymorphisms, and in identifying the clinical utility of yoga therapy in those who have susceptible gene polymorphisms and are resistant to drug therapy with SSRIs. There is a continuing need for searching other genes that may modulate treatment response to MDD, including in YBLI. A highly heterogeneous condition such as MDD is likely to yield the most optimal outcomes through personalized treatment approaches based on the genetic and environmental information specific to patients.
In conclusion, YBLI provides MDD remission irrespective of the presence of 5-HTTLPR and MTHFR 677C>T polymorphisms. YBLI provides MDD remission in those who have susceptible gene polymorphisms and are resistant to SSRIs treatment. The SS homozygous genotype of 5-HTTLPR and TT homozygous genotype of MTHFR 677C>T polymorphism have been widely studied as a risk allele for depression pathobiology and treatment. This study, however, presents the first evidence of the increased YBLI therapy MDD remission in these functional polymorphisms. As such, this research addresses MDD interventions that provide remissions independent of heterogeneity in pathogenesis that previous research has suggested to be of great importance.[125] High incidence of complications, poor prognosis, and poor QOL all may show significant improvement post-YBLI therapy in MDD and may also result in increased disease-free intervals and delayed or few relapses.
The previous research has documented several therapy response biomarkers for the treatment of MDD with different modalities including drug therapy,[126] psychotherapy,[127] and mindfulness-based cognitive behavioral therapy.[128] The markers vary between modalities, and the findings are contradictory within each modality. These studies highlight the absence of precise and uniform therapy genetic markers and suggest that the pathobiology of MDD involve complex gene x environmental interactions. Future work should also attempt to identify the impact of other genes and gene-environment interactions that may impact prognosis and management of MDD and its remission. Finding out genes that differ in modifying passive MDD treatments such as pharmacotherapy, ECT, and rTMS and active treatments such as yoga-based MBIs will not only improve our understanding of the biological processes that underlie MDD remission but may also shed light on their basic functions, with increased insight on molecular evolution of health and disease.[129] It must be noted that there is no single “remission gene,” which can be targeted by a specific pharmacological or neurological intervention. Instead, MDD is a complex disorder and numerous genes, whose expression, in combination with environmental factors and epigenetic modifications, influence pathobiology, and treatment of MDD. Active interventions such as YBLI normalize the functions in MDD by dynamically influencing the molecular networks of the complex tissue environments of the human body and the brain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Specify contributions that need acknowledging but do not justify authorship, such as general support by a departmental chair and acknowledgments of technical, financial, and material support
The authors are thankful to Amit Tomar and Sudhir Choudary for Yoga instructions. We are thankful to ICMR, New Delhi for supporting with funds for the project. We remain grateful to all the participants enrolled in the study.
REFERENCES
- 1.World Health Organization. Geneva: World Health Organization; 2017. [Last accessed on 2018 Jan 11]. Depression and Other Common Mental Disorders: Global Health Estimates. Available from: http://www.apps.who.int/iris/handle/10665/254610 . [Google Scholar]
- 2.Verhoeven JE, Révész D, Epel ES, Lin J, Wolkowitz OM, Penninx BW. Major depressive disorder and accelerated cellular aging: Results from a large psychiatric cohort study. Mol Psychiatry. 2014;19:895–901. doi: 10.1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- 3.Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: Biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11:129. doi: 10.1186/1741-7015-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldessarini RJ, Forte A, Selle V, Sim K, Tondo L, Undurraga J, et al. Morbidity in depressive disorders. Psychother Psychosom. 2017;86:65–72. doi: 10.1159/000448661. [DOI] [PubMed] [Google Scholar]
- 5.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 6.Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller K, et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatry. 2017;17:58. doi: 10.1186/s12888-016-1173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang YJ, Lane HY, Lin CH. New treatment strategies of depression: Based on mechanisms related to neuroplasticity. Neural Plast 2017. 2017:4605971. doi: 10.1155/2017/4605971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Liu J, Wang M, Zhang Y, Li L. From serotonin to neuroplasticity: Evolvement of theories for major depressive disorder. Front Cell Neurosci. 2017;11:305. doi: 10.3389/fncel.2017.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong DF, Wagner HN, Jr, Dannals RF, Links JM, Frost JJ, Ravert HT. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984;226:1393–6. doi: 10.1126/science.6334363. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Suhara T, Okubo Y, Ichimiya T, Sudo Y, Inoue M, et al. Age-related decline of serotonin transporters in living human brain of healthy males. Life Sci. 2002;71:751–7. doi: 10.1016/s0024-3205(02)01745-9. [DOI] [PubMed] [Google Scholar]
- 11.Iyo M, Yamasaki T. The detection of age-related decrease of dopamine D1, D2 and serotonin 5-HT2 receptors in living human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:415–21. doi: 10.1016/0278-5846(93)90075-4. [DOI] [PubMed] [Google Scholar]
- 12.Kraus C, Castrén E, Kasper S, Lanzenberger R. Serotonin and neuroplasticity-links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev. 2017;77:317–26. doi: 10.1016/j.neubiorev.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Prathikanti S, Rivera R, Cochran A, Tungol JG, Fayazmanesh N, Weinmann E. Treating major depression with yoga: A prospective, randomized, controlled pilot trial. PLoS One. 2017;12:e0173869. doi: 10.1371/journal.pone.0173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villemure C, Čeko M, Cotton VA, Bushnell MC. Neuroprotective effects of yoga practice: Age-, experience-, and frequency-dependent plasticity. Front Hum Neurosci. 2015;9:281. doi: 10.3389/fnhum.2015.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahn BR, Goodman MS, Peterson CT, Maturi R, Mills PJ. Yoga, meditation and mind-body health: Increased BDNF, cortisol awakening response, and altered inflammatory marker expression after a 3-month yoga and meditation retreat. Front Hum Neurosci. 2017;11:315. doi: 10.3389/fnhum.2017.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolahunase MR, Sagar R, Faiq M, Dada R. Yoga- and meditation-based lifestyle intervention increases neuroplasticity and reduces severity of major depressive disorder: A randomized controlled trial. Restor Neurol Neurosci. 2018;36:423–42. doi: 10.3233/RNN-170810. [DOI] [PubMed] [Google Scholar]
- 17.Hölzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epel ES, Puterman E, Lin J, Blackburn EH, Lum PY, Beckmann ND, et al. Meditation and vacation effects have an impact on disease-associated molecular phenotypes. Transl Psychiatry. 2016;6:e880. doi: 10.1038/tp.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson CT, Lucas J, John-Williams LS, Thompson JW, Moseley MA, Patel S, et al. Identification of altered metabolomic profiles following a panchakarma-based ayurvedic intervention in healthy subjects: The self-directed biological transformation initiative (SBTI) Sci Rep. 2016;6:32609. doi: 10.1038/srep32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolahunase M, Sagar R, Dada R. Impact of yoga and meditation on cellular aging in apparently healthy individuals: A prospective, open-label single-arm exploratory study. Oxid Med Cell Longev 2017. 2017:7928981. doi: 10.1155/2017/7928981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens I. Medical yoga therapy. Children (Basel) 2017;4 pii: E12. [Google Scholar]
- 22.Yu X, Fumoto M, Nakatani Y, Sekiyama T, Kikuchi H, Seki Y, et al. Activation of the anterior prefrontal cortex and serotonergic system is associated with improvements in mood and EEG changes induced by zen meditation practice in novices. Int J Psychophysiol. 2011;80:103–11. doi: 10.1016/j.ijpsycho.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Walton KG, Pugh ND, Gelderloos P, Macrae P. Stress reduction and preventing hypertension: Preliminary support for a psychoneuroendocrine mechanism. J Altern Complement Med. 1995;1:263–83. doi: 10.1089/acm.1995.1.263. [DOI] [PubMed] [Google Scholar]
- 24.Bujatti M, Riederer P. Serotonin, noradrenaline, dopamine metabolites in transcendental meditation-technique. J Neural Transm. 1976;39:257–67. doi: 10.1007/BF01256514. [DOI] [PubMed] [Google Scholar]
- 25.Daniele A, Divella R, Paradiso A, Mattioli V, Romito F, Giotta F, et al. Serotonin transporter polymorphism in major depressive disorder (MDD), psychiatric disorders, and in MDD in response to stressful life events: Causes and treatment with antidepressant. In Vivo. 2011;25:895–901. [PubMed] [Google Scholar]
- 26.Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: A HuGE review. Am J Epidemiol. 2007;165:1–3. doi: 10.1093/aje/kwj347. [DOI] [PubMed] [Google Scholar]
- 27.Hensler JG, Ferry RC, Labow DM, Kovachich GB, Frazer A. Quantitative autoradiography of the serotonin transporter to assess the distribution of serotonergic projections from the dorsal raphe nucleus. Synapse. 1994;17:1–5. doi: 10.1002/syn.890170102. [DOI] [PubMed] [Google Scholar]
- 28.Clarke H, Flint J, Attwood AS, Munafò MR. Association of the 5- HTTLPR genotype and unipolar depression: A meta-analysis. Psychol Med. 2010;40:1767–78. doi: 10.1017/S0033291710000516. [DOI] [PubMed] [Google Scholar]
- 29.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA. 2009;301:2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frodl T, Meisenzahl EM, Zill P, Baghai T, Rujescu D, Leinsinger G, et al. Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Arch Gen Psychiatry. 2004;61:177–83. doi: 10.1001/archpsyc.61.2.177. [DOI] [PubMed] [Google Scholar]
- 31.Tatham EL, Ramasubbu R, Gaxiola-Valdez I, Cortese F, Clark D, Goodyear B, et al. White matter integrity in major depressive disorder: Implications of childhood trauma, 5-HTTLPR and BDNF polymorphisms. Psychiatry Res Neuroimaging. 2016;253:15–25. doi: 10.1016/j.pscychresns.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Kiyohara C, Yoshimasu K. Association between major depressive disorder and a functional polymorphism of the 5-hydroxytryptamine (serotonin) transporter gene: A meta-analysis. Psychiatr Genet. 2010;20:49–58. doi: 10.1097/YPG.0b013e328335112b. [DOI] [PubMed] [Google Scholar]
- 33.Wilhelm K, Mitchell PB, Niven H, Finch A, Wedgwood L, Scimone A, et al. Life events, first depression onset and the serotonin transporter gene. Br J Psychiatry. 2006;188:210–5. doi: 10.1192/bjp.bp.105.009522. [DOI] [PubMed] [Google Scholar]
- 34.Verhagen M, van der Meij A, Janzing JG, Arias-Vásquez A, Buitelaar JK, Franke B, et al. Effect of the 5-HTTLPR polymorphism in the serotonin transporter gene on major depressive disorder and related comorbid disorders. Psychiatr Genet. 2009;19:39–44. doi: 10.1097/YPG.0b013e3283208061. [DOI] [PubMed] [Google Scholar]
- 35.Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–57. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 36.Murphy DL, Moya PR. Human serotonin transporter gene (SLC6A4) variants: Their contributions to understanding pharmacogenomic and other functional G×G and G×E differences in health and disease. Curr Opin Pharmacol. 2011;11:3–10. doi: 10.1016/j.coph.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22:239–58. doi: 10.1016/j.euroneuro.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Klein-Fedyshin M, Stevenson JM. Serotonin transporter gene polymorphisms and selective serotonin reuptake inhibitor tolerability: Review of pharmacogenetic evidence. Pharmacotherapy. 2017;37:1089–104. doi: 10.1002/phar.1978. [DOI] [PubMed] [Google Scholar]
- 39.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002, 12. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 40.Frodl T, Koutsouleris N, Bottlender R, Born C, Jäger M, Mörgenthaler M, et al. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Mol Psychiatry. 2008;13:1093–101. doi: 10.1038/mp.2008.62. [DOI] [PubMed] [Google Scholar]
- 41.Zhao J, Goldberg J, Bremner JD, Vaccarino V. Association between promoter methylation of serotonin transporter gene and depressive symptoms: A monozygotic twin study. Psychosom Med. 2013;75:523–9. doi: 10.1097/PSY.0b013e3182924cf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nazki FH, Sameer AS, Ganaie BA. Folate: Metabolism, genes, polymorphisms and the associated diseases. Gene. 2014;533:11–20. doi: 10.1016/j.gene.2013.09.063. [DOI] [PubMed] [Google Scholar]
- 43.Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One. 2010;5:e12201. doi: 10.1371/journal.pone.0012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peerbooms OL, van Os J, Drukker M, Kenis G, Hoogveld L MTHFR in Psychiatry Group. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: Evidence for a common genetic vulnerability? Brain Behav Immun. 2011;25:1530–43. doi: 10.1016/j.bbi.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Lok A, Bockting CL, Koeter MW, Snieder H, Assies J, Mocking RJ, et al. Interaction between the MTHFR C677T polymorphism and traumatic childhood events predicts depression. Transl Psychiatry. 2013;3:e288. doi: 10.1038/tp.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul RT, McDonnell AP, Kelly CB. Folic acid: Neurochemistry, metabolism and relationship to depression. Hum Psychopharmacol. 2004;19:477–88. doi: 10.1002/hup.614. [DOI] [PubMed] [Google Scholar]
- 47.Eszlari N, Kovacs D, Petschner P, Pap D, Gonda X, Elliott R, et al. Distinct effects of folate pathway genes MTHFR and MTHFD1L on ruminative response style: A potential risk mechanism for depression. Transl Psychiatry. 2016;6:e745. doi: 10.1038/tp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder.Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–5. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 49.Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive behavior therapy for depression and self-care in heart failure patients: A randomized clinical trial. JAMA Intern Med. 2015;175:1773–82. doi: 10.1001/jamainternmed.2015.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reeves GM, Rohan KJ, Langenberg P, Snitker S, Postolache TT. Validation of BDI-II response and remission cut-points for assessment of seasonal affective disorder patients. J Affect Disord. 2012;138:123–7. doi: 10.1016/j.jad.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang YP, Gorenstein C. Assessment of depression in medical patients: A systematic review of the utility of the beck depression inventory-II. Clinics (Sao Paulo) 2013;68:1274–87. doi: 10.6061/clinics/2013(09)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Y, Chan W, Lo BC. Comparing five depression measures in depressed chinese patients using item response theory: An examination of item properties, measurement precision and score comparability. Health Qual Life Outcomes. 2017;15:60. doi: 10.1186/s12955-017-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunt M, Auriemma J, Cashaw AC. Self-report bias and underreporting of depression on the BDI-II. J Pers Assess. 2003;80:26–30. doi: 10.1207/S15327752JPA8001_10. [DOI] [PubMed] [Google Scholar]
- 54.Beck AT, Steer RA, Brown G. Washington, DC: American Psychological Association; 1996. Beck Depression Inventory–II. PsycTESTS Dataset. [Google Scholar]
- 55.Green KL, Brown GK, Jager-Hyman S, Cha J, Steer RA, Beck AT. The predictive validity of the beck depression inventory suicide item. J Clin Psychiatry. 2015;76:1683–6. doi: 10.4088/JCP.14m09391. [DOI] [PubMed] [Google Scholar]
- 56.Sheehan DV. Mini International Neuropsychiatric Interview 7.0. Jacksonville, FL: Medical Outcomes Systems; 2015. [Google Scholar]
- 57.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22, 33. [PubMed] [Google Scholar]
- 58.Cramer H, Anheyer D, Lauche R, Dobos G. A systematic review of yoga for major depressive disorder. J Affect Disord. 2017;213:70–7. doi: 10.1016/j.jad.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, Harish MG, Subbakrishna DK, Vedamurthachar A. Antidepressant efficacy of Sudarshan Kriya Yoga (SKY) in melancholia: A randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57:255–9. doi: 10.1016/s0165-0327(99)00079-8. [DOI] [PubMed] [Google Scholar]
- 60.Sharma VK, Das S, Mondal S, Goswampi U, Gandhi A. Effect of sahaj yoga on depressive disorders. Indian J Physiol Pharmacol. 2005;49:462–8. [PubMed] [Google Scholar]
- 61.Kinser PA, Bourguignon C, Whaley D, Hauenstein E, Taylor AG. Feasibility, acceptability, and effects of gentle Hatha yoga for women with major depression: Findings from a randomized controlled mixed-methods study. Arch Psychiatr Nurs. 2013;27:137–47. doi: 10.1016/j.apnu.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kinser PA, Elswick RK, Kornstein S. Potential long-term effects of a mind-body intervention for women with major depressive disorder: Sustained mental health improvements with a pilot yoga intervention. Arch Psychiatr Nurs. 2014;28:377–83. doi: 10.1016/j.apnu.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarubin N, Nothdurfter C, Schüle C, Lieb M, Uhr M, Born C, et al. The influence of hatha yoga as an add-on treatment in major depression on hypothalamic-pituitary-adrenal-axis activity: A randomized trial. J Psychiatr Res. 2014;53:76–83. doi: 10.1016/j.jpsychires.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Schuver KJ, Lewis BA. Mindfulness-based yoga intervention for women with depression. Complement Ther Med. 2016;26:85–91. doi: 10.1016/j.ctim.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Uebelacker LA, Battle CL, Sutton KA, Magee SR, Miller IW. A pilot randomized controlled trial comparing prenatal yoga to perinatal health education for antenatal depression. Arch Womens Ment Health. 2016;19:543–7. doi: 10.1007/s00737-015-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma A, Barrett MS, Cucchiara AJ, Gooneratne NS, Thase ME. A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: A randomized pilot study. J Clin Psychiatry. 2017;78:e59–63. doi: 10.4088/JCP.16m10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmitt A, Benninghoff J, Moessner R, Rizzi M, Paizanis E, Doenitz C, et al. Adult neurogenesis in serotonin transporter deficient mice. J Neural Transm (Vienna) 2007;114:1107–19. doi: 10.1007/s00702-007-0724-6. [DOI] [PubMed] [Google Scholar]
- 68.Benninghoff J, Gritti A, Rizzi M, Lamorte G, Schloesser RJ, Schmitt A, et al. Serotonin depletion hampers survival and proliferation in neurospheres derived from adult neural stem cells. Neuropsychopharmacology. 2010;35:893–903. doi: 10.1038/npp.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gard T, Taquet M, Dixit R, Hölzel BK, de Montjoye YA, Brach N, et al. Fluid intelligence and brain functional organization in aging yoga and meditation practitioners. Front Aging Neurosci. 2014;6:76. doi: 10.3389/fnagi.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kjaer TW, Bertelsen C, Piccini P, Brooks D, Alving J, Lou HC. Increased dopamine tone during meditation-induced change of consciousness. Brain Res Cogn Brain Res. 2002;13:255–9. doi: 10.1016/s0926-6410(01)00106-9. [DOI] [PubMed] [Google Scholar]
- 71.Nagendra RP, Maruthai N, Kutty BM. Meditation and its regulatory role on sleep. Front Neurol. 2012;3:54. doi: 10.3389/fneur.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Micale V, Arezzi A, Rampello L, Drago F. Melatonin affects the immobility time of rats in the forced swim test: The role of serotonin neurotransmission. Eur Neuropsychopharmacol. 2006;16:538–45. doi: 10.1016/j.euroneuro.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Valdés-Tovar M, Estrada-Reyes R, Solís-Chagoyán H, Argueta J, Dorantes-Barrón AM, Quero-Chávez D, et al. Circadian modulation of neuroplasticity by melatonin: A target in the treatment of depression. Br J Pharmacol. 2018;175:3200, 8. doi: 10.1111/bph.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonda X, Hullam G, Antal P, Eszlari N, Petschner P, Hökfelt TG, et al. Significance of risk polymorphisms for depression depends on stress exposure. Sci Rep. 2018;8:3946. doi: 10.1038/s41598-018-22221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boland JR, Duffy B, Myer NM. Clinical utility of pharmacogenetics-guided treatment of depression and anxiety. Pers Med Psychiatry. 2018;7:7–13. [Google Scholar]
- 76.Manoharan A, Shewade DG, Rajkumar RP, Adithan S. Serotonin transporter gene (SLC6A4) polymorphisms are associated with response to fluoxetine in South Indian major depressive disorder patients. Eur J Clin Pharmacol. 2016;72:1215–20. doi: 10.1007/s00228-016-2099-9. [DOI] [PubMed] [Google Scholar]
- 77.Arias B, Catalán R, Gastó C, Gutiérrez B, Fañanás L. 5-HTTLPR polymorphism of the serotonin transporter gene predicts non-remission in major depression patients treated with citalopram in a 12-weeks follow up study. J Clin Psychopharmacol. 2003;23:563–7. doi: 10.1097/01.jcp.0000095350.32154.73. [DOI] [PubMed] [Google Scholar]
- 78.Zhong H, Haddjeri N, Sánchez C. Escitalopram, an antidepressant with an allosteric effect at the serotonin transporter – A review of current understanding of its mechanism of action. Psychopharmacology (Berl) 2012;219:1–3. doi: 10.1007/s00213-011-2463-5. [DOI] [PubMed] [Google Scholar]
- 79.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet. 2018;391:1357–66. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woo YS, McIntyre RS, Kim JB, Lee MS, Kim JM, Yim HW, et al. Paroxetine versus venlafaxine and escitalopram in Korean patients with major depressive disorder: A randomized, rater-blinded, six-week study. Clin Psychopharmacol Neurosci. 2017;15:391–401. doi: 10.9758/cpn.2017.15.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perlis RH, Mischoulon D, Smoller JW, Wan YJ, Lamon-Fava S, Lin KM, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54:879–83. doi: 10.1016/s0006-3223(03)00424-4. [DOI] [PubMed] [Google Scholar]
- 82.Crawford AA, Lewis G, Lewis SJ, Munafò MR. Systematic review and meta-analysis of serotonin transporter genotype and discontinuation from antidepressant treatment. Eur Neuropsychopharmacol. 2013;23:1143–50. doi: 10.1016/j.euroneuro.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zintzaras E. C677T and A1298C methylenetetrahydrofolate reductase gene polymorphisms in schizophrenia, bipolar disorder and depression: A meta-analysis of genetic association studies. Psychiatr Genet. 2006;16:105–15. doi: 10.1097/01.ypg.0000199444.77291.e2. [DOI] [PubMed] [Google Scholar]
- 84.Mischoulon D, Lamon-Fava S, Selhub J, Katz J, Papakostas GI, Iosifescu DV, et al. Prevalence of MTHFR C677T and MS A2756G polymorphisms in major depressive disorder, and their impact on response to fluoxetine treatment. CNS Spectr. 2012;17:76–86. doi: 10.1017/S1092852912000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen X, Wu Y, Guan T, Wang X, Qian M, Lin M, et al. Association analysis of COMT/MTHFR polymorphisms and major depressive disorder in Chinese Han population. J Affect Disord. 2014;161:73–8. doi: 10.1016/j.jad.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Lok A, Mocking RJ, Assies J, Koeter MW, Bockting CL, de Vries GJ, et al. The one-carbon-cycle and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in recurrent major depressive disorder; influence of antidepressant use and depressive state? J Affect Disord. 2014;166:115–23. doi: 10.1016/j.jad.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 87.Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61:631–7. doi: 10.1136/jech.2006.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lanctôt KL, Rapoport MJ, Chan F, Rajaram RD, Strauss J, Sicard T, et al. Genetic predictors of response to treatment with citalopram in depression secondary to traumatic brain injury. Brain Inj. 2010;24:959–69. doi: 10.3109/02699051003789229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castro R, Rivera I, Ravasco P, Camilo ME, Jakobs C, Blom HJ, et al. 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C-->T and 1298A-->C mutations are associated with DNA hypomethylation. J Med Genet. 2004;41:454–8. doi: 10.1136/jmg.2003.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bjelland I, Tell GS, Vollset SE, Refsum H, Ueland PM. Folate, Vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: The Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60:618–26. doi: 10.1001/archpsyc.60.6.618. [DOI] [PubMed] [Google Scholar]
- 91.Cho K, Amin ZM, An J, Rambaran KA, Johnson TB, Alzghari SK. Methylenetetrahydrofolate reductase A1298C polymorphism and major depressive disorder. Cureus. 2017;9:e1734. doi: 10.7759/cureus.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watts AW, Rydell SA, Eisenberg ME, Laska MN, Neumark-Sztainer D. Yoga's potential for promoting healthy eating and physical activity behaviors among young adults: A mixed-methods study. Int J Behav Nutr Phys Act. 2018;15:42. doi: 10.1186/s12966-018-0674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–20. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 94.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berrett AN, Gale SD, Erickson LD, Brown BL, Hedges DW. Helicobacter pylori moderates the association between 5-MTHF concentration and cognitive function in older adults. PLoS One. 2018;13:e0190475. doi: 10.1371/journal.pone.0190475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bonaz B, Sinniger V, Pellissier S. The vagus nerve in the neuro-immune axis: Implications in the pathology of the gastrointestinal tract. Front Immunol. 2017;8:1452. doi: 10.3389/fimmu.2017.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Streeter CC, Gerbarg PL, Saper RB, Ciraulo DA, Brown RP. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571–9. doi: 10.1016/j.mehy.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 99.Terruzzi I, Senesi P, Montesano A, La Torre A, Alberti G, Benedini S, et al. Genetic polymorphisms of the enzymes involved in DNA methylation and synthesis in elite athletes. Physiol Genomics. 2011;43:965–73. doi: 10.1152/physiolgenomics.00040.2010. [DOI] [PubMed] [Google Scholar]
- 100.Lee M, Moon W, Kim J. Effect of yoga on pain, brain-derived neurotrophic factor, and serotonin in premenopausal women with chronic low back pain. Evid Based Complement Alternat Med 2014. 2014:203173. doi: 10.1155/2014/203173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: II. Suicidal behavior. Mol Psychiatry. 2003;8:646–53. doi: 10.1038/sj.mp.4001336. [DOI] [PubMed] [Google Scholar]
- 102.Lin PY, Tsai G. Association between serotonin transporter gene promoter polymorphism and suicide: Results of a meta-analysis. Biol Psychiatry. 2004;55:1023–30. doi: 10.1016/j.biopsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 103.Gressier F, Bouaziz E, Verstuyft C, Hardy P, Becquemont L, Corruble E, et al. 5-HTTLPR modulates antidepressant efficacy in depressed women. Psychiatr Genet. 2009;19:195–200. doi: 10.1097/YPG.0b013e32832cef0d. [DOI] [PubMed] [Google Scholar]
- 104.Wang X, Fu J, Li Q, Zeng D. Geographical and ethnic distributions of the MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in Chinese populations: A meta-analysis. PLoS One. 2016;11:e0152414. doi: 10.1371/journal.pone.0152414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saraswathy KN, Asghar M, Samtani R, Murry B, Mondal PR, Ghosh PK, et al. Spectrum of MTHFR gene SNPs C677T and A1298C: A study among 23 population groups of India. Mol Biol Rep. 2012;39:5025–31. doi: 10.1007/s11033-011-1299-8. [DOI] [PubMed] [Google Scholar]
- 106.Taylor AG, Goehler LE, Galper DI, Innes KE, Bourguignon C. Top-down and bottom-up mechanisms in mind-body medicine: Development of an integrative framework for psychophysiological research. Explore (NY) 2010;6:29–41. doi: 10.1016/j.explore.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16:213–25. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 108.Streeter CC, Whitfield TH, Owen L, Rein T, Karri SK, Yakhkind A, et al. Effects of yoga versus walking on mood, anxiety, and brain GABA levels: A randomized controlled MRS study. J Altern Complement Med. 2010;16:1145–52. doi: 10.1089/acm.2010.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naveen GH, Varambally S, Thirthalli J, Rao M, Christopher R, Gangadhar BN. Serum cortisol and BDNF in patients with major depression-effect of yoga. Int Rev Psychiatry. 2016;28:273–8. doi: 10.1080/09540261.2016.1175419. [DOI] [PubMed] [Google Scholar]
- 110.Morgan N, Irwin MR, Chung M, Wang C. The effects of mind-body therapies on the immune system: Meta-analysis. PLoS One. 2014;9:e100903. doi: 10.1371/journal.pone.0100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmalzl L, Powers C, Henje Blom E. Neurophysiological and neurocognitive mechanisms underlying the effects of yoga-based practices: Towards a comprehensive theoretical framework. Front Hum Neurosci. 2015;9:235. doi: 10.3389/fnhum.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sullivan MB, Erb M, Schmalzl L, Moonaz S, Noggle Taylor J, Porges SW, et al. Yoga therapy and polyvagal theory: The convergence of traditional wisdom and contemporary neuroscience for self-regulation and resilience. Front Hum Neurosci. 2018;12:67. doi: 10.3389/fnhum.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gomez-Pinilla F, Ying Z, Zhuang Y. Brain and spinal cord interaction: Protective effects of exercise prior to spinal cord injury. PLoS One. 2012;7:e32298. doi: 10.1371/journal.pone.0032298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heck DH, McAfee SS, Liu Y, Babajani-Feremi A, Rezaie R, Freeman WJ, et al. Breathing as a fundamental rhythm of brain function. Front Neural Circuits. 2016;10:115. doi: 10.3389/fncir.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zelano C, Jiang H, Zhou G, Arora N, Schuele S, Rosenow J, et al. Nasal respiration entrains human limbic oscillations and modulates cognitive function. J Neurosci. 2016;36:12448–67. doi: 10.1523/JNEUROSCI.2586-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field: I. Comparative aspects of mesencephalic and pontine connections. J Comp Neurol. 1990;301:618–42. doi: 10.1002/cne.903010410. [DOI] [PubMed] [Google Scholar]
- 117.Fung SJ, Manzoni D, Chan JY, Pompeiano O, Barnes CD. Locus coeruleus control of spinal motor output. Prog Brain Res. 1991;88:395–409. doi: 10.1016/s0079-6123(08)63825-x. [DOI] [PubMed] [Google Scholar]
- 118.Haxhiu MA, Kc P, Neziri B, Yamamoto BK, Ferguson DG, Massari VJ. Catecholaminergic microcircuitry controlling the output of airway-related vagal preganglionic neurons. J Appl Physiol (1985) 2003;94:1999–2009. doi: 10.1152/japplphysiol.01066.2002. [DOI] [PubMed] [Google Scholar]
- 119.Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Träskman-Bendz L, Guillemin GJ, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110–7. doi: 10.1016/j.bbi.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 120.Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 121.Harkin A. Muscling In on Depression. N Engl J Med. 2014;371:2333–4. doi: 10.1056/NEJMcibr1411568. [DOI] [PubMed] [Google Scholar]
- 122.Elias AN, Guich S, Wilson AF. Ketosis with enhanced GABAergic tone promotes physiological changes in transcendental meditation. Med Hypotheses. 2000;54:660–2. doi: 10.1054/mehy.1999.0921. [DOI] [PubMed] [Google Scholar]
- 123.Blier P, El Mansari M. Serotonin and beyond: Therapeutics for major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120536. doi: 10.1098/rstb.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19:63–80. doi: 10.1038/nrn.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gartlehner G, Wagner G, Matyas N, Titscher V, Greimel J, Lux L, et al. Pharmacological and non-pharmacological treatments for major depressive disorder: Review of systematic reviews. BMJ Open. 2017;7:e014912. doi: 10.1136/bmjopen-2016-014912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fabbri C, Tansey KE, Perlis RH, Hauser J, Henigsberg N, Maier W, et al. New insights into the pharmacogenomics of antidepressant response from the GENDEP and STAR*D studies: Rare variant analysis and high-density imputation. Pharmacogenomics J. 2018;18:413–21. doi: 10.1038/tpj.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lester KJ, Eley TC. Therapygenetics: Using genetic markers to predict response to psychological treatment for mood and anxiety disorders. Biol Mood Anxiety Disord. 2013;3:4. doi: 10.1186/2045-5380-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bakker JM, Lieverse R, Menne-Lothmann C, Viechtbauer W, Pishva E, Kenis G, et al. Therapygenetics in mindfulness-based cognitive therapy: Do genes have an impact on therapy-induced change in real-life positive affective experiences? Transl Psychiatry. 2014;4:e384. doi: 10.1038/tp.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fitzpatrick MJ, Ben-Shahar Y, Smid HM, Vet LE, Robinson GE, Sokolowski MB, et al. Candidate genes for behavioural ecology. Trends Ecol Evol. 2005;20:96–104. doi: 10.1016/j.tree.2004.11.017. [DOI] [PubMed] [Google Scholar]