Abstract
Background
UV-related skin disease such as actinic keratosis is a major concern in public health. In view of the cell injury induced by UVB, Klotho protein it is an ideal therapy to eliminate UVB-induced cell damages and the associated signaling pathways.
Material/Methods
To gain insights into the potential role of Klotho and the underlying molecular mechanism, we constructed a Klotho-overexpress HaCaT cell line and assessed the protection against UVB insults. The effects of exposure to UVB radiation on the human keratinocyte HaCaT cells, including cell growth, apoptosis, and changes of selected biomarkers, were measured by CCK-8, flow cytometry, Quantitative real-time PCR, and Western blot analysis.
Results
We found that enhanced NF-κB activity was accompanied by decreased expression of the anti-aging protein Klotho upon UVB stimulation, which was further confirmed with in vivo experiments. Overexpression of Klotho was able to considerably alleviate the UVB-induced damages to cells and reversed the UVB-caused biomarker changes to a great extent, which was comparable to the effects of administration of NF-κB inhibitor PDTC, suggesting the inhibition of nuclear translocation and DNA-binding activity of NF-κB. Furthermore, Klotho overexpression was proved to decrease the nuclear expression of NF-κB as much as the treatment with PDTC, which provides support for the direct regulation of NF-κB by Klotho.
Conclusions
Collectively, our work provides new insight into the potential role of Klotho in the context of UVB-induced injuries in human keratinocytes, as well as providing the basis for future study of new therapies against UV-related skin disease.
MeSH Keywords: Keratinocytes; Keratosis, Actinic; NF-kappa B; Ultraviolet Rays
Background
Actinic keratosis (AK) is a common skin condition caused by chronic ultraviolet (UV) radiation exposure [1–3]. AK is a major concern in public health and can progress to keratinocyte carcinoma (AC) due to the impaired defensive antioxidant and DNA-repair mechanisms caused by prolonged UVB damage [4,5]. As a self-protection, UVB can induce cell death through distinct pathways, including DNA damage, reactive oxygen species (ROS) production, and death receptor activation, all of which contribute to the overall UV-induced apoptosis [6,7]. Thus, the ideal therapies should be able to eliminate UVB-induced cell damages and impaired cells, as well as being able to interfere with the associated signaling pathways [8].
The nuclear factor-κB (NF-κB) family of transcription factors, which consist of 5 different members, plays essential roles in response to stress, such as UVB radiation [9–11]. Upon stimulation, NF-κB is able to translocate from the cytoplasm, where it is normally sequestered, into the nucleus to exert its transcriptional functions [12]. It is recognized that NF-κB is a context-dependent apoptosis regulator having both pro-apoptotic and anti-apoptotic functions within a single cell type [13], and controls a wide variety of genes activated upon inflammation, such as IL-6 [14]. Considerable progress has been made in understanding the details of the signaling pathways that regulate NF-κB activity, particularly those responding to the proinflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) [12]. Chun et al. reported NF-κB signaling protects keratinocytes from DNA damage-induced death and facilitates the establishment of a tumor-nurturing proinflammatory microenvironment [15].
In recent decades, Klotho has attracted increasing attention due to its pleiotropic functions in aging-related diseases and disorders. Recently, numerous studies indicated the relationship between Klotho and NF-κB. For instances, Yoshihiro et al. found NF-κB activation can be attenuated by Klotho protein administration in endothelial cells [16] and Buendía et al. also reported that Klotho suppresses nuclear NF-κB activation and the subsequent transcription of proinflammatory genes [17]. Given the central role of NF-κB in UVB-induced injuries, these results encouraged us to explore the potential protective role of Klotho against UVB radiation. To the best of our knowledge, there is no previously published report on the promise of Klotho protein in this context.
In the present study, we exposed human keratinocytes HaCaT cells to high-intensity UVB radiation and studied the effects on the expressions of NF-κB as well as Klotho. Both in vitro and in vivo results consistently indicated the simultaneous changes of both proteins, suggesting the direct association between them. Use of the Klotho-overexpressing cell model helped us to investigate the influence of Klotho on the expression and cytoplasm/nucleus shuttling of NF-κB, as well as confirming the protective function of Klotho against UVB radiation in human keratinocytes. Our findings provide the basis for future study on prevention of actinic keratosis and carcinoma.
Material and Methods
Cell line and cell culture
The human immortalized and non-tumorigenic keratinocyte cell line (HaCaT) was purchased from the Cell Bank of Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Inc., Rochester, NY, USA) and 1% penicillin/streptomycin solution (Solarbio, Shanghai, China) at 37°C.
Plasmids construction and gene overexpression
The cDNA of Homo sapiens Klotho (KL) (GenBank accession number NM_004795.3) was inserted into the EcoRI and BamHI sites of pLVX-Puro. The insert was confirmed by sequencing. Virus particles carrying Klotho were produced by transfecting 293T cells with pLVX-Puro-Klotho together with viral packaging vectors (psPAX2, pMD2G) as previously described [18], and then HaCat cells were infected with virus-containing supernatants. The alteration of expression of Klotho was confirmed by real-time RT-PCR and Western blotting.
Cell proliferation assay
Cell proliferation was monitored by the colorimetric water-soluble tetrazolium salt assay using a Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. Cells were seeded onto 96-well plates (2×103 cells/well), and cell proliferation was documented every 24 h. The number of viable cells was assessed by measurement of the absorbance at 450 nm using a microplate reader.
Apoptosis assay
Apoptotic cells were assessed by staining with the Annexin V-FITC Apoptosis Detection Kit (Beyotime Institute of Biotechnology, China) following the instructions of the standard manual. Specifically, 1×106 HaCaT cells were collected, washed, and resuspended in 195 μL Annexin V-FITC-binding buffer. Then, the samples were co-stained with Annexin V-FITC (5 μL) and propidium iodide PI (5 μL) in the dark for 15 min. Next, apoptotic cells were determined by flow cytometry. Apoptosis was detected using a Guava easyCyte HT flow cytometer (Millipore, Bedford, MD, USA).
Western blot analysis
Western blotting was performed as described before [19]. Briefly, cells were harvested and lysed and the supernatants were collected. For NF-κB evaluation, cytoplasmic and nuclear fractionation was extracted separately. Then, the protein samples were separated with 12.5% SDS/PAGE. The proteins were transferred to PVDF membranes and probed with anti-Klotho, anti-NF-κB (Abcam, Cambridge, MA, USA), anti-Bcl-2, and anti-Bax (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies (1: 1000) at room temperature for 2 h, and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rat, goat anti-rabbit, or donkey anti-goat Ig (1: 1000) (Beyotime Biotechnology, China) for 15 min. GAPDH, β-actin, and H3 were used as internal controls (CST, Danvers, MA, USA). The X-ray film was developed by an enhanced chemo-luminescence system.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cultured cells with TRIzol reagents (Life Technologies, Grand Island, NY, USA) according to the standard manual. RNA was reversely transcribed using the RevertAid First Strand cDNA Synthesis Kit (Fermentas, St Leon-Rot, Germany) before quantification with spectrophotometry. Quantitative real-time PCR was carried out on the Applied Biosystems Prism 7300 sequence detection system with Maxima SYBR Green/ROX qPCR Master Mix (Fermentas, St Leon-Rot, Germany) according to the manual. PCR conditions were: 50°C for 2 min and 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 60°C for 45 s. GAPDH was considered as an internal control. The specific primers used in real-time PCR are shown as below (Table 1).
Table 1.
Real-Time PCR primer sequences.
| Gene | Accession number | Primer sequence | Length |
|---|---|---|---|
| Homo sapiens Klotho | XM_006719895.2 | F: 5′ TCTTCAGCCTTGTTCTAC 3′ R: 5′ TTTACTGTGGTGGACATC 3′ |
192 bps |
| NF-κB | NM_001145138.1 | 5′ GGAGCACAGATACCACCAAGAC 3′ 5′ CTCAGCCTCATAGAAGCCATCC 3′ |
161 bps |
| GAPDH | NM_001256799.2 | F: 5′ AATCCCATCACCATCTTC 3′ R: 5′ AGGCTGTTGTCATACTTC 3′ |
218 bps |
Measurement of Caspase-3 Activity
Cells (4×106) were lysed with 200 μL pre-cooled lysis buffer supplemented with DTT (Sigma, MO, USA) (volume ratio 100: 1), which were then centrifuged (14 000 g) for 15 min at 4°C and collected with supernatant for the following study. Caspase-3 activity was measured with caspase-3 colorimetric assay kit (Medical&Biological Laboratories, Nagoya, Japan) following the manufacturer’s protocol [20].
Enzyme-linked immunosorbent assay (ELISA) analysis
After being collected, the concentrations of TNF-α, IL-6, and IL-1β in the supernatant were measured using a standard ELISA Kit (JRDUN Biotechnology, Shanghai, China) following the instructions of the manufacturer [21]. For each well, absorbance at 450 nm was measured with a microplate reader. Concentrations were determined by comparing the optical density with the standard curve.
UVB irradiation and tissue staining
To construct a UVB-induced skin impairment in vivo model, 12 female Hairless SKH-1 mice (Charles River Laboratories, Saint-Germain-sur-L’Arbresle, France) were used and were fed with standard chow. All mice (6–8 weeks old, weighing 18–20 g) in individual cages (1 mouse/cage) were UVB-exposed 4 times per week for 12 weeks. UV-B radiation was generated by a UVB device (SUV1000, Shanghai SIGMA), range 280–320 nm, with an energy peak at 312 nm. Gradual UVB exposure was performed for 10 min each time in SKH-1 mice. After 12 weeks, mice were sacrificed and 2 cm2 of back skin was removed for histological analyses. Hematoxylin and eosin (HE) staining was carried out on sections following the standard protocol reported in previous studies [22–24]. Immunohistochemistry (IHC) staining of Klotho and NF-κB was performed on paraffin-embedded sections of both normal and UVB irradiated skin of mice with anti-Klotho and anti-NF-κB (Abcam, Cambridge, MA, USA) according to the method described in a previous report [25]. All experiments were approved by the Animal Studies Subcommittee of our institution.
Statistical analysis
All experiments were performed in triplicate and the results are expressed as the mean ±SD. Data were analyzed using SPSS12.0 statistical analytical software (Chicago, IL, USA). Comparison between different experimental groups was performed with t tests. Differences with p-values <0.05 were considered statistically significant.
Results
In vitro study of UVB effects on NF-κB and Klotho expressions in HaCaT cells
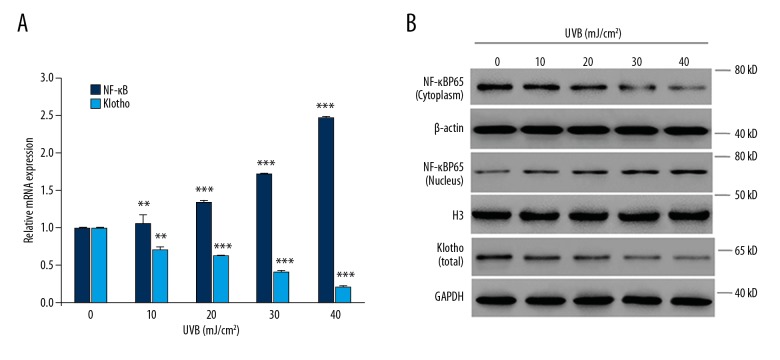
We found that UVB exposure significantly enhanced the mRNA expression of NF-κB in human keratinocytes, in an intensity-dependent (10, 20, 30, 40 mJ/cm2) manner (Figure 1A). Given the nuclear/cytoplasmic shuttling of NF-κB, we also determined the protein expressions in cytoplasm and nuclei, separately. As a result, we found that UVB exposure dramatically decreased the protein level of NF-κB in cytoplasm while increased its production in nuclei, both of which occurred in an intensity-dependent (10, 20, 30, 40 mJ/cm2) manner (Figure 1B). Moreover, it was found that UVB exposure significantly decreased the mRNA expression of Klotho in an intensity-dependent manner in HaCaT cells (Figure 1A), which was confirmed by the Western blot analysis, which showed similar changes in the protein production of Klotho (Figure 1B).
Figure 1.
In vitro study of the effects of UVB exposure on the expressions of NF-κB and Klotho proteins. (A) The mRNA expressions of NF-κB and Klotho in the human HaCaT cell line treated with UVB radiation at varying intensities (0–40 mJ/cm2). (B) The protein expressions of NF-κB and Klotho in UVB-treated HaCaT cells as indicated above. The amount of NF-κB protein in cytoplasm and nucleus were determined separately with Western blot analysis. Statistical significance was confirmed with ** P<0.01, *** P<0.001 compared with 0 mJ/cm2.
Klotho overexpression inhibited the nuclear translocation of NF-κB in HaCaT cells
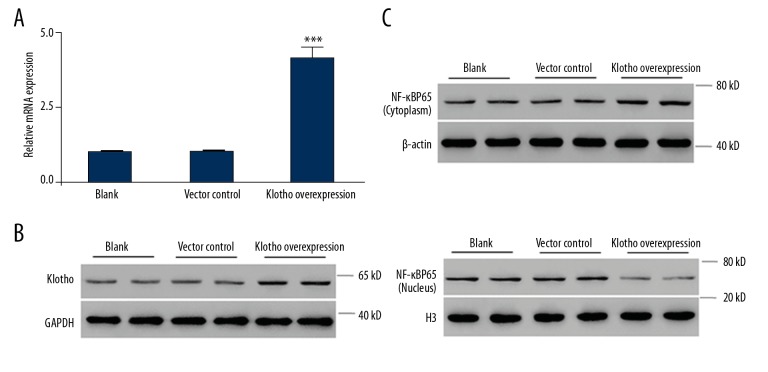
The above results encouraged us to hypothesize that Klotho can regulate the expression and nuclear translocation of NF-κB in HaCaT cells, and thus may be closely involved in the cellular response to UVB radiation. We constructed Klotho-overexpressed HaCaT cells. The expressions of Klotho were confirmed with qRT-PCR and Western blot analysis (Figure 2A, 2B) and the result was statistically significant (P<0.001). On this basis, we determined the protein level of NF-κB in cytoplasm and nuclei, separately. The Western blotting results clearly showed that compared with the vector control cells, which are similar to the normal control cells, the cytoplasmic NF-κB in the Klotho-overexpressing HaCaT cells was significantly increased while the nuclear NF-κB was significantly reduced (Figure 2C). These data support our hypothesis and suggest a direct relationship between Klotho and NF-κB in human keratinocytes, which is in agreement with previous reports [26].
Figure 2.
Effects of Klotho overexpression on the cytoplasmic and nuclear amount of NF-κB protein. (A) The quantification of Klotho production in Klotho-overexpressing HaCaT cells. (B) Western blot analysis of Klotho production in Klotho-overexpressing HaCaT cells. (C) Western blot analysis of NF-κB expression in cytoplasm and nucleus. Statistical significance was confirmed with *** P<0.01 compared with vector control.
Klotho overexpression protected HaCaT cells from UVB damage
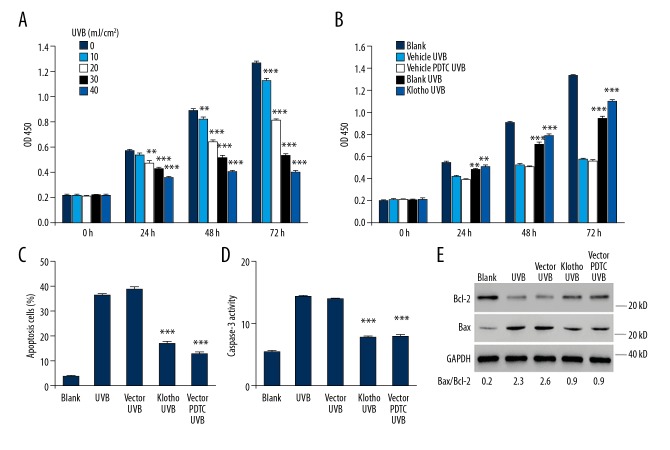
We examined the cytotoxic effects of UVB radiation (10, 20, 30, 40 mJ/cm2) on normal HaCaT cells for different exposure times (24, 48, 72 h), and our results showed significant cell growth inhibition in both intensity- and time-dependent manners (Figure 3A). On this basis, we exposed Klotho-overexpressing HaCaT cells to 30 mJ/cm2 UVB radiation and investigated the potential of Klotho to protect cells from UVB-induced injuries. Our results show that Klotho overexpression greatly alleviated the cell growth inhibition, which also follows a time-dependent manner (Figure 3B). Moreover, the protective effects of Klotho overexpression are comparable to the effects of administration of the NF-κB inhibitor pyrrolidine dithiocarbonate (PDTC) at a dose of 10 μM, which was reported to inhibit nuclear translocation and DNA-binding activity of NF-κB [27].
Figure 3.
Protective effects of Klotho overexpression on HaCaT cells against UVB radiation exposure. (A) Effects of UVB radiation on the cell growth in HaCaT cells. (B) The protection against UVB-induced cell growth inhibition by Klotho overexpression and NF-κB inhibitor PDTC in HaCaT cells. We assessed the effects of Klotho overexpression and NF-κB inhibition with PDTC on UVB-induced (C) apoptosis, (D) Caspase-3 activity, and (E) expression of apoptotic markers in HaCaT cells. Asterisks indicate significant difference ** P<0.01, *** P<0.001 compared with 0 mJ/cm2 or vector_UVB.
We also assessed the apoptosis in normal and Klotho-overexpressing HaCaT cells, showing that 24-h exposure of UVB radiation at the intensity of 30 mJ/cm2 resulted in dramatically enhanced apoptosis in HaCaT cells. But Klotho overexpression largely reversed the cytotoxic effect by reducing the apoptotic cells, which is comparable to the results of pre-treatment with PDTC (10 μM) (Figure 3C). To gain insights into the molecular mechanism, we evaluated the changes in selected protein markers. We found that UVB radiation enhanced the Caspase-3 activity (Figure 3D) and largely increased the amount of pro-apoptotic factor Bax, while decreasing the anti-apoptotic protein Bcl-2 (Figure 3E). Furthermore, Klotho overexpression was able to reverse these effects, which is comparable with that of PDTC administration. Further densitometric analysis revealed the significant decrease of Bax/Bcl-2 ratio from 2.6 to 0.9 by the protection of Klotho and PDTC, respectively.
Klotho overexpression decreased the production of TNF-α, IL-6, and IL-1β
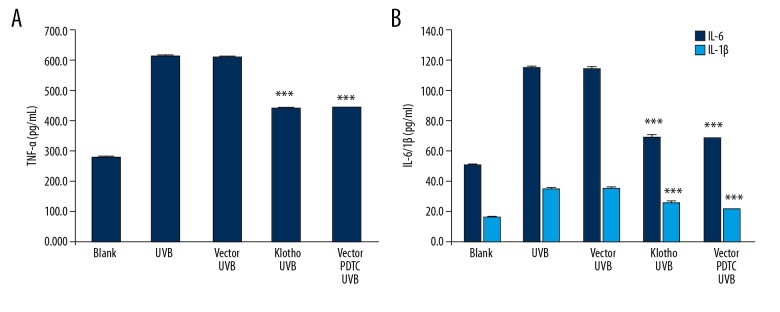
We examined the effects of UVB as well as the protection of Klotho and PDTC on the production of these factors. Consistent with the above findings, exposure to UVB enhanced the production of TNF-α, IL-6, and IL-1β in HaCaT cells, while Klotho overexpression and PDTC pre-treatment reversed the effect to a similar extent (Figure 4A, 4B).
Figure 4.
Effects of Klotho overexpression and NF-κB inhibition on UVB-induced expression of proinflammatory cytokines in HaCaT cells. (A) Production of TNF-α determined by ELISA assay. (B) Production of IL-6 and IL-1β determined by ELISA assay. Statistical significance was confirmed with *** P<0.001 compared with vector_UVB.
Klotho overexpression reduced the nuclear translocation of NF-κB
As shown in Figure 5, in the absence of UVB irritation, NF-κB primarily remains in inactive state by locating in the cytoplasm. However, with 24-h exposure to 30 mJ/cm2 UVB radiation, NF-κB becomes active by translocating to the nucleus, where it can start transcription. This is supported by the significant increase in nuclear NF-κB protein. In contrast, Klotho overexpression, very similar to the administration of PDTC, was able to reduce the shuttling of NF-κB between cytoplasm and nucleus, which was evidenced by the decreased protein expressions. Collectively, our results indicate that Klotho plays a protective role in HaCaT cells against UVB radiation by directly regulating the activation of NF-κB, thus alleviating the inflammatory reactions and cellular impairment.
Figure 5.
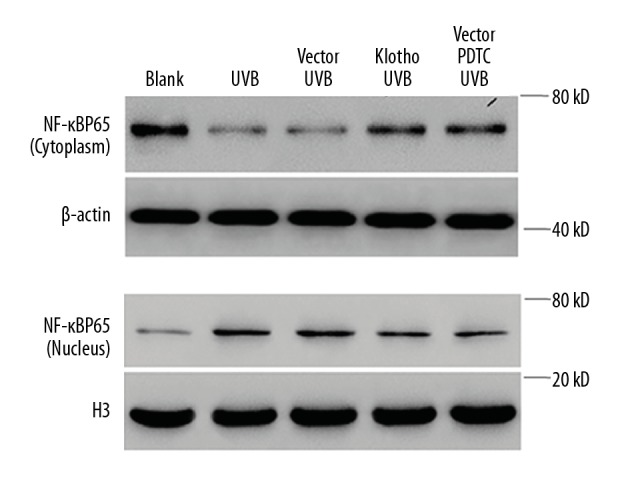
Effects of Klotho overexpression and NF-κB inhibition on UVB-induced expression of NF-κB protein in both cytoplasm and nucleus in HaCaT cells.
In vivo study of UVB effects on the epidermis and expressions of NF-κB and Klotho
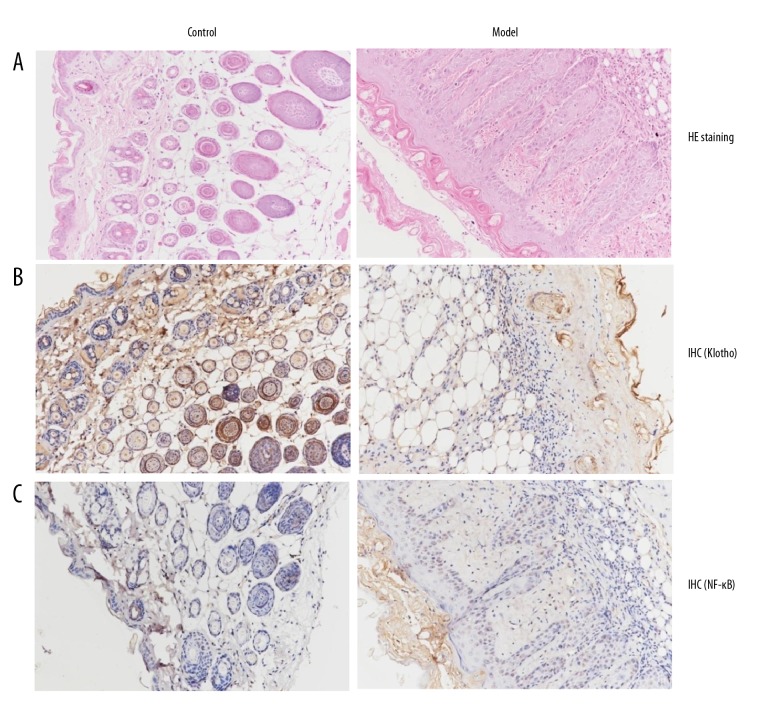
To confirm the above findings made in human keratinocytes, the effect of UVB mouse skin was evaluated. As shown by hematoxylin and eosin (HE) staining results (Figure 6A), the granular and spinous layers of UVB-treated mice were significantly thickened, with increased numbers of inflammatory cells compared to the control. We also determined the protein amounts of Klotho and NF-κB with immunohistochemistry (IHC) staining. From our results, it can be seen that there is significant decrease in the expression of Klotho (Figure 6B) and increase in NF-κB expression (Figure 6C) upon exposure to UVB radiation, which is in agreement with the above findings in the HaCaT cell line. These data strongly suggest a direct association between both targets, which was previously recognized in other cells [16,17].
Figure 6.
In vivo study of the effects of UVB exposure on the epidermis and protein expressions. (A) Hematoxylin and eosin (HE) staining of skin tissues in the SKH-1 mouse model treated with UVB radiation for 12 weeks. Immunohistochemistry (IHC) staining of (B) Klotho and (C) NF-κB proteins in the SKH-1 mouse model treated with UVB radiation 12 weeks. Representative histological pictures of each group are depicted.
Discussion
Cumulative UV radiation from sun exposure is the primary cause for a variety of skin disorders [28], including actinic keratoses and premature aging of the skin, which are common in Western countries [29]. There has been no agreement on the most efficacious treatment [4,30]. Therefore, it is necessary to gain new insight into the molecular mechanism underlying the stress responses. Klotho is a gene involved in premature aging syndromes and cell senescence [31,32], and has been found to be able to protect cells from a variety of insults and injuries [33]. Our investigation explored the potential role of the anti-aging protein Klotho in protection against UVB radiations, which could provide the basis for new therapies for UV-related skin diseases.
Previous reports showed that NF-κB plays an essential role in response to UVB radiation, which induces increased expression and DNA-binding activity of NF-κB [34,35]. For instance, the pioneering work by Nanxin et al. reported that exposure of mammalian cells to UV radiation induced significant DNA-binding activity of NF-κB and nuclear translocation of its p65 (RelA) subunit [36]. Actually, NF-κB plays essential role in response to stressful situation [9–11]. It has been established that the cytoplasmic NF-κB sequestering prevents its transcriptional activity and it must be translocated into the nucleus to function [12]. In our experiments, it was found UVB irradiation largely increased the mRNA expression and nuclear protein amount of NF-κB, which is consistent with previous reports. Moreover, it was also found that UVB significantly reduced the mRNA and protein expressions of Klotho in HaCaT cells. These findings were further confirmed by in vivo research, which showed similar changes in the UVB-impaired hyper-thickened epidermis, suggesting a direct relationship between both targets.
Our studies with Klotho overexpression directly validate that Klotho exerts effective protection of HaCaT cells against damage caused by high-intensity UVB radiation, such as alleviating cell growth inhibition and apoptosis. The molecular mechanism study revealed that Klotho overexpression reduced Caspase-3 activity and induced decreased expression of pro-apoptotic Bax, as well as increasing production of anti-apoptotic Bcl-2. It is well-established that the indicator of Bax/Bcl-2 is important for the induction and processing of apoptosis [37], whose elevation generally corresponds with the onset of cell apoptosis [38]. The significant decrease suggests the effective inhibition of cell apoptosis. These results are consistent with observations of cell growth inhibition. These effects were found to be comparable to that of NF-κB inhibition with administration of PDTC, which was reported to inhibit nuclear translocation and DNA-binding activity of NF-κB [27]. This result suggests that the protection of cells against UVB by Klotho overexpression is most likely mediated by the inhibition of NF-κB activity. Consistent with the above, Klotho has been found to be associated with activation of NF-κB in different cell types [16,17]. Accumulating evidence suggests that the anti-aging protein Klotho plays an important role in mediating inhibition of NF-κB and prevention of nuclear translocation [17,39].
Recently, considerable progress has been made in understanding the details of the signaling pathways that regulate NF-κB activity, particularly those responding to the proinflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6 [12,40]. According to previous reports, NF-κB can be activated by a wide variety of different stimuli, including TNF-α, IL-6, and IL-1 [12,40]. Subsequently, it was reported that the activated NF-κB also controls these genes and strengthens the response to inflammation [14]. We showed that Klotho overexpression caused the enhanced production of proinflammatory cytokines, including TNF-α, IL-6, and IL-1β. Subsequently, the activated NF-κB also controls these genes and strengthens the response to inflammation [14]. Our data prove the anti-inflammation effects of Klotho protein in UVB-treated HaCaT cells.
Conclusions
In conclusion, the present study confirmed that Klotho overexpression increased mRNA expression and the nuclear translocation of NF-κB, which shows its anti-UVB potential in human keratinocytes. Our work provides the basis for future study of new therapies against UV-related skin diseases and disorders.
Footnotes
Source of support: This study was supported by the Science and Technology Innovation Project of Shanghai Jiao Tong University School of Medicine (16XJ21011), the Shanghai Jiaotong University “Medical Professionals Cross Fund” (YG2017QN59), the Changning District Committee of Science and Technology (CNKW2016Y04), and the Introduction of Talent Research Start-up Fund of Tongren Hospital (TR2014rc15)
Conflict of interest.
None.
References
- 1.Uhlenhake EE. Optimal treatment of actinic keratoses. Clin Interv Aging. 2013;8:29–35. doi: 10.2147/CIA.S31930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey I, Frankel S, Marks R, et al. Non-melanoma skin cancer and solar keratoses II analytical results of the South Wales Skin Cancer Study. Br J Cancer. 1996;74:1308–12. doi: 10.1038/bjc.1996.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt JV, Miot HA. Actinic keratosis: A clinical and epidemiological revision. An Bras Dermatol. 2012;87:425–34. doi: 10.1590/s0365-05962012000300012. [DOI] [PubMed] [Google Scholar]
- 4.Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: A review. Br J Dermatol. 2017;177:350–58. doi: 10.1111/bjd.14852. [DOI] [PubMed] [Google Scholar]
- 5.Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895–904. doi: 10.1111/j.1365-4632.2007.03166.x. [DOI] [PubMed] [Google Scholar]
- 6.Assefa Z, Van Laethem A, Garmyn M, Agostinis P. Ultraviolet radiation-induced apoptosis in keratinocytes: on the role of cytosolic factors. Biochim Biophys Acta. 2005;1755:90–106. doi: 10.1016/j.bbcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Kulms D, Schwarz T. Independent contribution of three different pathways to ultraviolet-B-induced apoptosis. Biochem Pharmacol. 2002;64:837–41. doi: 10.1016/s0006-2952(02)01146-2. [DOI] [PubMed] [Google Scholar]
- 8.Vitale N, Kisslinger A, Paladino S, et al. Resveratrol couples apoptosis with autophagy in UVB-irradiated HaCaT cells. PLoS One. 2013;8:e80728. doi: 10.1371/journal.pone.0080728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–28. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 11.Tak PP, Firestein GS. NF-kappaB: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 13.Lin B, Williams-Skipp C, Tao Y, et al. NF-kappaB functions as both a proapoptotic and antiapoptotic regulatory factor within a single cell type. Cell Death Differ. 1999;6:570–82. doi: 10.1038/sj.cdd.4400528. [DOI] [PubMed] [Google Scholar]
- 14.Yasumoto K, Okamoto S, Mukaida N, et al. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-κB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992;267:22506–11. [PubMed] [Google Scholar]
- 15.Kim C, Pasparakis M. Epidermal p65/NF-kappaB signalling is essential for skin carcinogenesis. EMBO Mol Med. 2014;6:970–83. doi: 10.15252/emmm.201303541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maekawa Y, Ishikawa K, Yasuda O, et al. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35:341–46. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 17.Buendía P, Ramírez R, Aljama P, Carracedo J. Klotho prevents translocation of NFκB. Vitam Horm. 2016;101:119–50. doi: 10.1016/bs.vh.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Zufferey R, Nagy D, Mandel RJ, et al. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–75. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 19.Burnette WN. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate – polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 20.Li CY, Lee JS, Ko YG, et al. Heat shock protein 70 inhibits apoptosis downstream of cytochrome C release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–71. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Mi Y, Yang H, et al. The activation of HMGB1 as a progression factor on inflammation response in normal human bronchial epithelial cells through RAGE/JNK/NF-kappaB pathway. Mol Cell Biochem. 2013;380:249–57. doi: 10.1007/s11010-013-1680-0. [DOI] [PubMed] [Google Scholar]
- 22.Braun S, Krampert M, Bodó E, et al. Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J Cell Sci. 2006;119:4841–49. doi: 10.1242/jcs.03259. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Rudic RD, Sessa WC. Nitric oxide-releasing aspirin decreases vascular injury by reducing inflammation and promoting apoptosis. Lab Invest. 2002;82:825–32. doi: 10.1097/01.lab.0000018828.61722.bd. [DOI] [PubMed] [Google Scholar]
- 24.Murakami K, Inagaki J, Saito M, et al. Skin atrophy in cytoplasmic SOD-deficient mice and its complete recovery using a vitamin C derivative. Biochem Biophys Res Commun. 2009;382:457–61. doi: 10.1016/j.bbrc.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 25.Athar M, An KP, Morel KD, et al. Ultraviolet B (UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001;280:1042–47. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- 26.Farrington Daniels M, Doris Brophy LES, Lobitz, Walter C. Histochemical responses of human skin following ultraviolet irradiation 1. J Invest Dermatol. 1961;37:351–57. [PubMed] [Google Scholar]
- 27.Nurmi A, Vartiainen N, Pihlaja R, et al. Pyrrolidine dithiocarbamate inhibits translocation of nuclear factor kappa-B in neurons and protects against brain ischaemia with a wide therapeutic time window. J Neurochem. 2004;91:755–65. doi: 10.1111/j.1471-4159.2004.02756.x. [DOI] [PubMed] [Google Scholar]
- 28.Virasa BC, Taylor HR, Strickland PT, et al. Association of nonmelanoma skin cancer and actinic keratosis with cumulative solar ultraviolet exposure in Maryland watermen. Cancer. 1990;65:2811–17. doi: 10.1002/1097-0142(19900615)65:12<2811::aid-cncr2820651234>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 29.Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 30.Weinstock MA, Bingham SF, Digiovanna JJ, et al. Tretinoin and the prevention of keratinocyte carcinoma (basal and squamous cell carcinoma of the skin): A veterans affairs randomized chemoprevention trial. J Invest Dermatol. 2012;132:1583–90. doi: 10.1038/jid.2011.483. [DOI] [PubMed] [Google Scholar]
- 31.Li SA, Watanabe M, Yamada H, et al. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 32.German DC, Khobahy I, Pastor J, et al. Nuclear localization of Klotho in brain: An anti-aging protein. Neurobiol Aging. 2012;33:1483.e25. doi: 10.1016/j.neurobiolaging.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji-Hee K, Kyu-Hee H, Kyu-Sang P, et al. Biological role of anti-aging protein Klotho. J Lifestyle Med. 2015;5:1–6. doi: 10.15280/jlm.2015.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brach MA, Hass R, Sherman ML, et al. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991;88:691–95. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell KJ, Chapman NR, Perkins ND. UV stimulation induces nuclear factor kappaB (NF-kappaB) DNA-binding activity but not transcriptional activation. Biochem Soc Trans. 2001;29:688–91. doi: 10.1042/0300-5127:0290688. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-κB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95:13012–17. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim ME, Ha TK, Yoon JH, Lee JS. Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Res. 2014;34:701–6. [PubMed] [Google Scholar]
- 38.Perlman H, Zhang X, Chen MW, et al. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ. 1999;6:48–54. doi: 10.1038/sj.cdd.4400453. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Banerjee S, Dey N, et al. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (Serine)536 phosphorylation. Diabetes. 2011;60:1907–16. doi: 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Walia B, Evans J, et al. IL-6 induces NF-κB activation in the intestinal epithelia. J Immunol. 2003;171:3194–201. doi: 10.4049/jimmunol.171.6.3194. [DOI] [PubMed] [Google Scholar]