Abstract
Background
The aim of this paper was to investigate the association between clinicopathological factors and the coagulation test in lung cancer patients during follow-up care after treatment.
Material/Methods
Ninety-five medical patients with histologically proven advanced lung carcinoma (LC) who had undergone radiotherapy were prospectively reviewed between January 2014 and December 2016. The study investigated the relationship between the biochemical results, the disease stage, and the survival rate in lung cancer patients. Post-treatment coagulation-based D-dimer (DD), fibrinogen (Fib), and complete blood count (CBC) were evaluated during the follow-up over a period of 2 years after treatment or until the patient’s death.
Results
An increase of D-dimer generates an increased chance of early death by approximately 0.03% per 1 D-dimer unit. In cases when the difference in the D-dimer concentration equals 1000, the risk of an early death increases by (1.00031000−1)×100%=35%.
Conclusions
High levels of D-dimer are associated with an advanced form of disease with metastasis and higher risk of early death in lung cancer patients.
MeSH Keywords: Biological Markers; Blood Coagulation Tests; Carcinoma, Non-Small-Cell Lung
Background
Many researchers have suggested a correlation between the hemostatic system and cancer cells [1,2] and have presented evidence that the activation of coagulation and the fibrinolytic system by cancer cells facilitates cancer invasiveness and metastases [3]. This activation has been associated with the tumor stage and prognosis in malignancies such as breast, colorectal, and lung cancer [4–6]. The connection between venous thromboembolism (VTE) and cancer (the worsening of the clinical course of the disease and thus the survival rate of patients) has also been proven [7]. The association between VTE risk and the effects of cancer treatment can be described as follows:
Patients with chemotherapy treatment: non-metastatic patients hazard ratio (HR)=2; metastatic patients HR=2.3;
Patients with radiotherapy treatment: non-metastatic patients HR=0.8, metastatic patients=HR−0.7 [8].
Radiotherapy treatment alone does not increase the risk of VTE [9]. Hence, the aim of this study was to investigate whether simple laboratory blood tests, such as morphology, D-dimer (DD), and fibrinogen (Fib), can show changes over time after radiotherapy treatment. The relationship between those changes, the early VTE symptoms, and/or the overall survival (OS) was investigated. Post-treatment DD, Fib, and a complete blood count (CBC) were evaluated during the follow-up period for up to 2 years after the treatment or until the patient’s death.
Material and Methods
Subjects’ characteristics
This prospective study analyzed a total of 95 patients with lung cancer confirmed histologically or cytologically, who were treated at the Regional Clinical Hospital in Zielona Góra between 2015 and 2016. The disease stage was defined based on certain clinical and physical examinations: thoracic computed tomography (CT), brain CT or magnetic resonance imaging (MRI), abdominal ultrasonography, bone scintigraphy, and/or positron emission tomography-CT. The histopathological data was accessed in accordance with the Union for International Cancer Control (UICC) TNM classification [10]. The exclusion criteria were as follows: (i) a history of secondary tumor(s), (ii) an active infection, (iii) a familial coagulopathy, (iv) a peripheral vascular disease (thrombophlebitis and thromboembolism), (v) any treatment with anticoagulants and anti-aggregants, (vi) patients with a World Health Organization performance status of 4 (i.e., completely disabled, unable to care for themselves, confined to bed or a wheelchair). The serum specimens of DD, Fib, and complete blood count CBC – hemoglobin (Hb), platelets (PLT), white blood cells (WBC), neutrophiles (NEU), mean platelets volume (MPV) – were collected after radiotherapy treatment every 3–4 months during the follow-up. The subjects were divided into 2 groups: Group 1 was non-metastatic patients (58 patients) and Group 2 was metastatic patients (37 patients). Seventy patients had undergone palliative radiotherapy in 5 separate doses with the total dose of 20 Gy and 25 patients received radical radiotherapy. The group was 75% male and 25% female. According to the histopathology profile, 17% had non-small cell carcinoma, 28% had adenocarcinoma, 35% had squamous, and 20% had small cell carcinoma. Information pertaining to the date of death of a patient was obtained from the National Health Fund (Polish Civil Registration System). This study was approved by our institutional Ethics Committee (No. 2/57/2015). The last follow-up date was August 30, 2016.
Biochemical assays
Venous blood samples were drawn from peripheral blood and evaluated by measuring the CBC with a hematology analyzer (Abbott CD3700, CD RUBY, USA). The reference values at our hospital for these parameters are Hg: 12–18 g/dl; WBC: 4–10.2×103/μl, NEU: 2–6.9×103/μl; LYM: 0.6–3.4×103/μl, PLT: 140–420×103/μl; MPV: 7–11 fl; DD: 0.00–278 μg/L; and Fib: 200–472 mg/dl.
Statistical analysis
The patients’ clinical characteristics and features of the tumors are shown in Table 1. A survival analysis was done based on the Cox regression model. Additionally, the receiver operating characteristic (ROC) curves [11] were plotted to estimate the threshold value for a binary classifier of analyzed risk factors. Computations were performed using a “pROC” package [12] in the R platform [13]. A p-value of <0.05 was considered as significant in this analysis. Overall survival (OS) was defined as the time from radiotherapy to the time of a patient’s death by any cause or up to the last follow-up date when the patient was known to be alive.
Table 1.
Patients and clinical tumour characteristics.
| Characteristics | 95 patients (100%) |
|---|---|
| Age, years | |
| Median (range) | 67 (40–81) |
| Gender | |
| Male | 71 (75) |
| Female | 24 (25) |
| Histology | |
| Non-small cell carcinoma | 16 (17) |
| Adenocarcinoma | 27 (28) |
| Squamous | 33 (35) |
| Small cell carcinoma | 19 (20) |
| TNM factor | |
| T1a/T1b/T2/T3/T4 | 2/4/23/35/31 |
| N0 | 18 |
| N1N2/N3 | 9/51/17 |
| M0 | 58 |
| M1 | 37 |
T – tumour; N – nodes; M – metastases.
Results
The cut-off specificity and sensitivity values of DD, Fib, and CBC are shown in Tables 2, 3. The DD level of 1000 μg/L was arbitrarily set as 3.5 times the value of a normal DD level. The results with statistical significance from ROC analysis are presented, without any outcomes not deemed significant. By taking the estimated area under the curve (AUC) as 68.9% for the DD threshold value concentration=308 (μg/L), it can be established that 7 out of 10 patients were correctly classified to live or to die (Figure 1). Tables 4 and 5 show univariate analysis of the risk factor for lung cancer patients. The interpretation of the results above is as follows: each additional unit (mg/dl) of Fib resulted in nearly a 2% increase of the risk of an early death in patients; if the difference was 100 (mg/dl), then the risk increased by up to (1.0018100−1)×100%=20%. A DD increase results in an increased chance of an early death by approximately 0.03% per 1 DD unit (Figures 2, 3). In the case of a difference in DD concentration equal to 1000, the risk of an early death increases by (1.00031000−1)×100%=35%. For MPV, a difference of 2 units results in (1−0.72)×100%=51% risk reduction in the death of patients (Table 4).
Table 2.
Risk factors in Group without metastases.
| Risk factor | Treshlod value | Specificity (%) | Sensitivity (%) | AUC (95%CI) |
|---|---|---|---|---|
| D-dimer (μg/L) | 308.0 | 50.5 | 50.5 | 68.9% (61.1–76.6) |
| Fib (mg/dl) | 430.5 | 76.8 | 76.8 | 60.4% (51.2–69.9) |
| PLT (tys/μl), | 225.5 | 60.4 | 60.4 | 68.7% (60.6–76.9) |
| Hg (g/dl) | 12.8 | 62.0 | 62.0 | 61.2% (52.2–70.2) |
| WBC (tys/μl) | 7.9 | 70.0 | 70.0 | 74.5% (67–82) |
| NEU (tys/μl) | 5.6 | 73.3 | 73.3 | 75.8% (68.6–83.1) |
| MPV/PLT ratio | 2.7 | 72.4 | 72.4 | 66.0% (56.9–75) |
AUC – area under curves; Fib – fibrinogen; PLT – platelets; Hg – haemoglobin; WBC – white blood cells; NEU – neutrophils; MPV – mean platelets volume.
Table 3.
Risk factors in Group with metastases.
| Risk factor | Treshlod value | Specificity (%) | Sensitivity (%) | AUC (95%CI) |
|---|---|---|---|---|
| Hg (g/dl) | 12.9 | 71.8 | 68.9 | 76.6% (85.1–88) |
| MPV (fl) | 5.9 | 80 | 56.8 | 68.9% (57.2–80.6) |
AUC – area under curves; Hg – haemoglobin; MPV – mean platelets volume.
Figure 1.
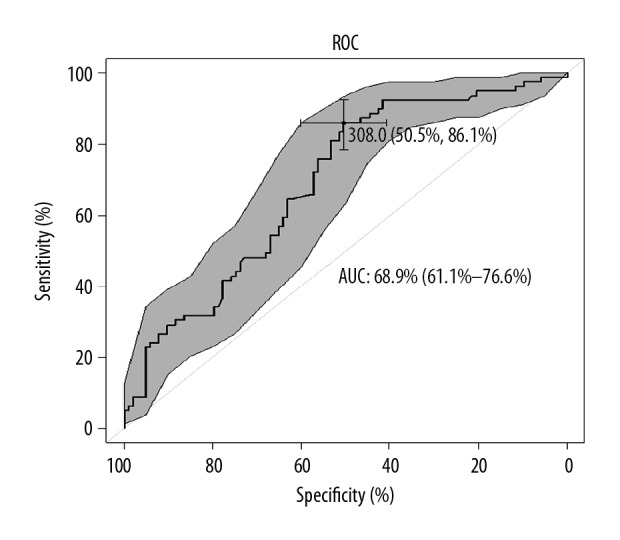
Receiver Operating Characteristic (ROC) curve analysis with area under curve (AUC) estimates of DD in group without metastases.
Table 4.
Univariate analysis of risk factor for lung cancer patients.
| Group | Risk factor | HR | (95%CI) | p-Value |
|---|---|---|---|---|
| Without metastases | Fibrynogen | 1.0018 | (1.0003, 1.0033) | 0.0196 |
| PLT | 1.0045 | (1.0027, 1.0064) | <0.0001 | |
| Hg | 0.76 | (0.66, 0.88) | 0.0003 | |
| WBC | 1.13 | (1.08, 1.19) | <0.0001 | |
| NEU | 1.17 | (1.1, 1.24) | <0.0001 | |
| D-dimer (1000) | 1.81 | (1.09, 2.98) | 0.0207 | |
| With metastases | D-dimer | 1.0003 | (1.0001,1.0005) | 0.0075 |
| MPV | 0.7 | (0.52, 0.93) | 0.0158 | |
| Hg | 0.69 | (0.56, 0.84) | 0.0003 | |
| D-dimer (1000) | 3.52 | (1.85, 6.67) | 0.0001 |
HR – hazard ratio; CI – confidence interval; Hg – haemoglobin; PLT – platelets; WBC – white blood cells; NEU – neutrophils; MPV – mean platelets volume.
Table 5.
Risk factors in group: radical and palliative treatment.
| Group | Risk factor | HR | 95% CI | p-Value |
|---|---|---|---|---|
| Radical | Fib | 1.013 | (1.003, 1.022) | 0.0076 |
| Palliative | D-dimer | 1.0002 | (1.0001, 1.0003) | 0.0111 |
| PLT | 1.003 | (1.002, 1.005) | <0.0001 | |
| Hg | 0.76 | (0.68, 0.85) | <0.0001 | |
| WBC | 1.10 | (1.05, 1.15) | <0.0001 | |
| NEU | 1.13 | (1.07, 1.19) | <0.0001 |
HR – hazard ratio; CI – confidence interval; Fib – fibrinogen; PLT – platelets; Hg – haemoglobin; WBC – white blood cells; NEU – neutrophils.
Figure 2.
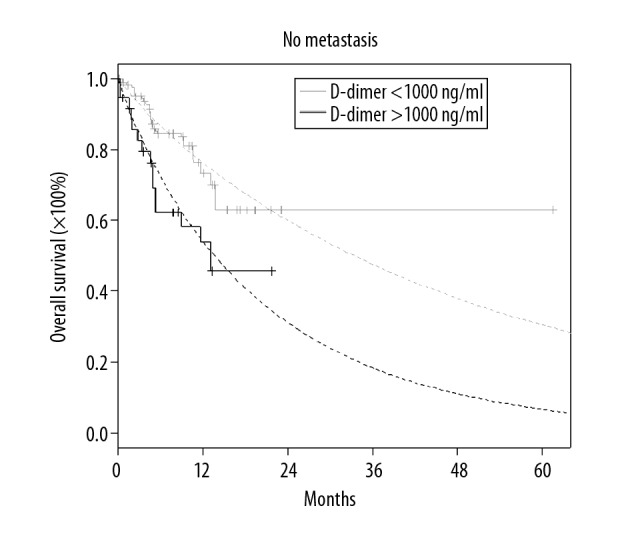
Overall survival for patients without metastasis in case of a difference in D-dimer concentration equal 1000 or above (Kaplan-Meier’s curves with Weibull’s approximations).
Figure 3.
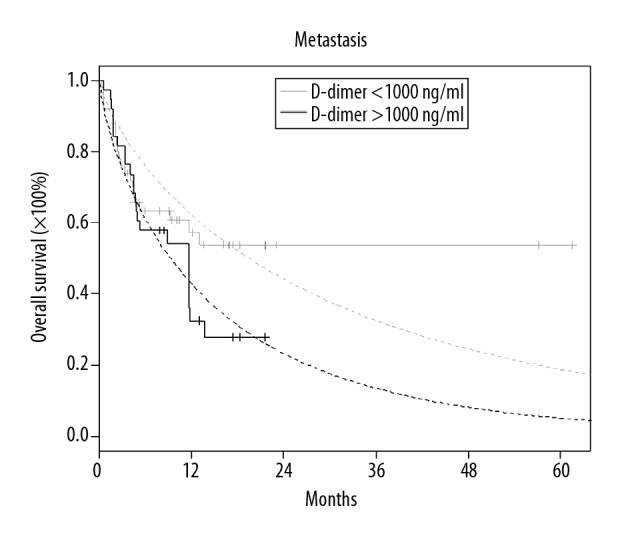
Overall survival for patients with metastasis in case of a difference in D-dimer concentration equal 1000 or above (Kaplan-Meier’s curves with Weibull’s approximations).
Discussion
The aim of this study was to investigate the association between clinicopathological factors and coagulation tests in lung cancer patients during the follow-up period after treatment. The smallest degradation product of fibrin is DD which is a sensitive indicator of the proteolytic actions of plasmin on fibrin [14,15]. Many researchers have suggested that DD is a valuable marker for prognosis and the treatment response evaluation in lung cancer cases [16–18]. It should be mentioned that DD level is increased in cancer patients without VTE, but a latent VTE may occur. In other studies, the following scoring system was improved on by expanding the score: DD ≥1.44 g/mL (1 point) and sP selectin ≥53.1 ng/mL (1 point) was used to predict VTE in cancer patients [19]. In the present study, however, only simple and economical laboratory tests were used (other tests have the status of science projects, such as sP selectin). An increased DD concentration was noted and correlated to patients with cancer progression. Patients with active cancer are more prone to thromboembolism and bleeding [7]. It was found that most patients had an increased plasma DD level, which was associated with a bad prognosis [20–25]. In the present study, we show that a high plasma DD level of over 1000 μg/L is associated with decreased OS in patients with advanced LC – for the group without metastases: HR 1.81 (95% confidence interval (CI): 1.09, 2.98, p=0.0207) and for the group with metastases: HR 3.52 (95% CI: 1.85, 6.67, p=0.0001). Similar to our study, Wang et al. [26] indicated that high D-dimer levels lead to a poor prognosis by reducing progression-free survival (PFS) and OS. Also, other studies showed a shorter OS (e.g., Ma et al. [27], Zhou et al. [28], and Taguchi et al. [29]). This is in accordance with our results showing that higher DD levels are associated with metastases, similar to the studies by Kilica et al. [30] and Yu et al. [31]. In our study, when the patients were divided into 2 groups – radical treatment and palliative treatment – the risk factor HR for palliative patients was 7.68 (95% CI, 1.87, 31.63; p=0.0048) times higher than for radical patients. The most important risk-related factors were the size of tumor (T) – HR: 1.37 (95% CI: 1.04, 1.8; p=0.024) and metastases (M) – HR: 1.51 (95% CI: 1.11, 2.04; p=0.0086). Therefore, elevated plasma DD levels are associated with T and M, as indicated by the results of other previous studies [32,33]. Another coagulation test factor – fibrinogen and fibrin – are localized in the tumor-host cells. Fibrinogen regulates inflammatory cell recruitment, supplies structure to the tumor, and induces angiogenesis [34–36]. In our study, we observed that increased Fib increased the chance of early death by approximately 2% per unit of Fib mg/dl in the group without metastases. The values of inflammatory factors tested (WBC and NEU) increased in both the palliative group and the group without metastases. Therefore, the elevated levels of Fib, WBC, and NEU were associated with an increased risk of lung cancer, thus suggesting that cancer promotes inflammatory processes [37–39]. Similarly, Simpson-Haidaris et al. stated in their study on breast cancer that Fib is a dynamic, multifunctional protein that influences many cellular processes during neogenesis [40]. Another study [41] suggested the use of plasma DD levels as a method of predicting clinical outcomes in patients with lung cancer and as a factor worsening the prognosis due to the persistence of high concentrations of DD during anticancer therapy. As can be concluded from the above, high DD, elevated Fib and other inflammatory-based factors are correlated with a risk of an early death in lung cancer patients.
Conclusions
High levels of DD are associated with advanced state of disease with metastases indicate the chance of early death in lung cancer patients. This study provides evidence that there are very high DD levels in patients with cancer who do not have clinical symptoms of VTE.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Trousseau A. Clinique Medicale de I’Hotel Dieu de Paris. 2nd ed. Vol. 3. Paris: Ballière; 1865. Phlegmasia alba dolens; pp. 654–712. [in French] [Google Scholar]
- 2.Gouin-Thibault I, Samama MM. Laboratory diagnosis of the thrombophilic state in cancer patients. Semin Thromb Hemost. 1999;25:167–72. doi: 10.1055/s-2007-994918. [DOI] [PubMed] [Google Scholar]
- 3.Gabazza EC, Taguchi O, Yamakami T, et al. Evaluating prethrombotic state in lung cancer using molecular markers. Chest. 1993;103:196–200. doi: 10.1378/chest.103.1.196. [DOI] [PubMed] [Google Scholar]
- 4.Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31:388–94. doi: 10.1093/jjco/hye075. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell K, Haroon Z, Broadwater G, et al. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol. 2000;18:600–8. doi: 10.1200/JCO.2000.18.3.600. [DOI] [PubMed] [Google Scholar]
- 6.Taguchi O, Gabazza EC, Yasui H, et al. Prognostic significance of plasma D-dimer levels in patient with lung cancer. Thorax. 1997;52:563–68. doi: 10.1136/thx.52.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tafur AJ, Wysokinski WE, McBane RD, et al. Cancer effect on periprocedural thromboembolism and bleeding in anticoagutaled patients. Ann Oncol. 2012;23:1998–2005. doi: 10.1093/annonc/mds058. [DOI] [PubMed] [Google Scholar]
- 8.Blom JW, Vanderschoot JPM, Oostindier MJ, et al. Incidence of venous thrombosis in a large cohort of 66 329 cancer patients: results of a record linkage study. J Thromb Haemostat. 2006;4:529–35. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 9.Khorana AA. Cancer-associated thrombosis: Updates and controversies. Hematology. 2012;2012:626–30. doi: 10.1182/asheducation-2012.1.626. [DOI] [PubMed] [Google Scholar]
- 10.Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC): TNM classification of malignant tumors. 7th ed. John Wiley and Sons, Ltd; UK: 2010. [Google Scholar]
- 11.Fawcett T. An introduction to ROC analysis. Pattern Recognition Letters. 2006;8:861–74. [Google Scholar]
- 12.Robin Z. pROC: Display and analyze ROC Curves. 2017. URL. http://web.expasy.org/pROC.
- 13.R Core Team R. A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. URL. https://www.R-project.org/ [Google Scholar]
- 14.Bick RL. Coagulation abnormalities in malignancy: A review. Semin Tromb Hemost. 1992;18:596–602. doi: 10.1055/s-2007-1002575. [DOI] [PubMed] [Google Scholar]
- 15.Francis CW, Marder VJ. Wiliams Hematology. ed. 5. McGraw Hill; 1995. Mechanism of fibrynolisis; pp. 1252–60. [Google Scholar]
- 16.Unsal E, Atalay F, Atikcan S, Yılmaz A. Prognostic significance of hemostatic parameters in patients with lung cancer. Resp Med. 2004;98:93–98. doi: 10.1016/j.rmed.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Altiay G, Ciftci A, Demir M, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol. 2007;19:494–98. doi: 10.1016/j.clon.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Komurcuoglu B, Ulusoy S, Gayaf M, et al. Prognostic value of plasma D-dimer levels in lung carcinoma. Tumori. 2011;97:743–48. doi: 10.1177/030089161109700611. [DOI] [PubMed] [Google Scholar]
- 19.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377–82. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 20.Ferrigno D, Buccheri G, Ricca I. Prognostic significance of blood coagulation tests in lung cancer. Eur Respir J. 2001;17:667–73. doi: 10.1183/09031936.01.17406670. [DOI] [PubMed] [Google Scholar]
- 21.Buccheri G, Torchio P, Ferrigno D. Plasma levels of D-dimer in lung cancer. Cancer. 2003;97:3044–52. doi: 10.1002/cncr.11432. [DOI] [PubMed] [Google Scholar]
- 22.Altiay G, Ciftci A, Demir M, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol. 2007;19:494–98. doi: 10.1016/j.clon.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Kömürcüoğlu B, Ulusoy S, Gayaf M, et al. Prognostic value of plasma D-dimer levels in lung carcinoma. J Thorac Oncol. 2007;2:S556. doi: 10.1177/030089161109700611. [DOI] [PubMed] [Google Scholar]
- 24.Antoniou D, Pavlakou G, Stathopoulos GP, et al. Predictive value of D-dimer plasma levels in response and progressive disease in patients with lung cancer. Lung Cancer. 2006;53:205–10. doi: 10.1016/j.lungcan.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Ursavaş A, Karadağ M, Uzaslan E, et al. Prognostic significance of plasma D-dimer levels in patients with lung cancer. Eur J Gen Med. 2010;7:155–60. [Google Scholar]
- 26.Wang Y, Wang Z. Predictive value of plasma D-dimer levels in patients with advanced non-small-cell lung cancer. Onco Targets Ther. 2015;8:805–8. doi: 10.2147/OTT.S78154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X, Li Y, Zhang J, et al. Prognostic role of D-dimer in patients with lung cancer: A meta-analysis. Tumor Biol. 2014;35:2103–9. doi: 10.1007/s13277-013-1279-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhou YX, Yang ZM, Feng J, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer: A meta-analysis. Tumor Biol. 2013;34:3701–4. doi: 10.1007/s13277-013-0953-2. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi O, Gabazza EC, Yasui H, et al. Prognostic significance of plasma D-dimer levels in lung cancer. Thorax. 1997;52:563–65. doi: 10.1136/thx.52.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilica L, Yildizb I, Karagoz Senb F, et al. D-dimer and international normalized ratio (INR) are correlated with tumor markers and disease stage in colorectal cancer patients. Cancer Biomarkers. 2015;15:405–11. doi: 10.3233/CBM-150477. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Li D, Lei D, et al. Tumor-specific D-dimer concentration ranges and influencing factors: A cross-sectional study. PLoS One. 2016;11:e0165390. doi: 10.1371/journal.pone.0165390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knowlson L, Bacchu S, Paneesha S, et al. Elevated D-dimers are also a marker of underlying malignancy and increased mortality in the absence of venous thromboembolism. J Clin Pathol. 2010;63:818–22. doi: 10.1136/jcp.2010.076349. [DOI] [PubMed] [Google Scholar]
- 33.Raj SD, Zhou X, Bueso-Ramos CE, et al. Prognostic significance of elevated D-dimer for survival in patients with sarcoma. Am J Clin Oncol. 2012;35:462–67. doi: 10.1097/COC.0b013e31821d4529. [DOI] [PubMed] [Google Scholar]
- 34.Costantini V, Zacharski LR, Memoli VA, et al. Fibrinogen deposition without thrombin generation in primary human breast cancer tissue. Cancer Res. 1991;51:349–53. [PubMed] [Google Scholar]
- 35.Brown LF, Van De Water L, Harvey VS, Dvorak HF. Fibrinogen influx and accumulation of cross-linked fibrin in healing wounds and in tumor stroma. Am J Pathol. 1988;130:455–65. [PMC free article] [PubMed] [Google Scholar]
- 36.Brown LF, Dvorak AM, Dvorak HF. Leaky vessels, fibrin deposition, and fibrosis: A sequence of events common to solid tumors and to many other types of disease. Am Rev Respir Dis. 1989;140:1104–7. doi: 10.1164/ajrccm/140.4.1104. [DOI] [PubMed] [Google Scholar]
- 37.Allin KH, Bojesen SE, NordestgaardInt BG. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. J Cancer. 2016;139:1493–500. doi: 10.1002/ijc.30194. [DOI] [PubMed] [Google Scholar]
- 38.Wolny-Rokicka E, Brzeźniakiewicz-Janus K, Wydmański J, et al. Analysis of haemostasis biomarkers in patients with advanced stage lung cancer during hypofractionated radiotherapy treatment. J Int Med Res. 2018;46:1876–83. doi: 10.1177/0300060517750976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolny-Rokicka E, Wydmański J, Tukiendorf A, et al. The correlation of blood parameters with size in cases of neoplastic tumor. Asian Pac J Cancer Prev. 2018 doi: 10.31557/APJCP.2019.20.1.53. [accepted for publication] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson-Haidaris PJ, Rybarczyk BJ, Sahni A. Gunduz Mehmet., editor. The role of Fibrin(ogen) in transendothelial cell migration during breast cancer metastasis, breast cancer – focusing tumor microenvironment, stem cells and metastasi. InTech. 2011. Available from: http://www.intechopen.com/books/breast-cancer-focusing-tumor-microenvironment-stem-cells-and-metastasis/the-role-of-fibrin-ogen-in-transendothelial-cell-migration-during-breast-cancer-metastasis.
- 41.Inal T, Anar C, Polat G, et al. The prognostic value of D-dimer in lung cancer. Clin Respir J. 2015;9:305–13. doi: 10.1111/crj.12144. [DOI] [PubMed] [Google Scholar]


