Abstract
Matrix solid phase dispersion (MSPD) has proven to be an efficient sample preparation method for solid, semi-solid, and viscous samples. Applications of MSPD have covered biological, food, and environmental samples, including both organic and inorganic analytes. This review presents an update on the development of MSPD in the period 2015~June 2018. In the first part of this review, we focus on the latest development in MSPD sorbent, including molecularly imprinted polymers, and carbon-based nanomaterials etc. The second part presents the miniaturization of MSPD, discussing the progress in both micro-MSPD and mini-MSPD. The on-line/in-line techniques for improving the automation and sample throughput are also discussed. The final part summarizes the success in the modification of original MSPD procedures.
Keywords: sample preparation, matrix solid phase dispersion, sorbent, miniaturization, on-line
1. Introduction
Sample preparation is the key step in analytical workflow [1]. For solid, semi-solid, and viscous samples, procedures of sample preparation generally start with extracting analytes from matrix into homogeneous liquid solvents. Then a consequent clean-up step may be performed to reduce interference compounds in the extract. Finally, an additional enrichment or concentration step may also be required to meet the sensitivity of the analytical technique. Limitations in the classical method are the use of large volumes of solvent, labor-intensive, and time-consuming through the manipulation. Matrix solid phase dispersion (MSPD), first introduced by Barker et al. [2], provides an alternative approach to reduce solvent use and analysis time for preparing solid, semi-solid, and viscous samples [3].
In a typical MSPD procedure, samples are blended with sorbent to obtain homogeneous mixture. The resulting mixture is transferred and packed into an extraction column. Then solvent is passed through the column to carry out washing and elution step for the extraction and isolation of analytes from the matrix. In some case, an additional co-sorbent could be loaded at the bottom of the column to further clean-up the eluent. Generally, the final extract can be analyzed by chromatography based analytical techniques. Compared with classical solvent extraction method, MSPD eliminates steps of repeated centrifugation and/or filtration, and procedures of re-extraction. Different with solid phase extraction (SPE), in which separated solvent extraction procedure is required to make solid samples suitable for loading into a SPE column, MSPD eliminates the solvent extraction step. These would dramatically reduce the consumption of solvent and the required manipulation time for the preparation. There have been extensive reviews regarding the trends and developments of MSPD [3,4,5,6,7,8,9,10]. In this review, we focus attention on the latest developments in MSPD sorbent, miniaturization of MSPD, on-line/in-line techniques, and the modification of original MSPD procedure. Literatures during 2015 and June 2018 are reviewed to avoid the overlap with recent excellent reviews [9,10].
2. Latest Developments in MSPD Sorbent
Molecularly imprinted polymers (MIPs), the synthetic sorbents which exhibit selective binding of target molecular, have been widely used for the extraction of specific compounds [11,12]. In MSPD, sorbent requires to be blended with sample to obtain homogeneous mixture. To improve mechanical strength of MIPs materials, imprinted polymers can be synthesized using other sorbents as carrier. For example, MIPs were prepared on carbon nanotubes (CNTs) for the MSPD preparation of malachite green in aquatic products [13]. Silica gel [14], silica nanoparticles [15], and mesoporous silica [16] also have been reported as the carrier of MIPs to improve the selectivity of MSPD sorbents. Additionally, Wang et al. reported the synthesis of mixed-template MIPs for the extraction of multi-class veterinary drugs [17]. This novel MIPs sorbent was used for the simultaneous MSPD extraction of 20 drugs in meat, including 8 fluoroquinolones, 8 sulfonamides and 4 tetracyclines.
Graphene is one of the carbon-based nanomaterials which shows great promise in sample preparation [18,19]. Graphene provides large surface area and nanosheets morphology for improving adsorption capacity. In addition, the delocalized π electron system in graphene could make it form strong π-stacking interaction with compounds containing aromatic rings. These properties make graphene a good candidate for the adsorption of benzenoid compounds. Sun et al. reported a graphene-encapsulated silica sorbent for the analysis of flavonoids in the leaves of Murraya panaculata (L.) Jack [20]. Immobilized on the of surface of silica gel avoided the aggregation and maintained the large surface area and π-electron rich structure graphene during the mechanical blending. Compared with five sorbents (graphene, silica gel, C18, diatomaceous earth, and neutral alumina), graphene-encapsulated silica showed the better extraction efficiency for the target flavonoid compounds.
Phenyltrichlorosilane-functionalized magnesium oxide microspheres were designed by Tan et al. for the extraction of polycyclic aromatic hydrocarbons (PAHs) in soils [21]. This material takes advantage of the high affinity between magnesium oxide and PAHs to enhance the retention of target molecules. Grafting the microspheres with phenyltrichlorosilane reduced the competitive adsorption of chlorine-contained interferences which are widely exist in soil samples. Using hexane and DCM as rinsing and eluting solvent, respectively, seven PAHs were successfully determined in HPLC-FLD with limits of detection (LODs) of 0.02–0.12 µg/kg.
The use of polyethyleneimine (PEI)-modified attapulgite material as MSPD sorbent was reported by Wang et al. for the determination of cadmium in seafood products [22]. Introducing of PEI, which is a cationic polymer with high affinity to cadmium ion, resulted in the high recovery of target ion in complex matrices. High concentration of HNO3 (50%, v/v) was required to release the cadmium. Determined by atomic absorption spectrometry (AAS), the LOD of cadmium in fish sample was found to be 2.5 μg/kg.
Additionally, sorbents such as mussel shell [23,24], molecular sieve [25,26], microcrystalline cellulose [27], and metal-organic framework materials [28,29] also have been reported. These emerging sorbents are summarized in Table 1.
Table 1.
Selected representative studies involving developments in MSPD sorbent.
| Sorbent | Analytes | Matrix | MSPD Parameters | Detection | LOD (μg/kg) | LOQ (μg/kg) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Amounts (g) | Sorbent Amounts (g) | Blend Time (min) | Co-Sorbent | Washing Solvent | Elution Solvent | |||||||
| MIPs | Veterinary drugs | Meat | 0.2 | 0.15 | 3 | 0.05 g MIPs | 3 mL MeOH/H2O (2:8, v/v) | 4 mL MeOH/acetic acid (9:1, v/v) | UPLC-DAD | 0.5–3 | 1.5–6 | [17] |
| CNTs-MIPs | Malachite green | Aquatic products | 0.3 | 0.2 | 15 | None | 4 mL 50% aqueous MeOH | 3 mL MeOH-acetic acid (98:2, v/v) | HPLC-UV | 0.7 | n.r. | [13] |
| CNTs-MIPs | Camptothecin | Herb (Camptotheca acuminate) | 0.1 | 0.1 | 5 | None | 5 mL 10% aqueous MeOH | 4 mL MeOH-acetic acid (95:5, v/v) | HPLC-UV | 130 μg/L | n.r. | [30] |
| Silica gel -MIPs | Degradation products of penicillin | Milk | 0.3 mL | 0.2 | n.r. | None | 2 mL DCM | 3 mL MeOH-10% acetic acid (9:1, v/v) | HPLC-UV | 40/50 | 130/170 | [14] |
| SiO2-MIP | Acrylamide | Biscuit and bread | 0.1 | 0.15 | n.r. | None | 1 mL hexane | 2.5 mL ACN-MeOH (50:50, v/v) | HPLC-UV | 14.5/16.1 | 40.5/40.1 | [15] |
| Mesoporous silica-MIPs | Ketoprofen | Powder milk | 0.05 | 0.025 | n.r. | None | None | 1 mL ACN | HPLC-MS/MS | n.r. | n.r. | [16] |
| Graphene-encapsulated silica | Flavonoids | Herb (Murraya panaculata (L.) Jack) | 0.025 | 0.05 | 3 | None | None | 5 mL MeOH | UPLC-UV | 4–12 μg/L | 10–40 μg/L | [20] |
| PTS-MgO | PAHs | Soils | 0.1 | 0.1 | n.r. | 0.05 g PTS-MgO | 4 mL hexane | 4 mL DCM | HPLC-FLD | 0.02–0.12 | 0.07–0.40 | [21] |
| PEI-attapulgite | Cadmium | Seafood | 0.21 | 0.13 | n.r. | None | 6 mL H2O | 8 mL 50%HNO3/H2O (v/v) | AAS | 2.5 | 8.3 | [22] |
| Golden mussel shell | Pesticides and PPCPs | Mussel tissue | 0.5 | 0.5 | 5 | None | None | 5 mL ethyl acetate | LC-MS/MS | 3–30 | 10–100 | [23] |
| Mussel shell | Booster biocides | Fish tissue | 0.5 | 0.5 | 5 | None | None | 5 mL EtOH | LC-MS/MS | 1.5/15 | 5/50 | [24] |
| Molecular sieves | Flavonoids | Fruit peels | 0.025 | 0.025 | 2.5 | None | None | 0.5 mL MeOH | UPLC-UV | 20–30 μg/L | 70–90 μg/L | [25] |
| Molecular sieve | Sesquiterpenes | Herb (Curcuma wenyujin) | 0.2 | 0.2 | 2.5 | None | None | 1 mL MeOH | MEEKC | 5–34 μg/mL | 16–78 μg/mL | [26] |
| Microcrystalline cellulose | Triterpenoid acids | Herb (loquat leaves) | 0.024 | 0.024 | 1 | None | None | 0.2 × 3 mL EtOH | UHPLC-Q-TOF | 19.6–51.6 | 65.3–171.8 | [27] |
| MOFs | Pesticides | Coconut palm | 0.25 | 1 | 3 | None | None | 20 mL ACN | HPLC-DAD | 10–50 | 50–100 | [28] |
| MOFs | Pesticides | Peppers (Capsicum annuum L.) | 0.5 | 0.35 | n.r. | 1 g Na2SO4 + 0.5 g silica | None | 10 mL DCM | GC-MS | 16.0–67.0 | 50.3–200.0 | [29] |
DCM, dichloromethane; CNTs, carbon nanotubes; MIPs, molecularly imprinted polymers; PPCPs, pharmaceutical and personal care products; MOFs, metal-organic frameworks; PTS, phenyltrichlorosilane; PAHs, polycyclic aromatic hydrocarbons; PEI, polyethyleneimine; ACN. acetonitrile. n.r., not reported.
3. Miniaturization of MSPD
In classical MSPD protocol, the sample amount is typically 0.5 g [3]. The miniaturization of MSPD (micro/mini-MSPD) can significantly reduce the sample amount, and consequently the consumption of sorbent, solvent, and preparation time. Developed micro/mini-MSPD methods are summarized in Table 2. For instance, Guerra et al. developed a method based on micro-MSPD combined with LC-MS/MS for the simple and rapid determination of dyes in cosmetic products [31]. The proposed micro-MSPD was carried by grounding 0.1 g cosmetic sample with 0.3 g anhydrous Na2SO4 (drying agent) and 0.4 g of Florisil. After transferring the mixture into a glass Pasteur pipette, 2 mL of methanol was eluted to extract nine water-soluble dyes. By using micro-MSPD method, time and solvent consumption in the sample preparation could be reduced.
Table 2.
Selected representative studies using miniaturized MSPD.
| Analytes | Matrix | MSPD Parameters | Detection | LOD (μg/kg) | LOQ (μg/kg) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Amounts (g) | Sorbent Amounts | Blend Time (min) | Co-Sorbent | Washing Solvent | Elution Solvent | ||||||
| Dyes | Cosmetic products | 0.1 | 0.4 g Florisil + 0.3 g Na2SO4 | n.r. | 0.1 g Florisil | None | 2 mL MeOH | LC-MS/MS | 0.01–11 | n.r. | [31] |
| Photoproducts of cosmetic preservatives | Personal care products | 0.1 | 0.4 g Florisil + 0.4 g Na2SO4 | 5 | 0.2 g Florisil | None | 1 mL hexane-acetone (1:1, v/v) | GC-MS/MS | 31–170 | n.r. | [34] |
| Flavonoids | Lime fruit | 0.05 | 0.15 g Florisil | 1 | None | None | 0.4 mL [Bmin]BF4 aqueous solution (250 mM) | UPLC-UV | 4.08/5.04 μg/g | 14.01/14.56 μg/g | [35] |
| Phenolic isomers | Honeysuckle | 0.025 | 0.075 g β-cyclodextrin | 2 | None | None | 0.5 mL MeOH-H2O (80:20, v/v) | UPLC-UV-Q-TOF | 1.62–3.33 ng/mL | 5.52–11.40 ng/mL | [36] |
| Inorganic iodine and iodinated amino acids | Seaweed | 0.05 | 0.05 g molecular sieve SBA-15 | 0.5 | None | None | 0.4 mL [C12mim] Br (200 mM) | UHPLC-UV | 3.7–16.7 ng/mL | 12.4–55.8 ng/mL | [37] |
| Phenols | Olive fruits | 0.05 | 0.025 g chitosan | 1 | None | None | 0.5 mL × 3 MeOH-H2O (6:4, v/v) | UHPLC-Q-TOF | 69.6–358.4 | 232–1240.8 | [38] |
| Mercury species | Fish organs | 1 mg | 0.5 mg MWCNTs | 5 | 0.15 g C18 | None | 0.1 mL × 2 0.5% l-cysteine and 4% HCOOH | AFS | 0.01 | n.r. | [22] |
| Gibberellins | Plant | 0.3–0.8 mg | 2 mg C18 | n.r. | None | None | 0.2 mL ACN | UPLC-MS/MS | 0.16–1.31 pg/mL | 0.53–4.37 pg/mL | [33] |
| Synthetic dyes | Cosmetics and foodstuffs | 0.1 | 0.4 g C18 + 0.3 g Na2SO4 | n.r. | 0.1 g C18 | None | 2 mL MeOH | LC-MS/MS | 14.2–95.2 | n.r. | [39] |
| Phenolic acids | Plant preparation (Danshen tablets) | 0.024 | 0.024 g graphene nanoplatelets | 1 | None | None | 0.2 mL H2O | UHPLC-ECD | 1.19–4.62 ng/mL | 3.91–15.23 ng/mL | [40] |
| Lignans | Herbs (Schisandrae Chinensis Fructus) | 0.025 | 0.05 g molecular sieve TS-1 | 2.5 | None | None | 0.5 mL MeOH | MEEKC | n.r. | 2.77 μg/mL | [41] |
MWCNTs, multiwall carbon nanotubes; AFS, atomic fluorescence spectrometry. n.r., not reported.
Taking advantage of high sensitive detection methods, sample amount in recently published mini-MSPD could be reduced to the scale of milligram. Chen et al. reported a sensitive quantification of mercury distribution in fish organ based on the mini-MSPD [32]. The sample amount in this research was as low as 1 mg of organ sample. Multiwall carbon nanotubes (MWCNTs) were used as the sorbent, with amount of 0.5 mg. Mercury species were eluted by 100 µL eluent containing HCOOH and l-cysteine. When combined with a sensitive mercury determination method named single-drop solution electrode glow discharge-induced cold vapor generation combined with atomic fluorescence spectrometry, LOD of 0.01 µg/L was achieved. The consumption of sample, adsorbent, and solvent were all dramatically decreased in this mini-MSPD.
Another example of mini-MSPD was reported by Deng et al. [33], in which only 0.30–0.80 mg of plant samples were ground with 2 mg C18 sorbent in liquid nitrogen to obtain the homogenous mixture. Based on this mini-MSPD and the precolumn derivatization coupled with UPLC-MS/MS determination, phytohormone gibberellins were detected with the limits of quantification (LOQs) of 0.54–4.37 pg/mL. As only sub-milligram sample was required for the determination, a spatial distribution of gibberellins in a single Arabidopsis thaliana leaf with resolution of 2 × 2 mm2 was profiled.
4. On-Line/In-Line MSPD
On-line/in-line sample preparation techniques that couple sample preparation step and chromatography separation are regarded as a promising technique with advantages of automatable high sample throughput, reducing sample manipulation and contamination, improving precision, and lower regent consumption [42]. On-line/in-line MSPD provides a potential automated way for the sample preparation of solid, semi-solid, and viscous samples.
Rajabi et al. reported an in-line micro-MSPD method for the determination of Sudan dyes in spices [43]. In this in-line MSPD, the filled MSPD column was placed in the mobile phase pathway before the analytical column. Then the mobile phase passed through the MSPD column to elute analytes and subsequently separated in a reverse-phased HPLC. Since the in-line method integrated extraction and separation into one step, this proposed approach was much faster than other reported methods for the determination of Sudan dyes.
Gutiérrez-Valencia et al. developed an on-line MSPD-SPE sample preparation method combined with HPLC-FLD for the analysis of PAHs in bovine tissues [44]. The bovine liver sample (50 mg) was dispersed on C18 sorbent (200 mg). Then the obtained homogenous mixture was packed into a stainless steel cartridge which was connected to a MSPD-SPE-HPLC-FLD system. The SPE column was used to trap and pre-concentrate the target compounds eluted from the MSPD cartridge. Acetonitrile (ACN)-water mixture and pure ACN solution were applied to wash and elute the MSPD cartridge, respectively. However, ACN extract exhibited poor retention of analytes in C18 SPE column. Thus a dynamic mixing chamber was required to dilute the ACN extract with water before pre-concentration to quantitatively transfer PAHs from MPSD cartridge to the SPE column. Finally, the analytes pre-concentrated on the SPE column were eluted through the guard-column and the analytical column with mobile phase and detected by FLD. Compared with off-line MSPD, the on-line MSPD method showed advantages of lower consumption of sample amount and saving of analysis time.
Additionally, an on-line MSPD-HPLC-ICP-MS method for the determination of mercury speciation in fish was reported by Deng et al. [45]. In this on-line MSPD performance, 1 mg fish sample was blended with 2 mg of MWCNTs, then the mixture was transferred into a stainless steel column which was prior loaded with 0.20 g of C18. The eluent solution containing HCl (2%, v/v) and l-cysteine (1.5%, m/v) was loaded by a 100 µL loop through the six-port valve. Then mobile phase flushed the eluent to pass through the MSPD column for the extraction of analytes, which were further separated and detected by HPLC-ICP-MS. It is interesting to notice that the on-line MSPD system consisting of two sequential valves and six stainless steel MSPD columns to improve sample throughput. This on-line system shows the potential of automatable high sample throughput in MSPD method.
5. Modification of Original MSPD
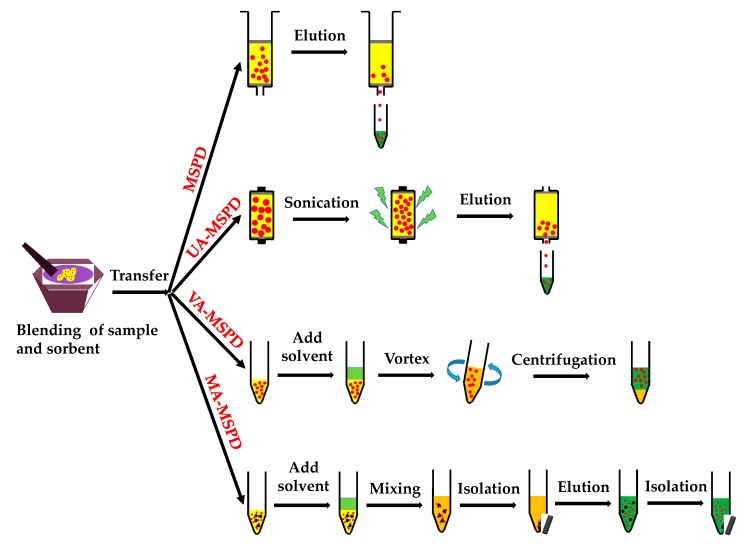
The original MSPD can be modified or combined with other extraction methodologies to improve the extraction yields or simplify the MSPD procedures. The schematic procedure of the original and representative modification of MSPD is shown in Figure 1. For instance, ultrasonic-assisted MSPD (UA-MSPD) was first reported by Ramos et al. to improve the extraction yields by putting MSPD column into ultrasonic bath or sonoreactor after the extraction solvent was loaded into the MSPD column [46]. As summarized in Table 3, UA-MSPD has been introduced for the analysis of multi-class organic contaminants. For example, Albero et al. developed an UA-MSPD method for the analysis of 17 emerging contaminants in vegetables [47]. In this modified method, vegetable samples (2 g) was blended with Florisil (4 g) and magnesium sulfate anhydrous (1 g), then the homogenous mixture was transferred into a 20 mL glass column. Extraction solution of 8 mL EtAc:MeOH (9:1, v/v) containing 3% of NH4OH were added to the column. After that, column was sonicated for 15 min in an ultrasonic water bath at room temperature for the extraction. Finally, extract was collected under vacuum manifold. Results indicated that better recoveries were obtained with the assistance of sonication.
Figure 1.
Schematic procedure of original matrix solid phase dispersion (MSPD), ultrasonic-assisted MSPD (UA-MSPD), vortex-assisted MSPD (VA-MSPD), and magnetically-assisted MSPD (MA-MSPD).
Table 3.
Selected representative studies using ultrasonic assisted MSPD and vortex assisted MSPD.
| Modification | Analytes | Matrix | MSPD Parameters | Detection | LOD (μg/kg) | LOQ (μg/kg) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Amount (g) | Sorbent Amount | Grind Time (min) | Extraction Time (min) | Elution Solvent | |||||||
| Ultrasonic assisted | Emerging organic contaminants | Poultry manure | 0.5 | 2 g Florisil + 1 g MgSO4 | n.r. | 15 | 8 mL ACN with 3% NH4OH + 10 mL ACN with 4% formic acid | GC-MS/MS | 0.9–2.2 | 2.8–5.5 | [48] |
| Ultrasonic assisted | Emerging contaminants | Vegetables | 2 | 4 g Florisil + 1 g MgSO4 | n.r. | 15 | 8 mL EtAc:MeOH (9:1, v/v) containing 3% NH4OH | GC-MS/MS | 0.1–0.4 | n.r. | [47] |
| Ultrasonic assisted | Emerging contaminants | Aquatic plants | 1 | 4 g Florisil + 2 g MgSO4 | 5 | 15 | 8 mL EtAc with 3% NH4OH | GC-MS | 0.3–2.2 | 1.0–6.7 | [49] |
| Ultrasonic assisted | Aflatoxins | Rice | 1 | 1 g C18 | 5 | 11 | 4 mL ACN | HPLC-FLD | 0.04–0.14 ng/g | 0.12–0.56 ng/g | [50] |
| Vortex assisted | 5-HMF and iridoid glycosides | Herb (Fructus Corni) | 0.02 | 0.04 g silica | 3 | 3 | 6 mL [Domin]HSO4 | UHPLC-UV | 0.02–0.08 μg/mL | 0.07–0.24 μg/mL | [51] |
| Vortex assisted | Booster biocides | Marine sediments | 2 | 0.25 g C18 | n.r. | 1 | 10 mL MeOH | LC-MS/MS | n.r. | 0.5–5 | [52] |
| Vortex assisted | Phenol | Herb (Forsythiae Fructus) | 0.02 | 0.02 g Florisil | 3 | 2 | 2 mL10% (v/v) Triton X-114 | UHPLC-UV | 0.03–0.08 μg/mL | 0.08–0.25 μg/mL | [53] |
| Vortex assisted | Ibuprofen enantiomers | Milk | 0.5 | 0.30 g diatomaceous earth + 0.30 g Na2SO4 + 0.26 g PSA + 0.021 g β-cyclodextrin | 5 | 1 | 2 mL MeOH | HPLC-UV | 0.042/0.045 μg/g | 0.14/0.15 μg/g | [54] |
| Vortex assisted | Pesticides | Drinking water treatment sludge | 1.5 | 0.5 g Chitin | 5 | 1 | 5 mL ethyl acetate | GC-MS | n.r. | 5–500 | [55] |
| Vortex assisted | Pharmaceuticals | Fish tissue | 0.5 | 0.5 g diatomaceous earth + 0.5 g Na2SO4 | 5 | 1 | 5 mL MeOH | LC-MS/MS | 1.5–300 | 5–1000 | [56] |
5-HMF, 5-hydroxymethy furfurol. n.r., nor reported.
Vortex-assisted MSPD (VA-MSPD), in which the step of column elution is replaced by vortex, has been developed to reduce the solvent consumption and analysis time. This simplified MSPD procedure has been found applications in the analysis of phytochemical compounds and organic contaminants (Table 3). For instance, Caldas et al. reported the analysis of antifouling booster biocides in marine sediments by employing VA-MSPD [52]. In the sample preparation procedure, the homogenized mixture of sample and sorbent was added into a centrifuge tube. Then the extraction solvent was added, and the sample was vortexed for 1 min. Finally, the mixture was centrifuged, and the supernatant was collected for the LC-MS/MS analysis. Compared with other extraction methods including ultrasonic extraction, SPE, and microwave extraction, this VA-MSPD exhibited the advantages of shorter extraction time and less solvent consumption.
Another recent progress of the modification is the magnetically-assisted MSPD (MA-MSPD) developed by Fotouhi et al. for the extraction of parabens from breast milks [57]. Modified magnetic nanoparticles were used as the sorbent in the MA-MSPD. Milk sample (200 µL) was blended with poly(indole-thiophene) coated magnetic graphene oxide (MGO@PIT, 50 mg) and drying salt Na2SO4 (550 mg). After blending, the homogenous mixture was transferred into water solution and mechanically stirred for the adsorption of parabens. Then the MGO@PIT with target compounds were isolated from the solution by magnet. Subsequently, analytes were desorbed from the sorbent with methanol. Compared with the magnetic liquid-solid extraction (MLSE) [58,59], a hot topic of nanomaterials in sample preparation, the major difference between MA-MSPD and MLSE is the manipulation of sample. For MLSE, analytes in solid sample are extracted into the liquid solution prior to the introduction of magnetic sorbent. While in MA-MSPD, the solid sample is blended with magnetic nanoparticles to obtain the homogenous mixture. The similarity of these two methods is the replacement of column packing and elution with simple magnetic isolation. This would simplify the preparation step and reduce the extraction time. More importantly, magnetic nanoparticles have been demonstrated to be reusable in the liquid-solid extraction [58]. Thus MA-MSPD may provide a solution for the reusability of sorbent in MSPD.
Recently, we reported the combination of Soxhlet extraction and MSPD to develop a Soxhlet assisted MSPD (SA-MSPD) method [60,61]. In this modification method, sample was blended with silica gel following the original MSPD protocol and loaded into a column of constant pressure funnel. Then elution solvent was heated and continuous refluxed and passed through the column for the extraction and isolation of flavonoids. By comparing with conventional solvent extraction and Soxhlet extraction method, SA-MSPD showed the higher extraction yield with shorter extraction time and less consumption of solvent. Moreover, the introduction of sorbent into the Soxhlet enabled this classical method to be with clean-up ability. More recently, this SA-MSPD method was further combined with acid-hydrolysis for the quantification of flavonoid aglycones in bee pollen [61]. The acid hydrolysis SA-MSPD procedure accomplished the extraction and hydrolysis of flavonoid glycosides into one step, and provided a more efficient sample preparation method for the quantification of flavonoid aglycones.
6. Conclusion Remarks
Application fields of MSPD have been extended from the first reported drug residues in biological tissues to the food and environmental analysis, both for organic and inorganic analytes. Development of new sorbent materials for improving the capacity or selectivity is still the exciting research area in MSPD. One of the drawbacks of MSPD is the reusability of the extraction column. Among the emerging MSPD sorbents, modified magnetic nanoparticles are expected to provide the possibility of reusability. Combining high efficient sorbents with ultra-sensitive analytical technologies, miniaturization of MSPD might be found great interests in the analysis of limited or small size samples. Especially, the mini-MSPD may provide more information on the evolution or the spatial distribution of analytes in the sample matrices. In addition, on-line MSPD has shown the possibility of high-throughput analysis in MSPD. This would also be the trend of automation in MSPD. The modification of the original MSPD appears to be simplified the MSPD procedure and could be help for improving the reproducibility of the manipulation.
Author Contributions
X.T. organized the literatures and wrote the manuscript; W.C. conceived and designed the review, and wrote the manuscript. Both authors approved the submitted version.
Funding
This research was funded by Natural Science Foundation of China, grant number 31201861 and 51202030.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen Y., Guo Z., Wang X., Qiu C. Sample preparation. J. Chromatogr. A. 2008;1184:191–219. doi: 10.1016/j.chroma.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Barker S.A., Long A.R., Short C.R. Isolation of drug residues from tissues by solid phase dispersion. J. Chromatogr. 1989;475:353–361. doi: 10.1016/S0021-9673(01)89689-8. [DOI] [PubMed] [Google Scholar]
- 3.Barker S.A. Matrix solid phase dispersion (MSPD) J. Biochem. Biophys. Methods. 2007;70:151–162. doi: 10.1016/j.jbbm.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Barker S.A. Applications of matrix solid-phase dispersion in food analysis. J. Chromatogr. A. 2000;880:63–68. doi: 10.1016/S0021-9673(99)01290-X. [DOI] [PubMed] [Google Scholar]
- 5.Barker S.A. Matrix solid-phase dispersion. J. Chromatogr. A. 2000;885:115–127. doi: 10.1016/S0021-9673(00)00249-1. [DOI] [PubMed] [Google Scholar]
- 6.Bogialli S., Di Corcia A. Matrix solid-phase dispersion as a valuable tool for extracting contaminants from foodstuffs. J. Biochem. Biophys. Methods. 2007;70:163–179. doi: 10.1016/j.jbbm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Moreda-Pineiro J., Alonso-Rodriguez E., Lopez-Mahia P., Muniategui-Lorenzo S., Prada-Rodriguez D., Romaris-Hortas V., Miguez-Framil M., Moreda-Pineiro A., Bermejo-Barrera P. Matrix solid-phase dispersion of organic compounds and its feasibility for extracting inorganic and organometallic compounds. Trends Anal. Chem. 2009;28:110–116. doi: 10.1016/j.trac.2008.09.016. [DOI] [Google Scholar]
- 8.Capriotti A.L., Cavaliere C., Giansanti P., Gubbiotti R., Samperi R., Lagana A. Recent developments in matrix solid-phase dispersion extraction. J. Chromatogr. A. 2010;1217:2521–2532. doi: 10.1016/j.chroma.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 9.Capriotti A.L., Cavaliere C., Lagana A., Piovesana S., Samperi R. Recent trends in matrix solid-phase dispersion. Trends Anal. Chem. 2013;43:53–66. doi: 10.1016/j.trac.2012.09.021. [DOI] [Google Scholar]
- 10.Capriotti A.L., Cavaliere C., Foglia P., Samperi R., Stampachiacchiere S., Ventura S., Lagana A. Recent advances and developments in matrix solid-phase dispersion. Trends Anal. Chem. 2015;71:186–193. doi: 10.1016/j.trac.2015.03.012. [DOI] [Google Scholar]
- 11.Speltini A., Scalabrini A., Maraschi F., Sturini M., Profumo A. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. Anal. Chim. Acta. 2017;974:1–26. doi: 10.1016/j.aca.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 12.Ashley J., Shahbazi M.-A., Kant K., Chidambara V.A., Wolff A., Bang D.D., Sun Y. Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosens. Bioelectron. 2017;91:606–615. doi: 10.1016/j.bios.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Chen L. Analysis of malachite green in aquatic products by carbon nanotube-based molecularly imprinted—Matrix solid phase dispersion. J. Chromatogr. B. 2015;1002:98–106. doi: 10.1016/j.jchromb.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Luo Z., Du W., Zheng P., Guo P., Wu N., Tang W., Zeng A., Chang C., Fu Q. Molecularly imprinted polymer cartridges coupled to liquid chromatography for simple and selective analysis of penicilloic acid and penilloic acid in milk by matrix solid-phase dispersion. Food Chem. Toxicol. 2015;83:164–173. doi: 10.1016/j.fct.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Arabi M., Ghaedi M., Ostovan A. Development of dummy molecularly imprinted based on functionalized silica nanoparticles for determination of acrylamide in processed food by matrix solid phase dispersion. Food Chem. 2016;210:78–84. doi: 10.1016/j.foodchem.2016.04.080. [DOI] [PubMed] [Google Scholar]
- 16.Ganan J., Morante-Zarcero S., Perez-Quintanilla D., Sierra I. Evaluation of mesoporous imprinted silicas as MSPD selective sorbents of ketoprofen in powder milk. Mater. Lett. 2017;197:5–7. doi: 10.1016/j.matlet.2017.03.137. [DOI] [Google Scholar]
- 17.Wang G.N., Zhang L., Song Y.P., Liu J.X., Wang J.P. Application of molecularly imprinted polymer based matrix solid phase dispersion for determination of fluoroquinolones, tetracyclines and sulfonamides in meat. J. Chromatogr. B. 2017;1065:104–111. doi: 10.1016/j.jchromb.2017.09.034. [DOI] [PubMed] [Google Scholar]
- 18.De Toffoli A.L., Soares Maciel E.V., Fumes B.H., Lancas F.M. The role of graphene-based sorbents in modern sample preparation techniques. J. Sep. Sci. 2018;41:288–302. doi: 10.1002/jssc.201700870. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B.T., Zheng X., Li H.F., Lin J.M. Application of carbon-based nanomaterials in sample preparation: A review. Anal. Chim. Acta. 2013;784:1–17. doi: 10.1016/j.aca.2013.03.054. [DOI] [PubMed] [Google Scholar]
- 20.Sun T., Li X., Yang J., Li L., Jin Y., Shi X. Graphene-encapsulated silica as matrix solid-phase dispersion extraction sorbents for the analysis of poly-methoxylated flavonoids in the leaves of Murraya panaculata (L.) Jack. J. Sep. Sci. 2015;38:2132–2139. doi: 10.1002/jssc.201500002. [DOI] [PubMed] [Google Scholar]
- 21.Tan D., Jin J., Li F., Sun X., Dhanjai, Ni Y., Chen J. Phenyltrichlorosilane-functionalized magnesium oxide microspheres: Preparation, characterization and application for the selective extraction of dioxin-like polycyclic aromatic hydrocarbons in soils with matrix solid-phase dispersion. Anal. Chim. Acta. 2017;956:14–23. doi: 10.1016/j.aca.2016.12.040. [DOI] [PubMed] [Google Scholar]
- 22.Wang T., Chen Y., Ma J., Jin Z., Chai M., Xiao X., Zhang L., Zhang Y. A polyethyleneimine-modified attapulgite as a novel solid support in matrix solid-phase dispersion for the extraction of cadmium traces in seafood products. Talanta. 2018;180:254–259. doi: 10.1016/j.talanta.2017.12.059. [DOI] [PubMed] [Google Scholar]
- 23.Rombaldi C., de Oliveira Arias J.L., Hertzog G.I., Caldas S.S., Vieira J.P., Primel E.G. New environmentally friendly MSPD solid support based on golden mussel shell: Characterization and application for extraction of organic contaminants from mussel tissue. Anal. Bioanal. Chem. 2015;407:4805–4814. doi: 10.1007/s00216-015-8686-2. [DOI] [PubMed] [Google Scholar]
- 24.Vieira A.A., Caldas S.S., Venquiaruti Escarrone A.L., de Oliveira Arias J.L., Primel E.G. Environmentally friendly procedure based on VA-MSPD for the determination of booster biocides in fish tissue. Food Chem. 2018;242:475–480. doi: 10.1016/j.foodchem.2017.09.085. [DOI] [PubMed] [Google Scholar]
- 25.Cao W., Hu S.S., Ye L.H., Cao J., Pang X.Q., Xu J.J. Trace matrix solid phase dispersion using a molecular sieve as the sorbent for the determination of flavonoids in fruit peels by ultra-performance liquid chromatography. Food Chem. 2016;190:474–480. doi: 10.1016/j.foodchem.2015.05.133. [DOI] [PubMed] [Google Scholar]
- 26.Wei M., Chu C., Wang S., Yan J. Quantitative analysis of sesquiterpenes and comparison of three Curcuma wenyujin herbal medicines by micro matrix solid phase dispersion coupled with MEEKC. Electrophoresis. 2018;39:1119–1128. doi: 10.1002/elps.201700454. [DOI] [PubMed] [Google Scholar]
- 27.Cao J., Peng L.Q., Xu J.J. Microcrystalline cellulose based matrix solid phase dispersion microextration for isomeric triterpenoid acids in loquat leaves by ultrahigh-performance liquid chromatography and quadrupole time-of-flight mass spectrometry. J. Chromatogr. A. 2016;1472:16–26. doi: 10.1016/j.chroma.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 28.De Jesus J.R., Wanderley K.A., Alves Junior S., Navickiene S. Evaluation of a novel metal-organic framework as an adsorbent for the extraction of multiclass pesticides from coconut palm (Cocos nucifera L.): An analytical approach using matrix solid-phase dispersion and liquid chromatography. J. Sep. Sci. 2017;40:3327–3334. doi: 10.1002/jssc.201700501. [DOI] [PubMed] [Google Scholar]
- 29.Barreto A.S., da Silva Andrade P.D.C., Farias J.M., Menezes Filho A., de Sa G.F., Alves Junior S. Characterization and application of a lanthanide-based metal-organic framework in the development and validation of a matrix solid-phase dispersion procedure for pesticide extraction on peppers (Capsicum annuum L.) with gas chromatography-mass spectrometry. J. Sep. Sci. 2018;41:1593–1599. doi: 10.1002/jssc.201700812. [DOI] [PubMed] [Google Scholar]
- 30.Liu H., Hong Y., Chen L. Molecularly imprinted polymers coated on carbon nanotubes for matrix solid phase dispersion extraction of camptothecin from Camptotheca acuminate. Anal. Methods. 2015;7:8100–8108. doi: 10.1039/C5AY01721A. [DOI] [Google Scholar]
- 31.Guerra E., Celeiro M., Pablo Lamas J., Llompart M., Garcia-Jares C. Determination of dyes in cosmetic products by micro-matrix solid phase dispersion and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A. 2015;1415:27–37. doi: 10.1016/j.chroma.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q., Lin Y., Tian Y., Wu L., Yang L., Hou X., Zheng C. Single-drop solution electrode discharge-induced cold vapor generation coupling to matrix solid-phase dispersion: A robust approach for sensitive quantification of total mercury distribution in fish. Anal. Chem. 2017;89:2093–2100. doi: 10.1021/acs.analchem.6b04753. [DOI] [PubMed] [Google Scholar]
- 33.Deng T., Wu D., Duan C., Yan X., Du Y., Zou J., Guan Y. Spatial profiling of gibberellins in a single leaf based on microscale matrix solid-phase dispersion and precolumn derivatization coupled with ultraperformance liquid chromatography-tandem mass spectrometry. Anal. Chem. 2017;89:9537–9543. doi: 10.1021/acs.analchem.7b02589. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez-Rivera G., Llompart M., Garcia-Jares C., Lores M. Identification of unwanted photoproducts of. cosmetic preservatives in personal care products under ultraviolet-light using solid-phase microextraction and micro-matrix solid-phase dispersion. J. Chromatogr. A. 2015;1390:1–12. doi: 10.1016/j.chroma.2015.02.056. [DOI] [PubMed] [Google Scholar]
- 35.Xu J.J., Yang R., Ye L.H., Cao J., Cao W., Hu S.S., Peng L.Q. Application of ionic liquids for elution of bioactive flavonoid glycosides from lime fruit by miniaturized matrix solid-phase dispersion. Food Chem. 2016;204:167–175. doi: 10.1016/j.foodchem.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Xu J.J., Cao J., Peng L.Q., Cao W., Zhu Q.Y., Zhang Q.Y. Characterization and determination of isomers in plants using trace matrix solid phase dispersion via ultrahigh performance liquid chromatography coupled with an ultraviolet detector and quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A. 2016;1436:64–72. doi: 10.1016/j.chroma.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 37.Cao J., Peng L.Q., Xu J.J., Du L.J., Zhang Q.D. Simultaneous microextraction of inorganic iodine and iodinated amino acids by miniaturized matrix solid-phase dispersion with molecular sieves and ionic liquids. J. Chromatogr. A. 2016;1477:1–10. doi: 10.1016/j.chroma.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 38.Peng L.Q., Li Q., Chang Y.X., An M., Yang R., Tan Z., Hao J., Cao J., Xu J.J., Hu S.S. Determination of natural phenols in olive fruits by chitosan assisted matrix solid-phase dispersion microextraction and ultrahigh performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A. 2016;1456:68–76. doi: 10.1016/j.chroma.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Guerra E., Llompart M., Garcia-Jares C. Miniaturized matrix solid-phase dispersion followed by liquid chromatography-tandem mass spectrometry for the quantification of synthetic dyes in cosmetics and foodstuffs used or consumed by children. J. Chromatogr. A. 2017;1529:29–38. doi: 10.1016/j.chroma.2017.10.063. [DOI] [PubMed] [Google Scholar]
- 40.Peng L.Q., Yi L., Yang Q.C., Cao J., Du L.J., Zhang Q.D. Graphene nanoplatelets based matrix solid-phase dispersion microextraction for phenolic acids by ultrahigh performance liquid chromatography with electrochemical detection. Sci. Rep. 2017;7:7496. doi: 10.1038/s41598-017-07840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu C., Wei M., Wang S., Zheng L., He Z., Cao J., Yan J. Micro-matrix solid-phase dispersion coupled with MEEKC for quantitative analysis of lignans in Schisandrae Chinensis Fructus using molecular sieve TS-1 as a sorbent. J. Chromatogr. B. 2017;1063:174–179. doi: 10.1016/j.jchromb.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 42.Fumes B.H., Andrade M.A., Franco M.S., Lancas F.M. On-line approaches for the determination of residues and contaminants in complex samples. J. Sep. Sci. 2017;40:183–202. doi: 10.1002/jssc.201600867. [DOI] [PubMed] [Google Scholar]
- 43.Rajabi M., Sabzalian S., Barfi B., Arghavani-Beydokhti S., Asghari A. In-line micro-matrix solid-phase dispersion extraction for simultaneous separation and extraction of Sudan dyes in different spices. J. Chromatogr. A. 2015;1425:42–50. doi: 10.1016/j.chroma.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Gutierrez-Valencia T.M., Garcia de Llasera M.P. On-line MSPD-SPE-HPLC/FLD analysis of polycyclic aromatic hydrocarbons in bovine tissues. Food Chem. 2017;223:82–88. doi: 10.1016/j.foodchem.2016.11.099. [DOI] [PubMed] [Google Scholar]
- 45.Deng D., Zhang S., Chen H., Yang L., Yin H., Hou X., Zheng C. Online solid sampling platform using multi-wall carbon nanotube assisted matrix solid phase dispersion for mercury speciation in fish by HPLC-ICP-MS. J. Anal. At. Spectrom. 2015;30:882–887. doi: 10.1039/C4JA00436A. [DOI] [Google Scholar]
- 46.Ramos J.J., Rial-Otero R., Ramos L., Capelo J.L. Ultrasonic-assisted matrix solid-phase dispersion as an improved methodology for the determination of pesticides in fruits. J. Chromatogr. A. 2008;1212:145–149. doi: 10.1016/j.chroma.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 47.Albero B., Sanchez-Brunete C., Miguel E., Tadeo J.L. Application of matrix solid-phase dispersion followed by GC-MS/MS to the analysis of emerging contaminants in vegetables. Food Chem. 2017;217:660–667. doi: 10.1016/j.foodchem.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 48.Aznar R., Albero B., Ana Perez R., Sanchez-Brunete C., Miguel E., Tadeo J.L. Analysis of emerging organic contaminants in poultry manure by gas chromatography-tandem mass spectrometry. J. Sep. Sci. 2018;41:940–947. doi: 10.1002/jssc.201700883. [DOI] [PubMed] [Google Scholar]
- 49.Aznar R., Albero B., Sanchez-Brunete C., Miguel E., Martin-Girela I., Tadeo J.L. Simultaneous determination of multiclass emerging contaminants in aquatic plants by ultrasound-assisted matrix solid-phase dispersion and GC-MS. Environ. Sci. Pollut. Res. 2017;24:7911–7920. doi: 10.1007/s11356-016-6327-8. [DOI] [PubMed] [Google Scholar]
- 50.Manoochehri M., Asgharinezhad A.A., Safaei M. Determination of aflatoxins in rice samples by ultrasound-assisted matrix solid-phase dispersion. J. Chromatogr. Sci. 2015;53:189–195. doi: 10.1093/chromsci/bmu018. [DOI] [PubMed] [Google Scholar]
- 51.Du K., Li J., Bai Y., An M., Gao X. m., Chang Y.X. A green ionic liquid-based vortex-forced MSPD method for the simultaneous determination of 5-HMF and iridoid glycosides from Fructus Corni by ultra-high performance liquid chromatography. Food Chem. 2018;244:190–196. doi: 10.1016/j.foodchem.2017.10.057. [DOI] [PubMed] [Google Scholar]
- 52.Caldas S.S., Soares B.M., Abreu F., Castro I.B., Fillmann G., Primel E.G. Antifouling booster biocide extraction from marine sediments: A fast and simple method based on vortex-assisted matrix solid-phase extraction. Environ. Sci. Pollut. Res. 2018;25:7553–7565. doi: 10.1007/s11356-017-0942-x. [DOI] [PubMed] [Google Scholar]
- 53.Du K., Li J., Tian F., Chang Y.X. Non-ionic detergent Triton X-114 Based vortex- synchronized matrix solid-phase dispersion method for the simultaneous determination of six compounds with various polarities from Forsythiae Fructus by ultra high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2018;150:59–66. doi: 10.1016/j.jpba.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Leon-Gonzalez M.E., Rosales-Conrado N. Determination of ibuprofen enantiomers in breast milk using vortex-assisted matrix solid-phase dispersion and direct chiral liquid chromatography. J. Chromatogr. A. 2017;1514:88–94. doi: 10.1016/j.chroma.2017.07.072. [DOI] [PubMed] [Google Scholar]
- 55.Soares K.L., Rodrigues Cerqueira M.B., Caldas S.S., Primel E.G. Evaluation of alternative environmentally friendly matrix solid phase dispersion solid supports for the simultaneous extraction of 15 pesticides of different chemical classes from drinking water treatment sludge. Chemosphere. 2017;182:547–554. doi: 10.1016/j.chemosphere.2017.05.062. [DOI] [PubMed] [Google Scholar]
- 56.Hertzog G.I., Soares K.L., Caldas S.S., Primel E.G. Study of vortex-assisted MSPD and LC-MS/MS using alternative solid supports for pharmaceutical extraction from marketed fish. Anal. Bioanal. Chem. 2015;407:4793–4803. doi: 10.1007/s00216-015-8685-3. [DOI] [PubMed] [Google Scholar]
- 57.Fotouhi M., Seidi S., Shanehsaz M., Naseri M.T. Magnetically assisted matrix solid phase dispersion for extraction of parabens from breast milks. J. Chromatogr. A. 2017;1504:17–26. doi: 10.1016/j.chroma.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Yu X., Yang H.S. Pyrethroid residue determination in organic and conventional vegetables using liquid-solid extraction coupled with magnetic solid phase extraction based on polystyrene-coated magnetic nanoparticles. Food Chem. 2017;217:303–310. doi: 10.1016/j.foodchem.2016.08.115. [DOI] [PubMed] [Google Scholar]
- 59.Yu X., Li Y.X., Ng M., Yang H.S., Wang S.F. Comparative study of pyrethroids residue in fruit peels and fleshes using polystyrene-coated magnetic nanoparticles based clean-up techniques. Food Control. 2018;85:300–307. doi: 10.1016/j.foodcont.2017.10.016. [DOI] [Google Scholar]
- 60.Ma S.Q., Tu X.J., Dong J.T., Long P., Yang W.C., Miao X.Q., Chen W.B., Wu Z.H. Soxhlet-assisted matrix solid phase dispersion to extract flavonoids from rape (Brassica campestris) bee pollen. J. Chromatogr. B. 2015;1005:17–22. doi: 10.1016/j.jchromb.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 61.Tu X.J., Ma S.Q., Gao Z.S., Wang J., Huang S.K., Chen W.B. One-step extraction and hydrolysis of flavonoid glycosides in rape bee pollen based on Soxhlet-assisted matrix solid phase dispersion. Phytochem. Anal. 2017;28:505–511. doi: 10.1002/pca.2699. [DOI] [PubMed] [Google Scholar]