Abstract
Regulator of G-protein signaling 5 (RGS5), a tissue-specific signal-regulating molecule, plays a key role in the development of the vasculature. It was recently found that RGS5 is abundantly expressed in epithelial ovarian cancer (EOC) compared with the normal ovaries. However, the distribution of RGS5 in EOC and its significance require further investigation. The aim of the present study was to investigate the expression of RGS5 in EOC, as well as its association with cancer differentiation, metastasis and clinicopathological parameters. Immunohistochemistry (IHC), western blotting, RT-PCR, wound-healing, cell proliferation and flow cytometric assays were the methods used in the present study. RGS5 was highly expressed in the cytoplasm of ovarian carcinoma cells and in microvascular structures. The expression of RGS5 in EOC was negatively associated with peritoneal metastasis (P=0.004), but it was not found to be associated with age, tumor size, clinical stage or lymph node metastasis (P>0.05). EOC patients with high RGS5 expression had a prolonged progression-free survival (72.34±8.41 vs. 43.56±5.41 months, P<0.001). High expression of RGS5 was correlated with significantly lower microvascular density (MVD) as indicated by the expression of CD34, whereas the opposite was observed in tissues with low RGS5 expression (P<0.05). Hypoxia increased RGS5 expression in ovarian carcinoma-derived endothelial cells (ODMECs), whereas the proliferative capacity of ODMECs exhibited a significant increase following RNAi-mediated reduction of RGS5 expression. These data indicated that RGS5 plays a key role in angiogenesis in ovarian carcinoma. In addition, RGS5 downregulated the expression of the downstream proteins CDC25A, CDK2 and cyclin E, which are mediated by the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway, causing ODMEC arrest in the G1 phase of the cell cycle under hypoxic conditions. Collectively, our data indicated that RGS5 is crucial for the occurrence and development of ovarian cancer, and that RGS5 and its signaling pathway may serve as anti-angiogenesis targets for the treatment of ovarian cancer.
Keywords: regulator of G-protein signaling 5, ovarian carcinoma, peritoneal metastasis, microvascular density
Introduction
Epithelial ovarian cancer (EOC) is characterized by multifocal intraperitoneal dissemination and the accumulation of ascitic fluid coupled with intense neovascularization (1). Similar to the majority of other solid tumors, angiogenesis occurs in the early stages of EOC development, and may precede neoplastic transformation (2,3). In addition, surgical stress may enhance ovarian cancer angiogenesis (4). Therefore, inhibition of angiogenesis is one of the most promising approaches to EOC treatment. Bevacizumab, a monoclonal antibody directed against vascular endothelial growth factor (VEGF)-A, has been approved for preliminary treatment of ovarian cancer (5). However, the clinical benefits are limited and recurrent ovarian cancer has been reported in a proportion of the patients (6). Hypoxic stress generated by successful preliminary therapy may cause upregulation of selective pro-angiogenic factors (7). Overcoming this evasive resistance further supports the need for a novel therapeutic approach in this field. In recent years, G-protein-coupled receptors (GPCRs) have been implicated in the initiation and progression of a variety of tumors (8). Thus, regulators of GPCR signaling are likely important in the pathophysiology of cancer (9). Regulator of G-protein signaling-5 (RGS5) is a member of the RGS family that consists of a diverse group of multifunctional proteins and has been reported to be expressed in vascular, cardiac, and skeletal muscle tissues (10). RGS5 was recently identified as a major upregulated gene in pericytes, and is responsible for several morphological changes in tumor vessels. Studies have found that the levels of RGS5 can decrease following increased expression of anti-VEGF antibody as a result of inhibition of angiogenesis. In addition, RGS5 protein expression was increased after blocking the VEGFR signaling pathway in mice during corneal neovascularization, and the effects of inhibiting angiogenesis are superior to those of blocking the VEGFR pathway (11). Thus, targeting RGS5 may affect tumor vessels.
Endothelial cells in malignant tumors are genetically variable and differ from endothelial cells derived from normal vessels at the molecular as well as the functional level (12,13). It is essential to study the precise effect of RGS5 on the biological characteristics of ovarian carcinoma-derived endothelial cells (ODMECs) prior to using them experimentally and performing drug research on anti-angiogenesis in EOC. RGS5 plays a vital role in the development of the vasculature. RGS5 was found to be abundantly expressed in EOC compared with normal ovaries. However, the distribution of RGS5 in EOC and its significance require further investigation. Therefore, in the present study, we observed the expression of RGS5 in EOC, as well as its association with clinicopathological parameters. We also assessed the status of RGS5 in primary endothelial cells derived from human EOC.
The aim of the present study was to examine the RGS5 protein expression in ovarian cancer cells and microvascular structures and determine whether the expression of RGS5 was significantly associated with peritoneal metastasis in patients with ovarian cancer.
Materials and methods
Patients and tissue specimens
A total of 127 human paraffin-embedded EOC tissue samples that had been collected from consecutive patients (The average age of the patients was approximately 50 years) undergoing standard surgical procedures for primarily diagnosed ovarian cancer at the Department of Obstetrics and Gynecology, Southwest Hospital (Chongqing, China) between December 2004 and July 2009, were used in this study. Relevant data were obtained via retrospective review of the medical files of patients. These data included demographic information, histopathological diagnosis, tumor grade, disease stage, ascites status, CA125 level, chemotherapy regimen and response to clinical treatment or chemotherapy. The tumors were histopathologically characterized according to the WHO criteria, tumor grade was determined based on the Gynecological Oncology Group criteria, and disease stage was assessed according to the International Federation of Gynecology and Obstetrics staging system (14). None of the patients received any preoperative anticancer treatment, including chemotherapy, radiotherapy or biotherapy. Patients who died from unknown causes or emergency were excluded from this study. The Ethics Committee of the First Affiliated Hospital of the Third Military Medical University approved the procedures and all patients provided written informed consent.
Patients with EOC were interviewed by telephone. The majority of the patients had been reviewed after completing treatment every 3–6 months over a 2-year period, and annually thereafter. Patients' follow-up information was updated until May 2016 by reviewing medical records. Progression-free survival (PFS) and overall survival (OS) were estimated from the date of the first cytoreduction to the date of recurrence/death or at the last follow-up, whichever occurred first. The progression (recurrence or metastasis) of ovarian cancer was confirmed by radiological examination. Recurrence was indicated clinically by the appearance of new lesions or an increase in the serum CA125 level to more than twice the upper limit of the reference range. Patients who remained alive without recurrence at the last follow-up were censored. Patients in the progressive disease group were those in whom no disease remission had been observed after treatment. Patients in the relapsed disease group were those in whom disease remission had been clinically documented. The extent of cytoreduction was defined as optimal if the largest diameter of any residual lesion from surgery was <1 cm, or as suboptimal if it was >1 cm.
Immunohistochemistry
Immunohistochemical (IHC) examination of RGS5 in the paraffin-embedded samples was performed using a standard streptavidin-peroxidase method as previously described (13). EOC sections (4-µm) were deparaffinized and unmasked for 5 min. After blocking endogenous peroxidase activity, the sections were incubated for 10 min with 20% normal rabbit serum to block non-specific binding sites. The primary antibody used was a polyclonal antibody against human RGS5 protein (dilution 1:100; cat. no. HPA001821; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The samples were incubated overnight at 4°C in a moist chamber. Following three washes, the sections were incubated for 30 min with goat anti-rabbit secondary IgG (dilution 1:400; cat. no. SP-9001; ZSGB-BIO, Beijing, China). The 3,5-diaminobenzidine (DAB) Detection kit (ZSGB-BIO) was used for staining. Negative controls with phosphate-buffered saline (PBS; 0.01 mol/l, pH 7.4) replacing the primary antibody were also included. Finally, the tissue sections were counterstained with hematoxylin, dehydrated and mounted in resinous mountant. Digital images were captured using a BH-2 light microscope (Olympus Corp., Tokyo, Japan) at an ×200 magnification.
RGS5 expression was determined semi-quantitatively by assessing the immunostaining intensity and the percentage and distribution of positively stained cells, as previously reported. Briefly, the tissue sections were screened at a high power (×200) and the mean of five visual fields was estimated. The mean percentage of immunoreactive cells was described as follows: 0, <5%; 1, 6–25%; 2, 26–50%; 3, 51–75% and 4, >75%. The intensity of RGS5 immunostaining was scored as follows: 0, negative; 1, weakly positive; 2, positive; 3, strongly positive. For sections with heterogeneous staining, the predominant pattern was taken into consideration for scoring. A staining index (with values of 0–12) was obtained by multiplying the staining intensity with the proportion of immunopositive tumor cells. For the statistical analysis, the patients were classified into three groups: Negative or low reactive cases (score 0–1), cases with moderate (scores 2–5) and cases with high expression (scores 6–12). All histological evaluations were independently performed in a double-blinded manner by two expert pathologists (Dr Jiang Zhu and Dr Feng Wu). Any differences in the scores were resolved by discussion between the two pathologists.
Cell culture
Primary human ODMECs were isolated from EOC tissue as previously described (13) and cultured in complete EGM-2MV medium (Lonza, Basel, Switzerland). Human aortic smooth muscle cells (HASMCs), A2780 and SKOV3 cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). A2780 and SKOV3 cells were cultured in RPMI-1640 media (Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal bovine serum (FBS) and used as negative controls. HASMCs were cultured in DMEM with 20% FBS. The angiogenic ability of ODMECs was analyzed by using a two-dimensional fibrin gel assay.
Ability of ODMECs to form capillary networks and RGS5 and endoglin (CD105) levels assay
Capillary network formation by ODMECs was analyzed using a two-dimensional fibrin gel assay as previously described (15). Whole-cell extracts were prepared for the different tubular structure stages (7 and 14 days) with mammalian protein extraction reagent (Pierce, Thermo Fisher Scientific, Inc., Waltham, MA, USA). Western blotting was performed to monitor RGS5 (1:60) and endoglin (1:2,000; both from Abcam, Shanghai, China) protein levels.
ODMEC culture under hypoxic conditions
ODMECs confluent to ~80% in the cell culture plate were harvested by trypsinization and EDTA. After 170 × g centrifuging and washing, the cell re-suspension solution was placed into the hypoxic incubator (oxygen concentration of 1%). Then, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting were used to detect the mRNA and protein levels of RGS5 at 1, 3, 6, 12 and 24 h under hypoxic conditions.
Construction and production of lentiviral vectors
To demonstrate the specificity of siRNA against human RGS5 (GenBank Accession no. NM_000118), the following oligonucleotides were used: Target sequences: 5′-GAACCTTCCCTGAGCAGCT-3′, 5′-ATATTGACCACTTCACTAA-3′ and 5′-GGAAAAGGATTCTCTGCCT-3′. A scrambled siRNA was used as a negative control. RGS5 siRNA and control siRNA bearing no sequence homology with any known human mRNA sequences were also used. Double-stranded DNA containing the interference sequences was synthesized and inserted into a linearized pGenesil-GFP viral vector (Gikai Gene Company, Shanghai, China). All the constructs were cloned and sequenced to confirm their proper construction. Lentivirus-encoded siRNA against RGS5 (LV-siRGS5) and control (LV-H) were produced by co-transfecting 293T cells with Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h, the supernatant was harvested and concentrated and the viral titers were measured.
Transfection of cells
ODMECs were plated into 6-well tissue culture plates at 1×105 cells/well under hypoxic conditions (continuous oxygen concentration of 1%). Three parallel wells were used for each group of cells: Non-transfected cells (control group), LV-H-transfected cells (negative control group) and LV-R-transfected cells (knockdown group). When the cells reached ~60–70% confluence, the media was replaced with DMEM containing lentivirus at a multiplicity of infection (MOI) of 20, and the cells were incubated overnight (16 h). Gene transfer efficiency was monitored by flow cytometry (FACStar plus; BD Biosciences, Franklin Lakes, NJ, USA) or confocal microscopy (Leica TCS SP5; Leica Microsystems, Wetzlar, Germany) at 48 h post-transfection.
RT-PCR
Total RNA was isolated at 48 h using TRIzol reagent (Invitrogen; Thermo Fisher Scientific). Aliquots of RNA were reverse-transcribed to cDNA using a Superscribe First-Strand synthesis system (Invitrogen; Thermo Fisher Scientific, Inc.). The following PCR primers were used: RGS5: 5′-AAGATGGCTGAGAAGGCAAA-3′ and 5′-TCAGGGCATGGATTCTTTTC-3′, with a product length of 396 bp; and GAPDH: 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′, with a product length of 220 bp. The thermal profile conditions were 30 sec at 95°C, 30 sec at 62°C and 30 sec at 72°C for 35 cycles, and a final extension at 72°C for 5 min.
Cell proliferation assays
After being transduced at an MOI of 50 under a continuous oxygen concentration of 1%, ODMECs were grown until confluent and plated again at an optimal density of 1×105 cells/well supplemented with EGM-2MV 20% FBS in 24-well plates. After 1, 2, 3, 4, 5 and 6 days, the cells were stained with 20 µl MTT (5 mg/ml) (Sigma-Aldrich; Merck KGaA) at 37°C for 4 h and subsequently solubilized in 200 µl dimethyl sulfoxide. The absorbance at 570 nm was measured using a microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA). Cell growth curves were calculated as mean values of triplicates/group.
Wound healing migration assay
ODMECs were seeded in 12-well plates, infected overnight (16 h) with either LV-siRGS5 or LV-H, and allowed to grow until confluent under a continuous oxygen concentration of 1%. A wound was created by using a pipette cone, and cells were allowed to migrate in the EGM-2MV media. The wounded area was monitored 16 h after injury. Wound-induced cell migration was measured by monitoring the distance between cells lining the wound edge and then normalized to time 0 h.
Flow cytometric assay
ODMECs cells infected overnight (16 h) with either LV-siRGS5 or LV-H under a continuous oxygen concentration of 1% were harvested by trypsinization, washed in ice-cold PBS, and fixed in 80% ice-cold ethanol in PBS. Prior to staining, the cells were pelleted using a chilled centrifuge and re-suspended in cold PBS. Bovine pancreatic RNase (Sigma-Aldrich; Merck KGaA) was added to a final concentration of 2 µg/ml and the cells were incubated at 37°C for 30 min, followed by incubation with 20 µg/ml propidium iodide (PI; Sigma-Aldrich; Merck KGaA) for 20 min at room temperature to analyze the cell cycle, or incubation with 61 µl FITC-Annexin V and 20 µl PI with 300 µl binding buffer for 15 min at room temperature to analyze the apoptotic rate. The profiles of 1×104 cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Western blot analysis
Whole-cell extracts were prepared with mammalian protein extraction reagent (Pierce; Thermo Fisher Scientific, Inc.), RIPA lysis buffer (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and an equal amount of protein (100 µg) from each cell line was loaded per lane and resolved by polyacrylamide gel electrophoresis (7.5% SDS-Tris glycine). Gels were electroblotted onto nitrocellulose membranes and blocked in 1X Tris-buffered saline containing 0.1% Tween and 5% non-fat dry milk overnight. The following primary antibodies were used for 1 h at room temperature: Polyclonal mouse anti-human RGS5 antibody (dilution 1:60; cat. no. ab196799) and rabbit anti-human CDC25A antibody (dilution 1:200; cat. no. ab47400; both from Abcam), mouse anti-human CDK2 antibody (dilution 1:500; cat. no. sc-6248; Santa Cruz Biotechnology, Inc.), mouse anti-human p53 antibody (dilution 1:1,000; cat. no. AF0255) and mouse anti-human p21 antibody (dilution 1:500; cat. no. AP021; both from Beyotime Institute of Biotechnology, Haimen, China), mouse anti-human p-ERK1/2 antibody (dilution 1:1,000; cat. no. ab201015), mouse anti-human ERK1/2 antibody (dilution 1:1,000; cat. no. ab36991), mouse anti-human p-p38 antibody (dilution 1:200; cat. no. ab178867), mouse anti-human p38 antibody (dilution 1:200; cat. no. ab31828; all from Abcam) and mouse anti-human GAPDH (dilution 1:10,000; cat. no. KC-5G4; KangChen Bio-Tech, Shanghai, China). After 3 washes in TBS/0.1% Tween 20, the membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibody (dilution 1:10,000; cat. no. KC-MM-035; KangChen Bio-Tech). Protein was detected using chemiluminescence (Santa Cruz Biotechnology, Inc.) and autoradiography (Kodak, Rochester, NY, USA).
Statistical analysis
Statistical analysis was performed using the SPSS statistical software package (version 13.0; SPSS, IBM., Chicago, IL, USA). Associations of RGS5 expression with the clinical parameters of the patients were assessed by the Chi-squared test. Fisher's exact test was also used when necessary. The Kaplan-Meier method was used to estimate the probability of overall and disease-free survival, and the log-rank test was used to compare different survival curves. Multivariate survival analysis was performed on all factors using the Cox regression model. Receiver operating characteristics curve analysis was used to compare the clinicopathological characteristics for estimation of the survival prediction. All P-values were the results of two-sided tests, and P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of RGS5 is associated with cancer metastasis in ovarian carcinoma
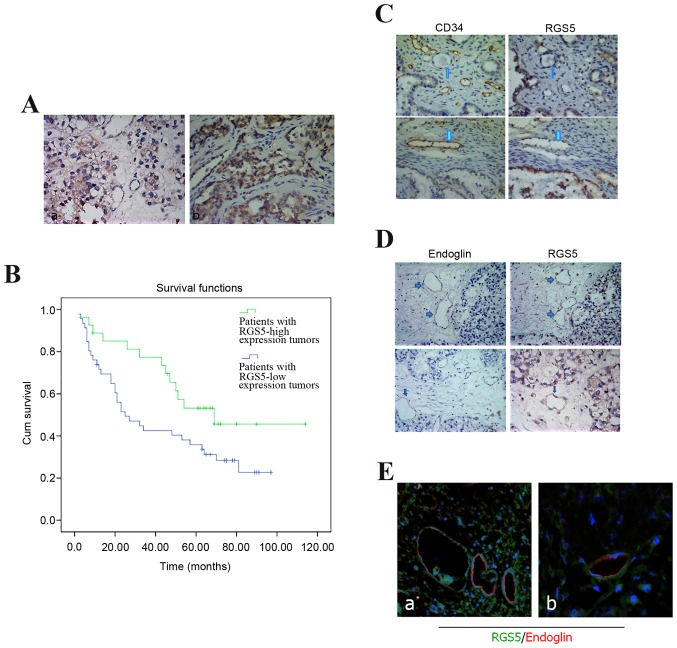
RGS5 was mainly expressed in the cytoplasm of ovarian carcinoma cells, and the highest levels were in the regions infiltrated by the tumor cells (Fig. 1A). In this study, according to the staining index described above, the protein expression with a scoring index of ≥4 (median score of RGS5 protein expression in the primary ovarian lesions) was defined as positive expression. A total of 42 cases were excluded due to significant discrepancies in the staining. Negative or low reactivity was observed in 55 cases (scores 0–1 and 2–5, respectively), and high expression in 30 cases (scores 6–12). RGS5 expression was not associated with younger age at diagnosis (<50 years; P=0.168), histological type (serous vs. not serous; P=0.325), early-stage disease (I and II vs. III and IV; P=0.466), low ascites incidence (P=0.198), and low-grade tumors (1 and 2 vs. 3; P=0.820). We examined the peritoneal metastasis of ovarian carcinoma with different expression of RGS5: A strong association was identified between the expression of RGS5 and intraperitoneal metastasis in ovarian carcinoma (P=0.004). Tumors highly expressing RGS5 were generally associated with a low incidence of intraperitoneal metastasis (Table I).
Figure 1.
Clinical significance of the expression of RGS5 in ovarian cancer. (A) Expression of RGS5 in endometrioid ovarian carcinoma. (a) Endometrioid ovarian carcinoma cells exhibited cytoplasmic staining for RGS5 (magnification, ×200). (b) Diffuse positive staining for RGS5 in endometrioid ovarian carcinoma (magnification, ×400). (B) Kaplan-Meier progression-free survival curves for the negative expression group (n=45) and the positive expression group (n=57). The difference in the progression-free survival rate between the two groups was statistically significant. ROC curve analysis for each clinicopathological variable and RGS5 expression was performed to evaluate the survival status of patients. (C) RGS5- and CD34-positive blood vessels did not overlap. (D) RGS5- and endoglin-positive blood vessels partially overlapped. (E) RGS5- and endoglin-positive vascular overlap with double immunofluorescence. RGS5, regulator of G-protein signaling 5; ROC, receiver operating characteristics.
Table I.
Association of RGS5 protein expression with clinicopathological parameters.
| Variables | All cases | Low expression | High expression | P-value |
|---|---|---|---|---|
| Age at surgery (years) | 0.168 | |||
| <50 | 34 | 23 | 11 | |
| ≥50 | 51 | 32 | 19 | |
| Histological type | 0.325 | |||
| Serous | 59 | 34 | 24 | |
| Other | 25 | 18 | 7 | |
| Histological grade (Silveberg) | 0.820 | |||
| G1 | 8 | 4 | 4 | |
| G2 | 32 | 20 | 12 | |
| G3 | 42 | 27 | 15 | |
| FIGO stage | 0.466 | |||
| I–II | 27 | 15 | 12 | |
| III–IV | 52 | 34 | 18 | |
| Ascites | 0.198 | |||
| No | 22 | 11 | 11 | |
| Yes | 63 | 43 | 20 | |
| CA125 (U/ml) | 0.625 | |||
| <500 | 42 | 25 | 18 | |
| ≥500 | 29 | 19 | 10 | |
| Lymph node metastasis | 0.467 | |||
| Absent | 40 | 29 | 15 | |
| Present | 30 | 16 | 12 | |
| Intraperitoneal metastasis | 0.004 | |||
| Absent | 27 | 11 | 15 | |
| Present | 45 | 36 | 10 |
RGS5, regulator of G-protein signaling 5; FIGO, International Federation of Gynecology and Obstetrics; CA125, carbohydrate antigen 125.
Association between RGS5 expression and the 5-year survival rate
The 5-year survival rate of patients with ovarian cancer has important clinical implications and was estimated using Kaplan-Meier survival curves. The survival time was 72.34±8.41 months for patients with RGS5-positive tumors and 43.56±5.41 months for those with RGS5-negative tumors. Then, the survival rates were compared using the log-rank method in univariate survival analysis. The results of our survival analysis indicated that patients with RGS5-high tumors had higher survival rates (P<0.001) compared with patients with RGS5-low tumors (Fig. 1B).
Expression of RGS5 protein in ovarian cancer cells and its association with microvascular density (MVD) marker CD34
MVD is a measure of the degree of tumor angiogenesis, reflected by the expression of CD34 antibody in the vascular endothelial cell membrane as brown-yellow particles. We evaluated the activity of angiogenesis in ovarian cancer by continuous counting of the microvessels in 5 high-power microscopic fields (×200). In order to study whether there is a statistical association between the expression of MVD and RGS5, we classified RGS5 expression in ovarian cancer cells according to the level and compared it with the difference in MVD within the respective tumors. The results demonstrated that the tumors expressing high levels of RGS5 also exhibited a significantly lower MVD, which co-localized with CD34 expression. The opposite was observed in tissues with low expression of RGS5, which indicated that the expression of RGS5 in ovarian cancer cells was negatively associated with MVD (P<0.05; Table II).
Table II.
The relationship between RGS5 protein and MVD.
| Variable | All cases | MVD (mean ± SD) | P-valuea |
|---|---|---|---|
| Expression of RGS5 | 0.037 | ||
| High | 18 | 17.64±11.29 | |
| Low | 33 | 28.82±17.30 |
P<0.05. RGS5, regulator of G-protein signaling 5; MVD, microvascular density.
Expression of RGS5 protein in ovarian cancer microvasculature is associated with blood vessels co-localized with endoglin, but not with blood vessels co-localized with CD34
RGS5 was expressed in the ovarian cancer microvasculature, whereas weaker expression, by comparison of serial sections, was observed for CD34. RGS5- and CD34-positive blood vessels rarely overlap (Fig. 1C). However, they are visible in some microvessels where endoglin expression is observed, but the expression of the two genes is weaker (Fig. 1D and E). Even in the tube-like structures, expression of CD34 or endoglin is not usually detected together with expression of RGS5 (Fig. 1C).
ODMECs do not stably express RGS5 in a conventional in vitro culture
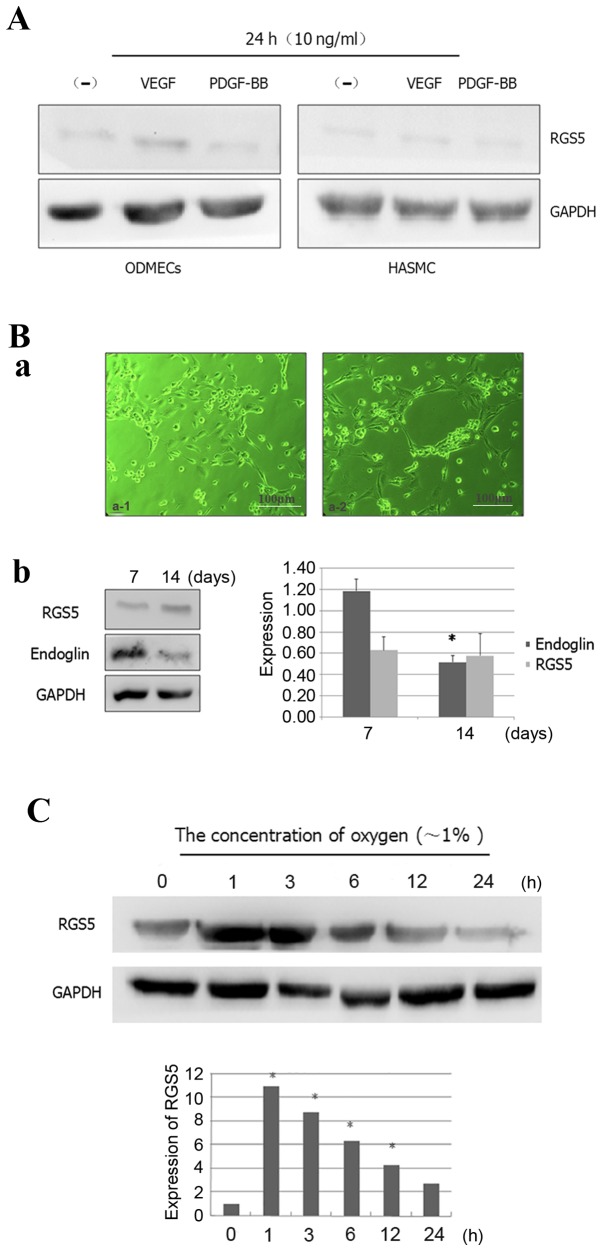
There is normally increased expression of VEGF, transforming growth factor-β, platelet-derived growth factor (PDGF)-BB and other growth factors in tumor cells and their microenvironment. However, it is highly likely that the microenvironment of ODMECs cultured in vitro lacks these factors. Previous studies reported that the expression of endoglin was significantly higher in ODMECs (15), but in the present study we found that in vitro ODMECs did not significantly express RGS5, which is a crucial factor for their biological function with respect to PDGF-BB (16) (Fig. 2A). Some scholars believe that the high expression of RGS5 in the tumor microenvironment in vivo is a result of the presence of high levels of angiogenic factors. Hence, in the cell culture system, when angiogenesis-related factors, such as VEGF and PDGF-BB, are added at a concentration of 10 ng/ml, it leads to stimulation of RGS5 expression, which can be detected in ODMECs and human aortic smooth muscle cells (HASMCs) after 24 h. However, the results of the present study revealed no promoting effect of VEGF or PDGF-BB on the expression of RGS5 (Fig. 2A). This indicated that the high expression of RGS5 in tumor tissue may not depend on regulation via the VEGFR and PDGF-BB signaling pathways in this model system.
Figure 2.
Expression patterns of RGS5 in ODMECs. (A) RGS5 was not obviously expressed in ODMECs and HASMCs in a conventional in vitro culture, since VEGF and PDGF-BB cannot effectively stimulate the expression of RGS5. (B) RGS5 did not play a decisive role in the formation of the lumen-like structures by ODMECs. (a) ODMECs form lumen-like structures on the FN-gel (phase contrast microscope, ×100). (b) The expression of endoglin was different at different stages of lumen-like structure formation by ODMECs (n=3, P<0.05), but the expression of RGS5 exhibited no statistically significant difference (n=3, P>0.05). (C) The expression of RGS5 was detected in ODMECs at different time-points (1, 3, 6, 12 and 24 h) under hypoxic conditions (~1% oxygen). *P<0.05. RGS5, regulator of G-protein signaling 5; ODMECs, ovarian carcinoma-derived endothelial cells; HASMCs, human aortic smooth muscle cells; FN, fibronectin; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor.
RGS5 does not play a decisive role in the formation of the lumen-like structures by ODMECs
In ODMECs, high expression of the angiogenesis-related proteins VEGFR-2 and endoglin was observed, regardless of the enhancement of the in vitro angiogenesis ability or lack thereof. In the preliminary study, we found that ODMECs spontaneously formed lumen-like structures on a single cell layer in a culture plate with no extracellular matrix, such as gelatin or fibronectin (FN), when subjected to long-term culture of 5–6 weeks. Therefore, this part of the experiment aimed to further investigate the tube-forming ability of ODMECs in vitro by the FN-collagen system (fibrin gel-based) (17). In the FN-gel package, serum-free EGM-2MV culture medium was used for 3–4 days, by which time ODMECs exhibited budding growth of adjacent formations (Fig. 2B-a). Then, the cells appeared to join and form tubular structures after ~1 week, and connected to form a network which was observed for ~10–12 days, after which time they began to break into the lumen (Fig. 2B-a). Under the same conditions, human umbilical vein endothelial cells failed to form any complete lumen-like structures. The size of the tube-like formations of ODMECs was not uniform, with large-diameter lumina or tufted cell masses both visible.
In order to determine the role of RGS5 in ODMECs in in vitro culture, we detected the expression of RGS5 and endoglin at different stages of lumen-like structure formation by ODMECs. FN-gel cells were seeded on the seventh day, when adjacent cells exhibited budding growth and connected into a tubular structure. The results revealed that the expression of endoglin was found to be significantly higher compared with that of cells seeded on FN-gels for ~14 days, when the formation of lumen-like structures was stable and had started to disintegrate (n=3, P<0.05; Fig. 2B). In addition, no significant change in the expression of RGS5 was observed with prolonged incubation time. This indicated that endoglin was involved in tube formation by ODMECs in vitro, while RGS5 did not play a decisive role in the formation of the lumen-like structures by ODMECs.
Expression of RGS5 increases in ODMECs during hypoxia
The expression of RGS5 in vitro exhibited little change with time prolongation. This is presumably due to the tumor vascular endothelial cells being extracted from the tumor microenvironment, and the high expression of the pro-angiogenic factors VEGF and PDGF-BB in the tumor microenvironment, which in turn decreased the expression of RGS5 (Fig. 2). This indicated that the high expression of RGS5 in tumor tissue may not rely on regulation via the VEGFR and PDGF-BB signaling pathways.
Tumor vasculature is usually heterogeneous, with luminal enlargement and distortion, while tumor neovascularization is prominent, but is often in a state of hypoxia. Hence, we detected the expression of RGS5 at different time-points (1, 3, 6, 12 and 24 h) under hypoxic conditions (1% oxygen). The results demonstrated that hypoxia can enhance the expression of RGS5 in ODMECs. The expression of RGS5 was significantly higher in hypoxia at 1 h, but it decreased as hypoxia was prolonged (Fig. 2C), which indicated that the expression of RGS5 was significantly induced in the early stages of hypoxia.
LV-siRGS5 effectively inhibits the expression of RGS5 at the mRNA and protein level in primary ODMECs
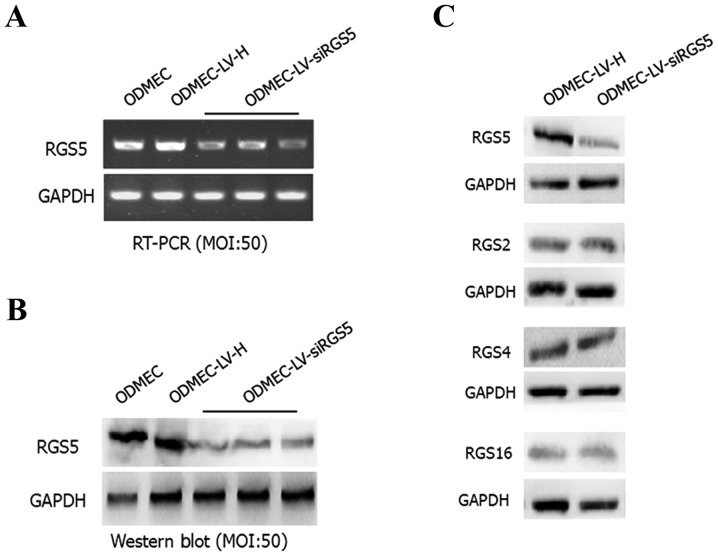
After 48 h, the total RNA and protein were extracted from the blank control group as well as the transfected LV-siRGS5 and negative control LV-H ODMECs after hypoxia for 6 h. Using qPCR and western blotting, the expression level of RGS5 mRNA and protein, respectively, was measured. The results demonstrated that LV-siRGS5 inhibited the expression of RGS5 mRNA and protein in ODMECs. At the mRNA level, the blank group and negative control group LV-H-siRNA and LV-siRGS5 three groups of quantitative analysis were 1.07±0.05, 1.32±0.09 and 0.52±0.06, 0.60±0.04 and 0.54±0.02, respectively, with the expression of negative control LV-H not shown to affect the RGS5 mRNA (n=3, P<0.05; Fig. 3A). At the protein level, the blank group and negative control group LV-H-siRNA and LV-siRGS5 quantitative analysis were 0.92±0.08, 0.95±0.21 and 0.17±0.04, 0.22±0.08 and 0.19±0.12, respectively, with the negative control of LV-H-siRNA not affecting the expression of RGS5 (n=3, P<0.05; Fig. 3B). As the RGS5 protein belongs to the B/R4 subfamily, and the family includes RGS1, RGS2, RGS3, RGS4, RGS5, RGS8, RGS13, RGS16, RGS 18 and RGS21, the expression of members of the same subfamily of RGS proteins is basically identical; therefore, to elucidate the effect of LV-siRGS5 on the expression of RGS4, RGS2 and RGS16 in endothelial cells, we detected the expression of RGS5, RGS2, RGS4 and RGS16 in ODMECs following transfection with LV-siRGS5 and negative control LV-H. The results demonstrated that LV-siRGS5 did not interfere with the expression of RGS2, RGS4 or RGS16 (Fig. 3C).
Figure 3.
LV-siRGS5 effectively inhibits the expression of RGS5 at the mRNA and protein level in ODMECs. (A) At the mRNA level, the blank group, the negative control group LV-H-siRNA and the LV-siRGS5 three groups of quantitative analysis were 1.07±0.05, 1.32±0.09 and 0.52±0.06, 0.60±0.04, 0.54±0.02, respectively; the expression of negative control LV-H did not affect the RGS5 mRNA (n=3, P<0.05). (B) At the protein level, the blank group, the negative control group LV-H-siRNA and the LV-siRGS5 three groups of quantitative analysis was 0.92±0.08, 0.95±0.21 and 0.17±0.04, 0.22±0.08, 0.19±0.12, respectively; the negative control LV-H-siRNA did not affect the expression of RGS5 (n=3, P<0.05). (C) LV-siRGS5 did not interfere with the expression of RGS2, RGS4 and RGS16 in the B/R4 subfamily. RGS5, regulator of G-protein signaling 5; ODMECs, ovarian carcinoma-derived endothelial cells; LV, lentiviral vector.
RGS5 inhibits the proliferation ability of ODMECs
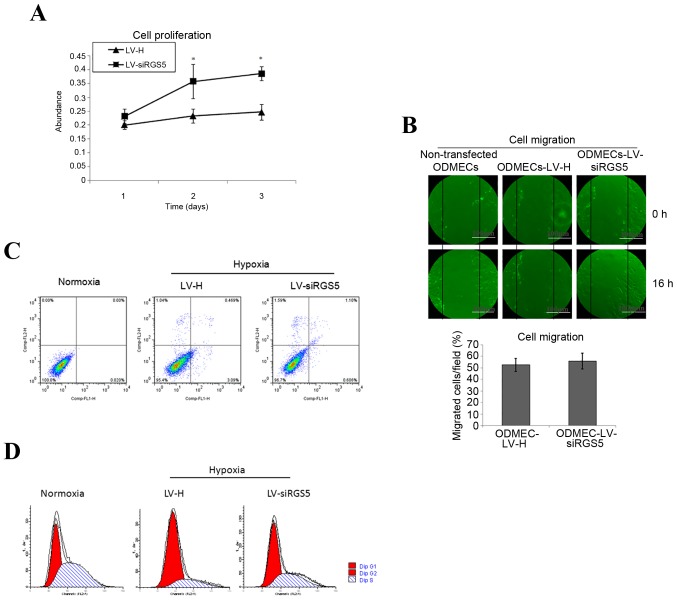
A hypoxic environment can induce changes of protein expression in vascular endothelial cells, in order to modify the corresponding biological characteristics. The high expression of RGS5 in tumor angiogenesis overlaps with the vascular endoglin marker. The vascular endothelial cells express highly the ‘active’ state of the vascular pro-growth factor receptor. Although it was previously found that RGS5 does not play a decisive role in the formation of the lumen-like structures observed when ODMECs are grown in vitro, endoglin is known to participate in angiogenesis, and is associated with cell proliferation. Therefore, this experiment determined the effect of RGS5 on the proliferation ability of ODMECs by MTT method under hypoxic conditions. The results demonstrated that the proliferation ability of the LV-siRGS5 ODMEC group increased by 61.36% (n=3, P<0.05; Fig. 4A) compared with the negative control group (Fig. 4A), indicating that the RGS5 protein was able to inhibit the proliferative ability of ODMECs.
Figure 4.
The effect of ODMECs with RGS5 under hypoxic conditions. (A) The proliferation ability of LV-siRGS5 ODMECs increased by 61.36% (n=3, P<0.05) compared with the negative control group. (B) The healing rate in the LV-H-ODMEC and LV-siRGS5-ODMEC groups was 52.62±5.63 and 55.83±6.89%, respectively; the difference was not statistically significant (n=3, P>0.05). (C) The apoptosis rate of the LV-H and LV-siRGS5 groups after 12 h of hypoxia was 3.09 and 0.606%, respectively. (D) RGS5 induced ODMECs arrest at the G1 phase of the cell cycle under anoxic conditions. The number of G1 phase cells in the LV-H and LV-siRGS5 groups were 74.24±8.33 and 46.33±4.53%, respectively (n=3, P<0.05). *P<0.05. RGS5, regulator of G-protein signaling 5; ODMECs, ovarian carcinoma-derived endothelial cells; LV, lentiviral vector.
RGS5 does not affect the migration of ODMECs under hypoxic conditions
The ultrastructure of ODMECs was observed under an electron microscope, and the cytoplasm was found to contain abundant microtubules and microfilaments. This finding indicated that ODMECs may possess a strong ability for movement. In order to determine whether the overexpression of RGS5 induced by hypoxia would affect the migration ability of ODMECs, we detected the rate of cell migration using the scratch test. At 16 h after scratching, the healing rate in the LV-H-ODMEC and LV-siRGS5-ODMEC groups were 52.62±5.63 and 55.83±6.89%, respectively, with no statistically significant difference (n=3, P>0.05; Fig. 4B), indicating that RGS5 did not participate in ODMEC migration under hypoxic conditions.
RGS5 can induce apoptosis of ODMECs under hypoxic conditions
Tumor hypoxia often promotes angiogenesis and is conducive to tumor development and metastasis; on the other hand, it also induces cell apoptosis, thereby inhibiting tumor growth. The balance of these two effects is important in tumor development and outcome. Hypoxia can induce the expression of RGS5 and it is crucial to understand whether RGS5 participates in the apoptosis of ODMECs. This may be achieved by interfering with the expression of RGS5 and detection of the ODMEC apoptosis rate in the LV-H and LV-siRGS5 groups under normoxic and hypoxic conditions at 12 h. The present study revealed that hypoxia can induce apoptosis of ODMECs (0.020 vs. 3.09%), and the apoptosis rate of the LV-H and LV-siRGS5 groups at 12 h after hypoxia was 3.09 and 0.606%, respectively (Fig. 4C), indicating that RGS5 promoted the apoptosis of ODMECs under hypoxic conditions.
RGS5 enables ODMECs to remain in the G1 phase of the cell cycle under anoxic conditions
By interfering with the expression of RGS5 under hypoxia, observations of the ODMEC cell cycle in the LV-H and LV-siRGS5 groups revealed that, under hypoxic conditions, RGS5 can arrest ODMECs in the G1 phase of the cell cycle (43.02 vs. 77.75%), The number of G1 cells in the LV-H and LV-siRGS5 groups was 74.24±8.33 and 46.33±4.53%, respectively (n=3, P<0.05; Fig. 4D), indicating that RGS5 can arrest the cell cycle at the transition of the G1 to the S phase by reducing the proliferation rate of ODMECs under anaerobic conditions.
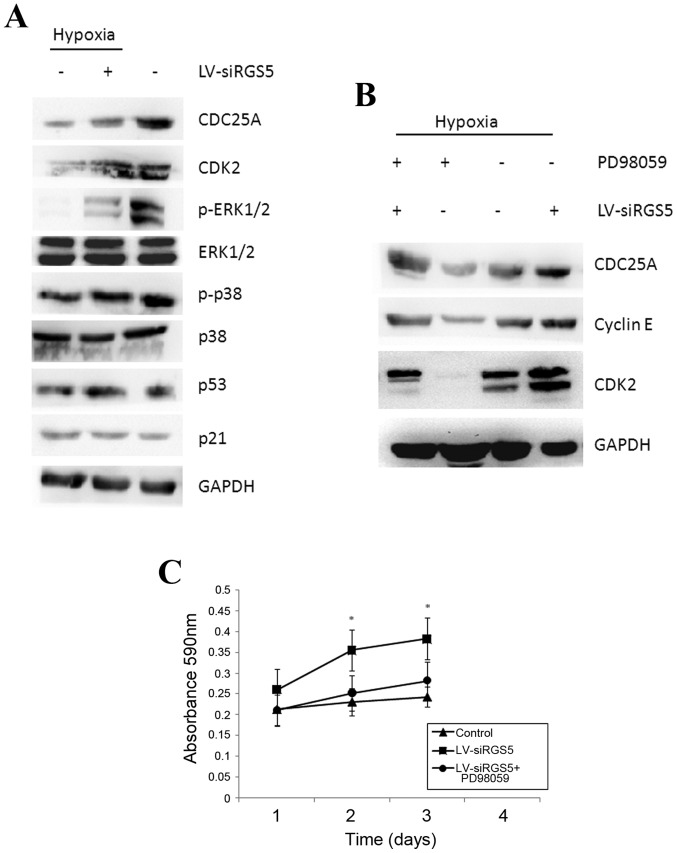
RGS5 inhibits the generation of CDC25A mediated by the MAPK/ERK signaling pathway, which causes cell cycle arrest at the G1 phase
Under hypoxic conditions, RGS5 arrested ODMECs at the G1 phase, and the rate of cell proliferation was decreased. The regulation of the cell cycle is strict and orderly, and the cell division cycle protein, CDC25A (ras-GRF1), plays a key role in the regulation of the cell cycle and the response to DNA damage.
We downregulated the expression of the RGS5 protein with RNAi under hypoxic conditions and demonstrated that the RGS5 inhibited the expression of CDC25A (Fig. 5A). The expression of CDC25A was higher under normoxic conditions compared with that under hypoxia (Fig. 5A) and it was positively associated with cell proliferation. Furthermore, upon assessment of the CDK2 and cyclin E proteins, which are closely linked to the cell cycle, it was found that both proteins increased when the RGS5 protein was decreased (Fig. 5B), indicating that the high expression of RGS5 was associated with cell cycle arrest.
Figure 5.
RGS5 inhibits the expression of the CDC25A protein induced by the MAPK/ERK signaling pathway. (A) The expression of the CDK2, CDC25A and cyclin E proteins was higher compared with the LV-H negative control group under hypoxic conditions, and the expression of the p53 and p21 proteins exhibited no change; the expression of phosphorylated ERK protein in the LV-siRGS5 group was markedly decreased and phosphorylation of p38 was not affected. (B) When the activity of ERK1/2 was inhibited with PD98059, the expression of the CDC25A, CDK2 and cyclin E proteins was decreased compared with the control group, and the effect of PD98059 on downregulating the expression of these proteins was in coordination with RGS5. (C) Treating ODMECs with PD98059 and LV-siRGS5 concurrently significantly reduced the cell proliferation rate (n=3; *P<0.05). RGS5, regulator of G-protein signaling 5; ODMECs, ovarian carcinoma-derived endothelial cells; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; LV, lentiviral vector.
The MAPK signaling pathway generally participates in cell proliferation and differentiation. In the present study, we found that the expression of the phosphorylated ERK protein significantly decreased when the RGS5 protein was decreased with RNAi, but p38 phosphorylation exhibited no obvious changes (Fig. 5A). To determine whether RGS5 participates in cell proliferation and cell cycle regulation of ODMECs by ERK kinase, we inhibited the ERK1/2 phosphorylation protein using the ERK1/2 inhibitor, PD98059. When the activity of ERK1/2 was inhibited, the expression of the CDC25A, CDK2 and cyclin E proteins were decreased compared with the control group, and the effect of PD98059 on downregulating the expression of these proteins was associated with the expression levels of RGS5 (Fig. 5B). In addition, treatment of ODMECs with PD98059 and LV-siRGS5 concurrently significantly reduced the cell proliferation rate (Fig. 5C), indicating that RGS5 downregulated the expression of the downstream proteins CDC25A, CDK2 and cyclin E, and that this effect was mediated by the MAPK/ERK pathway. In this manner, the ODMEC cell cycle was arrested at the G1 phase, which decreased the cell proliferation ability. In addition, careful surveillance of the DNA integrity during the G1 phase also revealed that the cell DNA repair apparatus and the apoptosis-inducing p53 protein were not affected by the change in the expression of RGS5 (Fig. 5A). The expression of cyclin-dependent kinase inhibitor, p21, which is located downstream of the p53 gene, and that can coordinate the association between the cell cycle, DNA replication and repair, did not significantly differ between the LV-H and LV-siRGS5 groups (Fig. 5A).
Discussion
RGS5 was recently revealed to be involved in tumor angiogenesis and metastasis (18,24). Thus, targeting RGS5 may affect both tumor cells and tumor vessels. In this study, using IHC, we found that RGS5 was weakly expressed in EOC microvessels expressing endoglin, with no expression in CD34-labeled blood vessels. Similarly, weak expression of both CD34 and endoglin was also found in lumen-like structures. Some scholars have reported that the expression of RGS5 is consistent with CD31 expression in the vasculature, and also that the expression of RGS5 and CD31 in blood vessels overlap (19,25). However, we used CD34 as a marker for the blood vessels in EOC, and there was no overlap between the RGS5 and CD34 markers. Although CD31 and CD34 are markers of macrovascular and microvascular endothelial cells, the two were revealed to be mainly expressed in mature endothelial cells, indicating that RGS5 is only expressed during early angiogenesis.
Studies have confirmed that the expression of RGS5 in mature or large blood vessels was significantly reduced, but was still higher compared with that in normal vascular endothelial cells, suggesting that RGS5 may be used as a potential anti-angiogenic target. Endoglin is highly expressed in tumor-derived vascular endothelial cells, and is of great value as a marker of tumor angiogenesis (20,26). Using IHC, we found that endoglin was mainly expressed in new microvascular endothelial cells in the peripheral regions of EOC compared with CD34-labeled blood vessels, whereas endoglin-expressing blood vessels in the central part of the tumor were significantly fewer, or even absent. In addition, RGS5 and endoglin staining of EOC microvessels overlapped, indicating that RGS5 may be involved in the regulation of early tumor angiogenesis. Although studies have revealed that RGS5 is highly expressed in early angiogenesis, participates in pericyte accumulation and differentiation through the PDGF-BB/PDGFR pathway and plays an important role in vascular remodeling, the mechanisms by which tumor vascular endothelial cells are regulated remain unclear.
MVD is a measure of the degree of tumor angiogenesis (21). Therefore, we found that MVD (reflected by CD34 expression) in tissues with high expression of RGS5 was significantly lower compared with that in tissues with low expression of RGS5. The two proteins were inversely correlated, indicating that the RGS5 protein may be involved in the regulation of ovarian cancer angiogenesis, thus affecting the MVD in ovarian cancer. This result is consistent with the expression of RGS5 and MVD in gastric carcinoma. The increase of the mean MVD was revealed to be associated with tumor metastasis. Although the expression of RGS5 was not found to be associated with ovarian cancer lymph node metastasis, it was associated with peritoneal metastasis, indicating that RGS5 plays an important role in the invasion and metastasis of ovarian cancer to the peritoneum.
Further analysis of the association between the expression of the RGS5 protein and clinicopathological characteristics revealed that the expression of the RGS5 protein in ovarian cancer cells exhibited no significant correlation with patient age, level of serum tumor markers such as CA125, the presence of ascitic fluid, tumor size, tumor differentiation, histological type or clinical stage of ovarian carcinoma. Ovarian cancer patients were followed up for 5–10 years, and using a univariate survival analysis we demonstrated that the expression of the RGS5 protein was associated with prognosis. The postoperative 5-year survival rate in patients with high expression of the RGS5 protein was higher compared with that in patients with low RGS5 expression. The log-rank test demonstrated that there were significant differences between the two groups of patients, with those exhibiting high expression of RGS5 having a better prognosis. The prognosis of ovarian cancer patients was associated with tumor histological type, pathological grade, clinical stage, patient age, as well as several other factors. Previous studies also found that intratumoral MVD was a prognostic risk factor for ovarian cancer (21,22,27,28), although MVD was an independent factor in the prognosis of patients with endometrial carcinoma (23), cervical cancer (24,29), and kidney and breast cancer (25,30). This indicated that the regulation of MVD is of major research value in ovarian cancer.
In order to determine whether RGS5 is associated with the tube-forming ability of ODMECs in vitro, the expression of RGS5 was detected at different stages of lumen-like structure formation. However, although the expression of RGS5 was high in tumor vessels, it was low in ODMECs in vitro, suggesting that it may be associated with the high expression of angiogenic factors in the tumor microenvironment. This revealed that RGS5 does not play a key regulatory role in the formation of lumen-like structures by ODMECs in vitro, but does not exclude the possibility of its involvement in the regulation of angiogenesis in other ways. A previous study reported that the expression of RGS5 in tumors highly expressing VEGF was higher compared with that in tumors with low VEGF expression in mice. In a mouse corneal neovascularization model, blocking the VEGF-mediated signaling pathway before the upregulation of the RGS5 gene effectively reduced neovascularization, whereas blocking the signaling pathway after upregulation of the RGS5 gene did not have the same effect, indicating that there is a connection between RGS5 and VEGF (11). In addition, the expression profiles of RGS5 and the PDGF-BB receptor are consistent in solid tumors (30). However, adding the angiogenesis-related factors, VEGF and PDGF-BB, at concentrations of 10 ng/ml for 24 h to the culture system stimulated ODMECs. The results indicated that VEGF and PDGF-BB cannot significantly promote the expression of RGS5 in ODMECs and HASMCs, suggesting that the high expression of RGS5 in tumor tissue does not depend entirely on the regulation of the VEGFR and PDGF-BB signaling pathways.
Animal experiments have confirmed that RGS5 gene expression is significantly increased in the brain cortex and hippocampus of rats under chronic hypoxic conditions. The response to hypoxia when the hypoxia inducible factor-1 (HIF-1) gene was knocked down in mice in vivo was to decrease the expression of RGS5. This revealed that the expression of RGS5 was regulated by hypoxia in tumor tissues and may be regulated by the HIF-1α signaling pathway (31,35). Hence, through regulation of the balance between angiogenic and anti-angiogenic factors, RGS5 may affect the progression of tumor vascularization under anoxic conditions.
In the present study, we downregulated the expression of the RGS5 protein in ODMECs under hypoxic conditions with a specific RGS5-siRNA lentiviral vector in order to explore the mechanism underlying its role in the angiogenesis of ovarian cancer. We found that ODMECs contained abundant microtubules and microfilaments in the cytoplasm when examined under an electron microscope, indicating strong motility. Subsequently, by using the scratch test we investigated whether the high expression of RGS5 induced by hypoxia was involved in cell migration. The healing rate at 16 h after scratching in the LV-H-ODMEC and LV-siRGS5-ODMEC groups did not differ significantly, indicating that the high expression of RGS5 does not affect the migration ability of ODMECs under hypoxic conditions. The tumor microenvironment contains numerous factors, and the function of proteins in tumors is often affected by the tumor microenvironment. We found that RGS5 does play a decisive role in the formation of lumen-like structures by ODMECs; therefore, it may affect ODMEC angiogenesis through other mechanisms, such as regulation of cell proliferation (32,36). Upon investigating the effect of RGS5 on ODMECs by MTT method under anoxic conditions, it was found that the RGS5 protein inhibited the proliferation of ODMECs. Upon further investigation, it was revealed that RGS5 also promoted cell apoptosis under hypoxic conditions, arresting ODMECs at the G1 phase of the cell cycle, thus inhibiting progression into the S phase.
The regulation of the cell cycle is strict and orderly (33,37), and the cell division cycle protein, CDC25A (ras-GRF1), plays a key role in the regulation of the cell cycle and the response to DNA damage (34,38). The CDC25 gene is highly conserved and has three subtypes CDC25A, CDC25B and CDC25C, among which CDC25A is considered to be involved in the G1/S and G2/M checkpoints, whereas CDC25B and CDC25C are mainly involved in the regulation of the G2/M checkpoint (35,39). We determined that RGS5 inhibited the expression of CDC25A through the downregulation of RGS5 protein expression with an RGS5-specific siRNA slow virus vector. The expression of CDC25A was higher under normoxic conditions compared with hypoxia, and it was positively associated with cell proliferation. CDK2 and cyclin E are closely linked to the cell cycle, and we found that the expression of the RGS5 protein was inversely associated with both proteins, indicating that a high expression of RGS5 was associated with cell cycle arrest. The MAPK signaling pathway, including the ERK, p38 and JNK pathways, participates in cell proliferation and differentiation (36). The JNK pathway is mainly involved in cell inflammatory reactions, whereas ERK-2 and p38 act through the receptors of a variety of growth factors, mainly mediating the signal of peptide growth factors. Their phosphorylation can promote cell proliferation, differentiation and migration (37,40). To determine whether RGS5 participated in the regulation of ODMEC proliferation and the cell cycle by ERK kinase, we used the ERK1/2 inhibitor, PD98059, to inhibit the phosphorylation of ERK1/2. The results demonstrated that the expression of the CDC25A, CDK2 and cyclin E proteins was decreased compared with that in the control group, and the effect of PD98059 on the downregulation of these proteins was coordinated with RGS5. Furthermore, treating ODMECs with PD98059 and LV-siRGS5 simultaneously can significantly reduce the cell proliferation rate, indicating that RGS5 can downregulate the expression of the CDC25A, CDK2 and cyclin E downstream proteins, which is mediated by the MAPK/ERK pathway, causing ODMEC cycle arrest at the G1 phase, thereby decreasing cell proliferation ability.
Acknowledgements
The authors are grateful to Mrs. YuDi Li and Mrs. ChunDong Lu for the collection of epithelial ovarian carcinoma samples, and to Professor XueFeng Jiang and Dr Qiao Wu for technical assistance. We would like to thank Mr. Jiang Zhu and Mr. Feng Wu for their help in evaluating the immunohistochemistry results. The authors would like to thank Dr Dev Sooranna, Imperial College London, for helping to edit the manuscript.
Glossary
Abbreviations
- ODMECs
ovarian carcinoma-derived endothelial cells
- EOC
epithelial ovarian cancer
- RGS5
regulator of G-protein signaling 5
- MVD
microvascular density
- RNAi
RNA interference
- CDK2
cyclin-dependent protein kinase 2
- MAPK/ERK
mitogen-activated protein kinase/extracellular signal-regulated kinase
- GPCRs
G-protein-coupled receptors
- HASMCs
human aortic smooth muscle cells
Funding
The present study was supported by grants from the National Natural Science Foundation of China (nos. 81101994 and 81072125).
Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
DW, PYa and ZL conceived and designed the study. DW and YX performed the experiments. LF and PYi collected the patient samples and the information. SSS and FW contributed to the statistical analysis. PYi wrote the manuscript and YX and PYa reviewed and edited the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The Ethics Committee of the First Affiliated Hospital of the Third Military Medical University approved the procedures and all patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen Q, Su Y, He X, Zhao W, Wu C, Zhang W, Si X, Dong B, Zhao L, Gao Y, et al. Plasma long non-coding RNA MALAT1 is associated with distant metastasis in patients with epithelial ovarian cancer. Oncol Lett. 2016;12:1361–1366. doi: 10.3892/ol.2016.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 3.Tang HS, Feng YJ, Yao LQ. Angiogenesis, vasculogenesis, and vasculogenic mimicry in ovarian cancer. Int J Gynecol Cancer. 2009;19:605–610. doi: 10.1111/IGC.0b013e3181a389e6. [DOI] [PubMed] [Google Scholar]
- 4.Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15:2695–2702. doi: 10.1158/1078-0432.CCR-08-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroep JR, Nortier JW. The role of bevacizumab in advanced epithelial ovarian cancer. Curr Pharm Des. 2012;18:3775–3783. doi: 10.2174/138161212802002689. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, Itamochi H. Bevacizumab and ovarian cancer. Curr Opin Obstet Gynecol. 2012;24:8–13. doi: 10.1097/GCO.0b013e32834daeed. [DOI] [PubMed] [Google Scholar]
- 7.Tejpar S, Prenen H, Mazzone M. Overcoming resistance to antiangiogenic therapies. Oncologist. 2012;17:1039–1050. doi: 10.1634/theoncologist.2012-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst JH, Hooks SB. Regulator of G-protein signaling (RGS) proteins in cancer biology. Biochem Pharmacol. 2009;78:1289–1297. doi: 10.1016/j.bcp.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Lappano R, Maggiolini M. G protein-coupled receptors: Novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 10.Perschbacher KJ, Deng G, Fisher RA, Gibson-Corley KN, Santillan MK, Grobe JL. Regulators of G-protein signaling in cardiovascular function during pregnancy. Physiol Genomics. 2018;50:590–604. doi: 10.1152/physiolgenomics.00037.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell TS, Bradley J, Robinson GS, Shima DT, Ng YS. RGS5 expression is a quantitative measure of pericyte coverage of blood vessels. Angiogenesis. 2008;11:141–151. doi: 10.1007/s10456-007-9085-x. [DOI] [PubMed] [Google Scholar]
- 12.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, Klagsbrun M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–8255. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network (NCCN): NCNN clinical practice guidelines in Oncology. www.nccn.org/patients. [Mar 9;2018 ];Ovarian cancer including fallopian tube cancer and primary peritoneal cancer (V2.2018)
- 15.Xu Y, Wang D, Zhao LM, Zhao XL, Shen JJ, Xie Y, Cao LL, Chen ZB, Luo YM, Bao BH, Liang ZQ. Endoglin is necessary for angiogenesis in human ovarian carcinoma-derived primary endothelial cells. Cancer Biol Ther. 2013;14:937–948. doi: 10.4161/cbt.25940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. Pericyte-specific expression of Rgs5: Implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J. 2003;17:440–442. doi: 10.1096/fj.02-0340fje. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci USA. 2008;105:11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z, Zuo Y, Wang J, Yu Z, Peng F, Chen Y, Dong Y, Hu X, Zhou Q, Ma H, et al. Overexpression of the regulator of G-protein signaling 5 reduces the survival rate and enhances the radiation response of human lung cancer cells. Oncol Rep. 2015;33:2899–2907. doi: 10.3892/or.2015.3917. [DOI] [PubMed] [Google Scholar]
- 19.Cheng WL, Wang PX, Wang T, Zhang Y, Du C, Li H, Ji Y. Regulator of G-protein signalling 5 protects against atherosclerosis in apolipoprotein E-deficient mice. Br J Pharmacol. 2015;172:5676–5689. doi: 10.1111/bph.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan L, Yang H, Xu C, Chen S, Meng Z, Li K, Chen H. ZNF750 inhibited the malignant progression of oral squamous cell carcinoma by regulating tumor vascular microenvironment. Biomed Pharmacother. 2018;105:566–572. doi: 10.1016/j.biopha.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 22.Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: Interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18:579–591. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4-activated G(i alpha1): Stabilization of the transition state for GTP hydrolysis. Cell. 1997;89:251–261. doi: 10.1016/S0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang JH, Huang WS, Hu CR, Guan XX, Zhou HB, Chen LB. Relationship between RGS5 expression and differentiation and angiogenesis of gastric carcinoma. World J Gastroenterol. 2010;16:5642–5646. doi: 10.3748/wjg.v16.i44.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silini A, Ghilardi C, Figini S, Sangalli F, Fruscio R, Dahse R, Pedley RB, Giavazzi R, Bani M. Regulator of G-protein signaling 5 (RGS5) protein: A novel marker of cancer vasculature elicited and sustained by the tumor's proangiogenic microenvironment. Cell Mol Life Sci. 2012;69:1167–1178. doi: 10.1007/s00018-011-0862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K, Lloyd RV. Endoglin (CD105): A review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–2290. [PubMed] [Google Scholar]
- 27.Taskiran C, Erdem O, Onan A, Arisoy O, Acar A, Vural C, Erdem M, Ataoglu O, Guner H. The prognostic value of endoglin (CD105) expression in ovarian carcinoma. Int J Gynecol Cancer. 2006;16:1789–1793. doi: 10.1111/j.1525-1438.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 28.Raspollini MR, Amunni G, Villanucci A, Baroni G, Boddi V, Rossi Degl'innocenti D, Taddei GL. Microvessel density in ovarian carcinoma: Computer image analysis in patients with shorter and longer survival. Int J Gynecol Cancer. 2005;15:844–849. doi: 10.1111/j.1525-1438.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 29.Cantu De León D, Lopez-Graniel C, Frias Mendivil M, Chanona Vilchis G, Gomez C, De La Garza Salazar J. Significance of microvascular density (MVD) in cervical cancer recurrence. Int J Gynecol Cancer. 2003;13:856–862. doi: 10.1111/j.1525-1438.2003.13399.x. [DOI] [PubMed] [Google Scholar]
- 30.Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: A systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–2955. doi: 10.1158/0008-5472.CAN-03-1957. [DOI] [PubMed] [Google Scholar]
- 31.Huang G, Song H, Wang R, Han X, Chen L. The relationship between RGS5 expression and cancer differentiation and metastasis in non-small cell lung cancer. J Surg Oncol. 2012;105:420–424. doi: 10.1002/jso.22033. [DOI] [PubMed] [Google Scholar]
- 32.Ladds G, Goddard A, Hill C, Thornton S, Davey J. Differential effects of RGS proteins on G alpha(q) and G alpha(11) activity. Cell Signal. 2007;19:103–113. doi: 10.1016/j.cellsig.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Kurrey NK, K A, Bapat SA. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97:155–165. doi: 10.1016/j.ygyno.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 34.Friedl P, Wolf K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y, An X, Ye Z, Cully B, Wu J, Li J. RG S5, a hypoxia-inducible apoptotic stimulator in endothelial cells. J Biol Chem. 2009;284:23436–23443. doi: 10.1074/jbc.M109.032664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cimpean AM, Saptefrati L, Ceausu R, Raica M. Characterization of endoglin and Ki-67 expression in endothelial cells from benign and malignant lesions of the uterine cervix. Pathol Int. 2009;59:695–700. doi: 10.1111/j.1440-1827.2009.02431.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Qiu H, Song Y, Liu Y, Wang H, Lu M, Deng M, Gu Y, Yin J, Luo K, et al. Cip2a promotes cell cycle progression in triple-negative breast cancer cells by regulating the expression and nuclear export of p27Kip1. Oncogene. 2017;36:1952–1964. doi: 10.1038/onc.2016.355. [DOI] [PubMed] [Google Scholar]
- 38.Zhao S, Wang Y, Guo T, Yu W, Li J, Tang Z, Yu Z, Zhao L, Zhang Y, Wang Z, et al. YBX1 regulates tumor growth via CDC25a pathway in human lung adenocarcinoma. Oncotarget. 2016;7:82139–82157. doi: 10.18632/oncotarget.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson I, Hoffmann I. Cell cycle regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res. 2000;4:107–114. doi: 10.1007/978-1-4615-4253-7_10. [DOI] [PubMed] [Google Scholar]
- 40.Anger T, El-Chafchak J, Habib A, Stumpf C, Weyand M, Daniel WG, Hombach V, Hoeher M, Garlichs CD. Statins stimulate RGS-regulated ERK 1/2 activation in human calcified and stenotic aortic valves. Exp Mol Pathol. 2008;85:101–111. doi: 10.1016/j.yexmp.2008.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.