Abstract
Objective
To evaluate the diagnostic accuracy of high-risk human papillomavirus (hrHPV) assays on self samples and the efficacy of self sampling strategies to reach underscreened women.
Design
Updated meta-analysis.
Data sources
Medline (PubMed), Embase, and CENTRAL from 1 January 2013 to 15 April 2018 (accuracy review), and 1 January 2014 to 15 April 2018 (participation review).
Review methods
Accuracy review: hrHPV assay on a vaginal self sample and a clinician sample; and verification of the presence of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) by colposcopy and biopsy in all enrolled women or in women with positive tests. Participation review: study population included women who were irregularly or never screened; women in the self sampling arm (intervention arm) were invited to collect a self sample for hrHPV testing; women in the control arm were invited or reminded to undergo a screening test on a clinician sample; participation in both arms was documented; and a population minimum of 400 women.
Results
56 accuracy studies and 25 participation trials were included. hrHPV assays based on polymerase chain reaction were as sensitive on self samples as on clinician samples to detect CIN2+ or CIN3+ (pooled ratio 0.99, 95% confidence interval 0.97 to 1.02). However, hrHPV assays based on signal amplification were less sensitive on self samples (pooled ratio 0.85, 95% confidence interval 0.80 to 0.89). The specificity to exclude CIN2+ was 2% or 4% lower on self samples than on clinician samples, for hrHPV assays based on polymerase chain reaction or signal amplification, respectively. Mailing self sample kits to the woman’s home address generated higher response rates to have a sample taken by a clinician than invitation or reminder letters (pooled relative participation in intention-to-treat-analysis of 2.33, 95% confidence interval 1.86 to 2.91). Opt-in strategies where women had to request a self sampling kit were generally not more effective than invitation letters (relative participation of 1.22, 95% confidence interval 0.93 to 1.61). Direct offer of self sampling devices to women in communities that were underscreened generated high participation rates (>75%). Substantial interstudy heterogeneity was noted (I2>95%).
Conclusions
When used with hrHPV assays based on polymerase chain reaction, testing on self samples was similarly accurate as on clinician samples. Offering self sampling kits generally is more effective in reaching underscreened women than sending invitations. However, since response rates are highly variable among settings, pilots should be set up before regional or national roll out of self sampling strategies.
Introduction
Cervical cancer rates in western Europe, North America, Australia, and New Zealand are relatively low compared with rates in less developed countries.1 However, demographic and social disparities in the burden of disease exist. In the United States, incidence is higher among Hispanic (8.9 per 100 000 women years in 2011-15, age adjusted using the 2000 US population as reference) and black (8.4) populations, versus the white population (7.4).2 The contrasts can be explained by differences in access to screening. In western and northern Europe, both cervical cancer incidence and mortality have decreased after widespread screening.3 In eastern Europe, where the coverage or quality, or both, of screening often is low to moderate, incidence has not dropped to the same extent and in some countries trends are even rising.4 5 6 To be noted, 85% of cases of cervical cancers occur in less developed countries, with incidence rates reaching 35 per 100 000 women years in eastern Africa.1
Most cervical cancer cases occur in women who have never been screened for cervical cancer, or do not participate in routine screening.7 However, recent trend analyses reveal an increasing burden of cervical cancer, even in countries with well organized screening programs based on cytology and good coverage.8 9 10 11 12 These observations can be reasonably explained by three arguments. First, the prevalence of exposure to the main etiologic factor, high-risk human papillomavirus (hrHPV) infection, has increased over time.13 14 Second, certain groups of the target population do not attend screening.15 16 17 Third, a proportion of screened women with cervical precancer and cancer show false negative Pap tests.18 19 We now have high level evidence that screening by hrHPV testing is more effective than screening by cytology for protecting against future cervical precancer and cancer.18 20 hrHPV testing provides another advantage: it can be done on vaginal samples collected by the patient (self samples), whereas cytology on self samples shows poor accuracy.21
hrHPV testing on self samples could be one way to increase access to cervical cancer screening for women not participating in routine screening. hrHPV testing also removes the need for a pelvic exam. In order to assess whether vaginal self sampling could improve cervical cancer prevention among underscreened populations, we updated two meta-analyses: one on the accuracy of self samples tested for hrHPV to detect cervical precancer; and one on the potential of strategies to reach women who were not screened or underscreened by offering them self sampling devices.22 23
The previous accuracy meta-analysis concluded that hrHPV testing was less sensitive on self samples than on clinician samples, but also found that the reason for lower sensitivity was the use of assays based on signal amplification.22 In this review, we separately pooled the accuracy of hrHPV assays based on signal amplification from hrHPV assays based on polymerase chain reaction. We also included subgroup meta-analyses and multivariable analyses assessing the variation in hrHPV testing accuracy on self samples by assay, self sampling device, and storage medium.
Two previous participation meta-analyses showed that sending self sampling kits to a woman’s home address generated greater response rates than invitation or reminder letters.23 24
New assays, self sampling devices, storage media, and participation strategies have entered the market since the publication of these two meta-analyses; and more accuracy and participation studies have been conducted. Moreover, countries such as Australia and the Netherlands have introduced self sampling to national screening guidelines. Other countries, such as the US, Canada, and some European countries, have called for rigorous comparative accuracy and participation studies.25 26 27
Methods
Study designs
Two different aspects of hrHPV testing on self samples were addressed (accuracy to detect cervical precancer and the participation of women who were underscreened) through two joint reviews. The reviews comprised of previous meta-analyses that were updated with new references published up to 15 April 2018.22 23
The first meta-analysis included diagnostic test accuracy studies that answered the following questions: what is the relative accuracy a hrHPV assay on a self sample compared with a clinician sample; and does the relative accuracy vary by clinical setting (screening population, high-risk population, follow-up for previous abnormalities, and monitoring after treatment), assay, self sampling device, and storage medium? We distinguished hrHPV assays based on a principle of signal amplification from hrHPV assays based on polymerase chain reaction and included only assays that were clinically validated for cervical cancer screening on clinician samples.28 However, we also performed more comprehensive analyses that included non-clinically validated assays (supplementary materials). The targeted disease was cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and CIN3+.
The second meta-analysis included randomized clinical trials and aimed to answer whether offering self sampling kits to women who were underscreened generated higher response rates than sending invitation or reminder letters. Secondary outcomes were test positivity rates, adherence to follow-up in women who were screened, and detection of CIN2+.
Criteria for study selection
Diagnostic studies for the accuracy review were eligible if the following criteria were met: a vaginal sample was collected by a woman herself (self sample) followed by a cervical sample collected by a clinician (clinician sample); the same hrHPV assay was performed on both samples; and the presence or absence of CIN2+ was verified by colposcopy and biopsy in all enrolled women, or in women with one or more positive tests. Studies with cytological follow-up for women with negative colposcopy results at baseline assessment were accepted as well, but were indexed for sensitivity analyses.
Randomized clinical trials for the participation review were eligible if the following criteria were met: the study population involved women who were irregularly screened, never screened, or did not respond to invitation or reminder letters for conventional screening for cervical cancer; women in the intervention group (self sampling arm) were invited to collect a self sample for hrHPV testing; women in the control arm were invited or reminded to undergo conventional cytology screening or hrHPV testing, or both, on a sample taken by a clinician; participation in the self sampling arm and the control arm was documented; and a minimum of 400 women were included in the study.
Study selection, data extraction, and checking
Search strategies are explained in the supplementary materials. To ensure that there were at least 12 months of overlap with the previous meta-analyses, the search period was 1 January 2013 to 15 April 2018 for the accuracy review and 1 January 2014 to 15 April 2018 for the participation review.22 23 Newly retrieved studies from each search were added to those already included in the corresponding review. We restricted the retrieval of references to published literature that had been peer reviewed. MA and one other author (SBS, ST, FS, or a Collaboration on Self-Sampling and HPV Testing group member) independently performed the study selection and data extraction. PC judged any unresolvable discordances. We assessed the quality of the diagnostic studies for the accuracy review by using the QUADAS-2 check list.29 We assessed the quality of the randomized clinical trials for the participation review by using the Cochrane Collaboration’s tool for risk of bias in randomized trials.30
Statistical methods
The meta-analyses followed PRISMA guidelines for reporting of meta-analyses, and recommendations established by the Cochrane Collaboration for diagnostic test accuracy and intervention trials.31 32 We used the bivariate normal model for the logit transforms of sensitivity and specificity taking the intrinsic correlation between true and false rates of positivity and the variability between studies into account for the pooling of accuracy data.33 34 We estimated relative accuracy of tests on self samples versus clinician samples by incorporating assay category as a covariate in the model.32 35 The same type of analysis was performed to assess the variation of the accuracy according to clinical setting (screening population, high-risk population, follow-up for previous abnormalities, and monitoring after treatment), assay, self sampling device, and storage medium. We assessed publication bias by using Deeks' and Harbord's regression tests for the pooled absolute and relative accuracy estimates, respectively.36 37
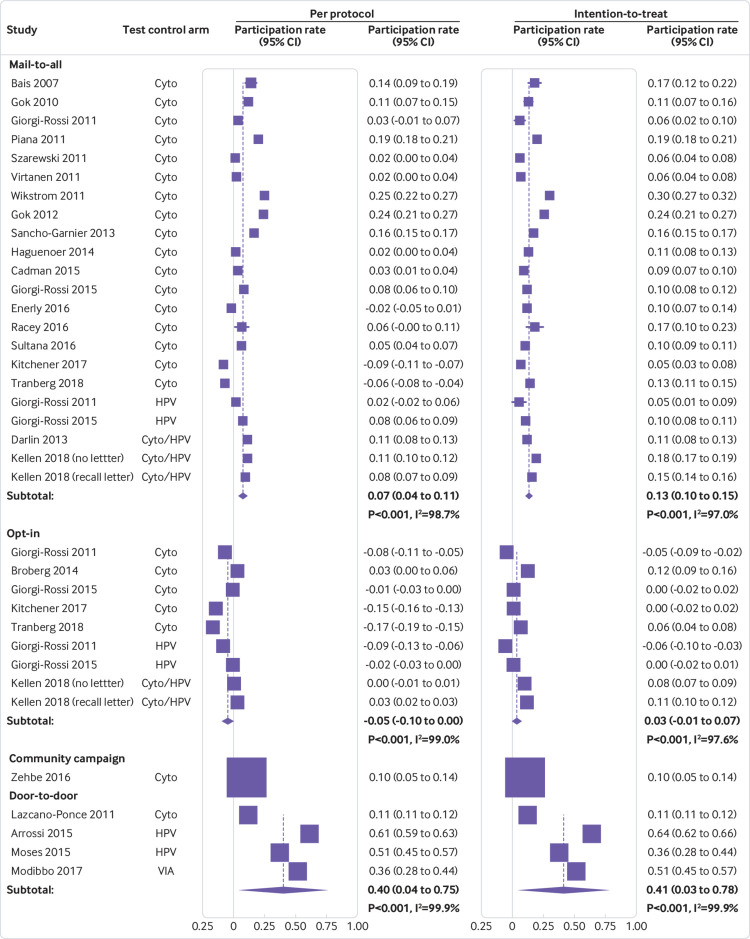
In the per protocol analyses of randomized clinical trials, only women who took a self sample in the experimental arm were counted. In the intention-to-treat analyses, which reflected the overall public health effect in a real world situation, we additionally included women in the experimental arm who had been offered self sampling but choose to have a clinician sample taken instead. We included the following invitation scenarios: mail-to-all, opt-in, community campaign, and door-to-door. In mail-to-all studies, self sampling kits were mailed directly to a woman’s home address for her to return by mail or in person to a local clinic. In opt-in studies, women had to request a self sampling kit. Community campaigns included community supported actions and outreach supported by mass media. In door-to-door interventions, community health workers delivered self sampling kits to women’s homes or workplaces. Given the intrinsic strategic differences, participation rates were pooled separately by invitational scenario.
We ran a random effects model using Metaprop, a statistical procedure for meta-analysis of binomial data, to pool proportions.38 We assessed statistical heterogeneity by using the I2 statistic, which measures the variation across studies that is due to interstudy heterogeneity.39 Relative participation rates (self/control) and absolute participation differences (self−control) were assessed by applying random effects models for ratios of proportions.40 41 We used meta-regression to assess the influence of study characteristics on study outcomes.42
Statistical significance was defined as P<0.05. We used STATA/SE 14 (STATA Corp, College Station, TX) for statistical analyses, except for the bivariate normal model, which was run in SAS 9.3 (SAS Institute Inc, Cary, NC).
Patient and public involvement
Since the meta-analyses only included published reports, no patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community. It was not evaluated whether the studies included in the review had any patient involvement.
Results
This update contains 19 new reports containing 22 diagnostic studies and nine new randomized participation trials, 26 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 which were added to the 34 accuracy studies and to the 16 participation trials already included in the previous meta-analyses.70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 The updated meta-analyses finally comprised 56 diagnostic test accuracy studies and 25 randomized trials. The PRISMA flowcharts and study characteristics are available in the supplementary materials.
Quality of diagnostic studies in the accuracy review
The risk of bias for enrolment of women was considered low in 59%, moderate in 39%, and high in 2% of the 56 diagnostic studies (supplementary materials). Reporting and execution of tests (description of cut-off, blinding of the index test toward comparator and reference test) was adequate in 73% and unclear in 27%, but the risk of bias was never assessed as high. The quality of verification with a reference standard (acceptable validity, blinding toward tests, and avoidance of incorporating test results in final conclusion of disease outcome) was good in 84%, moderate in 13%, and problematic in 4% of the studies. The delay between self sampling, clinician sampling, and verification with the reference standard was short in 61%, unreported in 38%, and long in 2% of the studies. Partial verification was avoided in 70% but clearly present in 25%, whereas differential verification was absent in 89% but unclear or present in 11% of the studies. Withdrawal of patients was explained appropriately in 70%, but not in 18%, and unclear in 12% of the studies. In most papers, uninterpretable results were poorly reported for the evaluated tests and for the reference standard (39% and 45%, respectively).
Quality of randomized clinical trials in the participation review
Three (12%) of the 25 trials met the criteria for low risk of bias in all categories.114 115 117 In eight trials (32%) allocation was random and concealed,26 63 64 65 104 114 115 117 in 14 trials (56%) allocation was unclear,66 67 102 103 105 106 107 108 109 110 111 112 113 116 and in three trials (12%) allocation was problematic.62 68 69 All trials had complete data for the participation outcome and were therefore at low risk of attrition bias. The quality of reporting was adequate in 12 trials (48%),26 67 102 103 104 107 108 109 114 115 116 117 118 incomplete in one trial,106 and intermediate in the other trials.
Accuracy of hrHPV assay
The pooled absolute sensitivity and specificity for outcomes CIN2+ and CIN3+, varied substantially by clinical setting and, therefore, accuracy measures were pooled separately by setting. Figure 1 shows that in screening studies, the pooled absolute sensitivity of hrHPV assays for CIN2+ based on signal amplification was substantially lower in self samples (77%, 95% confidence interval 69% to 82%) than in clinician samples (93%, 89% to 96%). The pooled absolute specificity to exclude CIN2+ was 84% (95% confidence interval 77% to 88%) in self samples and 86% (81% to 90%) in clinician samples. The pooled absolute sensitivity of hrHPV assays for CIN2+ based on polymerase chain reactions was 96% for both self samples and clinician samples. The specificity to exclude CIN2+ was similar for both self samples and clinician samples (79%).
Fig 1.
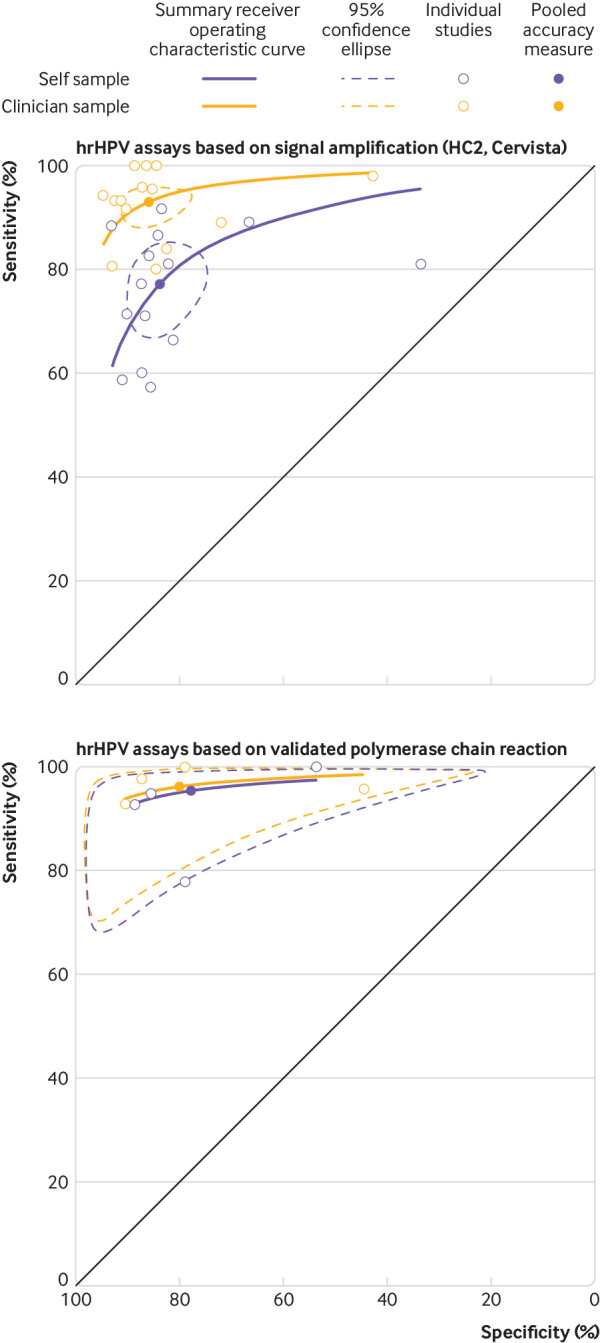
Meta-analysis of the accuracy, for hrHPV assays for CIN2+ based on signal amplification and polymerase chain reaction for self samples and clinician samples in primary cervical cancer screening. Estimates are derived from a bivariate model for pooling of diagnostic data
Supplementary table 10 shows the pooled absolute accuracy values for all clinical settings (screening population, high-risk population, follow-up for previous abnormalities, and monitoring after treatment). The absolute specificity of all hrHPV assays on self samples and clinician samples was substantially lower in the other clinical settings than with screening.
The relative accuracy of hrHPV assays on self samples versus clinician samples did not vary substantially by clinical setting and therefore we could estimate overall relative sensitivity and specificity under the condition to separate hrHPV assays based on signal amplification from hrHPV assays based on validated polymerase chain reaction.
Table 1 shows that hrHPV assays based on signal amplification were less sensitive (ratio 0.85, 95% confidence interval 0.80 to 0.89 for CIN2+; 0.86, 0.76 to 0.98 for CIN3+) and less specific (0.96, 0.93 to 0.98 to exclude CIN2+) on self samples versus clinician samples. The test positivity rate was, on average, 14% higher and the positive predictive value was significantly lower for both CIN2+ and CIN3+ for self samples (positive predictive value< 1).
Table 1.
Pooled relative sensitivity and specificity of high-risk human papillomavirus (hrHPV) assays based on signal amplification (SA) and polymerase chain reaction (PCR) on self samples versus clinician samples
| Assay | Outcome | No of studies | Ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Test positivity | PPV | ||||
| SA | CIN2+ | 23 | 0.85 (0.80 to 0.89)* | 0.96 (0.93 to 0.98)* | 1.14 (1.05 to 1.24) | 0.71 (0.62 to 0.82) | |
| CIN3+ | 9 | 0.86 (0.76 to 0.98)* | 0.97 (0.95 to 0.99)* | 0.65 (0.57 to 0.78) | |||
| PCR | CIN2+ | 17 | 0.99 (0.97 to 1.02) | 0.98 (0.97 to 0.99)* | 1.00 (0.94 to 1.06) | 0.97 (0.90 to 1.04) | |
| CIN3+ | 8 | 0.99 (0.96 to 1.02) | 0.98 (0.97 to 0.99)* | 0.90 (0.78 to 1.05) | |||
PPV=positive predictive value; CIN2+=cervical intraepithelial neoplasia of grade 2 or worse; CIN3+=cervical intraepithelial neoplasia of grade 3 or worse.
Statistically significantly different from unity.
hrHPV assays based on polymerase chain reaction were equally sensitive (ratio 0.99, 95% confidence interval 0.97 to 1.02 for CIN2+; 0.99, 0.96 to 1.02 for CIN3+) and slightly less specific (0.98, 0.97 to 0.99 to exclude CIN2+) on self samples versus clinician samples (table 1 and supplementary figs 3-6). The test positivity rate was similar in self samples versus clinician samples. Table 1 shows that the positive predictive values for CIN2+ or CIN3+ were not significantly lower for self samples.
Supplementary table 11 shows the pooled relative sensitivity and specificity for individual hrHPV assays for CIN2+ on self samples versus clinician samples. Each hrHPV assay based on signal amplification (HC2, careHPV, and Cervista) as well as the hrHPV E6/E7 mRNA test with APTIMA were at least 15% less sensitive for CIN2+ on self samples versus clinician samples. Each hrHPV assay based on polymerase chain reaction was equally sensitive for CIN2+ and for CIN3+ on self samples versus clinician samples.
Table 2 shows that the pooled sensitivity of hrHPV assays based on signal amplification was 10% to 16% lower on a self sample versus a clinician sample for all self sampling device and storage medium categories.
Table 2.
Variation in relative sensitivity and specificity of high-risk human papillomavirus (hrHPV) assays on self samples versus clinician samples, by self sampling device and storage medium
| Covariate | No of studies | Relative sensitivity (95% CI) | Relative specificity (95% CI) |
|---|---|---|---|
| Self sampling device | |||
| hrHPV assay based on signal amplification | |||
| Brush | 13 | 0.84 (0.78 to 0.90)* | 0.93 (0.91 to 0.96)* |
| Swab | 7 | 0.85 (0.78 to 0.91)* | 0.93 (0.90 to 0.95)* |
| Lavage† | 2 | 0.84 (0.69 to 1.04) | 0.74 (0.55 to 0.98)* |
| Tampon | 1 | 0.86 (0.78 to 0.96)* | 1.02 (1.00 to 1.03) |
| hrHPV assay based on polymerase chain reaction | |||
| Brush | 12 | 0.98 (0.95 to 1.02) | 0.95 (0.91 to 0.99)* |
| Swab | 4 | 0.98 (0.93 to 1.03) | 0.93 (0.89 to 0.98)* |
| Lavage† | 4 | 0.95 (0.87 to 1.04) | 1.09 (0.91 to 1.30) |
| Tampon | 0 | NA | NA |
| Storage medium | |||
| hrHPV assay based on signal amplification | |||
| Cell preserving† | 3 | 0.84 (0.78 to 0.90)* | 0.93 (0.91 to 0.96)* |
| Virological† | 15 | 0.86 (0.81 to 0.91)* | 0.95 (0.92 to 0.98)* |
| Dry samples | 0 | NA | NA |
| Other | 1 | 0.90 (0.71 to 1.13) | 0.92 (0.71 to 1.21) |
| hrHPV assay based on polymerase chain reaction | |||
| Cell preserving | 6 | 1.00 (0.96 to 1.04) | 0.92 (0.88 to 0.97)* |
| Virological† | 3 | 0.97 (0.91 to 1.04) | 0.94 (0.89 to 0.99)* |
| Dry samples† | 7 | 0.96 (0.90 to 1.02) | 1.01 (0.94 to 1.10) |
| Other | 1 | 0.95 (0.80 to 1.13) | 1.05 (0.69 to 1.58) |
Relative values were computed by using a bivariate normal model, separating studies using a hrHPV assay based on signal amplification or a hrHPV assay based on polymerase chain reaction. Pooling was performed using a bivariate normal model.
NA=not available.
Relative accuracy statistically significantly different from unity.
When the bivariate model containing covariates did not fit or when the number of studies <4, a separate pooling of the relative sensitivity and relative specificity using a model for ratios of proportions was run.
Table 2 shows that the pooled sensitivity of hrHPV assays based on polymerase chain reaction on a self sample was similar (the 95% confidence interval for the ratio included unity) to a clinician sample for all of the self sampling devices and storage media. In general, hrHPV assays were less specific on self samples except for the HC2 assay on a tampon self sample (n=1) or an assay based on polymerase chain reaction on a vaginal lavage self sample (2). All hrHPV assays were less specific on self samples stored in cell preserving media (9) or virological media (18). Pooled relative accuracy data for each self sampling device and storage medium can be found in supplementary tables 12 and 13.
No important patterns in the relation between the accuracy and QUADAS items or small study effects could be discerned (supplementary materials). However, in screening studies, where partial verification bias was avoided, the specificity of the hrHPV assay based on signal amplification on self samples was significantly lower (83%) than in studies where verification was not avoided or unclear (87%; P=0.03).
Efficacy of invitation scenarios
Participation in the self sampling arm
Table 3 and supplementary figure 9 show that the percentage of women in the self sampling arm that had a hrHPV test done on a self sample, when the self sampling kit was mailed to a woman’s home (mail-to-all), varied in the per protocol analysis between 6.4% and 34.0%, with a pooled average of 19.2% (95% confidence interval 15.7% to 23.0%).
Table 3.
Absolute participation in self sampling arm and control arm, participation difference and relative participation in self sampling versus control arm, by invitation scenario
| Invitation scenario | No of studies | Absolute participation | Participation difference % (95% CI) | Relative participation (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Self sampling % (95% CI) | Control % (95% CI) | |||||||
| Per protocol | ||||||||
| Mail-to-all | 19/21* | 19.2 (15.7 to 23.0) | 11.5 (8.3 to 15.1) | 7.3 (4.1 to 10.6) | 1.87 (1.43 to 2.44) | |||
| Opt-in | 6/8* | 7.8 (5.2 to 10.9) | 13.4 (10.2 to 16.9) | −5.1 (−10.0 to −0.2) | 0.73 (0.51 to 1.04) | |||
| Community campaign | 1 | 15.6 (12.4 to 19.5) | 6.0 (4.2 to 8.7) | 9.5 (5.4 to 13.7) | 2.58 (1.67 to 3.99) | |||
| Door-to-door | 4 | 94.2 (80.2 to 100.0) | 53.3 (10.5 to 93.2) | 39.7 (4.0 to 75.4) | 1.99 (0.68 to 5.85) | |||
| Intention-to-treat† | ||||||||
| Mail-to-all | 19/21* | 24.8 (21.6 to 28.1) | 11.5 (8.3 to 15.1) | 12.8 (10.4 to 15.1) | 2.33 (1.86 to 2.91) | |||
| Opt-in | 6/8* | 17.7 (12.3 to 23.9) | 13.4 (10.2 to 16.9) | 3.3 (−0.7 to 7.3) | 1.22 (0.93 to 1.61) | |||
| Community campaign | 1 | 15.6 (12.4 to 19.5) | 6.0 (4.2 to 8.7) | 9.5 (5.4 to 13.7) | 2.58 (1.67 to 3.99) | |||
| Door-to-door | 4 | 94.6 (83.0 to 99.9) | 53.3 (10.5 to 93.2) | 40.5 (3.0 to 78.0) | 2.01 (0.66 to 6.15) | |||
Giorgi-Rossi, 2011 and Giorgi-Rossi, 2015 had two control groups (one in which a Pap smear was taken by a clinician and another in which a sample for hrHPV testing was taken by a clinician). Kellen et al, 2018 also had two control arms (one with and one without recall letters).
Certain studies reported that some women, allocated to the self sampling, had a Pap smear taken by a clinician. The sum of the number of self samples taken and Pap smears taken, were counted in the intention-to-treat analyses. In studies, where no such cases were reported, the number of events in the per protocol and intention-to-treat analyses analyses were considered as equal.
The pooled participation rate was 7.8% (95% confidence interval 5.2% to 10.9%) when women had to request a self sampling kit (opt-in), 15.6% (12.4% to 19.5%) in one trial where women were invited through community campaigns, and 94.2% (80.2% to 100.0%) when community health workers delivered self sample kits directly to women’s homes or workplaces (door-to-door). In the trials in which additional Pap tests were reported in the mail-to-all group (intention-to-treat analysis), the overall participation rate was slightly or substantially higher, ranging from 10.2% to 39.0%, with an average rate of 24.8% (21.6% to 28.1%). The pooled percentage of participating women, in the intention-to-treat analysis of the self sampling arm, was 17.7% (12.3% to 23.9%) in the opt-in scenario and 94.6% (83.0 to 99.9%) in the door-to-door scenario.
Participation in the control arm
Table 3 and supplementary figure 10 show that the average percentage of women who participated in the control arm was 11.5% (95% confidence interval 8.3% to 15.1%) in the mail-to-all scenario, 13.4% (10.2% to 16.9%) in the opt-in scenario, 6.0% (4.2% to 8.7%) in the community campaigns scenario, and 53.3% (10.5% to 93.2%) in the door-to-door scenario. By default, the participation rates in the control arm were the same in the intention-to-treat analysis. In two Italian studies,104 117 there were groups of women who were offered hrHPV testing on clinician samples in the control arm. The participation was not significantly different when hrHPV testing was performed versus when cytology was performed (P=0.60 v P=0.76).104 117 In the Belgian randomized clinical trial,69 two control arms were included: one where the usual reminder letter was sent to non-responders and another one where no letter was sent to non-responders. In the first control arm (reminder letter) the response rate was 10.5% (95% confidence interval 9.9% to 11.2%), whereas in the second control arm (no letter), the response rate was slightly lower (8.0%, 95% confidence interval 7.5% to 8.6%).
Participation differences between self sampling and control arms
Table 3 and figure 2 show that in the per protocol analysis of the mail-to-all scenario, the pooled participation difference was 7.3% (95% confidence interval 4.1% to 10.6%, I2=99%). The participation difference was nearly always positive except for two studies where the difference was significantly lower than zero. In the opt-in scenario, the pooled difference tended to be negative in several studies and was also lower than zero when trials were pooled (participation difference −5.1%, 95% confidence interval −10.0% to −0.2%). In the community campaign, the participation difference was 9.5% (95% confidence interval 5.4% to 13.7%), whereas in the door-to-door scenario the pooled difference was 39.7% (95% confidence interval 4.0% to 75.4%, range 11% to 64%, I2=99.9%).
Fig 2.
Difference in participation rate between the self sampling and the control arms of randomized trials. Cyto=cytology; HPV=human papillomavirus; VIA=visual inspection after application of acetic acid
In the intention-to-treat analysis of the mail-to-all scenario, the pooled participation difference was 12.8% (95% confidence interval 10.4% to 15.1%, range 5% to 30%, I2=97%), whereas in the opt-in scenario the difference was not significantly different from zero (participation difference 3.3%, 95% confidence interval −0.7% to 7.3%). For the other scenarios, the intention-to-treat analyses showed similar participation differences as in the per protocol analyses.
Relative participation in self sampling versus control arms
Table 3 and supplementary figure 11 show that, on average, the relative participation rate was 1.87 times (95% confidence interval 1.43 to 2.44) and 2.33 times (1.86 to 2.91) higher in the self sampling arm versus the control arm of the trials with a mail-to-all scenario, in the per protocol and intention-to-treat analyses, respectively. In the per protocol analysis of the trials with opt-in scenarios, the average relative participation was lower in the self sampling versus the control arm, although not significantly (0.73, 95% confidence interval 0.51 to 1.04), whereas on average in the intention-to-treat analysis, the pooled relative participation exceeded unity, although not significantly (1.22, 0.93 to 1.61). The relative participation was on average twice as high in the self sampling versus the control arms in the community campaign and door-to-door scenarios.
We found five studies that provided response rates in both trial arms stratified by categories defined on the screening history status.26 65 103 113 114 Since the categorization was different throughout studies, subgroup meta-analysis or meta-regressions were not possible and, therefore, we analyzed participation rates and relative participation separately for the trials with stratified data (supplementary table 16).
We observed that participation rates decreased generally with length of time since last screening in both trial arms. The lowest rates were in women with no screening records except for one study.65 However, the relative participation tended to increase with time since last screening, not taking the women with no screening records into account.
We did not find evidence of publication bias in the mail-to-all or opt-in scenarios for relative participation (supplementary table 17).
Sample adequacy, test positivity rate, adherence to follow-up, detection of CIN2+
Sixteen of the trials reported on the adequacy of self collected samples. Table 4 and supplementary figure 12 show that, on average, 0.7% of self samples (95% confidence interval 0.4% to 1.0%, range 0.0% to 2.7%, I2=77.5%) were unsatisfactory for hrHPV testing.
Table 4.
Absolute proportion in self sampling arm and contrasts between self sampling and control arms
| Parameter | No of studies* | Absolute proportion self sampling % (95% CI) | No of studies† | Relative proportion (95% CI) | Proportion difference % (95% CI) |
|---|---|---|---|---|---|
| Unsatisfactory sample | 16 | 0.7 (0.4 to 1.0) | NA | NA | NA |
| Test positivity‡ | 22 | 11.1 (9.8 to 12.4) | NA | NA | NA |
| Adherence to follow-up | 20 | 80.6 (67.0 to 91.5) | 10 | 0.91 (0.80 to 1.04) | −4.8 (−13.1 to 3.5) |
| CIN2+ detection per thousand invited §¶ | 18 | 2.6 (1.4 to 4.1) | 14 | 2.28 (1.44 to 3.61) | 1.6 (0.1 to 3.1) |
| CIN2+ detection per thousand screened**¶ | 18 | 9.8 (7.1 to 13.0) | 14 | 1.13 (0.63 to 2.04) | 2.9 (−1.7 to 7.5) |
NA=Not available; CIN2+=cervical intraepithelial neoplasia grade 2 or worse.
Reporting the parameter in both the self sampling and control arms.
Reporting the parameter in the self sampling arm.
Of high-risk human papillomavirus (hrHPV) assay in the self sampling arm (per protocol).
Depends on participation, adherence to follow-up, prevalence of disease among participants, and sensitivity of tests (screening and follow-up).
Restricted to data where a Pap smear was taken in the control arm.
Depends on adherence to follow-up, prevalence of disease among participants and sensitivity of tests (screening and follow-up).
The hrHPV test positivity varied between 6.0% and 29.4%. The hrHPV positivity rate, pooled from 22 trials, was 11.1% (95% confidence interval 9.8% to 12.4%, I2=92.2%) (table 4 and supplementary fig 14).
In 20 trials in which adherence to follow-up among women with self samples that tested positive for hrHPV was reported, on average, 80.6% (95% confidence interval 67.0% to 91.5%, range 41% to 100%, I2=98.7%) had a follow-up examination. The rate of adherence to follow-up varied by applied triage policy, with higher adherence in studies with direct referral compared with studies with a triage policy (supplementary fig 14). The adherence to follow-up was lower in women who tested positive for hrHPV in the self sampling arm versus women in the control arm, but the difference was not significant in 10 trials in which the follow-up adherence was reported in both arms (relative proportion of 0.91, 95% confidence interval 0.80 to 1.04; proportion difference of −4.8%, 95% confidence interval −13.1% to 3.5%).
The CIN2+ detection rate in the self sampling arm varied between 0 to 11 per 1000 invited women (supplementary fig 15). On average, the detection rate was 2.28 times (95% confidence interval 1.44 to 3.61, I2=41.4%) higher in the self sampling arm versus the control arm. The detection rate ratio varied by triage policy, with a greater detection rate ratio (P=0.031) when women with a self sample that tested positive for hrHPV were directly referred to colposcopy (relative detection for the self sampling v control arm of 3.03 v 1.79; supplementary fig 16). The detection rate per 1000 screened women varied between 0 and 35. On average, the detection of CIN2+ per number of screened women was similar in the two trial arms (relative proportion 1.13, 95% confidence interval 0.63 to 2.04, range 0.05-4.31, I2=64.8%; table 4 and supplementary fig 17). No detection rate heterogeneity by self sample triage strategy was observed.
Discussion
Our first meta-analysis, on test accuracy, showed that hrHPV testing with an hrHPV assay based on polymerase chain reaction is as sensitive for detection of CIN2+ and CIN3+ and slightly less specific on self samples compared with clinician samples. On the other hand, Hybrid Capture II and Cervista, two hrHPV assays based on signal amplification, have lower sensitivity to detect CIN2+ and CIN3+ and are less specific to exclude CIN2+ when applied to self samples. mRNA testing with APTIMA and hrHPV DNA testing with careHPV were less sensitive but as specific on self samples compared with clinician samples. No strong effects of self sampling devices or storage media could be shown. However, most studies compared accuracy of self samples with clinician samples using certain sampling devices or storage media. Differences could be assessed by subgroup meta-analyses. More comparative diagnostic studies are needed, comparing combinations of assays, sampling devices, and storage media to generate more robust data in order to develop more precise guidelines.119
Our first meta-analysis also confirmed that the estimates of the relative accuracy of hrHPV testing of self samples compared with clinician samples is a robust outcome that does not vary substantially across clinical setting. This finding justifies the choice of a colposcopy clinic as an appropriate research setting in which combinations of hrHPV tests, self sampling devices (including instruments to collect urine), and storage media can be evaluated.119
Comparison with other studies
Recent randomized trials have shown that cytology triage after hrHPV screening results in lower incidence of cancer.18 20 Since reflex cytology triage is not recommended on self samples because of its poor accuracy,21 an additional visit is needed to collect a cervical sample for cytological assessment. If follow-up to the cytology visit is poor, the overall gain in screening coverage could be partly compromised. Therefore, we consider that finding a molecular test that permits reflex testing of samples that test positive for hrHPV should be a priority for future research. Hyper-methylation of certain viral or human genes involved in carcinogenesis has shown promising accuracy profiles and could be applied to self samples, but needs to be validated.120 121 Recent studies have revealed that hrHPV testing is also feasible on urine.122 Collection of urine could be easier for women who dislike vaginal collection. However, most studies document only virological outcomes.123 Studies assessing the clinical accuracy of hrHPV testing on urine are urgently needed.124
The relative participation, the main effect size of participation trials, is determined by the response rate in the control arm. An absolute gain in participation (participation difference) will yield a larger relative effect when the participation in the control intervention is low. For instance, relative participation tended to be highest among women who were never screened or who were last screened five or more years ago (supplementary table 16).103 113 114
Mailing self sampling kits to women’s home address is more effective in reaching populations that are underscreened compared with sending invitation or reminder letters for clinician sampling. The size of effect is highly variable among the included trials. Therefore, we recommend the set up of local trials to assess feasibility, effectiveness, and cost effectiveness before rolling out programs that include self sampling at regional or national levels.
The opt-in scenario, in which women request a self sampling kit, looks interesting from an economic and ecological point of view, but is not significantly more efficacious in generating responses in women who do not attend the regular screening program than routine invitations. Also, the results were very heterogeneous (P<0.001) for the opt-in scenario, warning against generalization. In two observational studies conducted in Sweden and Denmark, women who were underscreened had the option to request a self sampling kit. In the Swedish study, 63% of the invited women, who did not have a screening record in the previous six years, requested a kit and 39% took a self sample and sent it to the laboratory.125 In the Copenhagen cohort, 32% of women, not screened in the previous four to six years, requested self sampling kits and 20% took a self sample and sent it to the laboratory.126 These response rates correspond with the better rates observed in the mail-to-all randomized clinical trials. All randomized clinical trials conducted in less developed countries included home visits in the self sampling arm and noted excellent participation rates (>80%, fig 2). However, high participation was also noted in demonstration studies when women had to contact health centers to obtain or return the self sampling kit, as observed in rural Bhutan (average participation of 71%, with a negative trend by distance).127
The proportion of hrHPV tests applied on inadequate self samples in participation studies was low, showing that this sampling method is suitable for hrHPV testing. The adherence to follow-up among women with a self sample that tested positive for hrHPV was remarkably high (81% on average), probably owing to measures foreseen in the randomized clinical trials. It remains to be elucidated whether these high adherence rates observed in trials could be reproduced in routine screening programmes, in which resources to maximize follow-up are more limited.
Study limitations
A limitation, inherent among meta-analyses based on aggregated results extracted from published reports, is the lack of detailed data equally stratified according to potentially influencing factors related to the target population. A meta-regression, including only eight studies, did not reveal age effects. An Italian study showed a greater participation difference (self sampling arm minus the control arm of 11% to 12%) in urban than in rural areas (participation difference of 3%, with zero included in the confidence interval).104 Certain studies observed a lower response to the offer of a self sampling kit in more socially deprived groups,128 whereas others did not observe a social gradient.65 114 Several trials reported lower response rates in the self sampling arm in women who were never screened than in women who had been screened,26 114 126 whereas others observed an opposite trend.65 Subgroup analyses and meta-regressions were not possible in the current systematic review because covariates were categorized differently. In order to evaluate the impact of influential factors more efficiently, we propose pooling individual patient data from the best trials.
Future research
Future research could explore whether primary care providers could contribute to raising screening coverage. Primary care providers could verify the screening status of their patients belonging to the target screening population and offer self sampling kits to those who have not been screened recently.129 130 Primary care providers could be an effective alternative in settings where mailing sampling kits as part of an organized screening program is not feasible and door-to-door invitation is too costly. Self sampling could also become the new paradigm for primary cervical cancer screening in the general population as an alternative for the collection of a clinician sample.131 This idea is currently being explored in the IMPROVE trial in the Netherlands.132 A more detailed list of study proposals on self sampling is included in the research agenda in the supplementary materials.
Policy implications
For the first time, test accuracy of hrHPV screening and participation in programmes offering self sampling kits have been assessed in two joint reviews. This enabled simulating program sensitivity, determined as program participation multiplied by test sensitivity, as well as other parameters needed for cost effectiveness modeling. When adequate resources and infrastructure are available, a hrHPV assay based on polymerase chain reaction test should be used to achieve optimal program sensitivity. When funds are limited, using a less expensive hrHPV assay based on signal amplification could still provide an overall gain in program sensitivity despite the loss in test sensitivity.
The highest participation rates in our systematic review were observed in studies that included door-to-door invitation scenarios conducted in Latin America or Africa. However, the direct offer of a self sampling kit by a health professional might also be an effective strategy to reach non-responders in countries with established screening programmes. Point-of-care hrHPV testing on self samples could also be appropriate in a context of providing preventive care to medically underserved communities.
Conclusions
hrHPV testing with an appropriate assay offers a promising new strategy that could increase population coverage substantially. Whereas accuracy of new combinations of assays and self sampling devices can be evaluated in a diagnostic setting, acceptance and participation should be shown locally in a screening setting before general roll out.
What is already known on this topic
Tests performed on self samples are less sensitive and less specific than tests performed on clinician samples when using a high-risk human papillomavirus (hrHPV) assay based on signal amplification
Response rates for hrHPV testing are higher for self sampling kits than for conventional invitations
What this study adds
Tests performed on self samples are similarly sensitive and slightly less specific than tests performed on clinician samples when using a hrHPV assay based on polymerase chain reaction
Response rates for hrHPV testing continue to be higher for self sampling kits than for conventional invitations
Acknowledgments
The Collaboration on Self-Sampling and HPV Testing group members: Mona Saraiya and Virginia Senkomago, Centers for Disease Control and Prevention; Francisco AR García, Pima County Department of Health; David Chelmow, Virginia Commonwealth University Medical Center; Alan Waxman, University of New Mexico Health Sciences Center; Julia C Gage, US National Cancer Institute; Nicolas Wentzensen, US National Cancer Institute; Christopher M Zahn, American College of Obstetricians and Gynecologists; Warner K Huh, University of Alabama at Birmingham; Jennifer S Smith, University of North Carolina at Chapel Hill; Thomas C Randall, Massachusetts General Hospital; Mark H Einstein, Rutgers New Jersey Medical School; Michael A Gold, Tulsa Cancer Institute; Kathleen M Schmeler, The University of Texas MD Anderson Cancer Center; Robert A Smith, American Cancer Society; Sarah Temin, American Society of Clinical Oncology; Jose Jeronimo, Global Coalition Against Cervical Cancer; and Philip E Castle, Albert Einstein School of Medicine.
Web Extra.
Extra material supplied by the author
Supplementary materials: Supplementary materials and supplementary figures 1-17 and tables 1-18
Contributors: MA designed the study concept and protocol. MA and PC formulated the clinical questions and the definition of PICOS components. MA elaborated data extraction forms for the review update. MA, SBS, ST, and FS extracted data. MA conducted the statistical analysis. MA, SS, and PC wrote the manuscript. SBS, ST, FS, and PC conducted a critical revision of the manuscript. MA, SBS, and PC revised and approved the manuscript before submission. Members of the Collaboration on Self sampling and HPV Testing (MS, VS, DC, AW, JG, NW, CMZ, JJ, FARG, WKH, JSS, TCR, MHE, KMS, RAS, and MAG) provided feedback on the study protocol, performed and verified the initial part of study selection, and reviewed the manuscript. All authors had full access to the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. MA is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This systematic review was supported by Global Coalition Against Cervical Cancer (New York, NY, USA) through a grant by the US Centers for Disease Control and Prevention through its cooperative agreement with the National Network of Public Health Institutes and subaward to GC3, and by High Authority for Health (Paris, France). MA was supported by the COHEAHR Network (grant No 603019), funded by the 7th Framework Programme of DG Research and Innovation, European Commission (Brussels, Belgium) and the German Guideline Program in Oncology (GGPO, Hannover, Germany). The funder had no role in the study design, data collection, data interpretation, or writing of the report.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: MA is principal investigator of the VALGENT (Validation of HPV GENotyping Test) and VALHUDES (VALidation of HUman papillomavirus assays and collection DEvices for HPV testing on Self samples and urine samples) framework. Both protocols provide a template for HPV test comparison and validation on clinician samples and self samples, respectively. Manufacturers of HPV assays and devices for self collection can participate, under the condition of provision of test kits and funding for laboratory testing and statistical analyses to the employing institutions. Researchers did not receive any personal funding. SS was supported in part by unrestricted educational grants to the Global Coalition Against Cervical Cancer from Rovers, BD, QIAGEN, and Roche; a contract from Chengdu Genegle Biotechnology Co, Ltd; and has received cervical screening tests and diagnostics at a reduced or no cost for research from BD, Hologic, Rovers, Arbor Vita Corp, and Trovagene. PC has received cervical screening tests and diagnostics at a reduced or no cost for research from Roche, BD, Cepheid, and Arbor Vita Corporation.
Ethical approval: Not required.
Patient consent: Patient consent not required.
Data sharing: No additional data are available.
The manuscript's guarantor (MA) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1. Arbyn M, Castellsagué X, de Sanjosé S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol 2011;22:2675-86. 10.1093/annonc/mdr015 [DOI] [PubMed] [Google Scholar]
- 2.Surveillance Epidemiology and End Results Program (SEER). Cancer Stat Facts: Cervical Cancer. National Cancer Institute. https://seer.cancer.gov/statfacts/html/cervix.html
- 3. Bray F, Loos AH, McCarron P, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev 2005;14:677-86. 10.1158/1055-9965.EPI-04-0569 [DOI] [PubMed] [Google Scholar]
- 4. Arbyn M, Raifu AO, Weiderpass E, Bray F, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. Eur J Cancer 2009;45:2640-8. 10.1016/j.ejca.2009.07.018 [DOI] [PubMed] [Google Scholar]
- 5. Arbyn M, Antoine J, Mägi M, et al. Trends in cervical cancer incidence and mortality in the Baltic countries, Bulgaria and Romania. Int J Cancer 2011;128:1899-907. 10.1002/ijc.25525 [DOI] [PubMed] [Google Scholar]
- 6. Bray F, Lortet-Tieulent J, Znaor A, Brotons M, Poljak M, Arbyn M. Patterns and trends in human papillomavirus-related diseases in Central and Eastern Europe and Central Asia. Vaccine 2013;31(Suppl 7):H32-45. 10.1016/j.vaccine.2013.02.071 [DOI] [PubMed] [Google Scholar]
- 7. IARC Cervix Cancer Screening. IARC Handbooks of Cancer Prevention. Vol. 10 IARCPress, 2005. [Google Scholar]
- 8. Sasieni P, Castanon A. Dramatic increase in cervical cancer registrations in young women in 2009 in England unlikely to be due to the new policy not to screen women aged 20-24. J Med Screen 2012;19:127-32. 10.1258/jms.2012.012081 [DOI] [PubMed] [Google Scholar]
- 9. Baldur-Felskov B, Munk C, Nielsen TS, et al. Trends in the incidence of cervical cancer and severe precancerous lesions in Denmark, 1997-2012. Cancer Causes Control 2015;26:1105-16. 10.1007/s10552-015-0603-7 [DOI] [PubMed] [Google Scholar]
- 10.Finnish Cancer Registry. Cancer Statistics. Cancer Society of Finland. https://cancerregistry.fi/statistics/cancer-statistics/
- 11.Nationellt Kvalitetsregister för Cervixcancerprevention (NKCx). Swedish National Cervical Screening Registry Analysis. Annual reports (in Swedish). http://www.nkcx.se/templates/_rsrapport_2017.pdf
- 12.The Netherlands Cancer Registry. Dutch cancer figures. Integraal Kankercentrum Nederland. https://www.cijfersoverkanker.nl/
- 13. Laukkanen P, Koskela P, Pukkala E, et al. Time trends in incidence and prevalence of human papillomavirus type 6, 11 and 16 infections in Finland. J Gen Virol 2003;84:2105-9. 10.1099/vir.0.18995-0 [DOI] [PubMed] [Google Scholar]
- 14. Lehtinen M, Kaasila M, Pasanen K, et al. Seroprevalence atlas of infections with oncogenic and non-oncogenic human papillomaviruses in Finland in the 1980s and 1990s. Int J Cancer 2006;119:2612-9. 10.1002/ijc.22131 [DOI] [PubMed] [Google Scholar]
- 15. Elit L, Saskin R, Raut R, Elliott L, Murphy J, Marrett L. Sociodemographic factors associated with cervical cancer screening coverage and follow-up of high grade abnormal results in a population-based cohort. Gynecol Oncol 2013;128:95-100 10.1016/j.ygyno.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 16. Ricardo-Rodrigues I, Jiménez-García R, Hernández-Barrera V, Carrasco-Garrido P, Jiménez-Trujillo I, López de Andrés A. Social disparities in access to breast and cervical cancer screening by women living in Spain. Public Health 2015;129:881-8. 10.1016/j.puhe.2015.02.021 [DOI] [PubMed] [Google Scholar]
- 17. Arbyn M, Fabri V, Temmerman M, Simoens C. Attendance at cervical cancer screening and use of diagnostic and therapeutic procedures on the uterine cervix assessed from individual health insurance data (Belgium, 2002-2006). PLoS One 2014;9:e92615. 10.1371/journal.pone.0092615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arbyn M, Ronco G, Anttila A, et al. Evidence regarding HPV testing in secondary prevention of cervical cancer. Vaccine 2012;30(Suppl 5):F88-99. 10.1016/j.vaccine.2012.06.095 [DOI] [PubMed] [Google Scholar]
- 19. Koliopoulos G, Nyaga VN, Santesso N, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev 2017;8:CD008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ronco G, Dillner J, Elfström KM, et al. International HPV screening working group Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014;383:524-32. 10.1016/S0140-6736(13)62218-7 [DOI] [PubMed] [Google Scholar]
- 21. Snijders PJ, Verhoef VM, Arbyn M, et al. High-risk HPV testing on self-sampled versus clinician-collected specimens: a review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J Cancer 2013;132:2223-36. 10.1002/ijc.27790 [DOI] [PubMed] [Google Scholar]
- 22. Arbyn M, Verdoodt F, Snijders PJF, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol 2014;15:172-83. 10.1016/S1470-2045(13)70570-9 [DOI] [PubMed] [Google Scholar]
- 23. Verdoodt F, Jentschke M, Hillemanns P, Racey CS, Snijders PJF, Arbyn M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur J Cancer 2015;51:2375-85. 10.1016/j.ejca.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 24. Racey CS, Withrow A, Gesink D. Self-collected HPV Testing Improves Participation in Cervical Cancer Screening: A Systematic Review and Meta-analysis. Can.J.Public Health 2013;104:e159-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Curry SJ, Krist AH, Owens DK, et al. US Preventive Services Task Force Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018;320:674-86. 10.1001/jama.2018.10897 [DOI] [PubMed] [Google Scholar]
- 26. Tranberg M, Bech BH, Blaakær J, Jensen JS, Svanholm H, Andersen B. Preventing cervical cancer using HPV self-sampling: direct mailing of test-kits increases screening participation more than timely opt-in procedures - a randomized controlled trial. BMC Cancer 2018;18:273. 10.1186/s12885-018-4165-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chao YS, Clark M, Ford C. HPV Self-Sampling for Primary Cervical Cancer Screening: A Review of Diagnostic Test Accuracy and Clinical Evidence. Canadian Agency for Drugs and Technologies in Health, 2018. [PubMed] [Google Scholar]
- 28. Arbyn M, Snijders PJ, Meijer CJLM, et al. Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin Microbiol Infect 2015;21:817-26. 10.1016/j.cmi.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 29. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2 Group QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264-9. [DOI] [PubMed] [Google Scholar]
- 32. Deeks JJ, Bossuyt PM, Gatsonis C. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. [Google Scholar]
- 33. Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. 10.1093/biostatistics/kxl004 [DOI] [PubMed] [Google Scholar]
- 34. Harbord RM, Whiting P. metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J 2009;9:211-29. [Google Scholar]
- 35.Takwoingi Y, Deeks J. METADAS: A SAS macro for meta-analysis of diagnostic accuracy studies. Quick reference and worked example. https://methods.cochrane.org/sites/methods.cochrane.org.sdt/files/public/uploads/MetaDAS%20Quick%20Reference%20v1.3%20May%202012.pdf
- 36. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. 10.1016/j.jclinepi.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 37. Harbord RM, Harris RJ, Sterne JAC. Updated tests for small-study effects in meta-analyses. Stata J 2009;9:197-210. [Google Scholar]
- 38. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014;72:39. 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. metan: fixed- and random-effects meta-analysis. Stata J 2008;8:3-28. [Google Scholar]
- 41. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 42. Harbord R, Higgins JP. Meta-regression in Stata. Stata J 2013;8:493-519. [Google Scholar]
- 43. Zhao FH, Jeronimo J, Qiao YL, et al. An evaluation of novel, lower-cost molecular screening tests for human papillomavirus in rural China. Cancer Prev Res (Phila) 2013;6:938-48. 10.1158/1940-6207.CAPR-13-0091 [DOI] [PubMed] [Google Scholar]
- 44. Chernesky M, Jang D, Gilchrist J, et al. Evaluation of a new APTIMA specimen collection and transportation kit for high-risk human papillomavirus E6/E7 messenger RNA in cervical and vaginal samples. Sex Transm Dis 2014;41:365-8. 10.1097/OLQ.0000000000000125 [DOI] [PubMed] [Google Scholar]
- 45. Hesselink AT, Berkhof J, van der Salm ML, et al. Clinical validation of the HPV-risk assay, a novel real-time PCR assay for detection of high-risk human papillomavirus DNA by targeting the E7 region. J Clin Microbiol 2014;52:890-6. 10.1128/JCM.03195-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jeronimo J, Bansil P, Lim J, et al. START-UP Study Group A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. Int J Gynecol Cancer 2014;24:576-85. 10.1097/IGC.0000000000000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang SM, Hu SY, Chen F, et al. Clinical evaluation of human papillomavirus detection by careHPV™ test on physician-samples and self-samples using the indicating FTA Elute® card. Asian Pac J Cancer Prev 2014;15:7085-90. 10.7314/APJCP.2014.15.17.7085 [DOI] [PubMed] [Google Scholar]
- 48. Zhang S, Kang L, Liu B, et al. [Evaluation of screening performance of HPV DNA test on specimens from different sites of the female genital tract]. Zhonghua Zhong Liu Za Zhi 2014;36:389-93. [PubMed] [Google Scholar]
- 49. Boggan JC, Walmer DK, Henderson G, et al. Vaginal Self-Sampling for Human Papillomavirus Infection as a Primary Cervical Cancer Screening Tool in a Haitian Population. Sex Transm Dis 2015;42:655-9. 10.1097/OLQ.0000000000000345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Porras C, Hildesheim A, Gonzalez P, et al. Performance of Self-Collected Cervical Samples in Screening for Future Precancer Using Human Papillomavirus DNA Testing. J Natl Cancer Inst 2014;107:400. 10.1093/jnci/dju400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen Q, Du H, Zhang R, et al. Evaluation of novel assays for the detection of human papilloma virus in self-collected samples for cervical cancer screening. Genet Mol Res 2016; 15 10.4238/gmr.15027896 [DOI] [PubMed] [Google Scholar]
- 52. Chen K, Ouyang Y, Hillemanns P, Jentschke M. Excellent analytical and clinical performance of a dry self-sampling device for human papillomavirus detection in an urban Chinese referral population. J Obstet Gynaecol Res 2016;42:1839-45. 10.1111/jog.13132 [DOI] [PubMed] [Google Scholar]
- 53. Jentschke M, Chen K, Arbyn M, et al. Direct comparison of two vaginal self-sampling devices for the detection of human papillomavirus infections. J Clin Virol 2016;82:46-50. 10.1016/j.jcv.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 54. Qin Y, Zhang H, Marlowe N, et al. Evaluation of human papillomavirus detection by Abbott m2000 system on samples collected by FTA Elute™ Card in a Chinese HIV-1 positive population. J Clin Virol 2016;85:80-5. 10.1016/j.jcv.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 55. Stanczuk G, Baxter G, Currie H, et al. Clinical validation of hrHPV testing on vaginal and urine self-samples in primary cervical screening (cross-sectional results from the Papillomavirus Dumfries and Galloway-PaVDaG study). BMJ Open 2016;6:e010660. 10.1136/bmjopen-2015-010660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aiko KY, Yoko M, Saito OM, et al. Accuracy of self-collected human papillomavirus samples from Japanese women with abnormal cervical cytology. J Obstet Gynaecol Res 2017;43:710-7. 10.1111/jog.13258 [DOI] [PubMed] [Google Scholar]
- 57. Asciutto KC, Henningsson AJ, Borgfeldt H, Darlin L, Borgfeldt C. Vaginal and Urine Self-sampling Compared to Cervical Sampling for HPV-testing with the Cobas 4800 HPV Test. Anticancer Res 2017;37:4183-7. [DOI] [PubMed] [Google Scholar]
- 58. Catarino R, Vassilakos P, Bilancioni A, et al. Accuracy of self-collected vaginal dry swabs using the Xpert human papillomavirus assay. PLoS One 2017;12:e0181905. 10.1371/journal.pone.0181905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leeman A, Del Pino M, Molijn A, et al. HPV testing in first-void urine provides sensitivity for CIN2+ detection comparable with a smear taken by a clinician or a brush-based self-sample: cross-sectional data from a triage population. BJOG 2017;124:1356-63. 10.1111/1471-0528.14682 [DOI] [PubMed] [Google Scholar]
- 60. Asciutto KC, Ernstson A, Forslund O, Borgfeldt C. Self-sampling with HPV mRNA analyses from vagina and urine compared with cervical samples. J Clin Virol 2018;101:69-73. 10.1016/j.jcv.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 61. Leinonen MK, Schee K, Jonassen CM, et al. Safety and acceptability of human papillomavirus testing of self-collected specimens: A methodologic study of the impact of collection devices and HPV assays on sensitivity for cervical cancer and high-grade lesions. J Clin Virol 2018;99-100:22-30. 10.1016/j.jcv.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 62. Enerly E, Bonde J, Schee K, Pedersen H, Lönnberg S, Nygård M. Self-Sampling for Human Papillomavirus Testing among Non-Attenders Increases Attendance to the Norwegian Cervical Cancer Screening Programme. PLoS One 2016;11:e0151978. 10.1371/journal.pone.0151978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moses E, Pedersen HN, Mitchell SM, et al. Uptake of community-based, self-collected HPV testing vs. visual inspection with acetic acid for cervical cancer screening in Kampala, Uganda: preliminary results of a randomised controlled trial. Trop Med Int Health 2015;20:1355-67. 10.1111/tmi.12549 [DOI] [PubMed] [Google Scholar]
- 64. Racey CS, Gesink DC, Burchell AN, Trivers S, Wong T, Rebbapragada A. Randomized Intervention of Self-Collected Sampling for Human Papillomavirus Testing in Under-Screened Rural Women: Uptake of Screening and Acceptability. J Womens Health (Larchmt) 2016;25:489-97. 10.1089/jwh.2015.5348 [DOI] [PubMed] [Google Scholar]
- 65. Sultana F, English DR, Simpson JA, et al. Home-based HPV self-sampling improves participation by never-screened and under-screened women: Results from a large randomized trial (iPap) in Australia. Int J Cancer 2016;139:281-90. 10.1002/ijc.30031 [DOI] [PubMed] [Google Scholar]
- 66. Zehbe I, Jackson R, Wood B, et al. Community-randomised controlled trial embedded in the Anishinaabek Cervical Cancer Screening Study: human papillomavirus self-sampling versus Papanicolaou cytology. BMJ Open 2016;6:e011754. 10.1136/bmjopen-2016-011754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kitchener H, Gittins M, Cruickshank M, et al. A cluster randomized trial of strategies to increase uptake amongst young women invited for their first cervical screen: The STRATEGIC trial. J Med Screen 2018;25:88-98. 10.1177/0969141317696518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Modibbo F, Iregbu KC, Okuma J, et al. Randomized trial evaluating self-sampling for HPV DNA based tests for cervical cancer screening in Nigeria. Infect Agent Cancer 2017;12:11. 10.1186/s13027-017-0123-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kellen E, Benoy I, Vanden Broeck D, et al. A randomized, controlled trial of two strategies of offering the home-based HPV self-sampling test to non- participants in the Flemish cervical cancer screening program. Int J Cancer 2018;143:861-8. 10.1002/ijc.31391 [DOI] [PubMed] [Google Scholar]
- 70. Morrison EAB, Goldberg GL, Hagan RJ, Kadish AS, Burk RD. Self-administered home cervicovaginal lavage: a novel tool for the clinical-epidemiologic investigation of genital human papillomavirus infections. Am J Obstet Gynecol 1992;167:104-7. 10.1016/S0002-9378(11)91637-8 [DOI] [PubMed] [Google Scholar]
- 71. Hillemanns P, Kimmig R, Hüttemann U, Dannecker C, Thaler CJ. Screening for cervical neoplasia by self-assessment for human papillomavirus DNA. Lancet 1999;354:1970. 10.1016/S0140-6736(99)04110-0 [DOI] [PubMed] [Google Scholar]
- 72. Sellors JW, Lorincz AT, Mahony JB, et al. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ 2000;163:513-8. [PMC free article] [PubMed] [Google Scholar]
- 73. Wright TC, Jr, Denny L, Kuhn L, Pollack A, Lorincz A. HPV DNA testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA 2000;283:81-6. 10.1001/jama.283.1.81 [DOI] [PubMed] [Google Scholar]
- 74. Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001;83:439-44. 10.1006/gyno.2001.6370 [DOI] [PubMed] [Google Scholar]
- 75. Lorenzato FR, Singer A, Ho L, et al. Human papillomavirus detection for cervical cancer prevention with polymerase chain reaction in self-collected samples. Am J Obstet Gynecol 2002;186:962-8. 10.1067/mob.2002.122390 [DOI] [PubMed] [Google Scholar]
- 76. Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, et al. Primary screening for high risk HPV by home obtained cervicovaginal lavage is an alternative screening tool for unscreened women. J Clin Pathol 2002;55:435-9. 10.1136/jcp.55.6.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Garcia F, Barker B, Santos C, et al. Cross-sectional study of patient- and physician-collected cervical cytology and human papillomavirus. Obstet Gynecol 2003;102:266-72. [DOI] [PubMed] [Google Scholar]
- 78. Salmerón J, Lazcano-Ponce E, Lorincz A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003;14:505-12. 10.1023/A:1024806707399 [DOI] [PubMed] [Google Scholar]
- 79. Brink AA, Meijer CJLM, Wiegerinck MA, et al. High concordance of results of testing for human papillomavirus in cervicovaginal samples collected by two methods, with comparison of a novel self-sampling device to a conventional endocervical brush. J Clin Microbiol 2006;44:2518-23. 10.1128/JCM.02440-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Daponte A, Pournaras S, Mademtzis I, et al. Evaluation of HPV 16 PCR detection in self- compared with clinician-collected samples in women referred for colposcopy. Gynecol Oncol 2006;103:463-6. 10.1016/j.ygyno.2006.03.021 [DOI] [PubMed] [Google Scholar]
- 81. Girianelli VR, Thuler LC, Szklo M, et al. Comparison of human papillomavirus DNA tests, liquid-based cytology and conventional cytology for the early detection of cervix uteri cancer. Eur J Cancer Prev 2006;15:504-10. 10.1097/01.cej.0000220630.08352.7a [DOI] [PubMed] [Google Scholar]
- 82. Holanda F, Jr, Castelo A, Veras TM, de Almeida FM, Lins MZ, Dores GB. Primary screening for cervical cancer through self sampling. Int J Gynaecol Obstet 2006;95:179-84. 10.1016/j.ijgo.2006.07.012 [DOI] [PubMed] [Google Scholar]
- 83. Seo SS, Song YS, Kim JW, Park NH, Kang SB, Lee HP. Good correlation of HPV DNA test between self-collected vaginal and clinician-collected cervical samples by the oligonucleotide microarray. Gynecol Oncol 2006;102:67-73. 10.1016/j.ygyno.2005.11.030 [DOI] [PubMed] [Google Scholar]
- 84. Szarewski A, Cadman L, Mallett S, et al. Human papillomavirus testing by self-sampling: assessment of accuracy in an unsupervised clinical setting. J Med Screen 2007;14:34-42. 10.1258/096914107780154486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol 2008;9:929-36. 10.1016/S1470-2045(08)70210-9 [DOI] [PubMed] [Google Scholar]
- 86. Bhatla N, Dar L, Patro AR, et al. Can human papillomavirus DNA testing of self-collected vaginal samples compare with physician-collected cervical samples and cytology for cervical cancer screening in developing countries? Cancer Epidemiol 2009;33:446-50. 10.1016/j.canep.2009.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Balasubramanian A, Kulasingam SL, Baer A, et al. Accuracy and cost-effectiveness of cervical cancer screening by high-risk human papillomavirus DNA testing of self-collected vaginal samples. J Low Genit Tract Dis 2010;14:185-95. 10.1097/LGT.0b013e3181cd6d36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gustavsson I, Sanner K, Lindell M, et al. Type-specific detection of high-risk human papillomavirus (HPV) in self-sampled cervicovaginal cells applied to FTA elute cartridge. J Clin Virol 2011;51:255-8. 10.1016/j.jcv.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 89. Taylor S, Wang C, Wright TC, Denny L, Kuhn L. A comparison of human papillomavirus testing of clinician-collected and self-collected samples during follow-up after screen-and-treat. Int J Cancer 2011;129:879-86. 10.1002/ijc.25731 [DOI] [PubMed] [Google Scholar]
- 90. Twu NF, Yen MS, Lau HY, Chen YJ, Yu BK, Lin CY. Type-specific human papillomavirus DNA testing with the genotyping array: a comparison of cervical and vaginal sampling. Eur J Obstet Gynecol Reprod Biol 2011;156:96-100. 10.1016/j.ejogrb.2010.12.023 [DOI] [PubMed] [Google Scholar]
- 91. Belinson JL, Du H, Yang B, et al. Improved sensitivity of vaginal self-collection and high-risk human papillomavirus testing. Int J Cancer 2012;130:1855-60. 10.1002/ijc.26202 [DOI] [PubMed] [Google Scholar]
- 92. Dijkstra MG, Heideman DA, van Kemenade FJ, et al. Brush-based self-sampling in combination with GP5+/6+-PCR-based hrHPV testing: high concordance with physician-taken cervical scrapes for HPV genotyping and detection of high-grade CIN. J Clin Virol 2012;54:147-51. 10.1016/j.jcv.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 93. Longatto-Filho A, Naud P, Derchain SF, et al. Performance characteristics of Pap test, VIA, VILI, HR-HPV testing, cervicography, and colposcopy in diagnosis of significant cervical pathology. Virchows Arch 2012;460:577-85. 10.1007/s00428-012-1242-y [DOI] [PubMed] [Google Scholar]
- 94. van Baars R, Bosgraaf RP, ter Harmsel BW, Melchers WJG, Quint WG, Bekkers RL. Dry storage and transport of a cervicovaginal self-sample using the Evalyn Brush(R): reliable HPV detection combined with women’s comfort. J Clin Microbiol 2012;50:3937-43. 10.1128/JCM.01506-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhao FH, Lewkowitz AK, Chen F, et al. Pooled analysis of a self-sampling HPV DNA Test as a cervical cancer primary screening method. J Natl Cancer Inst 2012;104:178-88. 10.1093/jnci/djr532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Darlin L, Borgfeldt C, Forslund O, Hénic E, Dillner J, Kannisto P. Vaginal self-sampling without preservative for human papillomavirus testing shows good sensitivity. J Clin Virol 2013;56:52-6. 10.1016/j.jcv.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 97. Geraets DT, van Baars R, Alonso I, et al. Clinical evaluation of high-risk HPV detection on self-samples using the indicating FTA-elute solid-carrier cartridge. J Clin Virol 2013;57:125-9. 10.1016/j.jcv.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 98. Guan Y, Gravitt PE, Howard R, et al. Agreement for HPV genotyping detection between self-collected specimens on a FTA cartridge and clinician-collected specimens. J Virol Methods 2013;189:167-71. 10.1016/j.jviromet.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jentschke M, Soergel P, Hillemanns P. Evaluation of a multiplex real time PCR assay for the detection of human papillomavirus infections on self-collected cervicovaginal lavage samples. J Virol Methods 2013;193:131-4. 10.1016/j.jviromet.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 100. Jentschke M, Lange V, Soergel P, Hillemanns P. Enzyme-linked immunosorbent assay for p16(INK4a) - a new triage test for the detection of cervical intraepithelial neoplasia? Acta Obstet Gynecol Scand 2013;92:160-4. 10.1111/aogs.12032 [DOI] [PubMed] [Google Scholar]
- 101. Nieves L, Enerson CL, Belinson S, et al. Primary cervical cancer screening and triage using an mRNA human papillomavirus assay and visual inspection. Int J Gynecol Cancer 2013;23:513-8. 10.1097/IGC.0b013e318280f3bc [DOI] [PubMed] [Google Scholar]
- 102. Bais AG, van Kemenade FJ, Berkhof J, et al. Human papillomavirus testing on self-sampled cervicovaginal brushes: an effective alternative to protect nonresponders in cervical screening programs. Int J Cancer 2007;120:1505-10. 10.1002/ijc.22484 [DOI] [PubMed] [Google Scholar]
- 103. Gök M, Heideman DA, van Kemenade FJ, et al. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ 2010;340:c1040. 10.1136/bmj.c1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Giorgi Rossi P, Marsili LM, Camilloni L, et al. Self-Sampling Study Working Group The effect of self-sampled HPV testing on participation to cervical cancer screening in Italy: a randomised controlled trial (ISRCTN96071600). Br J Cancer 2011;104:248-54. 10.1038/sj.bjc.6606040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet 2011;378:1868-73. 10.1016/S0140-6736(11)61522-5 [DOI] [PubMed] [Google Scholar]
- 106. Piana L, Leandri FX, Le RL, Heid P, Tamalet C, Sancho-Garnier H. L’auto-prélèvement vaginal à domicile pour recherche de papilloma virus à haut risque. Une solution de remplacement pour les femmes ne participant pas au dépistage cytologique des cancers du col de l’utérus. Campagne expérimentale du département des Bouches-du-Rhône. Bull Cancer 2011;98:723-31. [DOI] [PubMed] [Google Scholar]
- 107. Szarewski A, Cadman L, Mesher D, et al. HPV self-sampling as an alternative strategy in non-attenders for cervical screening - a randomised controlled trial. Br J Cancer 2011;104:915-20. 10.1038/bjc.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Virtanen A, Nieminen P, Luostarinen T, Anttila A. Self-sample HPV tests as an intervention for nonattendees of cervical cancer screening in Finland: a randomized trial. Cancer Epidemiol Biomarkers Prev 2011;20:1960-9. 10.1158/1055-9965.EPI-11-0307 [DOI] [PubMed] [Google Scholar]
- 109. Wikström I, Lindell M, Sanner K, Wilander E. Self-sampling and HPV testing or ordinary Pap-smear in women not regularly attending screening: a randomised study. Br J Cancer 2011;105:337-9. 10.1038/bjc.2011.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gök M, van Kemenade FJ, Heideman DA, et al. Experience with high-risk human papillomavirus testing on vaginal brush-based self-samples of non-attendees of the cervical screening program. Int J Cancer 2012;130:1128-35. 10.1002/ijc.26128 [DOI] [PubMed] [Google Scholar]
- 111. Darlin L, Borgfeldt C, Forslund O, et al. Comparison of use of vaginal HPV self-sampling and offering flexible appointments as strategies to reach long-term non-attending women in organized cervical screening. J Clin Virol 2013;58:155-60. 10.1016/j.jcv.2013.06.029 [DOI] [PubMed] [Google Scholar]
- 112. Sancho-Garnier H, Tamalet C, Halfon P, et al. HPV self-sampling or the Pap-smear: a randomized study among cervical screening nonattenders from lower socioeconomic groups in France. Int J Cancer 2013;133:2681-7. [DOI] [PubMed] [Google Scholar]
- 113. Broberg G, Gyrd-Hansen D, Miao Jonasson J, et al. Increasing participation in cervical cancer screening: offering a HPV self-test to long-term non-attendees as part of RACOMIP, a Swedish randomized controlled trial. Int J Cancer 2014;134:2223-30. 10.1002/ijc.28545 [DOI] [PubMed] [Google Scholar]
- 114. Cadman L, Wilkes S, Mansour D, et al. A randomized controlled trial in non-responders from Newcastle upon Tyne invited to return a self-sample for Human Papillomavirus testing versus repeat invitation for cervical screening. J Med Screen 2015;22:28-37. 10.1177/0969141314558785 [DOI] [PubMed] [Google Scholar]
- 115. Haguenoer K, Sengchanh S, Gaudy-Graffin C, et al. Vaginal self-sampling is a cost-effective way to increase participation in a cervical cancer screening programme: a randomised trial. Br J Cancer 2014;111:2187-96. 10.1038/bjc.2014.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Arrossi S, Thouyaret L, Herrero R, et al. EMA Study team Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Glob Health 2015;3:e85-94. 10.1016/S2214-109X(14)70354-7 [DOI] [PubMed] [Google Scholar]
- 117. Giorgi Rossi P, Fortunato C, Barbarino P, et al. HPV Self-sampling Italian Working Group Self-sampling to increase participation in cervical cancer screening: an RCT comparing home mailing, distribution in pharmacies, and recall letter. Br J Cancer 2015;112:667-75. 10.1038/bjc.2015.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schee K, Stefan L, Pedersen H, Bonde J, Nyganullrd M. Improving attendance to the cervical cancer screening program: Does self-sampling at home improve cervical cancer prevention. Cancer Res 2014;74(Suppl 19):4135 10.1158/1538-7445.AM2014-4135 [DOI] [Google Scholar]
- 119. Arbyn M, Peeters E, Benoy I, et al. VALHUDES: A protocol for validation of human papillomavirus assays and collection devices for HPV testing on self-samples and urine samples. J Clin Virol 2018;107:52-6. 10.1016/j.jcv.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 120. Snellenberg S, De Strooper LM, Hesselink AT, et al. Development of a multiplex methylation-specific PCR as candidate triage test for women with an HPV-positive cervical scrape. BMC Cancer 2012;12:551. 10.1186/1471-2407-12-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cuschieri K, Ronco G, Lorincz A, et al. Eurogin roadmap 2017: Triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer 2018;143:735-45. 10.1002/ijc.31261 [DOI] [PubMed] [Google Scholar]
- 122. Vorsters A, Van den Bergh J, Micalessi I, et al. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur J Clin Microbiol Infect Dis 2014;33:2005-14. 10.1007/s10096-014-2147-2 [DOI] [PubMed] [Google Scholar]
- 123. Pathak N, Dodds J, Zamora J, Khan K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ 2014;349:g5264. 10.1136/bmj.g5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Van Keer S, Tjalma WAA, Pattyn J, et al. Human papillomavirus genotype and viral load agreement between paired first-void urine and clinician-collected cervical samples. Eur J Clin Microbiol Infect Dis 2018;37:859-69. 10.1007/s10096-017-3179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sanner K, Wikström I, Strand A, Lindell M, Wilander E. Self-sampling of the vaginal fluid at home combined with high-risk HPV testing. Br J Cancer 2009;101:871-4. 10.1038/sj.bjc.6605194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lam JU, Rebolj M, Møller Ejegod D, et al. Human papillomavirus self-sampling for screening nonattenders: Opt-in pilot implementation with electronic communication platforms. Int J Cancer 2017;140:2212-9. 10.1002/ijc.30647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Baussano I, Tshering S, Choden T, et al. Cervical cancer screening in rural Bhutan with the careHPV test on self-collected samples: an ongoing cross-sectional, population-based study (REACH-Bhutan). BMJ Open 2017;7:e016309. 10.1136/bmjopen-2017-016309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Stanczuk GA, Baxter GJ, Currie H, et al. Defining optimal triage strategies for hrHPV screen positive women - an evaluation of HPV 16/18 genotyping, cytology and p16/Ki-67 cyto-immunochemistry. Cancer Epidemiol Biomarkers Prev 2017;26:1629-35. 10.1158/1055-9965.EPI-17-0534 [DOI] [PubMed] [Google Scholar]
- 129. Saville M, Hawkes D, Mclachlan E, Anderson S, Arabena K. Self-collection for under-screened women in a National Cervical Screening Program: pilot study. Curr Oncol 2018;25:27-32. 10.3747/co.25.3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. McLachlan E, Anderson S, Hawkes D, Saville M, Arabena K. Completing the cervical screening pathway: Factors that facilitate the increase of self-collection uptake among under-screened and never-screened women, an Australian pilot study. Curr Oncol 2018;25:17-26. 10.3747/co.25.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gustavsson I, Aarnio R, Berggrund M, et al. Randomised study shows that repeated self-sampling and HPV test has more than two-fold higher detection rate of women with CIN2+ histology than Pap smear cytology. Br J Cancer 2018;118:896-904. 10.1038/bjc.2017.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Polman NJ, Berkhof J. IMPROVE-study: Prospective randomized pilot implementation trial of HPV self-sampling in primary cervical screening. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5078.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials: Supplementary materials and supplementary figures 1-17 and tables 1-18