Abstract
Aims:
This study investigated the association between severe hypoglycemia (SH) and new onset atrial fibrillation (AF) and all-cause mortality in adult patients with type 2 diabetes mellitus (T2DM).
Methods:
Retrospective data on patients with T2DM aged between 30 and 75 years who received healthcare checkups from January 1, 2005 to December 31, 2008 were analyzed using the National Health Insurance Database in Korea. The primary outcome was newly diagnosed non-valvular AF occurring after SH episode using ICD-10 codes.
Results:
Among 1,509,280 subjects, 10,864 (0.72%) patients had experienced SH events in the three years prior to health examination, and a total of 48,916 (3.24%) first-time AF episodes occurred during the follow-up period of 8.5 years. The incidence of AF was significantly higher in the group with SH than the group without SH. After multivariable adjustment, previous SH was a significant risk factor for the development of AF (HR 1.10, 95% CI 1.01–1.19). All-cause mortality was also significantly increased in patients with previous SH events and prior SH with subsequent AF occurrence, compared to patients without SH events.
Conclusions:
Prior SH events were associated with a higher risk of new onset AF and all-cause mortality in patients with T2DM.
Keywords: Diabetes type 2, severe hypoglycemia, atrial fibrillation, all-cause mortality
1. Introduction
Many large epidemiological studies have shown that glycemic control within the target range is the only proven strategy for the prevention of diabetic vascular complications.1 To achieve this goal active use of oral antidiabetic agents or insulin should be initiated, along with lifestyle modification after the diagnosis of diabetes, matching the individual’s situation. 2 However, with intensive glycemic control with antidiabetic medication, patients with type 2 diabetes mellitus inevitably incur the risk of hypoglycemia.3,4
A hypoglycemic episode, regardless of its severity, is clinically important due to its association with increased cardiovascular (CV) events and mortality.5 A meta-analysis including 903,510 subjects with type 2 diabetes demonstrated that severe hypoglycemia (SH) was significantly associated with a higher risk of cardiovascular disease (CVD).6 Moreover, a strong association has been found between SH and an increased risk of all-cause and CV mortality in patients with type 2 diabetes mellitus.7
The pathophysiologic link between SH and CVD or CV death has not been fully elucidated. Hypoglycemia has the potential to trigger fatal arrhythmic events by more than one mechanism in patients with high CV risk.8 Previous studies have demonstrated that hypoglycemia directly or indirectly influences myocardial ischemia or QT interval prolongation by abnormal repolarization, enhanced adrenergic tone, and cardiac autonomic dysfunction.9,10 During an SH episode, the QTc interval has been known to be prolonged significantly, independent of diabetic duration, age, diabetic medications (insulin or sulfonylurea), and glycemic control status in type 2 diabetes.11 According to an Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial, SH was associated with an increased risk for arrhythmic death (HR 1.77) in patients with high CV risk and dysglycemia.12
Atrial fibrillation (AF) is the most common arrhythmia in the general population, with an estimated lifetime risk of 25% worldwide.13 Clinically, AF is important due to its association with increased risk of stroke, heart failure, myocardial infarction, and total mortality.14 Diabetes itself has been identified as a risk factor for AF, increasing the risk of new onset AF by 34–40%.15 With numerous metabolic defects, atrial structural, electrical, and electromechanical remodeling are suggested mechanisms in the relationship between diabetes and AF.16 AF has been reported as a complication of hypoglycemia in case reports 17,18 and severe hypoglycemia was reported in a single-center retrospective cohort study in patients with diabetes.19 However, there are few studies focusing on the relationship between SH and new onset AF in patients with type 2 diabetes mellitus.
In Korea, the national health care program, the National Health Insurance Service (NHIS), covers the entire Korean population as a social insurance benefits scheme.20 Based on the NHI program, the computerized database contains all of the national health examination data, claim data, including drug prescriptions, diagnostic codes for the International Classification of Disease-10 (ICD-10) disease coding system, and claimed treatment details.20,21 From this database, claim data regarding patients with type 2 diabetes mellitus with a prior history of SH within a certain period and subsequent development of CV events can be extracted.
The aim of this study was to investigate the association between previous SH episodes and the risk of AF and all-cause mortality in adult patients with type 2 diabetes mellitus using the NHIS claim database from January 1, 2002 to December 31, 2015 in Korea.
2. Subjects, Materials and Methods
2.1. Source of the database
In this retrospective cohort study, we used the NHI database maintained by the Korean NHIS, a government-affiliated agency under the Korean Ministry of Health and Welfare that supervises all medical services in Korea. All Korean people aged 30 years and older are encouraged to receive regular biennial or pre-employment healthcare checkups provided by the NHIS. This regular healthcare checkup includes anthropometric measurements, blood pressure, social habits, physical activity, and laboratory tests after overnight fasting, including serum glucose, total cholesterol, creatinine, liver function, and urinalysis. Quality control procedures for laboratory tests were performed in accordance with the Korean Association of Laboratory Quality Control.21 Past history, alcohol, smoking, and exercise habits were collected by standardized self-reporting questionnaires. Additionally, the NHIS contains information on the patients’ demographics, medical use, transaction information, healthcare checkups, and claim database.20,21
This study was approved by the institutional review board of the Catholic University of Korea (VIRB-0E237-001). The study was conducted in compliance with the Declaration of Helsinki.
2.2. Definition of type 2 diabetes, severe hypoglycemia, and atrial fibrillation
Among all of the NHI beneficiaries, the source population for this study consisted of patients who visited clinics or hospitals with an ICD-10 of type 2 diabetes mellitus (E11, E14) from January 1, 2002 to December 31, 2015. A diagnosis of type 2 diabetes mellitus based on ICD-10 codes included the principal diagnosis and up to four additional accompanying diagnoses to reflect clinical significance in the current condition. Patients were classified as having type 2 diabetes mellitus when they had at least one service claim with a diagnosis of type 2 diabetes, either in outpatient or inpatient care, and were prescribed at least one antidiabetic drug any time during a given year to exclude prediabetic or non-diabetic subjects.20
The inclusion criteria for patients with type 2 diabetes mellitus were as follows 1) between 30 and 75 years of age; 2) received healthcare checkups between January 1, 2005 and December 31, 2008; 3) treated with hypoglycemic agents (including insulin) on claim database or fasting plasma glucose ≥ 126 mg/dL on healthcare checkup examination (newly diagnosed diabetes); and 4) subjects who had not been diagnosed with AF before January 1, 2005 to exclude participants with a prior history of AF (Fig. 1). Type 1 diabetes, gestational diabetes, subjects with missing data, and those diagnosed with mitral stenosis (I050, I52, I059), or mechanical heart valves (Z952-Z954) were excluded.21
Fig. 1.
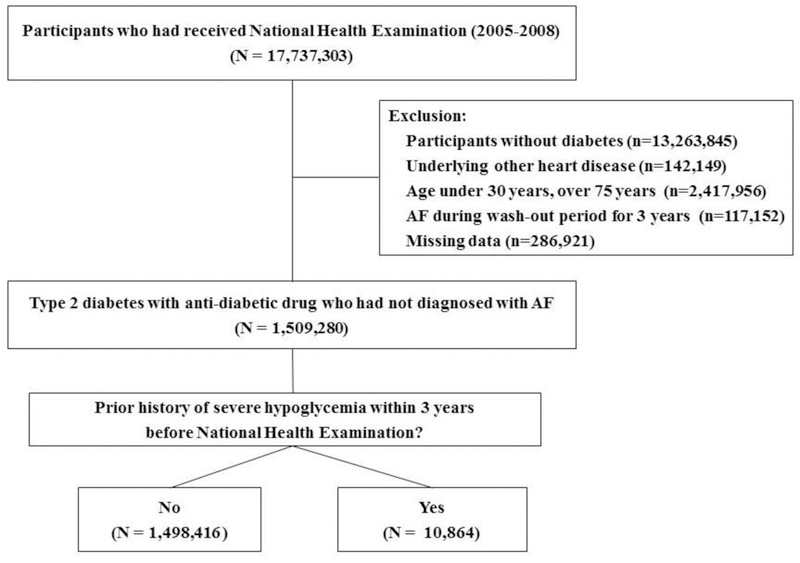
Sample recruitment from the database of National Health Insurance Service
Severe hypoglycemia (SH) was defined as any hypoglycemic events requiring the assistance of another person to actively administer carbohydrates, other corrective actions, hospitalization, or medical care.22 Plasma glucose levels may not be available, but neurological recovery following the return of plasma glucose to normal is considered sufficient evidence.22 Due to the limitation of claim data, in which glucose concentrations during SH events could not be confirmed, we used the ICD-10 codes of SH. To investigate the effect of previous SH events on the future development of AF, we collected all admission episodes reporting SH (ICD-10 codes of E16.x, E11.63, E13.63, E14.63) from the inpatients or emergency room claim dataset between January 1, 2002 and December 31, 2007 (Additional file, Figure 1) among the patients with type 2 diabetes mellitus. To obtain high enough number of SH events, we searched whether the participants had experienced at least one SH episode within the 3 years prior to the date of the current health examination using ICD-10 codes.
Hypertension was defined with the ICD-10 codes of I10-I13, and I15 and treatment with anti-hypertensive agents, or systolic or diastolic blood pressure ≥ 140 mmHg / ≥ 90 mmHg, hyperlipidemia as E78 with treatment with lipid-lowering agents or total cholesterol ≥ 240 mg/dL, congestive heart failure (CHF) as I50, and end-stage renal disease (ESRD) as N18–19, Z49, Z940, Z90, Z992.20,21,23 Previous medical history of ischemic stroke or myocardial infarction was defined as ICD-10 codes of I63-I64 or I21-I22 before the date of the health examination.23 Previous history of hyperthyroidism was defined as ICD-10 code of E05.
Demographic characteristics were abstracted, including age, sex, body mass index (BMI), and urbanization level of residence. We identified low socioeconomic status population as lowest quartile income status. Heavy drinking was defined as alcohol habits of ≥ 5 days/week, and physical activity was defined as ≥ 1 /week of moderate exercise.
The primary outcome was newly diagnosed non-valvular AF, either one diagnosis (ICD-10 code of I48.0-I48.4, I48.9) during hospitalization, more than twice at outpatient clinics, or until censoring by death.21 Development of AF after an SH event was considered to be newly developed AF if the patient had no previous diagnosis of AF before the healthcare checkup. For all-cause mortality, those patients who died of any cause were selected. Individuals with SH episodes occurring after the beginning of follow-up for AF were excluded.
2.3. Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Descriptive characteristics were presented as mean ± SD or 95% confidence interval. Data were also expressed as numbers and as a frequency percentage (%). The χ2 test was used to determine differences in the proportion of categorical variables, and independent Student’s t tests evaluated differences between the means of two continuous variables. Incidence rates were expressed as events per 1,000 patient-years and adjusted for age and sex using a direct method. Participants were followed until the first diagnosis of AF, death, or December 31, 2015. Cox proportional hazards regression analysis was used to estimate the hazard ratios (HRs) and 95% confidence interval (CIs) for the association between the prior SH events and development of AF after adjustment for sex, age, BMI, hypertension, heavy alcohol drinking, current smoking, exercise, presence of ESRD, history of ischemic stroke or myocardial infarction, insulin use, history of hyperthyroidism, and CHF. Potential confounders were identified a priori based on the literature review. The proportional hazards assumption was evaluated by Schoenfeld residuals, with the logarithm of the cumulative hazards function based on Kaplan-Meier curves estimated for the presence or absence of prior SH events. The potential effect modification by age, sex, and various accompanying clinical conditions was evaluated through the stratified analysis and interaction testing using the likelihood ratio test, and the P value for interaction was presented with a significance of < 0.25. All-cause mortality according to the absence of SH events, SH, and SH with AF was analyzed using the Kaplan-Meier curve. The multivariable-adjusted proportional hazards model was used to evaluate the association between prior SH events, incident AF, and all-cause mortality. A two-sided P value < 0.05 was considered statistically significant.
3. Results
3.1.1. Clinical characteristics of the study population according to SH events and the development of AF
From January 1, 2005 to December 31, 2008, 17,737,303 subjects had received a national health examination. After exclusion, 1,509,280 participants with type 2 diabetes mellitus who had not been diagnosed with AF were included in this study (Fig. 1). Among them, we identified 10,864 (0.72%) patients who had experienced SH episodes within the 3 years before the date of the current health examination.
Patients with a prior history of SH were older (61.3 ± 9.6 vs. 54.9 ± 10.9 years), had a lower BMI level (24.1 ± 3.5 vs. 25.0 ± 3.3 kg/m2) and a lower proportion of males (51.6% vs. 63.9%), as compared to those without SH. They also had a higher rate of the presence of hypertension (66.7% vs. 53.3%), myocardial infarction (6.5% vs. 1.5%), congestive heart failure (7.1 % vs. 2.2%), and insulin treatment (29.7% vs. 6.3%), compared to the patients without SH (Table 1).
Table 1.
Baseline characteristics of study participants according to prior hypoglycemic events
| Severe Hypoglycemia | P value | ||
|---|---|---|---|
| □ | NO | YES | |
| N (%) | 1,498,416 (99.3) | 10,864 (0.7) | |
| Age (year-old) | 54.9 ± 10.9 | 61.3 ± 9.6 | <0.001 |
| Age (≥55 year-old) (%) | 775,460 (51.8) | 8,213 (75.6) | <0.001 |
| Sex (male)(%) | 957,587 (63.9) | 5,600 (51.6) | <0.001 |
| Height (cm) | 162.9 ± 9.0 | 159.6 ± 9.1 | <0.001 |
| Weight (kg) | 66.5 ± 11.2 | 61.5 ± 10.9 | <0.001 |
| BMI (kg/m2) | 25.0 ± 3.3 | 24.1 ± 3.5 | <0.001 |
| BMI (≥ 25 kg/m2) (%) | 719,520 (48.0) | 4,171 (38.4) | <0.001 |
| Systolic blood pressure (mmHg) | 130.7 ± 17.0 | 130.7 ± 17.9 | 0.9919 |
| Diastolic blood pressure (mmHg) | 80.5 ± 10.8 | 78.8 ± 10.7 | <0.001 |
| Current smoking (%) | 412,025 (27.5) | 2,051 (18.9) | <0.001 |
| Drinker (heavy) (%) | 74,598 (5.0) | 486 (4.47) | 0.0159 |
| Exercise (yes) (%) | 759,217 (50.7) | 4,802 (44.2) | <0.001 |
| Hypertension (yes) (%) | 798,214 (53.3) | 7,251 (66.7) | <0.001 |
| Low economic status (%) | 397,663 (26.5) | 2,838 (26.1) | 0.328 |
| Rural area (%) | 872,444 (55.6) | 6,716 (62.5) | <0.001 |
| Hyperlipidemia (%) | 471,195 (31.5) | 3,853 (35.5) | <0.001 |
| Heart failure (%) | 33,280 (2.2) | 769 (7.1) | <0.001 |
| Myocardial infarction (%) | 23,035 (1.5) | 706 (6.5) | <0.001 |
| Ischemic stroke (%) | 83,825 (5.6) | 1,971 (18.1) | <0.001 |
| End stage renal disease (%) | 1,746 (0.1) | 250 (2.3) | <0.001 |
| Hyperthyroidism (%) | 54,969 (3.7) | 805 (7.4) | <0.001 |
| Total cholesterol (mg/dL) | 201.4 ± 43.0 | 191.2 ± 45.6 | <0.001 |
| Fasting glucose (mg/dL) | 149.6 ± 51.6 | 145.4 ± 67.7 | <0.001 |
| Antidiabetic therapy | |||
| Insulin (%) | 94,582 (6.3) | 3,224 (29.7) | <0.001 |
| Sulfonylurea (%) | 661,037 (44.1) | 7,037 (64.8) | <0.001 |
| Metformin (%) | 504,599 (33.7) | 5,785 (53.3) | <0.001 |
Data are mean ± SD or number (%). BMI, body mass index
During the follow-up period of 8.5 years, 48,916 individuals (3.24% of the total patients with type 2 diabetes) were newly diagnosed with AF, with an incidence rate of 3.5 per 1,000 person-years. The mean time from SH events to AF development was 7.7 years (median 8.3 years). The proportion of subjects who developed AF was significantly different between the no SH and prior SH groups (3.2% vs. 5.5%, P < 0.001). The incidences of AF in patients with type 2 diabetes with previous SH and without SH were 7.09 and 3.79 per 1,000 person-years, respectively (P < 0.001) (Table 2). The incidence rate of AF was significantly increased with increasing age, both in the no SH and prior SH groups (P value for trend < 0.001 both, Supplement Fig. 2).
Table 2.
Incidence rate and hazard ratios for the risk of atrial fibrillation in subgroups analysis
| SH | Event | Duration (year) | IR of AF* | Age, sex-adjusted HR (95% CI) | P value | P for interaction | |
|---|---|---|---|---|---|---|---|
| SH | No | 48,323 | 12,760,497 | 3.79 | 1 | ||
| Yes | 593 | 83,668 | 7.09 | 1.36 (1.26–1.48) | <.0001 | ||
| Age (years) | <.0001 | ||||||
| < 55 | No | 10,734 | 6,312,940 | 1.70 | 1 | ||
| Yes | 84 | 21,761 | 3.86 | 2.09 (1.69–2.59) | <.0001 | ||
| ≥ 55 | No | 37,589 | 6,447,558 | 5.83 | 1 | ||
| Yes | 509 | 61,907 | 8.22 | 1.30 (1.19–1.42) | <.0001 | ||
| Sex | 0.753 | ||||||
| Male | No | 31,568 | 8,101,323 | 3.90 | 1 | ||
| Yes | 321 | 41,445 | 7.75 | 1.35 (1.21–1.51) | <.0001 | ||
| Female | No | 16,755 | 4,659,174 | 3.60 | 1 | ||
| Yes | 272 | 42,223 | 6.44 | 1.37 (1.22–1.55) | <.0001 | ||
| Hypertension | 0.041 | ||||||
| No | No | 14,735 | 6,037,202 | 2.44 | 1 | ||
| Yes | 130 | 28,924 | 4.50 | 1.29 (1.08–1.53) | 0.004 | ||
| Yes | No | 33,588 | 6,723,295 | 4.50 | 1 | ||
| Yes | 463 | 54,744 | 8.46 | 1.36 (1.24–1.49) | <.0001 | ||
| BMI (kg/m2) | 0.041 | ||||||
| < 25 | No | 24,011 | 6,591,888 | 3.64 | 1 | ||
| Yes | 369 | 50,103 | 7.37 | 1.48 (1.34–1.64) | <.0001 | ||
| ≥ 25 | No | 24,312 | 6,168,609 | 3.94 | 1 | ||
| Yes | 224 | 33,564 | 6.67 | 1.24 (1.09–1.41) | 0.0014 | ||
| Heart failure | 0.649 | ||||||
| No | No | 44,575 | 12,502,302 | 3.57 | 1 | ||
| Yes | 507 | 78,626 | 6.45 | 1.33 (1.22–1.45) | <.0001 | ||
| Yes | No | 3,748 | 258,195 | 14.5 | 1 | ||
| Yes | 86 | 5,041 | 17.1 | 1.07 (0.86–1.32) | 0.563 | ||
| MI | 0.045 | ||||||
| No | No | 46,527 | 12,578,387 | 3.70 | 1 | ||
| Yes | 531 | 78,694 | 6.75 | 1.33 (1.22–1.45) | <.0001 | ||
| Yes | No | 1,796 | 182,110 | 9.86 | 1 | ||
| Yes | 62 | 4,974 | 12.5 | 1.14 (0.88–1.47) | 0.322 | ||
| Stroke | 0.181 | ||||||
| No | No | 42,950 | 12,092,206 | 3.55 | 1 | ||
| Yes | 452 | 69,701 | 6.49 | 1.35 (1.23–1.49) | <.0001 | ||
| Yes | No | 5,373 | 668,291 | 8.04 | 1 | ||
| Yes | 141 | 13,967 | 10.10 | 1.17 (0.99–1.38) | 0.0716 | ||
| Insulin use | 0.733 | ||||||
| No | No | 42,977 | 12,025,972 | 3.57 | 1 | ||
| Yes | 360 | 61,106 | 5.89 | 1.19 (1.07–1.32) | 0.0011 | ||
| Yes | No | 5,346 | 734,525 | 7.28 | 1 | ||
| Yes | 233 | 22,561 | 10.3 | 1.25 (1.10–1.43) | 0.0007 | ||
| SU use | 0.008 | ||||||
| No | No | 22,021 | 7,124,365 | 3.09 | 1 | ||
| Yes | 203 | 29,331 | 6.92 | 1.55 (1.35–1.78) | <.0001 | ||
| Yes | No | 26,302 | 5,636,132 | 4.67 | 1 | ||
| Yes | 390 | 54,337 | 7.18 | 1.27 (1.15–1.40) | <.0001 | ||
| ESRD | 0.356 | ||||||
| No | No | 48,098 | 12,750,093 | 3.77 | 1 | ||
| Yes | 561 | 82,323 | 6.82 | 1.31 (1.21–1.42) | <.0001 | ||
| Yes | No | 225 | 10,404 | 21.6 | 1 | ||
| Yes | 32 | 1,345 | 23.8 | 1.09 (0.75–1.57) | 0.663 | ||
| Hyperthyroidism | 0.222 | ||||||
| No | No | 45,682 | 12,300,520 | 3.71 | 1 | ||
| Yes | 542 | 77,520 | 6.99 | 1.36 (1.25–1.48) | <.0001 | ||
| Yes | No | 2,641 | 459,977 | 5.74 | 1 | ||
| Yes | 51 | 6,148 | 8.30 | 1.16 (0.88–1.53) | 0.2486 |
, incidence rate of AF (events/1,000 patient-years)
SH, severe hypoglycemia; BMI, body mass index; MI, myocardial infarction; SU, sulfonylurea; ESRD, end-stage renal disease
3.2. Risk factors for incident AF
The age and sex-adjusted HR for AF according to SH experience was 1.36 (95% CI 1.26–1.48) (Table 2). This significant association also persisted after adjusting for age, sex, BMI, smoking and drinking habits, socioeconomic status, the presence of hypertension or ESRD, history of heart failure, myocardial infarction, ischemic stroke, or hyperthyroidism, and insulin or sulfonylurea use (HR 1.10, 95% CI 1.01–1.19) (Table 3, Fig 2A). Additionally, in females (HR 1.56, 95% CI 1.53–1.59), the presence of hypertension (HR 1.39, 95% CI 1.36–1.42), congestive heart failure (HR 2.57, 95% CI 2.49–2.66), use of sulfonylurea (SU) (HR 1.07, 95% CI 1.05–1.09), and use of insulin (HR 1.51, 95% CI 1.46–1.55) were also significant predictive factors for the development of AF (Table 3).
Table 3.
Multivariate cox proportional hazard regression analysis of incident occurrence of atrial fibrillation in patients with type 2 diabetes
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| Severe hypoglycemia (yes) | 1.10 (1.01–1.19) | 0.0300 |
| Age (years) | < 0.0001 | |
| 30–39 | 1 | |
| 40–49 | 2.17 (2.02–2.33) | |
| 50–59 | 4.16 (3.88–4.45) | |
| 60–74 | 8.61 (8.04–9.22) | |
| Sex (male) | 1.56 (1.53–1.59) | < 0.0001 |
| BMI ≥ 25 kg/m2 | 1.11 (1.09–1.13) | < 0.0001 |
| Smoking (current) | 1.06 (1.03–1.08) | < 0.0001 |
| Alcohol (heavy) | 1.19 (1.15–1.24) | < 0.0001 |
| Exercise (yes) | 0.90 (0.89–0.92) | < 0.0001 |
| Urban place | 1.07 (1.05–1.09) | < 0.0001 |
| Low economic status (Q1) | 0.99 (0.97–1.01) | 0.218 |
| Hypertension | 1.39 (1.36–1.42) | < 0.0001 |
| Congestive heart failure | 2.57 (2.49–2.66) | < 0.0001 |
| Myocardial infarction | 1.55 (1.48–1.63) | < 0.0001 |
| Ischemic stroke | 1.32 (1.28–1.36) | < 0.0001 |
| End-stage renal disease | 2.85 (2.51–3.23) | < 0.0001 |
| Insulin use | 1.51 (1.46–1.55) | < 0.0001 |
| Sulfonylurea use | 1.07 (1.05–1.09) | < 0.0001 |
| Hyperthyroidism | 1.35 (1.30–1.41) | < 0.0001 |
Fig. 2.
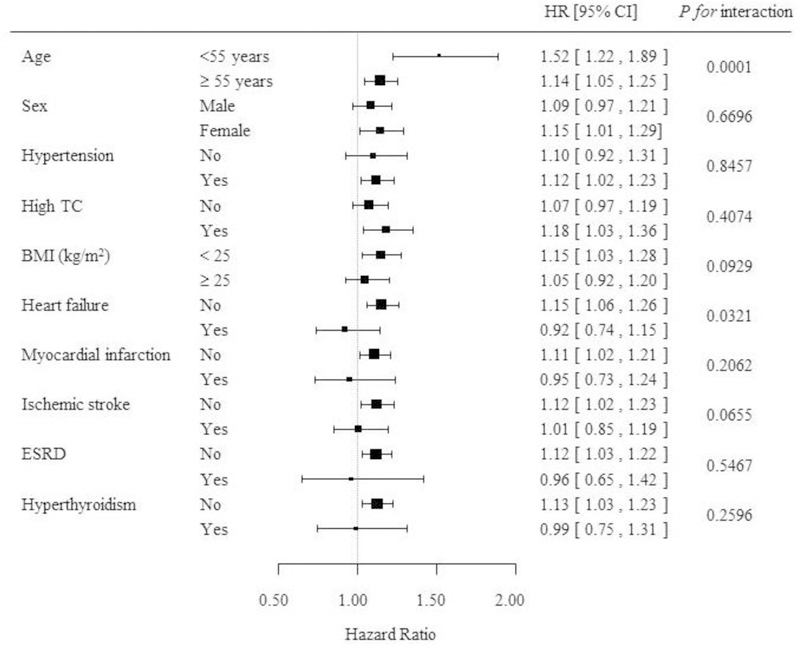
(A) Prior severe hypoglycemia (SH) events and the risk of atrial fibrillation (AF). (B) All-cause mortality according to the presence of SH events.
In subgroup analysis, the association between prior SH and incident AF was stronger in patients aged < 55 years (HR 1.52, 95% CI 1.22–1.89) than in aged ≥ 55 years (1.14, 95% CI 1.05–1.25) (P for interaction = 0.0001). Patients without underlying CHF had a stronger association with incident AF (HR 1.15, 95% CI 1.06–1.26) than incident AF with underlying CHF (HR 0.92, 95% CI 0.74–1.15) (P for interaction = 0.032). Also, patients without underlying myocardial infarction (HR 1.11, 95% CI 1.02–1.21) or ischemic stroke (HR 1.12, 95% CI 1.02–1.23) had a stronger association with incident AF than incident AF with underlying myocardial infarction (HR 0.95, 95% CI 0.73–1.24) or ischemic stroke (HR 1.01, 95% CI 0.85–1.19) (P for interaction = 0.206 and 0.066, respectively) (Fig 3).
Fig. 3.
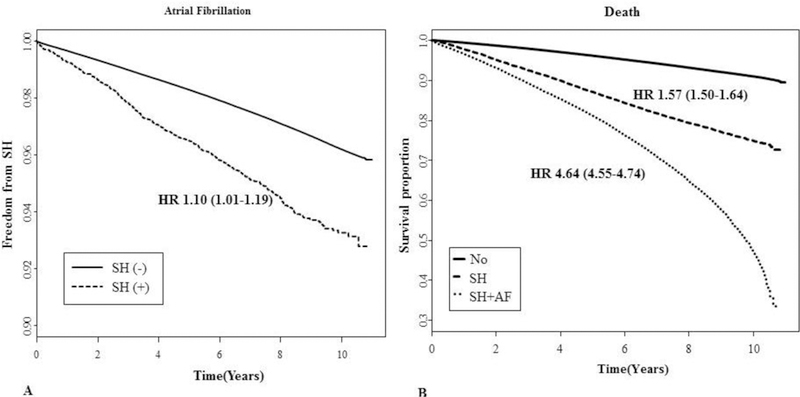
Hazard ratios for the risk of AF following severe hypoglycemia (SH) in subgroups.
Data are HRs (95% CI). TC, total cholesterol; BMI, body mass index; ESRD, end-stage renal disease.
3.3. All-cause mortality after SH
During the observation period 128,335 subjects (8.5%) died. More patients from the SH group died than in the non-SH group (23.4% vs. 8.4%, P < 0.001). The all-cause mortality in people with type 2 diabetes who had experienced SH was 52.9 per 1,000 patient-years. When the subjects were divided into 3 groups according to the presence of prior SH events and SH following AF events, the mortality rate was significantly higher in patients with prior SH events and prior SH with following AF events compared to that in patients from the non-SH episode group (28.6 vs. 63.9 vs. 9.1 per 1,000 patient-years, P < 0.001). Compared to patients without SH events, the all-cause mortality was significantly higher in patients with previous SH events (HR 1.57, 95% CI 1.50–1.64) and prior SH with subsequent AF occurrence (HR 4.64, 95% CI 4.55–4.74) after adjustment for multiple variables (Fig. 2B).
4. Discussion
In this analysis using a national database of health insurance claims in Korea, we found substantial and significant development of AF after SH episodes in patients with type 2 diabetes mellitus during the past decade. To the best of our knowledge, the present study is the first evidence of SH as a risk factor for AF in a large number of patients with type 2 diabetes mellitus. Importantly, the development of AF after SH was independent of preexisting cardiovascular disease.
Data has suggested that type 2 diabetes mellitus is strongly associated with an increased risk of AF.15 In a meta-analysis involving 108,703 cases of AF in 1,686,097 patients from cohort and case-control studies, diabetes was associated with an overall 34% increase in the risk of AF.15 Diabetic cardiomyopathy, cardiac autonomic dysfunction, sympathetic over-activity, electrical, electromechanical, and structural remodeling, oxidative stress, and glycemic fluctuations were considered pathophysiological mechanisms implicating AF in diabetes.24 Paroxysmal AF episodes are preceded by autonomic fluctuations, with a primary increased sympathetic tone followed by a marked modulation toward vagal predominance.25 Additionally, excess CV morbidity and mortality are attributable to AF in patients with diabetes. According to the ADVANCE (Action in Diabetes and Vascular Disease: PreterAx and Diamicron-MR Controlled Evaluation) study, AF was associated with an increased risk of all-cause mortality, CV death, stroke, and heart failure in patients with type 2 diabetes.26
Intensive glycemic control is inevitably accompanied by an increased frequency and severity of hypoglycemic episodes in patients with type 2 diabetes. According to the ADVANCE Collaborative group study, 231 of 11,140 patients with type 2 diabetes (2.1%) had at least one SH event.4 Moreover, SH was significantly associated with an increased risk of macrovascular complications, adverse clinical outcomes, and all-cause mortality.4–7 However, the effect of SH on new-onset AF in patients with type 2 diabetes is still unknown.
Hypoglycemia increased the risk of arrhythmia in patients with type 2 diabetes and high CV risk. Bradycardia, QTc prolongation, and atrial and ventricular ectopic beats were significantly higher during hypoglycemic or severe hypoglycemic events.27 This hypoglycemia-associated arrhythmia has been proposed as a predisposing factor for SH-induced CV morbidity and mortality. Although hypoglycemia or SH-associated fatal cardiac arrhythmia is difficult to confirm because simultaneous monitoring of glucose level and cardiac rhythm is rarely undertaken, arrhythmic deaths were reported as a direct cause of mortality.28 The arrhythmogenic effect of hypoglycemia is likely to be greatest in patients with pre-existent cardiac disease and diabetes.10
In this study, we demonstrated that previous SH episodes were significantly associated with an incident AF onset in patients with type 2 diabetes mellitus. After adjustment of multiple clinical covariates, a previous SH event was significantly associated with an incident AF. Additionally, old age, male sex, current smoking status, heavy alcohol use, presence of hypertension, ESRD, pre-existing myocardial infarction, ischemic stroke, hyperthyroidism or CHF, and the use of insulin or sulfonylurea were also associated with AF development after SH events. In subgroup analysis, the AF development after SH events was significantly increased in patients aged 55 years and younger and in patients without CHF or CVD experiences. Importantly, compared to patients without SH, patients with SH or SH with incident AF showed a significantly increased all-cause mortality during the observation period. With our study design, we can’t definitively say that there is a direct causal relationship between SH and AF due to the long lag time (7.7 years) from SH experience to AF occurrence. We showed only a close association between SH episode and AF or all-cause mortality. We believed that AF development combined with SH history could be an important risk factor for increased mortality in patients with type 2 diabetes mellitus.
There were some limitations in this analysis. First, because health insurance coverage is confined to the allowable range based on the recommendations of antidiabetic drug combinations made by the Health Insurance Review and Assessment Service in Korea, prescriptions not covered by health insurance were missed in this analysis. Second, subjects with disease codes E11–14 who were not on anti-diabetic medication were not included in this analysis. Third, this analysis relied only on claim data; therefore, we could not obtain clinical information on HbA1c levels, diabetic duration, glucose level during the SH event, or electrocardiographic findings. AF was diagnosed only by claim data. Also, SH episodes without claim, which were not presented to emergency departments or clinics, asymptomatic or mild to moderate hypoglycemia could not be detected. Therefore, some individuals with symptomatic or paroxysmal AF may have been missed. Finally, the cause of death could not be confirmed by claim data.
Despite these limitations, the major strength of this study was that the data, including anthropometric, clinical information, and baseline laboratory results, were based on a nationwide Korean population covering nearly 100% of Korean patients with type 2 diabetes, which provided evidence regarding real-world clinical practice.
In conclusion, previous SH events were important independent risk factors for incident onset of AF and all-cause mortality in patients with type 2 diabetes mellitus. Importantly, compared to patients with SH, subjects with SH with subsequent AF showed significantly higher mortality. Therefore, SH events should be avoided in patients with type 2 diabetes mellitus who are vulnerable to cardiac arrhythmia or preexisting cardiac disease. Future research should be conducted to define the mechanism between SH and cardiac arrhythmia.
Supplementary Material
Acknowledgements:
This work was performed by the cooperation with National Health Insurance Service (NHIS) and the Korean Diabetes Association. The National Health Information Database made by NHIS was used (No. NHIS-2016 −1–139).
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
List of Abbreviations:
- AF
atrial fibrillation
- BMI
body mass index
- CHF
congestive heart failure
- CVD
cardiovascular disease
- ESRD
end-stage renal disease
- ICD-10
International Classification of Diseases, 10th revision
- NHIS
National Health Insurance Service
- SH
severe hypoglycemia
Footnotes
Ethics approval and consent to participate: This study was approved by the institutional review board of the Catholic University of Korea (VIRB-0E237-001).
Conflicts of interest: None.
References
- 1.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med 2009; 151:394–403. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of Medical Care in Diabetes-2017. Diabetes Care 2017;40(Suppl 1):S1–S129. [DOI] [PubMed] [Google Scholar]
- 3.Action to Control Cardiovascular Risk in Diabetes Study Group., Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ADVANCE Collaborative Group., Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–72. [DOI] [PubMed] [Google Scholar]
- 5.Hsu PF, Sung SH, Cheng HM, Yeh JS, Liu WL, Chan WL, et al. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes: a nationwide population-based study. Diabetes Care 2013; 36:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ 2013; 347:f4533. [DOI] [PubMed] [Google Scholar]
- 7.Cha SA, Yun JS, Lim TS, Hwang S, Yim EJ, Song KH, et al. Severe Hypoglycemia and Cardiovascular or All-Cause Mortality in Patients with Type 2 Diabetes. Diabetes Metab J 2016;40:202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow E, Bernjak A, Williams S, Fawdry RA, Hibbert S, Freeman J, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014;63:1738–47. [DOI] [PubMed] [Google Scholar]
- 9.Reno CM, Daphna-Iken D, Chen YS, VanderWeele J, Jethi K, Fisher SJ. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes 2013; 62:3570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordin C The case for hypoglycaemia as a proarrhythmic event: basic and clinical evidence. Diabetologia 2010;53:1552–61. [DOI] [PubMed] [Google Scholar]
- 11.Cha SA, Yun JS, Lim TS, Kang YG, Lee KM, Song KH, et al. Baseline-Corrected QT (QTc) Interval Is Associated with Prolongation of QTc during Severe Hypoglycemia in Patients with Type 2 Diabetes Mellitus. Diabetes Metab J 2016;40:463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ORIGIN Trial Investigators., Mellbin LG, Rydén L, Riddle MC, Probstfield J, Rosenstock J, et al. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J 2013;34:3137–44. [DOI] [PubMed] [Google Scholar]
- 13.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Violi F, Soliman EZ, Pignatelli P, Pastori D. Atrial Fibrillation and Myocardial Infarction: A Systematic Review and Appraisal of Pathophysiologic Mechanisms. J Am Heart Assoc 2016;5:e003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tadic M, Cuspidi C. Type 2 diabetes mellitus and atrial fibrillation: From mechanisms to clinical practice. Arch Cardiovasc Dis 2015;108:269–76. [DOI] [PubMed] [Google Scholar]
- 17.Baxter MA, Garewal C, Jordan R, Wright AD, Nattrass M. Hypoglycaemia and atrial fibrillation. Postgrad Med J 1990;66:981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celebi S, Celebi OO, Aydogdu S, Diker E. A peculiar medical cardioversion of atrial fibrillation with glucose infusion--a rare cause of atrial fibrillation: hypoglycemia. Am J Emerg Med 2011;29:134.e1–3. [DOI] [PubMed] [Google Scholar]
- 19.Tsujimoto T, Yamamoto-Honda R, Kajio H, Kishimoto M, Noto H, Hachiya R, et al. Vital signs, QT prolongation, and newly diagnosed cardiovascular disease during severe hypoglycemia in type 1 and type 2 diabetic patients. Diabetes Care 2014;37:217–25. [DOI] [PubMed] [Google Scholar]
- 20.Lee YH, Han K, Ko SH, Ko KS, Lee KU; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data Analytic Process of a Nationwide Population-Based Study Using National Health Information Database Established by National Health Insurance Service. Diabetes Metab J 2016;40:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang SH, Choi EK, Han KD, Lee SR, Lim WH, Cha MJ, et al. Underweight is a risk factor for atrial fibrillation: A nationwide population-based study. Int J Cardiol 2016;215:449–56. [DOI] [PubMed] [Google Scholar]
- 22.Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36:1384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung CH, Chung JO, Han K, Ko SH, Ko KS, Park JY; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Improved trends in cardiovascular complications among subjects with type 2 diabetes in Korea: a nationwide study (2006–2013). Cardiovasc Diabetol 2017;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goudis CA, Korantzopoulos P, Ntalas IV, Kallergis EM, Liu T, Ketikoglou DG. Diabetes mellitus and atrial fibrillation: Pathophysiological mechanisms and potential upstream therapies. Int J Cardiol 2015;184:617–22. [DOI] [PubMed] [Google Scholar]
- 25.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 2002;105:2753–59. [DOI] [PubMed] [Google Scholar]
- 26.Du X, Ninomiya T, de Galan B, Abadir E, Chalmers J, Pillai A, et al. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur Heart J 2009;30:1128–35. [DOI] [PubMed] [Google Scholar]
- 27.Tsujimoto T, Yamamoto-Honda R, Kajio H, Kishimoto M, Noto H, Hachiya R, et al. High risk of abnormal QT prolongation in the early morning in diabetic and non-diabetic patients with severe hypoglycemia. Ann Med 2015;47:238–44. [DOI] [PubMed] [Google Scholar]
- 28.Clark AL, Best CJ, Fisher SJ. Even silent hypoglycemia induces cardiac arrhythmias. Diabetes 2014;63:1457–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


