Abstract
Neuroinflammatory diseases such as multiple sclerosis are characterized by infiltration of lymphocytes into the central nervous system followed by demyelination and axonal degeneration. While evidence suggests that activated T-lymphocytes induce neurotoxicity and impair function of neural stem cells, the effect of T-cells on oligodendrocyte progenitor cells (OPCs) is still uncertain, partly due to the difficulty in obtaining human OPCs. Here we studied the effect of activated T cells on OPCs using OPCs derived from human hematopoietic stem cells or from human fetal brain. OPCs were exposed to supernatants (sups) from activated T-cells. Cell proliferation was determined by EdU incorporation and CellQuanti-Blue assays. Surprisingly, we found that sups from activated T cells induced OPC proliferation by regulating cell cycle progression. Vascular endothelial growth factor A (VEGF-A) transcripts and protein were increased in T cells after activation. Immunodepletion of VEGF-A from activated T cell sups significantly attenuated its effect on OPC proliferation. Furthermore, VEGF receptor 2 (VEGF-R2) was expressed on OPCs and its inhibition also attenuated activated T cell-induced OPC proliferation. Thus activated T cells have a trophic role by promoting OPC proliferation via the VEGF-R2 pathway.
Keywords: Oligodendrocyte progenitor cells, T cells, neural inflammation, VEGF, neural stem cells
INTRODUCTION
Oligodendrocyte progenitor cells (OPCs) in the adult central nervous system (CNS) proliferate and differentiate to mature oligodendrocytes which protect and help maintain normal neuronal functions by myelinating axons (Franklin and ffrench-Constant 2008). The OPCs may thus play a critical role in the pathogenesis and recovery of neuroinflammatory diseases such as multiple sclerosis (MS), progressive multifocal leukoencephalopathy, neuromyelitis optica, acute disseminated encephalomyelitis and adrenoleukodystrophy where infiltration of lymphocytes into the CNS leads to demyelination and axonal degeneration (Gensert and Goldman 1997), (Zawadzka et al. 2010), (Levine and Reynolds 1999), (Franklin 2002). Further, increased numbers of OPCs and remyelination are usually observed in focal areas infiltrated with activated T cells but less commonly in chronic plaques (Prineas et al. 1993; Wolswijk 2002). However, the effect of activated T-cells on OPCs in adult brain is unknown. Developing appropriate models to study T cell-OPC interactions has been challenging since rodent OPC and oligodendrocytes behave differently compared to human cells in their gene expression, response to growth factors, mitotic competence, differentiation and remyelination potentials (Chandran et al. 2004). There are also logistical and technical challenges in obtaining and culturing adult human OPCs (Wang et al. 2014). Recent developments in cellular reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) and subsequent differentiation provide novel opportunities to generate cell types of interest. However, generation of iPSCs and the subsequent differentiation are usually labor intensive and time consuming. Therefore, direct conversion of somatic cells to other cell types by bypassing iPSCs provides another desirable option. Using this approach, neural stem cells have been directly transformed from adult fibroblasts (Thier et al. 2012), (Ring et al. 2012), (Han et al. 2012). Recently, we showed that induced neural stem cells (iNSCs) can be derived directly from human adult hematopoietic stem cells using Sendai virus containing Yamanaka factors and neural stem cell selective medium (Wang et al. 2013; Wang et al. 2015). The iNSCs derived from adult CD34+ cells were self-renewing and could be further differentiated into adequate numbers of neurons and glia including OPCs. This provides a unique model to study the effect and mechanism of activated T cells on human OPC functions.
While activated T cells are known to cause neuronal injury, to our surprise we found that these cells release factors that induce proliferation of OPCs. T lymphocytes, once activated release a variety of inflammatory factors including vascular endothelial growth factor A (VEGF-A)(Mor et al. 2004). VEGF-A is best known for its essential role in growth of blood vessels and regulation of proliferation and migration of endothelial cells. But VEGF-A has also been implicated in pathological conditions such as disruption of the blood brain barrier in CNS inflammatory diseases (Proescholdt et al. 1999), (Suidan et al. 2010), (Argaw et al. 2012), and there is increased expression of VEGF in acute and chronic MS plaques (Proescholdt et al. 2002). VEGF-A is also upregulated in other autoimmune diseases including diabetic retinopathy, macular degeneration, and systemic sclerosis (Pe’er et al. 1996), (Kvanta et al. 1996), (Kikuchi et al. 1998). There is emerging evidence that VEGF-A also plays a role in a wide range of neuronal functions and in neurogenesis (Mackenzie and Ruhrberg 2012). In this study, we used human OPCs differentiated from iNSCs to study the effect of inflammatory factors released from activated T-cells on OPC function. We observed that activated T cells-released VEGF-A which led to increased proliferation of OPCs through activation of the VEGF-receptor 2 (VEGF-R2).
MATERIALS AND METHODS
Materials:
Culture media and components were purchased from Invitrogen (Carlsbad, CA) and growth factors and cytokines were purchased from PeproTech (Rocky Hill, NJ) if not specified.
Blood was collected from deidentified adult healthy donors at the Transfusion Medicine Blood Bank of the National Institutes of Health. Signed informed consent was obtained in accordance with the NIH Institutional Review Board.
Generation of iNSCs from adult CD34+ cells
iNSCs were directly generated from CD34+ cells as previously published (Wang et al. 2013; Wang et al. 2015). Briefly, CD34+ cells were purified from cells obtained via leukophoresis using a CD34 MultiSort Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured in StemSpan SFEM medium (Stemcell technologies, Vancouver, Canada) containing human thrombopoietin (TPO, 100 ng/ml), fms-like tyrosine kinase 3 (Flt-3) ligand (100 ng/ml) and stem cell factor (SCF, 100 ng/ml), interleukin-6 (IL-6, 20 ng/ml) and interleukin-7 (IL-7, 20 ng/ml) in a 6 well plate (3×105 cells/well in 2 ml of medium per well) at 37oC in 5% CO2 incubator for five days. Cells were replated daily to exclude adherent non-hematopoietic population. Then the cells were infected with CytoTune Sendai viral particles (Invitrogen) containing transcription factor constructs of Oct 3/4, Sox-2, Klf-4, c-Myc with a multiplicity of infection of 20. Half medium exchanges were performed every other day. Five days after transfection, all non-adherent cells were removed and the adherent cells were collected and transferred into 6 well plates in neural progenitor cell medium: DMEM/F12 with 1×N2 supplement 0.1% (w/v) bovine serum albumin (Sigma, St. Louis, MO), 1% (v/v) antibiotics, 20 ng/ml of beta-fibroblast growth factor (b-FGF) and 20 ng/ml of epidermal growth factor. Cells were passaged at a ratio of 1: 3 by mechanical dissociation before reaching 60% confluence and cultured in StemPro NSC SFM - serum-free human neural stem cell culture medium (Invitrogen) for expansion. Immunostaining for nestin and Sox2 was used to determine the quality and purity of neural stem cells. The produced iNSC could be passaged at least 20 times without significant change of nestin positive cells. More than 99% of cells were positive for nestin when the iNSC were used for experiments.
Differentiation of iNSCs into OPCs
OPCs and myelin basic protein (MBP) positive oligodendrocyte were differentiated from iNSCs using a two-step process as previously published (Wang et al. 2013; Wang et al. 2015). For OPC differentiation, 2.5×104 iNSCs were plated on poly-L-ornithine (Sigma) coated 6-well plates in StemPro® NSC SFM. After 2 days, medium was replaced with OPC induction medium containing DMEM/F12 with 1×B27 supplement, 1×N2 supplement, 2 mM GlutaMAX, 20 ng/ml of platelet derived growth factor (PDGF)-AA, 10 ng/ml of bFGF, and 30 ng/ml of triiodothyronine (T3) (Sigma). Culture medium was changed every other day for 7 days. At this stage, 90%~99% of cells were positive for OPC positive markers O4 or NG2, while most of the other cells were positive for neuronal markers βIII-tubulin or MAP2 (supplemental figure 1) and 0~1% of cells were positive for astroglial marker GFAP (not shown). Cells were then replated for experiments to minimize the neuronal cell contamination following treatment with Accutase. For oligodendrocyte differentiation, the OPC cells were cultured for one more week in OPC induction medium. Then the medium was replaced with DMEM/F12 with 1×N2 supplement and 3 nM of T3 (oligodendrocyte medium) for an additional week for myelin basic protein (MBP) production.
Human fetal oligodendrocyte progenitor culture
Human fetal brain specimens of 7–8 weeks gestation were obtained from the Birth Defects Research Laboratory at the University of Washington, Seattle. Human fetal OPCs were cultured as published previously (Monaco et al. 2012) in accordance with NIH guidelines and following approval by the Office of Human Subjects Research and Protection at the National Institutes of Health.
T-cell culture and stimulation
Pan-T-cells, CD4+T cells and CD8+T cells were isolated from peripheral blood mononuclear cells of healthy human adult donors using isolation kits with MACS beads (Miltenyi Biotec) per manufacturer’s protocol. T-cells and T-cell subsets were incubated in IMDM with 5% (v/v) pooled human serum and 1% antibiotics at 37°C in a 5% CO2 incubator. The cells were activated by adding CD3/CD28 Dynabeads (Invitrogen) with a bead-to-cell ratio of 1:10 and incubated for 48 hrs. Culture sups were collected and used to treat OPCs or oligodendrocytes with a supernatant-to-medium (OPC induction medium or oligodendrocyte medium) ratio of 1:20.
Transfection of HEK293 cells
HEK 293 cells were plated (5.0×105 cells/well) on poly-D-lysine (Sigma) coated 24-well plates in DMEM containing 10% fetal bovine serum and 1% antibiotics and incubated for overnight at 37°C in 5% CO2 incubator. For transfection, 1 μl of lipofectamine (Invitrogen) was diluted in 50 μl of Opti-MEM I reduced serum medium and 2 μg of plasmid was diluted in 50 μl of Opti-MEM I reduced serum medium and incubated for 5 min at room temperature. Plasmid and lipofectamine were combined, mixed by pipetting, and incubated at room temperature for 20 min before adding drop-wise onto the cells. The cells were cultured for 48 hours, and culture sups were collected and used to treat OPCs with a sup-to-medium ratio of 1:20.
Immunocytochemistry
Immunostaining was performed as previously described (Wang et al. 2013). Briefly, cells were fixed in 4% (w/v) paraformaldehyde (Sigma) and permeabilized in 0.1% (v/v) Triton X-100 (Sigma). Non-specific antigenic sites were blocked with PBS-T containing 4% (v/v) goat serum (Sigma) and 1% (v/v) glycerol for 20 min at room temperature. Cells were immunostained with mouse anti-oligodendrocyte marker O4 (1:200, R&D system, Minneapolis, MN), rabbit anti-NG2 Chondroitin Sulfate Proteoglycan (1:200, Millipore, Billerica, MA), mouse monoclonal anti-nestin (1:1000, Millipore), mouse monoclonal anti-βIII-tubulin (1:1000, Promega, Madison, WI), rabbit anti-MAP2 (1:100 Sigma) and rabbit anti-GFAP (1:1000, Sigma) antibodies followed by corresponding secondary antibodies (anti-mouse IgG Alexa Fluor 488, 1:400; anti rabbit IgG Alexa Fluor 594, 1:400; anti-mouse IgM Alexa Fluor 488, 1:400; Invitrogen) and 4’,6-diamidino-2-phenylindole (DAPI, Sigma) nuclear staining. Images were acquired on an EVOS fluorescence microscope (AMG, Bothell, WA).
Cell proliferation assays
Cell numbers were determined using CellQuanti-Blue Cell Viability Assay kit (BioAssay Systems, Hayward, CA) per manufacturer’s instructions. OPCs were plated in 96-well plates in OPC medium and treated with T-cell or HEK293 cell sups (1:20 dilution) for 24 hours at 37°C. CellQuanti-blue reagent (10 μl/well) was added to each well. After incubation for another hour at 37°C, the fluorescence was quantified at an excitation wavelength of 530 nm and emission wavelength of 590 nm using a fluorescence plate reader. Cell proliferation was measured by EdU incorporation assay. After treatment with T-cell sups for 20 hours, OPCs were treated with 10 μM of EdU (Invitrogen) for another 4 hours. The cells were then fixed with 4% paraformaldehyde and EdU labeling was detected using Click-iT EdU Imaging Kits (Invitrogen) per the manufacturer’s instructions. DAPI was used for nuclear staining. Images from seven predetermined fields per well were taken and cell proliferation was determined by calculating the ratio of EdU positive cells to total DAPI positive cells.
Cell cycle evaluation
Fluorescence based cell cycle indicators were used to determine cells in G1 and S/G2/M phase. OPCs were plated in 96-well plates in OPC medium and transduced with the Premo FUCCI Cell Cycle Sensor (Invitrogen) according to the manufacturer’s instruction and incubated with T-cell sups (1:20) for 24 hours at 37°C in a 5% CO2 incubator. Cells were fixed in 4% paraformaldehyde for 15 min at room temperature, followed by 3 washes with PBS. Cdt 1-RFP was expressed in nuclei of cells in the G1 phase, and geminin-GFP was expressed in nuclei of cells in the S/G2/M phase. RFP and GFP positive cells in seven predetermined fields were counted.
Quantitative real time PCR
VEGF-A expression was determined by quantitative real-time PCR (qRT-PCR). CD4+ T cells and CD8+ T cells were activated by adding CD3/CD28 Dynabeads (Invitrogen) with a bead-to-cell ratio of 1:10 and incubated for 24–48 hours. Total RNA was extracted using NORGEN total RNA purification kit (NORGEN, Ontario, Canada), and the RNA samples were treated with DNase I (Invitrogen) for 20 min. qRT-PCR was conducted using SensiFAST SYBR kit (Bioline, Taunton, MA), QuantiTect VEGF-A primer (Qiagen, Valencia, CA), and ViiA7 real-time PCR system according to manufacturer’s instructions. Beta-actin (ACTB) was used as an internal control. PCR products were analyzed by 1.0% (w/v) agarose gel electrophoresis.
Western blot analysis for VEGF receptors
Cell culture lysates were prepared with RIPA lysis buffer for Western-blot analysis of VEGFR1 and VEGFR2. Protein concentrations were determined with BCA Assay kit (Thermo Scientific, Waltham, MA) following manufacturer’s instructions. Proteins were separated by SDS-PAGE and electro-transferred to PVDF membranes, which were blocked with 5% (w/v) non-fat milk in Tris-buffered saline with 0.1% (v/v) Tween-20 and 5% (w/v) BSA. Membranes were incubated overnight at 4 °C with anti-VEGFR1 (1:1000, Cell Signaling, Danvers, MA) and anti-VEGFR2 (1:1000, Cell Signaling). After washing, membranes were incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG for 1 hour at room temperature. Proteins were visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific) on a FlourChem M Imager (Proteinsimple, San Jose, CA).
Cell viability assay
The cell viability was measured by propidium iodide staining. OPCs were plated in 96-well plates in OPC medium and treated with T-cell or HEK293 cell sups (1:20 dilution) for 24 hours at 37°C. Culture plate was centrifuged for 5 min at 400× g at room temperature. 100 μl of assay binding buffer was added to each well and plate was centrifuged for 5 min at 400× g. Assay binding buffer was discarded and 50 μl of propidium iodide staining solution was added to each well. The plate was centrifuged for 5 min at 400× g, and propidium iodide staining solution was discarded. 100 μl of assay binding buffer was added to each well and the fluorescence was quantified at an excitation wavelength of 560 nm and emission wavelength of 595 nm using a fluorescence plate reader.
VEGF-A immunodepletion and VEGF receptor inhibition
To immunodeplete VEGF-A from T-cell sups, the sups were incubated with protein G Dynabeads (Invitrogen) conjugated to VEGF-A antibody (Acris, San Diego, CA) or an isotype-matched control antibody for 18 hours at 4°C. The Dynabead complex was removed using a magnet. The remaining supernatant after bead removal was used as to treat the OPC cultures. To assess the role of VEGF receptor (VEGFR) 1 and 2 on proliferation of OPCs the cells were pretreated with VEGFR1 antagonist, ZM306416 (2–10 μM) (Selleckchem, Houston, TX) or VEGFR2 antagonist SU1498 (2–10 μM) (Calbiochem, Billerica, MA) for 30 min followed by incubation with T-cell sups for 24 hours. Cell proliferation was assessed by Premo FUCCI Cell Cycle Sensor as described above.
Statistical Analysis
NIH Image J program was used for DAPI and EdU positive cell counting. The total number of cells and immunolabeled cells were counted in seven predetermined fields per well using an EVOS fluorescence microscope with a 20× objective. Three wells were counted in each group. At least three independent experiments were performed. Data are expressed as mean and SEM for each treatment group. Statistical analysis was performed with Graphpad Prism 6 (Graphpad software, La Jolla, CA). Differences were evaluated using either one-way ANOVA followed by Bonferroni’s test for multiple comparisons or Student’s t test for two-group comparisons. Two-tailed values of P < 0.05 were considered significant.
RESULTS
Activated T-cells increased proliferation of OPCs derived from iNSC
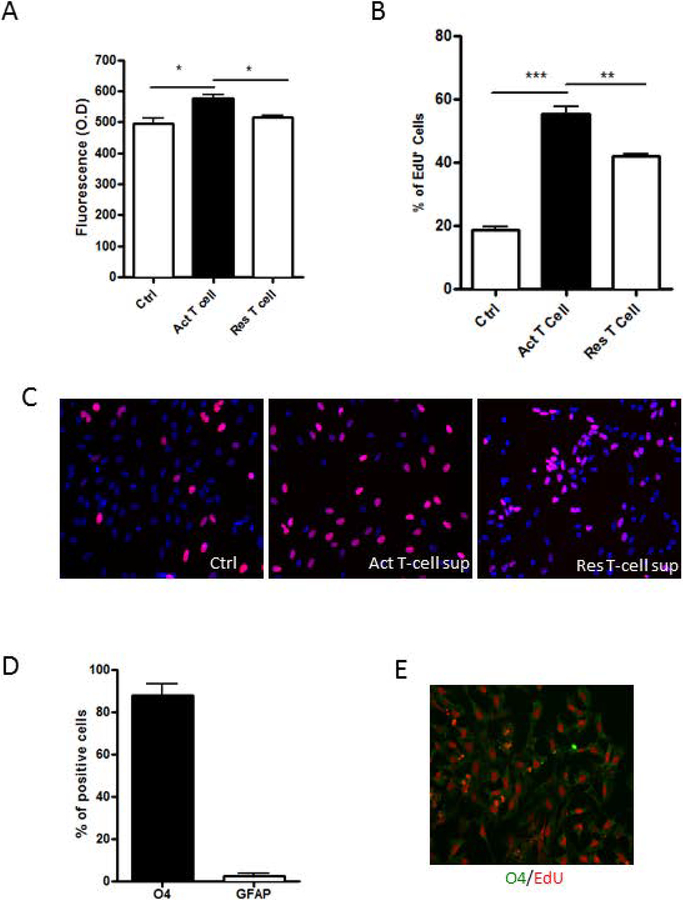
The effect of activated T-cells on OPCs was studied by exposing OPCs differentiated from iNSC to sups from cultured T-cells with or without CD3/CD28 co-activation. A small but statistically significant increase in the cell numbers of OPCs as determined by CellQuanti-Blue Assay was seen with sups from activated pan T cells compared to sups from non-stimulated pan-T-cells (Figure 1A). This was accompanied by a significant increase in EdU incorporation in OPCs treated with sups from activated pan-T-cells leading to a three-fold increase in proliferating cells compared to untreated controls. Resting T cell sups also increased proliferation of OPC resulting in a doubling of the proliferating cells compared to untreated controls likely due to baseline activation of T cells (Figure. 1B and C). To confirm the identity of the proliferating cells, we co-immunostained the EdU+ cells with OPC marker, O4 and astroglial marker, GFAP. Most EdU+ cells expressed O4 (Figure 1D and E). These results indicated that activated pan-T-cells released soluble factors that increased proliferation of OPCs.
Figure 1: Effect of T-cell sups on OPCs.
(A) OPCs were exposed to culture sups from T cells for 24 hrs. An increase in the number of OPCs was seen with activated (Act) T-cell sups compared to T-cell media control (Ctrl) or resting (Res) T-cell sup as determined by CellQuanti-blue assay. (B) An increase in the percentage of proliferating cells is seen with Act T sup as determined by uptake of EdU. (C) Representative photomicrographs show EdU staining (red) OPCs exposed to T-cell media Ctrl, Act T-cell sup or Res T-cell sup. The nuclei are stained blue with DAPI. (D) OPCs exposed to Act T-cell sup were dual stained for O4 or GFAP and EdU. While most of the cells were positive for O4 and only a few were positive for GFAP, nearly all proliferating cells were O4 positive. (E) Representative photomicrograph shows O4 cells (green) dual stained for EdU (red). Data represent mean + SEM of three independent experiments. N=3 for A, B and D, *P<0.05; **P<0.005 and ***P<0.0005.
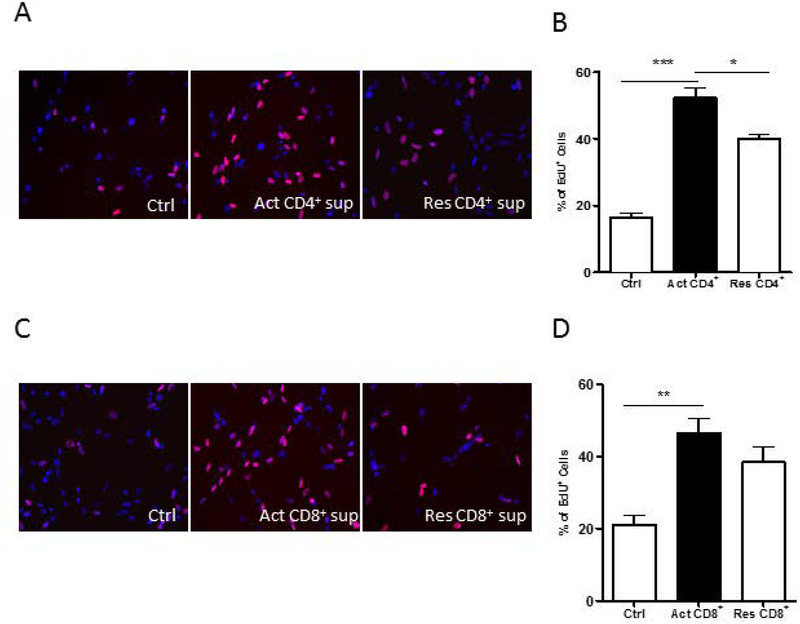
To further determine which subpopulations of T-cells were responsible for the effect on proliferation of OPCs, CD4+ cells and CD8+ cells were isolated from PBMCs. Flow cytometry assay was used to determine the purity of isolated CD4+ cells and CD8+ cells (Supplemental Figure 2). Purified CD4+ cells and CD8+ cells were separately activated with anti-CD3/CD28 antibodies and the OPCs were then exposed to the culture sups. In both instances, there was increased proliferation of OPCs suggesting that both activated CD4+ cells (Figure 2A and B) and CD8+ cells (Figure 2C and D) released soluble factors responsible for increasing the proliferation of OPCs.
Figure 2: Effect of CD4 and C8 lymphocytes on OPC proliferation.
(A) Representative photomicrographs of OPCs exposed to T cell media control (Ctrl), activated (Act) CD4+ cell sups or resting (Res) CD4+ cell sups. Nuclei are stained blue with DAPI and EdU+ cells are red. (B) Act -CD4+ cell sups induced increased proliferation of OPCs as determined by percentage of EdU positive cells. (C) Representative photomicrographs of OPCs exposed to Ctrl, Act CD8+ cell sup or Res CD4+ cell sup. Nuclei are stained blue with DAPI and EdU+ cells are red. (D) Act -CD8+ cell sups induced increased proliferation of the OPCs. Data represent mean + SEM of three independent experiments. N=3 for Band D, *P<0.05; **P<0.005 and ***P<0.0005
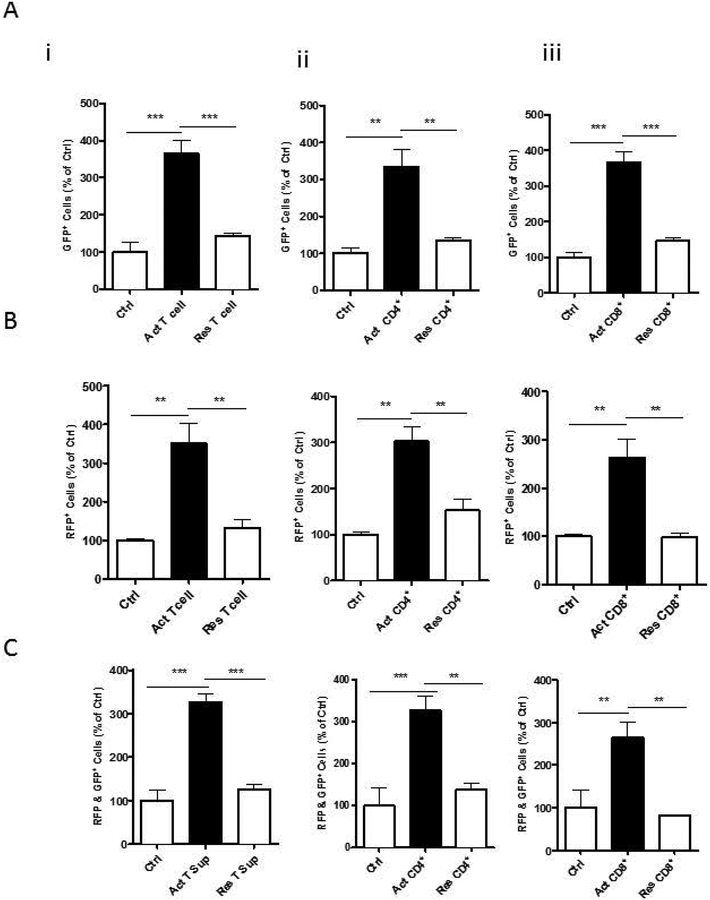
Activated T cells upregulated G1 to S phase transition in OPCs
We used FUCCI to study the effects of activated T-cells on cell cycle progression of proliferating OPCs. OPCs were transfected with FUCCI constructs, followed by treatment of sups from activated pan-T-cells, CD4+ cells, and CD8+ cells. Cells transduced with FUCCI constructs express Cdt1-RFP in G1 phase and Geminin-GFP in S/G2/M phases. Therefore, the indicator labels the nuclei of cells in G1 red and those in S/G2/M green. Treatment with activated sups from either pan-T cells, CD4+ or CD8+ cells significantly increased the number of cells in both G1 phase and S/G2/M phases compared to control while there were much more RFP+ cells than the GFP+ cells (Figures 3A and B). The number of cells expressing both RFP and GFP were increased as well (Figure 3C). These results indicate that activated T cell sups increased proliferation of OPCs by increasing the cell numbers in G1 phase and then transitioning them to S phase and eventually leading to cell division which was evidenced by an increase in the number of cells expressing GFP (Figure 3A).
Figure 3: Effect of T cells on OPC cell cycle phases:
FUCCI Cell Cycle Sensor was used to study the cell cycle phases. (A) GFP indicates number of OPCs in S/G2/M phase. A significant GFP increase in these cells is seen with treatment with sups from activated (Act) (i) pan T cells (ii) CD4 cells or (iii) CD8 cells compared to T cell media control (Ctrl) or resting (Res) pan T cells, CD4 or CD8 cells respectively. (B) RFP+ cells indicate number of OPCs in G1 phase and (C) dual GFP and RFP+ cells indicate cells in transition phase. Data represent mean + SEM of three independent experiments. N=3, *P<0.05; **P<0.005 and ***P<0.0005
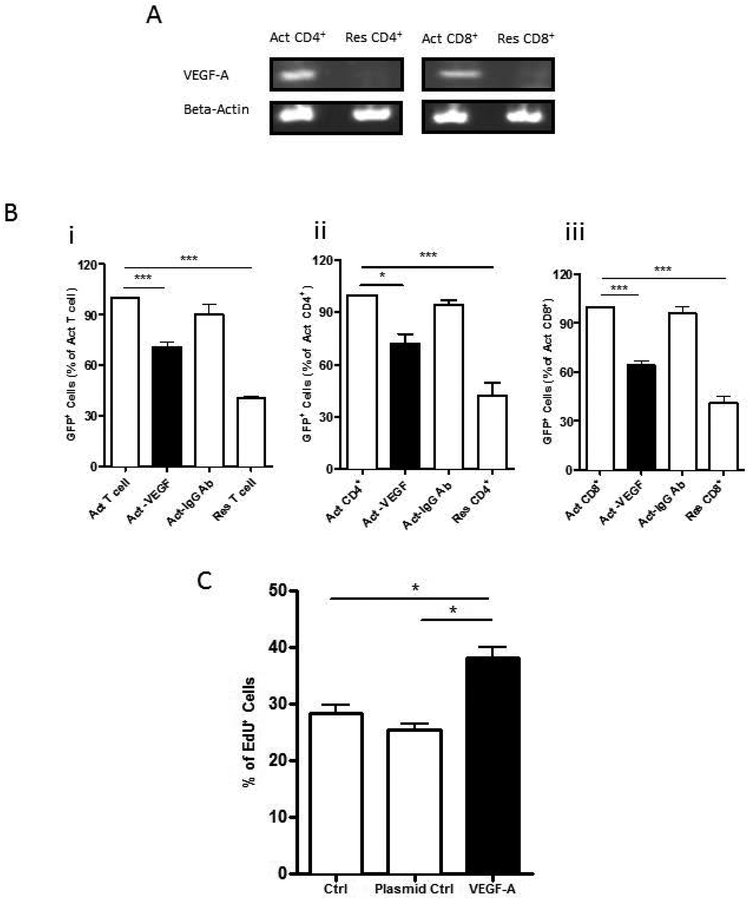
VEGF-A released from activated T-cells mediated proliferation of OPCs
Previously published gene arrays from activated T cells indicated that VEGF-A was a growth factor produced by these cells (Diehn et al. 2002). Hence, we used real-time polymerase chain reaction (PCR) to determine the levels of gene expression of VEGF-A in T-cells with or without activation. Following anti-CD3/CD28 co-activation for 24 hours, VEGF-A expression level was upregulated significantly in both CD4+ cells and CD8+ cells (Figure 4A). Further, immunodepletion of VEGF-A from activated pan T-cells, CD4+ cells, or CD8+ cells significantly attenuated their effect on enhanced proliferation of OPCs, as determined by the number of cells expressing FUCCI fluorescence (Figure 4B). OPCs also showed increased proliferation when incubated with culture sups from 293 cells transfected with VEGF-A plasmid as determined by EdU incorporation assay when compared to sups from 293 cells treated with transfection reagent and 293 cells transfected with a control plasmid (Figure. 4C).
Figure 4: Effect of VEGF on OPC proliferation.
(A) Expression of VEGF-A transcripts as determined by RT-PCR is increased in activated (Act) CD4+ and CD8+ cells. (B) Fluorescence ubiquitination cell cycle indicator was used to measure OPC proliferation and is indicated by GFP+ cells. Exposure to sups following immunodepletion of VEGF (Act-VEGF) from (i) Act Pan T cells (ii) Act CD4+ cells or (iii) Act CD8+ cells resulted in a significant decrease in proliferation of OPCs compared to each of the sups treated with an isotype antibody (Act-IgG Ab) or untreated sups from each of the Act cell types. (C) OPC proliferation was determined by EdU incorporation. Sups from 293 cells transfected with VEGF-A plasmid show increased proliferation compared to sups from cells treated with transfection reagent alone (Ctrl) or transfected with a control plasmid (Plasmid Ctrl). Data represent mean + SEM from three independent experiments. N=3 for B and C, *P<0.05; ***P<0.0005
VEGF-A induced OPC proliferation is VEGFR2 dependent
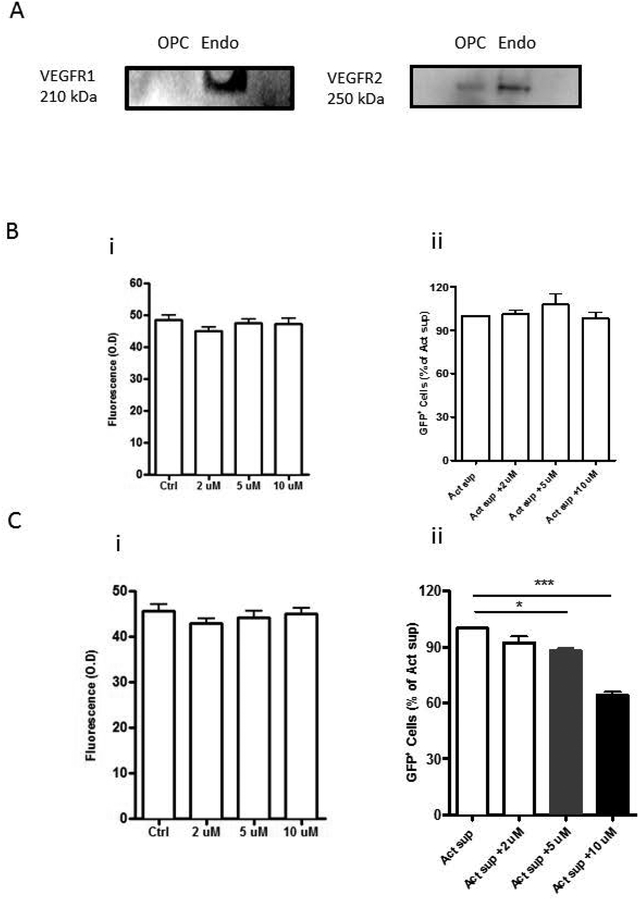
VEGF-A exerts its effects by activating receptor tyrosine kinases, VEGF receptor 1 (VEGFR1) and VEGF receptor 2 (VEGFR2). Hence, to determine which receptor on OPCs is responsible for VEGF-A mediated OPC proliferation, VEGFR1 and VEGFR2 expression on OPCs were determined by Western-blot analysis. It showed that OPCs expressed VEGFR2 but not VEGFR1 (Figure 5A). Further, pretreatment of OPCs with VEGFR1 specific inhibitor, ZM306416 (2–10 μM) had no effect on activated pan T cell supernatant mediated OPC proliferation (Figure 5B) while pretreatment with VEGFR2 specific inhibitor, SU1498 (2–10 μM) inhibited the proliferation in a dose-responsive manner (Figure 5Cii). No significant toxicity was observed when OPCs were treated with SU1498 at 2–10 μM for 24 hour and assessed by propidium iodide staining (Figure 5Ci). These results suggested that VEGF-A released from activated T-cells increased proliferation of OPCs through VEGR2 activation.
Figure 5: Effect of Activated T cell sups on OPCs is mediated by VEGFR-2.
(A) Western blot analysis for VEGF receptors shows lack of detection of VEGFR-1 but presence of VEGFR-2 on OPCs. Endothelial cells (Endo) were used as a positive control. Propidium iodide staining was used to measure cell viability and fluorescence ubiquitination cell cycle indicator was used to measure OPC proliferation and is indicated by GFP+ cells. (B) VEGFR-1 antagonist, ZM306416 (i) had no effect on OPC viability when incubated alone and (ii) did not inhibit the effect of activated (Act) T cell sup induced GFP+ cell numbers on OPCs. (C) VEGFR-2 antagonist, SU1498, (i) had no effect on OPC viability when incubated alone but (ii) inhibited OPC proliferation as indicated by GFP+ cell numbers in a dose-responsive manner in the presence of Act T cell sup. Data represent mean + SEM from three independent experiments. N=3 for B and C, **P<0.005; ***P<0.0005
VEGF-A induced proliferation of OPCs derived from human fetal brain
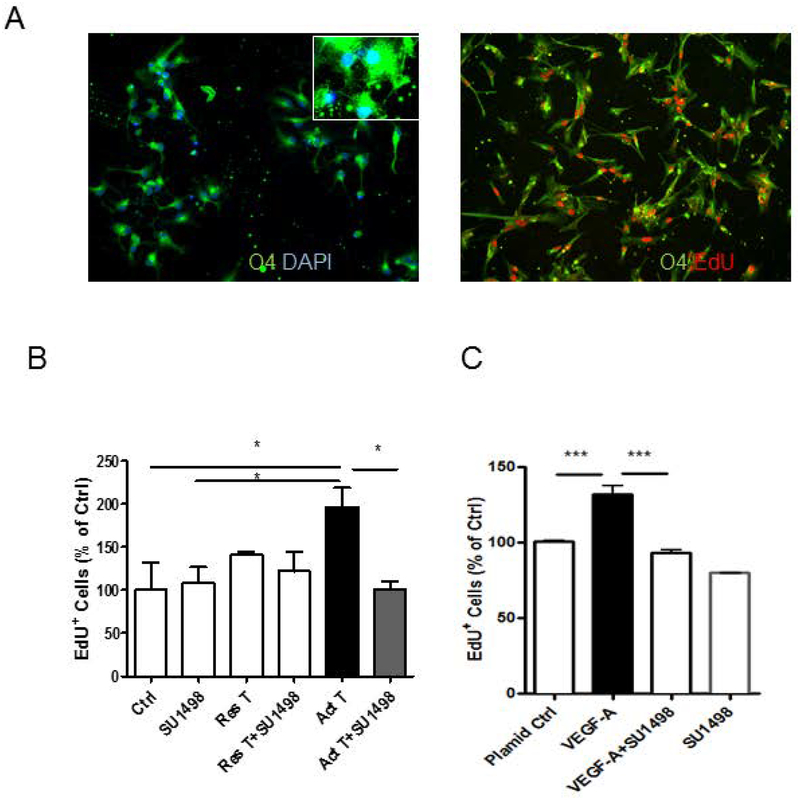
To determine whether activated T cells released VEGF-A has the same effect on primary OPCs, We first differentiated OPCs and oligodendrocytes from human fetal brain tissues. To confirm the identity of the proliferating cells, we co-immunostained the EdU+ cells with OPC marker, O4. As seen in Figure 6A, majority of the cells were O4 positive and most EdU+ cells expressed O4. We treated the OPCs with culture sups from activated T cells or 293 cells transfected with VEGF-A plasmid for 24 hours and added EdU during the last 6 hours. As seen in Figure 6B, only supernatants from activated T cells increased OPC proliferation significantly and the increased proliferation was attenuated by pretreatment with SU1498 significantly. Similarly, OPCs showed increased proliferation when incubated with culture sups from 293 cells transfected with VEGF-A plasmid as determined by EdU incorporation assay when compared to sups from 293 cells transfected with a control plasmid (Figure. 6C). Further, pretreatment with SU1498, attenuated the effect of sups from 293 cells transfected with VEGF-A plasmid (Figure. 6C). These results suggested that VEGF-A released from activated T-cells increased proliferation of primary OPCs through VEGR2 activation. However, activated T cell sups showed no increase in MBP production in primary oligodendrocytes (Supplemental Figure 3), indicating the trophic effect of activated T cells is specific to OPC proliferation with no effect on oligodendrocyte maturation.
Figure 6. Effect of VEGF-A on proliferation of primary human fetal OPCs.
Immunostaining for O4 was used for the identification of primary human OPCs. EdU incorporation assay was used to measure OPC proliferation. (A), almost all EdU positive cells were O4 positive cells. (B), OPCs treated with sup from activated T cells (Act T) show increased proliferation compared to control cells (Ctrl) while OPCs treated with sup from restive T cells (Res T) show no increased proliferation. The increased proliferation of OPCs was attenuated by VEGF-A inhibitor SU1498. (C), Sups from 293 cells transfected with VEGF-A plasmid show increased proliferation compared to sups from cells transfected with a control plasmid (Plasmid Ctrl), but was attenuated by VEGF-A inhibitor SU1498. Data represent mean ± SEM from three independent experiments. N=3 for B and C, *P<0.05, ***P<0.001
DISCUSSION
In the present study, we determined the effect of activated T cells on OPC proliferation using OPCs differentiated from human iNSCs and further confirmed the results using human fetal brain derived OPCs. We demonstrated that VEGF-A released from activated T-cells induce proliferation of OPCs by activating VEGFR2 which promotes G1 to S phase transition of OPCs.
In this study, we used OPCs derived from iNSC which has several advantages. iNSCs can be derived from CD34 cells obtained from blood. The iNSC can then be expanded to sufficient numbers for in vitro studies (Wang et al. 2015). Further, we showed that OPCs differentiated from iNSCs behave in a similar way as OPCs differentiated from human fetal brain tissue in response to inflammatory factors. Both forms of OPCs responded to VEGF-A released from activated T-cells with an increase in proliferation which could be inhibited by the VEGFR2-specific inhibitor, SU1498. However, we did observe that iNSC-derived OPCs were more sensitive to activated T-cell-induced proliferation. It indicates that the two OPCs may represent different cell stages and thus responded differently to pro-proliferation factors. Secondly, primary human OPCs were more heterogenous populations compared to the iNSC cells which are clonal and more homogeneous. This explains the inconsistency in the human primary OPC experiments. Further, as the resting T cells produce minimal VEGF-A but still induced cell proliferation of the iNSC-derived OPCs but not the human primary cultured OPCs, it is possible that there may be other factors the sups which may induce OPC proliferation. Nevertheless, in patients with neuroinflammatory disorders, using our in vitro model system, lymphocytes from the same patient could be used in an autologous manner to determine its effects on the OPCs derived from the same patient’s iNSC. This would allow for the study of the effects of cell-to-cell contact between the two cell types that cannot be done in an allogeneic system due to MHC mismatch.
T cell activation and infiltration in the brain has been observed in MS and other demyelinating diseases such as acute disseminated encephalomyelitis (Garg 2003), neuromyelitis optica (Pohl et al. 2013) and adrenoleukodystrophy (Ito et al. 2001). In these disorders, T cell activation has been implicated in causing neurotoxicity and injury to myelin (Garg 2003; Kutzelnigg and Lassmann 2014) and most therapeutic strategies prevent T cell infiltration into the brain. However, others have shown that infiltration of activated T cells alone is not sufficient to cause demyelination. In fact in some patients, a fulminant T cell encephalitis may occur in the setting of immune reconstitution syndrome with no evidence of demyelination (Venkataramana et al. 2006), (Johnson et al. 2013). It has been observed that OPCs can be frequently found in MS plaques in the brain but their relationship to T cells had not been studied (Chang et al. 2002), (Wolswijk 1998), (Chari and Blakemore 2002). Following treatment with glatiramer acetate Th2 cells release insulin growth factor-2 which is also trophic to OPCs (Zhang et al. 2010). Activated T cells have also been shown to increase proliferation of smooth muscle cells in the respiratory passages in patients with asthma. In this case the ligand for epidermal growth factor receptor is released by enzymatic cleavage by T cells (Al Heialy et al. 2013). Thus, activated T cells have a wide range of trophic effects on various cell types which may in part be driven by the factors released and types of receptors expressed on these cells. We now show that activated T cells, promote OPC proliferation. We found that there is a significant increase in transcript of bFGF but not PDGF-A (Supplemental Figure 4 A and 4B). While it is known that bFGF and PDGF-AA induce rodent OPC proliferation (Furusho et al. 2015), as bFGF and PDGF-AA were already included in the culture medium, adding more bFGF or PDGF-AA in the culture medium failed to further increase OPC proliferation (Supplemental Figure 4C). Our result is also in agreement with a report that bFGF and PDGF-AA support human OPC survival but not proliferation(Chandran et al. 2004). Instead, we found that activated T cells increased expression of VEGF-A which is consistent with other reports (Lyu and Park 2011; Mor et al. 2004). We found that OPCs express VEGFR2, activation which triggers a proliferative response in these cells. These observations indicate that the effect of T cells activation on neural cells could be very complicated. While some factors released from activated T cells such as granzyme B may cause neurotoxicity (Wang et al. 2006), other factors such as VEGF-A may enhance OPC proliferation and thus has the potential to protect neurons. This also implies that when targeting T cell related inflammation as a therapy for neuroinflammatory disorders, it may be better to target specific inflammatory factors or pathways rather than generally inhibiting T cell activation.
Using iNSC as a model, we found that both activated CD4 and CD8 T cells induced OPC proliferation. Among the soluble factors, VEGF-A was upregulated in both CD4 and CD8 cells following activation and was primarily responsible for induction of OPC proliferation through activation of VEGFR2. VEGF-A is well known as a potent and integral regulator of angiogenesis, which contributes to pathogenesis of many diseases, including cancer, rheumatoid arthritis, age-related macular degeneration, and diabetic retinopathy. VEGF is widely expressed in normal and disease conditions, and upregulation occurs at sites of angiogenesis and inflammation. Several lines of evidence have suggested that VEGF is also implicated in neuroinflammatory diseases such as MS. In both chronic and acute MS plaques, VEGF-positive reactive astroglia were widespread and disseminated (Proescholdt et al. 2002). However, VEGF-A may act as a double-edged sword. In a mouse model of experimental allergic encephalomyelitis, VEGF-A exacerbated the inflammatory response by inducing focal blood brain barrier (BBB) breakdown, lymphocyte infiltration, and migration of lymphocytes into the lesions (Proescholdt et al. 1999), (Suidan et al. 2010), (Argaw et al. 2012). On the other hand, VEGF-A has been shown to promote neurite growth in a nerve injury model (Li et al. 2011) and be neuroprotective in motor neuron disease models (Blackburn et al. 2009). Here our studies suggest that VEGF-A has trophic effects on OPCs. It has been reported that VEGF-C could enhance proliferation of NSCs and OPCs expressing VEGFR-3 (Le Bras et al. 2006). Others reported that VEGF-A promoted migration but not proliferation of OPC (Hayakawa et al. 2011). The observed differences maybe specie specific since prior studies were done with a murine model and we used human cells. It is also noteworthy that an increase in OPC numbers may not always be beneficial, as OPCs may also release pro-inflammatory factors such as MMP9 to disrupt the blood-brain-barrier (Seo et al. 2013). We evaluated MMP9 gene expression in OPCs after treatment with activated T cell sups and did not find a significant change (Supplemental Figure 5). Although the pathological significance of a single factor such as MMP9 may be limited due to abundance and disease conditions (Savarin et al. 2011), it still highlights the complexity in targeting inflammation as an intervention to control disease progression.
In summary, we report that OPCs generated from CD34-derived iNSC behave similarly as primary OPCs in response to treatment of T cell activation and could be used to study the physiological functions and pathogenesis of OPCs, providing a specific advantage especially by using patient specific blood samples. Further, using this model we found VEGF-A released from activated T cells could enhance OPC proliferation through activation of VEGF-R2, indicating a critical role for VEGF-A in T-cell mediated neuroinflammation. These finding add to the complexity of the neuropathogenesis of diseases that cause leukoencephalitis and may have important implications for therapeutic strategies that target activated T cells for treatment of these disorders.
Supplementary Material
Main points.
Activated T cells induce OPC proliferation.
Activation of T cells produce VEGF-A.
Depletion of VEGF-A attenuates activated T cell-induced OPC proliferation.
VEGF-R2 inhibition attenuates VEGF-A induced OPC proliferation.
Acknowledgements:
This project was supported by NIH intramural research funds.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
REFERENCES:
- Al Heialy S, Risse PA, Zeroual MA, Roman HN, Tsuchiya K, Siddiqui S, Laporte SA, Martin JG. 2013. T cell-induced airway smooth muscle cell proliferation via the epidermal growth factor receptor. Am J Respir Cell Mol Biol 49:563–70. [DOI] [PubMed] [Google Scholar]
- Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG and et al. 2012. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 122:2454–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn D, Sargsyan S, Monk PN, Shaw PJ. 2009. Astrocyte function and role in motor neuron disease: A future therapeutic target? Glia 57:1251–1264. [DOI] [PubMed] [Google Scholar]
- Chandran S, Compston A, Jauniaux E, Gilson J, Blakemore W, Svendsen C. 2004. Differential generation of oligodendrocytes from human and rodent embryonic spinal cord neural precursors. Glia 47:314–324. [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. 2002. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 346:165–73. [DOI] [PubMed] [Google Scholar]
- Chari DM, Blakemore WF. 2002. Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia 37:307–13. [PubMed] [Google Scholar]
- Diehn M, Alizadeh AA, Rando OJ, Liu CL, Stankunas K, Botstein D, Crabtree GR, Brown PO. 2002. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci U S A 99:11796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ. 2002. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci 3:705–14. [DOI] [PubMed] [Google Scholar]
- Franklin RJM, ffrench-Constant C. 2008. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9:839–855. [DOI] [PubMed] [Google Scholar]
- Furusho M, Roulois AJ, Franklin RJM, Bansal R. 2015. Fibroblast growth factor signaling in oligodendrocyte-lineage cells facilitates recovery of chronically demyelinated lesions but is redundant in acute lesions. Glia 63:1714–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg RK. 2003. Acute disseminated encephalomyelitis. Postgraduate medical journal 79:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. 1997. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 19:197–203. [DOI] [PubMed] [Google Scholar]
- Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S and et al. 2012. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 10:465–72. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, Arai K. 2011. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci 31:10666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Blumberg BM, Mock DJ, Goodman AD, Moser AB, Moser HW, Smith KD, Powers JM. 2001. Potential environmental and host participants in the early white matter lesion of adreno-leukodystrophy: morphologic evidence for CD8 cytotoxic T cells, cytolysis of oligodendrocytes, and CD1-mediated lipid antigen presentation. Journal of neuropathology and experimental neurology 60:1004–19. [DOI] [PubMed] [Google Scholar]
- Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A. 2013. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A 110:13588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Kubo M, Kadono T, Yazawa N, Ihn H, Tamaki K. 1998. Serum concentrations of vascular endothelial growth factor in collagen diseases. Br J Dermatol 139:1049–51. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Lassmann H. 2014. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handbook of clinical neurology 122:15–58. [DOI] [PubMed] [Google Scholar]
- Kvanta A, Algvere PV, Berglin L, Seregard S. 1996. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 37:1929–34. [PubMed] [Google Scholar]
- Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, Haiko P, Karkkainen MJ, Yuan L, Muriel MP and et al. 2006. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci 9:340–8. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R. 1999. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol 160:333–47. [DOI] [PubMed] [Google Scholar]
- Li Z, Burns AR, Han L, Rumbaut RE, Smith CW. 2011. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am J Pathol 178:1106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu S-Y, Park W-B. 2011. Gene network analysis on the effect of Viscum album var. coloratum in T cells stimulated with anti-CD3/CD28 antibodies. Archives of Pharmacal Research 34:1735–1749. [DOI] [PubMed] [Google Scholar]
- Mackenzie F, Ruhrberg C. 2012. Diverse roles for VEGF-A in the nervous system. Development 139:1371–80. [DOI] [PubMed] [Google Scholar]
- Monaco MC, Maric D, Bandeian A, Leibovitch E, Yang W, Major EO. 2012. Progenitor-derived oligodendrocyte culture system from human fetal brain. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor F, Quintana FJ, Cohen IR. 2004. Angiogenesis-Inflammation Cross-Talk: Vascular Endothelial Growth Factor Is Secreted by Activated T Cells and Induces Th1 Polarization. The Journal of Immunology 172:4618–4623. [DOI] [PubMed] [Google Scholar]
- Pe’er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. 1996. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol 80:241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl M, Kawakami N, Kitic M, Bauer J, Martins R, Fischer M-T, Machado-Santos J, Mader S, Ellwart J, Misu T and et al. 2013. T cell-activation in neuromyelitis optica lesions plays a role in their formation. Acta Neuropathologica Communications 1:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. 1993. Multiple sclerosis: remyelination of nascent lesions. Annals of neurology 33:137–51. [DOI] [PubMed] [Google Scholar]
- Proescholdt MA, Heiss JD, Walbridge S, Muhlhauser J, Capogrossi MC, Oldfield EH, Merrill MJ. 1999. Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J Neuropathol Exp Neurol 58:613–27. [DOI] [PubMed] [Google Scholar]
- Proescholdt MA, Jacobson S, Tresser N, Oldfield EH, Merrill MJ. 2002. Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J Neuropathol Exp Neurol 61:914–25. [DOI] [PubMed] [Google Scholar]
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. 2012. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 11:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C, Stohlman SA, Rietsch AM, Butchi N, Ransohoff RM, Bergmann CC. 2011. MMP9 deficiency does not decrease blood–brain barrier disruption, but increases astrocyte MMP3 expression during viral encephalomyelitis. Glia 59:1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, Kim KW, Lo EH, Arai K. 2013. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest 123:782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suidan GL, Dickerson JW, Chen Y, McDole JR, Tripathi P, Pirko I, Seroogy KB, Johnson AJ. 2010. CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions. J Immunol 184:1031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nothen MM and et al. 2012. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10:473–9. [DOI] [PubMed] [Google Scholar]
- Venkataramana A, Pardo CA, McArthur JC, Kerr DA, Irani DN, Griffin JW, Burger P, Reich DS, Calabresi PA, Nath A. 2006. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology 67:383–8. [DOI] [PubMed] [Google Scholar]
- Wang J, Pol SU, Haberman AK, Wang C, O’Bara MA, Sim FJ. 2014. Transcription factor induction of human oligodendrocyte progenitor fate and differentiation. Proceedings of the National Academy of Sciences 111:E2885–E2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Allie R, Conant K, Haughey N, Turchan-Chelowo J, Hahn K, Rosen A, Steiner J, Keswani S, Jones M and et al. 2006. Granzyme B mediates neurotoxicity through a G-protein-coupled receptor. The FASEB Journal 20:1209–1211. [DOI] [PubMed] [Google Scholar]
- Wang T, Choi E, Monaco MC, Campanac E, Medynets M, Do T, Rao P, Johnson KR, Elkahloun AG, Von Geldern G and et al. 2013. Derivation of neural stem cells from human adult peripheral CD34+ cells for an autologous model of neuroinflammation. PLoS ONE 8:e81720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Choi E, Monaco MC, Major EO, Medynets M, Nath A. 2015. Direct induction of human neural stem cells from peripheral blood hematopoietic progenitor cells. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G 1998. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci 18:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G 2002. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain 125:338–349. [DOI] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M and et al. 2010. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6:578–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jalili F, Ouamara N, Zameer A, Cosentino G, Mayne M, Hayardeny L, Antel JP, Bar-Or A, John GR. 2010. Glatiramer acetate-reactive T lymphocytes regulate oligodendrocyte progenitor cell number in vitro: role of IGF-2. J Neuroimmunol 227:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.