Abstract
Background –
Patch electrocardiographic (ECG) monitors permit extended noninvasive ambulatory monitoring. To guide use of these devices, information is needed about their performance. We sought to determine in a large general population sample the acceptability of patch ECG monitors, the yield of arrhythmia detection, and the consistency of findings in participants monitored twice.
Methods –
In the Multi-Ethnic Study of Atherosclerosis, 1122 participants completed one or two monitoring episodes using the Zio Patch XT, a single-channel ECG patch monitor capable of recording for 14 days. Recordings were analyzed for atrial fibrillation (AF), atrial flutter, atrioventricular block, pauses, and supraventricular and ventricular ectopy.
Results –
The mean(SD) age at the time of monitoring was 75(8) years, 52% were men, and 15% had a prior history of clinically-recognized AF/flutter. The median monitoring duration was 13.8 days. Among 804 participants with no prior clinical history of AF/flutter and at least 12 days of monitoring on a single device, AF/flutter was detected in 32 (4.0%); in 38% of these, AF/flutter was first detected during days 3 through 12 of monitoring. In participants monitored twice, findings from the two devices showed excellent agreement for supraventricular and ventricular ectopic beats per hour, but only fair agreement for high-grade atrioventricular block and pauses of greater than 3 seconds duration.
Conclusions –
In a general population of older individuals, new diagnoses of AF/flutter were made in 4.0% of participants without a prior history. A single monitoring episode accurately estimated rates of supraventricular and ventricular ectopy.
Keywords: ECG screening, atrial fibrillation, atrial flutter, supraventricular ectopy, ventricular ectopy
Introduction
Convenient electrocardiographic (ECG) patch monitors now make it practical to conduct extended noninvasive ambulatory monitoring for durations longer than conventional Holter monitors. The devices permit identification of atrial fibrillation (AF) and atrial flutter and estimation of AF burden, defined as the proportion of monitored time that the cardiac rhythm is AF. They also provide information on heart rate, pauses, high grade atrioventricular block, counts of isolated supraventricular and ventricular ectopic beats, and runs of supraventricular and ventricular tachycardia.
For both researchers and clinicians, it is of interest to understand the yield of AF detection for shorter versus longer monitoring periods. Studies in patients with major clinical indications for monitoring or indications for implanted devices have estimated the yield of AF detection for various monitoring durations and frequencies [1,2]. However, little information is available from a general population about yield and consistency of findings with various monitoring strategies, including ECG patch monitors. In the Multi-Ethnic Study of Atherosclerosis (MESA), we examined in a community-based sample of older individuals: 1) the yield of arrhythmia detection for various ECG monitoring periods up to 12 days, and 2) the consistency of results from two patch monitors worn one shortly after the other by the same person, to determine whether conducting two monitoring episodes of up to 14 days each adds important information beyond a single monitoring episode.
Material and methods
Setting
MESA is designed to investigate the pathogenesis of early cardiovascular disease and its progression. In 2000–2002, MESA enrolled 6814 participants 45–84 years of age and free of clinically-recognized cardiovascular disease from six US communities [3]. Participants self-identified with one of four race/ethnic groups: African-American (28%), white (38%), Hispanic (22%) and Asian, of Chinese descent (12%); 53% were women. After the initial baseline study visit, there have been five additional follow-up visits; the most recent study visit is in 2016–18. At study visits, height, waist circumference, and weight were measured, and smoking, current medications, and physician diagnoses of hypertension, diabetes, and AF were assessed by questionnaire [3]. Blood pressure was measured with the participant in a seated position; serum glucose was measured in a fasting blood sample.
Since baseline, participants have been contacted by telephone every 9–12 months to identify new hospitalizations and medical diagnoses during follow-up. Medical records were obtained for cardiovascular events; myocardial infarction and heart failure were adjudicated by the MESA Morbidity and Mortality Committee. Clinically-recognized AF and atrial flutter were identified by an International Classification of Disease (ICD) code for AF or atrial flutter (version 9: 427.31 or 427.32; version 10: I48) in any position assigned at hospital discharge; by 12-lead electrocardiogram at the 2010–2012 MESA exam; for those enrolled in fee-for-service Medicare, by an inpatient, outpatient, or physician claim with an AF or atrial flutter ICD diagnosis code in any position; or by a participant report of a physician diagnosis of AF. Details of the study protocol and procedures have been described [3].
In a pilot study, self-application by MESA participants of a patch monitor capable of recording for 14 days was found to be feasible and to result in wear time and analyzable time nearly equal to those from devices applied by MESA staff. For self-applied monitors, median wear time was 13.9 days and median analyzable time was 98% of total wear time; for staff-applied monitors; corresponding figures were 13.9 days and 99% [4].
Participants
During the 2016–2018 study visit, we enrolled a subset of MESA participants in an ancillary study designed to determine the prevalence of AF, atrial flutter, and other arrhythmias, and to study these arrhythmias in relation to cardiac and brain structure and function. The present analysis reports on the yield and consistency of arrhythmia detection from ECG monitoring of the first 1,122 MESA participants enrolled in the ancillary study between September 6, 2016 and October 12, 2017; the target enrollment is 1,500. Participants both with and without a prior history of heart disease or clinically-recognized AF were included in the ancillary study; those with a history of clinically-recognized AF were oversampled. Participants were not queried about or selected on the basis of symptoms suggestive of arrhythmia. Individuals with a history of skin allergy to tape or adhesives were excluded. At the 2016–2018 study visit, study staff at each field center applied an ECG patch monitor and asked the participant to wear it for the full 14 days. Study staff made one or more follow-up phone calls during the monitoring period to check on progress and answer participant questions. Participants removed the device at the end of the monitoring period and mailed it to the manufacturer (iRhythm Technologies, Inc, San Francisco, CA) for interpretation. By phone, participants were questioned about their willingness to complete a second monitoring period shortly after the first. A second monitor was mailed to those who agreed and they were asked to self-apply it. Processing and analysis of the ECG data was done by certified technicians at iRhythm and all reported arrhythmias were verified by the Epidemiological Cardiology Reading Center at Wake Forest University School of Medicine, Winston-Salem, NC. Approval for the study was obtained from the institutional review board on human research at each participating institution (University of Washington, Wake Forest University, Columbia University, Johns Hopkins University, University of Minnesota, Northwestern University, and University of California, Los Angeles) and all participants provided written informed consent. The ECG monitoring devices were purchased for the study and the device manufacturer had no role in the study design or statistical analysis. Dr. Heckbert had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
ECG patch monitors
For cardiac rhythm monitoring, we used the Zio Patch XT (iRhythm Technologies, Inc, San Francisco, CA). This device is an FDA-approved single-channel ECG patch monitor with a battery and memory capable of detecting and storing up to 14 days of cardiac rhythm. It does not communicate in real time with anyone; rather, at the end of the recording period, the participant mails the device back to the company, which processes and analyzes the ECG data. Wear time was defined as the interval from activation of the device until removal of the device. Analyzable time was defined as the total time during which the monitor provided a tracing adequate to determine cardiac rhythm. AF was defined as an irregularly irregular rhythm with absent P waves lasting at least 30 seconds. AF burden was quantified as the proportion of the analyzable time that the rhythm was AF. Isolated supraventricular ectopic beats, isolated ventricular ectopic beats, number of runs of ≥4 supraventricular ectopic beats (supraventricular tachycardia), and number of runs of ≥4 ventricular ectopic beats (ventricular tachycardia) were counted.
Measurement of arrhythmia
The total number of isolated supraventricular ectopic beats during the monitoring period was divided by the total analyzable time, and was expressed as the average rate of supraventricular ectopic beats per hour. The average rate of isolated ventricular ectopic beats was calculated similarly. The total number of runs of supraventricular tachycardia during the monitoring period was divided by the total analyzable time, and was expressed as the average rate of runs of supraventricular tachycardia per 24 hours; the average rate of runs of ventricular tachycardia was calculated similarly. The presence of high grade atrioventricular block (2nd degree Mobitz II and 3rd degree block) and pauses of 3 seconds or longer were also reported.
Assessment of participant characteristics
Participant age and history of past clinically-recognized AF were determined at the date the first ECG monitor was activated. For the present analysis, all other participant characteristics were assessed at the 2010–2012 MESA exam. Treated hypertension was defined as use of an antihypertensive medication in combination with self-report of a physician diagnosis of hypertension. Diabetes was defined by use of a diabetes medication or fasting glucose ≥ 126 mg/dL.
Statistical analysis
In analyses of the yield of arrhythmia diagnoses for various lengths of monitoring on a single device (Analysis 1), we included all participants with at least one monitoring period with analyzable time of at least 12 days. In analyses that examined the consistency of findings in participants monitored twice (Analysis 2), we limited consideration to participants who wore two devices with analyzable time of at least 2 days on each device and with an interval between monitoring periods of less than 60 days. In a sensitivity analysis, Analysis 2 was repeated for consistency of supraventricular ectopy and high-grade AV block after excluding 32 participants with any AF or flutter during either monitoring period, because supraventricular ectopy and high-grade AV block are not read during periods when the rhythm is AF/flutter.
The yield of participants with various findings was expressed as a percent. Agreement for binary characteristics was measured with the kappa statistic. For continuous variable characteristics, we examined frequency distributions and scatterplots. For both supraventricular and ventricular ectopy, rates of isolated beats and rates of runs were highly skewed and were ln-transformed for analysis; the smallest nonzero value for each variable was imputed for zero values before ln-transforming the data. Reliability for continuous variable characteristics was measured with the intraclass correlation coefficient, and 95% confidence intervals were calculated around each estimate.
Results
Among MESA participants offered enrollment in the ancillary study, which involved rhythm monitoring, cognitive function testing, and a brain MRI, 76% agreed to participate. During the study period, 1,122 participants completed the rhythm monitoring component of the study; 580 (52%) participants wore two devices. The median wear time for all devices was 14.0 days (interquartile range [IQR] 13.2–14.0 days) and the median analyzable time was 13.8 (IQR 12.8–14.0) days; the median proportion of wear time that was analyzable was 99.6%. The devices were well tolerated; skin irritation was reported in 4% of participants. Included in Analysis 1 were 946 participants (84% of 1,122) with at least 12 days of analyzable time from a single monitoring period. Included in Analysis 2 were 439 participants (39% of 1,122) who had two recording periods of at least 2 days duration on each device and an interval between recording periods of less than 60 days. The median analyzable time per device for all devices in Analysis 2 was 13.8 (IQR 13.1–14.0) days; 98% of participants in Analysis 2 had 15 or more total days of analyzable time for their two devices combined. The mean (SD) interval between monitoring periods was 23 (13) days. Of the 439 participants included in Analysis 2, 423 were also included in Analysis 1.
Table 1 shows the characteristics of participants included in the two analysis groups. In Analysis 1, the mean (SD) age was 75 (8) years, 52% of participants were men, 43% were white, 22% African American, 22% Hispanic, and 13% Chinese. Three percent or fewer had past histories of myocardial infarction or heart failure; 15% had a history of clinically-recognized AF or atrial flutter at the time of rhythm monitoring. In comparison, the prevalence of a history of clinically-recognized AF/flutter in the overall sample of MESA participants who attended the 2016–2018 exam was 10%. The characteristics of participants in Analysis 2 were generally similar to those in Analysis 1, although the proportion of Hispanic participants was lower.
Table 1.
Characteristics of MESA study participants with ECG patch monitoring
| Characteristic | Analysis 1: ≥ 12 days of analyzable time n=946 |
Analysis 2: ≥ 2 days of analyzable time on each of 2 monitors n=439 |
|---|---|---|
| Age at monitoring, yrs, N (%) | ||
| 57–69 | 320 (33.8) | 150 (34.2) |
| 70–79 | 322 (34.0) | 149 (33.9) |
| 80–89 | 278 (29.4) | 127 (28.9) |
| 90–99 | 26 (2.8) | 13 (3.0) |
| Race/ethnicity, N (%) | ||
| White | 409 (43.2) | 229 (52.2) |
| African American | 208 (22.0) | 88 (20.0) |
| Hispanic | 208(22.0) | 69 (15.7) |
| Chinese | 121 (12.8) | 53 (12.1) |
| Male sex, N (%) | 487 (51.5) | 219 (49.9) |
| Current smoking, N (%)* | 71 (7.5) | 32 (7.3) |
| Treated hypertension, N (%)* | 513 (54.2) | 239 (54.4) |
| Systolic blood pressure, mmHg, mean (SD)* | 121.1 (19.1) | 121.4 (19.6) |
| Diastolic blood pressure, mmHg, mean (SD)* | 68.2 (10.1) | 68.8 (10.0) |
| Body mass index, kg/m2, mean (SD) | 28.4 (5.1) | 28.4 (5.2) |
| Diabetes, N (%)* | 166 (17.6%) | 69 (15.7) |
| History of myocardial infarction, N (%)* | 26 (2.8) | 13 (3.0) |
| History of heart failure, N (%)* | 21 (2.2) | 9 (2.0) |
| History of clinically-recognized AF or atrial flutter, N (%) |
142 (15.0) | 69 (15.7) |
Assessed at the 2010–2012 MESA exam
Analysis 1: Yield of diagnoses of atrial fibrillation or atrial flutter
Among all 946 participants in Analysis 1, AF or atrial flutter was detected during the 12 days of monitoring in 69 (7.3%). Among the 69 with AF/flutter detected, the arrhythmia was detected at device activation in 38 and subsequently during the initial 24 hours in 11 (together, 71% of the 69 with AF/flutter), during the second 24 hours in 6 (9%), and during days 3 through 12 of monitoring in the remaining 14 (20%) participants (Figure 1). The overall duration of detected AF/flutter ranged from 30 seconds (the minimum duration required for defining AF) to the full 14 days of monitoring. Among the total of 1419 individual episodes of AF/flutter detected in 34 participants with paroxysmal AF/flutter, 74% lasted less than 6 minutes, and 26% lasted more than 6 minutes.
Figure 1.
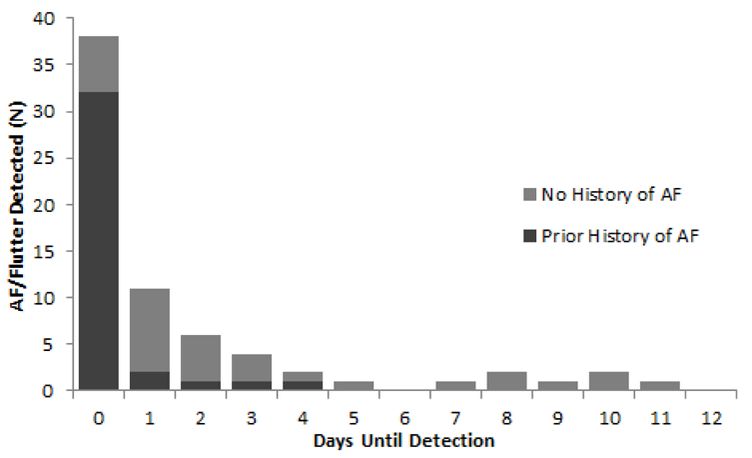
Analysis 1: Day on which atrial fibrillation (AF) or atrial flutter was first detected among 69 MESA participants with either arrhythmia and at least 12 days of analyzable time on an ECG patch monitor, in participants with and without a history of clinically-recognized AF/flutter. Those in the “Day 0” column had AF/flutter detected immediately at device activation, while those in the “Day 1” column had AF/flutter first detected after device activation but during the first 24 hours of monitoring.
Among the 804 participants in Analysis 1 with no prior history of clinically-recognized AF/flutter, 32 (4.0%) had AF/flutter detected during the monitoring period, representing a new diagnosis. Among the 32 with AF/flutter detected, the arrhythmia was detected at device activation or during the initial 24 hours in a total of 15 (47%), during the second 24 hours in 5 (16%), and during days 3–12 of monitoring in 12 (38%) (Figure 1, light gray columns). Among the 142 participants with a prior clinical history of AF, 37 (26%) had AF/flutter on the patch monitor and 105 (74%) had no AF/flutter detected.
Analysis 2: Consistency of results from two patch monitors
For the 439 participants with data from two monitoring episodes in Analysis 2, Table 2 shows the number of participants in whom AF/ flutter, AV block, and pauses greater than 3 seconds were detected and the consistency of findings in participants monitored twice. For AF/flutter, which was present during at least one monitoring episode in 7.3% of participants, the kappa statistic suggested excellent agreement, but for AV block and pauses greater than 3 seconds, which were considerably less common (each present during at least one monitoring episode in 3.0% of participants), the kappa statistics suggested only fair to moderate agreement.
Table 2.
Analysis 2: Among 439 MESA participants with data from 2 ECG patch monitors, number with selected findings on one or both devices, and kappa statistic for agreement in results
| ECG monitor finding | N (%) with finding on at least 1 patch |
N with Finding on both patches |
N with finding on patch 1 but not patch 2 |
N with finding on patch 2 but not patch 1 |
Kappa statistic for any finding vs. none (95% CI) |
|---|---|---|---|---|---|
| Atrial fibrillation/ flutter |
32 (7.3) | 24 | 6 | 2 | 0.85 (0.75–0.94) |
| AV block: 2nd degree Mobitz II and 3rd degree |
13 (3.0) * | 3 | 3 | 7 | 0.36 (0.27–0.45) |
| Pause > 3 seconds |
13 (3.0) | 4 | 5 | 4 | 0.46 (0.37–0.55) |
MESA = Multi-Ethnic Study of Atherosclerosis; ECG = electrocardiographic; CI = confidence interval; AV = atrioventricular
In the sensitivity analysis excluding the 32 participants with AF/flutter on either device, 10 (2.5%) participants had high grade AV block.
Figure 2 shows scatterplots of results for the two monitoring episodes for AF/flutter burden among those with paroxysmal AF/flutter during either monitoring episode, and for rates of isolated supraventricular ectopic beats, runs of supraventricular tachycardia, rates of isolated ventricular ectopic beats, and runs of ventricular tachycardia. Among the 13 participants with paroxysmal AF/flutter during at least one monitoring episode, the overall ICC for AF/flutter burden was −0.02, 95% CI −0.59, 0.53. The remarkably poor consistency was due to one participant with no AF/flutter burden during one monitoring episode and substantial burden during the second. When that individual was excluded, the ICC for the remaining 12 participants with paroxysmal AF/flutter was 0.82, 95% CI 0.25–0.95. Isolated supraventricular ectopic beats and runs of supraventricular tachycardia were present in most participants, and concordance for the two monitoring episodes for rates of these arrhythmias was high (Table 3). Isolated ventricular ectopic beats were also present in almost all participants and concordance was high for the two monitoring episodes, but runs of ventricular tachycardia were considerably less prevalent, and concordance was moderate (Table 3). Results for consistency of findings for supraventricular ectopy and high-grade AV block were not materially different in the sensitivity analysis that excluded the 32 participants with any AF or flutter during either monitoring episode.
Figure 2.
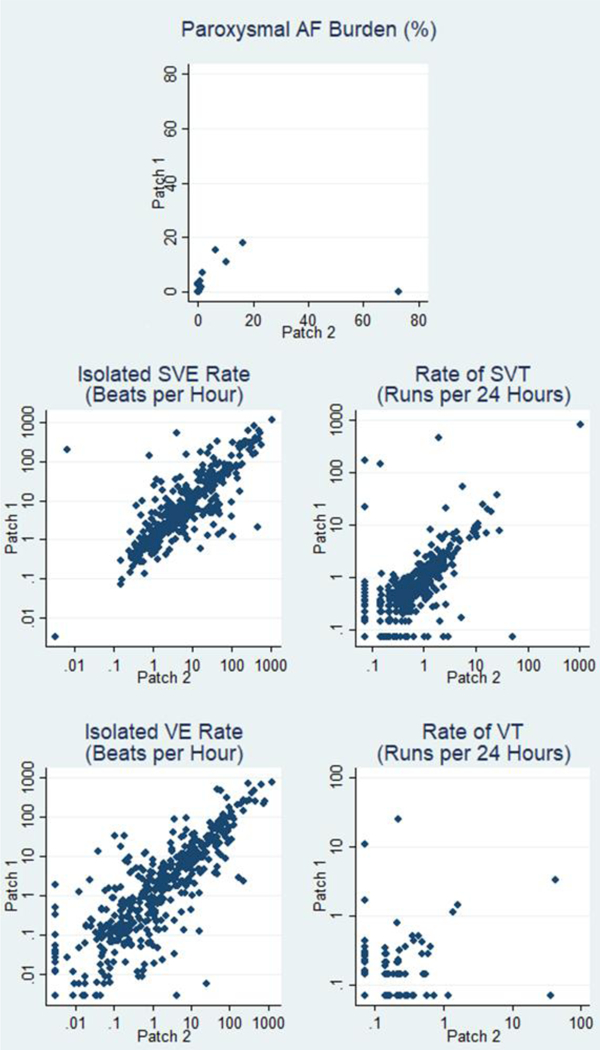
Analysis 2: Scatterplots of findings from two episodes of ECG patch monitoring per participant. (AF = atrial fibrillation; SVE = supraventricular ectopic beat; VE = ventricular ectopic beat; SVT = supraventricular tachycardia; VT = ventricular tachycardia)
Table 3.
Analysis 2: Among 439 MESA participants with data from 2 patch monitors, number and proportion with isolated beats and runs (greater than 4 beats) of supraventricular and ventricular tachycardia, and intraclass correction coefficients for agreement in results
| Measure of ectopy | N (%) of Participants with ectopy on at least 1 patch |
Median (IQR) for the measure of ectopy |
Individual intraclass Correlation coefficient for ln(ectopic rate) (95% CI) |
|---|---|---|---|
| Supraventricular ectopy | |||
| Isolated supraventricular ectopic beats per hour |
423 (96.4)† | 4.4 (1.4–26.2) | 0.88 (0.86–0.90) |
| Runs of supraventricular tachycardia (>4 beats) per 24 hours |
389 (88.6)† | 0.5 (0.2–1.4) | 0.71 (0.66–0.76) |
| Ventricular ectopy | |||
| Isolated ventricular ectopic beats per hour |
437 (99.5) | 1.9 (0.3–12.0) | 0.82 (0.79–0.85) |
| Runs of ventricular tachycardia (>4 beats) per 24 hours |
152 (34.6) | 0.1 (0.0–0.1) | 0.38 (0.29–0.45) |
CI = confidence interval
In the sensitivity analysis excluding the 32 participants with AF/flutter on either device, all 407 (100%) participants had supraventricular ectopic beats, and 377 (92.6%) had any runs of supraventricular tachycardia.
Discussion
In our study of older adults from the general population, 14 to 28 days of monitoring with a patch monitor was feasible, the devices were well-tolerated, and the median wear times and analyzable times were at or near the theoretical maximum of 14 days. With ambulatory ECG monitoring for at least 12 days, AF/flutter was detected in 7.3% of this multi-ethnic population with an average age of 75 years, 15% of whom had a history of prior clinically-recognized AF. Among the 804 participants with no prior clinical history of AF/flutter, 32 (4.0%) had AF/flutter detected during 12 days of monitoring, representing a new diagnosis.
Among the 32 participants with no prior clinical history of AF/flutter in whom a new diagnosis of AF/flutter was made, in 12 (38%) participants the arrhythmia was first detected during days 3 through 12 of monitoring, after the typical duration of Holter monitoring. The longer the monitoring period, the more participants with AF/flutter were identified, but the yield of additional cases per day of additional monitoring declined rapidly over the 12-day period. However, among 142 participants with a history of clinically-recognized AF/flutter, almost three quarters had no AF/flutter detected during at least 12 days of continuous monitoring, indicating that in population studies of AF prevalence, even extended ECG monitoring fails to identify a substantial proportion of those who have experienced clinical episodes of AF, and should be supplemented with investigation of medical history.
Among participants with two monitoring periods, AF/flutter burden was generally highly correlated on the two devices, with the notable exception of a single individual who had no AF/flutter on one device and substantial AF/flutter burden on the other. Rates of isolated supraventricular and ventricular ectopic beats per hour and of runs of supraventricular tachycardia were highly correlated for the two monitoring periods, while the rate of runs of ventricular tachycardia was moderately correlated. Our findings suggest that longer periods of monitoring and/or repeated monitoring periods are needed to increase sensitivity for identifying AF/flutter, high degree AV block and pauses, but that a single monitoring episode may suffice for estimating rates of supraventricular and ventricular ectopy and is adequate for studies designed to assess associations with future cardiovascular events.
In participants who wore two devices, the two patches monitored different time periods with a mean interval of 23 days between monitoring periods. Therefore, exact agreement on arrhythmia findings from the two devices would not be expected. The analyses examining concordance of findings between the two devices are thus intended to reveal the extent to which findings agree over a relatively short time interval and to inform decisions about how much additional clinical information is obtained with two versus a single monitoring episode.
Strengths of this study include the large number of participants representative of the race/ethnic diversity of Americans, the excellent analyzable time achieved with the monitoring devices, the previously demonstrated accuracy of the Zio Patch XT device compared with Holter monitoring [5,6], and the extensive, high quality information on pre-existing cardiovascular disease and risk factors. Our analysis is limited in its ability to determine the true prevalence of relatively infrequent arrhythmias including AF/flutter, high grade AV block, and pauses, which can be fully ascertained only with implanted devices. However, implanted devices are too invasive for use in a general population study.
Our findings for AF/flutter detection with increased duration of monitoring are in agreement with previous studies using implanted or inserted devices [1,7,8] and noninvasive devices [2,9,10] conducted in clinical populations. Like these studies, we found that longer duration of monitoring identified more participants with AF/flutter, but that the yield of new cases per day of additional monitoring decreased steadily over time.
Limited information is available from general population studies about the consistency of estimates of supraventricular and ventricular ectopy with extended or repeat recording. A previous analysis of data from two 48-hour Holter monitors worn an average of 38 days apart in the Atherosclerosis Risk in Communities study showed high agreement for PAC and PVC counts per hour when repeated recording periods of 3 hours were considered (ICCs 0.80 and 0.74, respectively) and even better agreement for repeated recording periods of 24 hours (ICCs 0.84 and 0.84, respectively) [11]. Our findings extend these observations to longer recording periods, demonstrating very high consistency in findings for two recording periods of an average of 12 days each (ICCs 0.88 and 0.82, respectively). In addition, our analysis provided information on consistency for runs of supraventricular tachycardia and ventricular tachycardia, and for less frequent arrhythmias including AF/flutter, high grade AV block, and pauses of greater than 3 seconds.
Conclusion
In an older general population sample, use of a 14-day patch monitor was feasible and provided analyzable rhythm data during nearly all of the wear time. The yield of AF/flutter increased with longer monitoring time and 38% of participants with newly-diagnosed AF/flutter had the arrhythmia first detected during days 3 through 12 of monitoring. New diagnoses of AF/flutter were made in 4.0% of participants who had no history of the arrhythmia, but in participants with a history of clinically-recognized AF/flutter, no AF/flutter was detected during 12 days of monitoring in 74%. A single monitoring episode of 12 days was adequate for estimating the extent of supraventricular and ventricular ectopy, but longer monitoring periods may be required to reliably estimate the prevalence of less common arrhythmias including high grade AV block and pauses of greater than 3 seconds.
Highlights.
In an epidemiologic study (n=1122), 14-day patch ECG monitoring was feasible.
Atrial fibrillation or flutter (AF) was detected in 4% of those with no prior history.
38% of newly-detected AF was first found on days 3–14 of monitoring.
In participants monitored twice, agreement was excellent for ectopic beats per hour.
Acknowledgments:
None.
Funding: This research was supported by the National Heart, Lung, and Blood Institute [contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169, and grant R01 HL127659]; and the National Center for Advancing Translational Sciences [grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Susan R. Heckbert, MD, PhD: Research Grant from NIH
Thomas R. Austin, MPH: Research Grant from NIH
Paul N. Jensen, PhD: Research Grant from NIH
James S. Floyd, MD, MS: None
Bruce M. Psaty, MD, PhD: Research Grant from NIH; serves on the DSMB of a clinical trial funded by Zoll LifeCor and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson
Elsayed Z. Soliman, MD, MSc, MS: Research Grant from NIH
Richard A. Kronmal, PhD: Research Grant from NIH
References
- [1].Charitos EI, Stierle U, Ziegler PD, Baldewig M, Robinson DR, Sievers HH, et al. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation 2012;126:806–14. [DOI] [PubMed] [Google Scholar]
- [2].Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. [DOI] [PubMed] [Google Scholar]
- [3].Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- [4].Chen LY, Roetker NS, Folsom AR, Alonso A, and Heckbert SR. Feasibility of using a leadless patch heart rhythm monitor to measure atrial fibrillation burden in community-based epidemiological studies: The Multi-Ethnic Study of Atherosclerosis. American Heart Association Scientific Sessions, Orlando FL: Circulation 2015. (abstract);132:A11721. [Google Scholar]
- [5].Rosenberg MA, Samuel M, Thosani A, and Zimetbaum PJ. Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing Clin Electrophysiol 2013;36:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, et al. Comparison of 24-hour Holter Monitoring with 14-day Novel Adhesive Patch Electrocardiographic Monitoring. Am J Med 2014;127:95.e11–95.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- [8].Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. [DOI] [PubMed] [Google Scholar]
- [9].Tung CE, Su D, Turakhia MP, and Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2014;5:266. doi:10.3389/fneur.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Turakhia MP, Hoang DD, Zimetbaum P, Miller JD, Froelicher VF, Kumar UN, et al. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol 2013;112:520–4. [DOI] [PubMed] [Google Scholar]
- [11].Meyer ML, Soliman EZ, Wruck LM, Mosley TH, Wagenknecht LE, Poon AK, et al. Repeatability of ectopic beats from 48-hr ambulatory electrocardiography: The Atherosclerosis Risk in Communities (ARIC) Study. Ann Noninvasive Electrocardiol 2017;22 10.1111/anec.12426 [DOI] [PMC free article] [PubMed]


