Abstract
α-Vinyl or α-acetylenyl azacycles were easily synthesized from 7- to 9-membered lactams and 6- to 9-membered lactams via N,O-acetal trimethylsilyl (TMS) ethers. Organocopper and organostannane reagents afforded reasonable yields for the respective N-acyliminium ion vinylation and acetylenylation intermediates generated from N,O-acetal TMS ethers in the presence of a Lewis acid.
Keywords: medium-sized lactam, amidoalkylation, α-vinyl or α-acetylenyl azacycles
1. Introduction
The conversion of lactams to their corresponding azacycles is an efficient route for the preparation of functionalized azacycles. It involves amide bond manipulations, however, the nucleophilic addition to amides is limited due to the poor electrophilicity of amide carbonyls. Therefore, α-amidoalkylation of reactive iminium ions derived from amides is an effective method for providing azacycles with scaffold diversity [1,2]. Many 5- and 6-membered lactams and azacycles exist in Nature, and the conversion of these lactams via α-amidoalkylation has been extensively studied. For instance, approaches involving thioamides [3,4,5], sulfonyl amides [6], N-alkoxylactams [7], formamidines [8], enamine triflates/phosphates [9,10] and N,O-acetals [11,12,13] have been developed for conversion to α-vinyl and α-acetylenyl azacycles. Moreover, these methods have been used for the preparation of key intermediates for the assembly of structurally diverse azacycles, ranging from a simple bicyclic to complex polycyclic systems [1] in combination with various synthetic methods including metathesis [6] and aza-Claisen rearrangements [14]. Recent developments in organometallic chemistry also have enabled concise and efficient synthesis of α-vinyl and α-acetylenyl azacycles from cycloalkylamines as surrogates for lactams via direct C–H activation [15] and photoredox reactions [16]. However, most of the reported methods have been limited to the synthesis of 5- and 6-membered azacycles. In contrast, the conversion of medium- to macrocyclic lactams to their corresponding azacycles has not been extensively investigated. Thus, a lack of concise and efficient methods for such conversions has been a major challenge in the synthesis of azacycles in which the high ring strain energy might give rise to undesirable ring openings. Conventional methods generate unstable intermediates from medium- to macrocyclic lactams, resulting in imines or enamines which would be readily hydrolyzed to the corresponding amino aldehyde [17]. In addition, a series of synthetic steps were required for the preparation of medium-sized 2-vinylazacycles even after extensive attempts to apply the reported methods [14]. In this regard, there is growing demand for efficient methods to convert medium-sized lactams to their corresponding azacycles with α-vinyl or α-acetylenyl functionality. Herein, we report a novel synthetic method for the conversion of medium-sized lactam rings to α-vinyl and α-acetylenyl azacycles using N,O-acetal trimethylsilyl (TMS) ethers as N-acyliminium ion precursors.
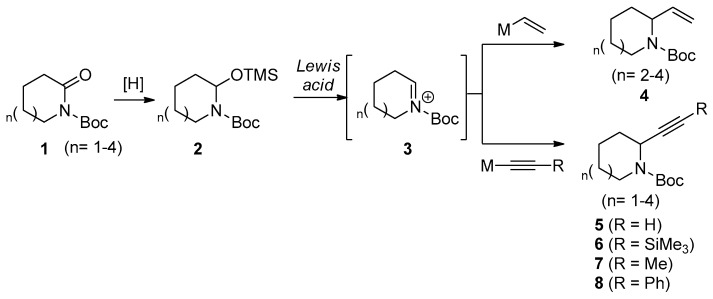
The conversion of lactams to α-substituted azacycles involves the partial reduction and addition of a given nucleophile. The order of the two reactions is likely an important factor. For the conversion of medium-sized lactams, the introduction of a vinyl or acetylene nucleophile after the partial reduction of the lactam is likely to be more effective than the reverse sequence because of ring opening at the hemiaminal intermediate of medium-sized lactam. Considering the stability of the hemiaminal intermediate, N,O-acetal TMS ethers have been shown to be excellent precursors for reactions with medium- to macrocyclic azacycles that proceed via intermediate N-acyliminium ions [17]. Thus, we expected that α-vinyl and α-acetylenyl azacycles 4 and 5 could be prepared using N,O-acetal TMS ether 2 with proper nucleophiles via N-acyliminium ions 3 in the presence of a Lewis acid, as shown in Scheme 1.
Scheme 1.
Conversion of medium-sized lactams to α-vinyl and α-acetylenyl azacycles via N,O-acetal TMS ethers.
2. Results and Discussion
2.1. Preparation of N,O-Acetal TMS Ether
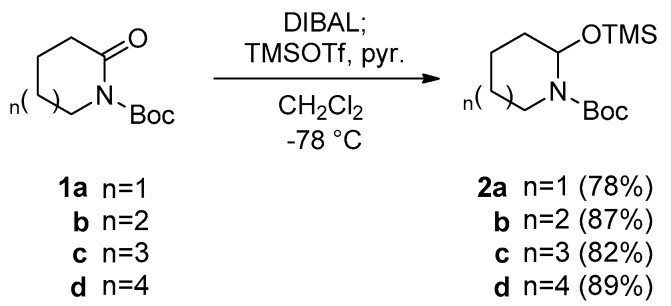
Our work commenced with the preparation of N,O-acetal TMS ethers. The requisite N,O-acetal TMS ethers were prepared from 6- to 9-membered lactams by using previously reported procedures [18], as shown in Scheme 2. In short, protected lactams 1a–d were reduced with DIBAL, and trapping of the resulting N,O-acetal with trimethylsilyl triflate (TMSOTf) afforded the desired N,O-acetal TMS ethers 2a–d in high yields.
Scheme 2.
Preparation of N,O-acetal TMS ethers [16].
2.2. Optimization of α-Vinylation
The vinylation of the 9-membered N,O-acetal TMS ether 2d was performed using various vinyl metals as nucleophiles. As shown in Table 1, reaction of 2d with the vinylcopper reagent derived from vinylmagnesium bromide and CuBr·SMe2 in the presence of BF3 OEt2 afforded the desired product 4d, while the use of vinyllithium or vinylsilane resulted in a mixture of side products. The yield was considerably higher in tetrahydrofuran than in diethyl ether solvent. Other copper salts such as CuI and CuOTf could not be used instead of CuBr·SMe2. The coordinating characteristics of the anion that accompanies the Cu(I) ion might be attributed to the reaction. Under the same conditions, the vinylcopper reagent was reacted with N,O-acetal TMS ethers of the 7- and 8-membered azacycles 2b and 2c affording the α-vinylated adducts 4b and 4c, respectively. The N,O-acetal TMS ether 2a did not undergo vinylation with the vinylcopper reagent. Previously, Neipp and Martin reported that an organocopper reagent derived from vinylmagnesium bromide could not produce a vinyl adduct from the 6-membered cyclic precursor of the N-acyliminium ion [6], whereas Collado et al. reported the highly efficient addition of Grignard-derived organocopper reagents to 5-membered cyclic N,O-acetals.
Table 1.
Vinylation of N,O-acetal TMS ethers.
| Entry a | n | M | Additive | Lewis Acid | Solvent | Yield b |
|---|---|---|---|---|---|---|
| 1 | 4 | Li | BF3·OEt2 | THF | ND c | |
| 2 | 4 | SiMe3 | BF3·OEt2 | CH2Cl2 | ND c | |
| 3 | 4 | MgBr | BF3·OEt2 | THF | ND c | |
| 4 | 4 | MgBr | CuBr·SMe2 | BF3·OEt2 | THF | 63 |
| 5 | 4 | MgBr | CuBr·SMe2 | BF3·OEt2 | Et2O | 32 |
| 6 | 4 | MgBr | CuI | BF3·OEt2 | THF | ND c |
| 7 | 4 | MgBr | CuOTf | BF3·OEt2 | THF | ND c |
| 8 | 3 | MgBr | CuBr·SMe2 | BF3·OEt2 | THF | 60 |
| 9 | 2 | MgBr | CuBr·SMe2 | BF3·OEt2 | THF | 65 |
| 10 | 1 | MgBr | CuBr·SMe2 | BF3·OEt2 | THF | ND c |
a All reactions were performed using 0.3 mmol of 2 in dry solvent. See, Experimental section for a representative procedure. b Isolated yields. c Not detected.
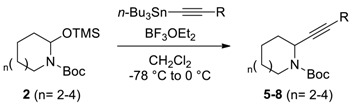
2.3. Optimization α-Acetylenylation
Next, various acetylene nucleophiles with N,O-acetal TMS ether 2d were reacted to afford α-acetylenyl azacycle 5d. As shown in Table 2, the organocopper, organomagnesium, and organolithium reagents yielded unsuccessful results (entries 1–3, respectively). Although the use of trimethylsilyl cyanide (TMSCN) as a nucleophile for the reaction of N,O-acetal TMS ethers to afford α-cyanated cycloalkylamines is reported by Suh et al. [17], the use of trimethylsilylacetylene could not provide the desired α-acetylenyl azacycle 5d (Entry 4). After extensive efforts, an organotin reagent was successful for the synthesis of α-acetylenyl azacycle 5d from N,O-acetal TMS ether 2d in the presence of a Lewis acid. Various solvents including dichloromethane, toluene, and diethyl ether except tetrahydrofuran (entries 5–8) were tolerable for the reaction. It was reported that tetrahydrofuran was not suitable for some reactions of N,O-acetal TMS ethers [19], even though tetrahydrofuran was the best solvent for vinylation of N,O-acetal TMS ethers in Table 1. BF3·OEt2 and TMSOTf were effective Lewis acids for the generation of the N-acyliminium ion, which readily reacted with the organotin reagent to afford the desired adduct 5d (entries 5,11). The treatment of N,O-acetal TMS ether 2d with the organotin reagent in the presence of SnCl4 or TiCl4 afforded a mixture of unidentified and possibly decomposed side products.
Table 2.
Optimization of the acetylenylation conditions for N,O-acetal TMS ethers.
| Entry a | M | Additive | Lewis acid | Solvent | Yield b |
|---|---|---|---|---|---|
| 1 | MgBr | CuBr·SMe2 | BF3·OEt2 | THF | ND c |
| 2 | MgBr | BF3·OEt2 | THF | ND c | |
| 3 | Li | BF3·OEt2 | THF | ND c | |
| 4 | SiMe3 | BF3·OEt2 | CH2Cl2 | ND c | |
| 5 | n-Bu3Sn | BF3·OEt2 | CH2Cl2 | 58 | |
| 6 | n-Bu3Sn | BF3·OEt2 | THF | ND c | |
| 7 | n-Bu3Sn | BF3·OEt2 | PhCH3 | 37 | |
| 8 | n-Bu3Sn | BF3·OEt2 | Et2O | 29 | |
| 9 | n-Bu3Sn | SnCl4 | CH2Cl2 | ND c | |
| 10 | n-Bu3Sn | TiCl4 | CH2Cl2 | ND c | |
| 11 | n-Bu3Sn | TMSOTf | CH2Cl2 | 46 |
a All reactions were performed using 0.2 mmol of 2d in dry solvent (2 mL) by adding the additive (if any) at −50 °C. The nucleophile was then added at the same temperature followed by the addition of BF3·OEt2 at −78 °C and stirring for 1 h. The reaction mixture was slowly warmed to r.t. over 2 h before quenching with Et3N. b Isolated yields. c Not detected.
After optimizing the reaction conditions, we explored the scope of the reaction by using various substituted acetylenes. The required stannylacetylene reagents were purchased from commercial vendors or prepared as previously reported [20]. As shown in Table 3, N,O-acetal TMS ether 2 derived from 6- to 9-membered lactams 1 were easily reacted with various substituted stannylacetylenes to afford the corresponding α-acetylenyl azacycles 5–8 in reasonable yields.
Table 3.
Acetylenylation of N,O-acetal TMS ethers.
| Entry a | n | R | Yield b |
|---|---|---|---|
| 1 | 1 | H | 30 |
| 2 | 1 | SiMe3 | 29 |
| 3 | 1 | Me | 35 |
| 4 | 1 | Ph | 31 |
| 5 | 2 | H | 28 |
| 6 | 2 | SiMe3 | 38 |
| 7 | 2 | Me | 89 |
| 8 | 2 | Ph | 88 |
| 9 | 3 | H | 84 |
| 10 | 3 | SiMe3 | 36 |
| 11 | 3 | Me | 82 |
| 12 | 3 | Ph | 68 |
| 13 | 4 | H | 58 |
| 14 | 4 | SiMe3 | 64 |
| 15 | 4 | Me | 80 |
| 16 | 4 | Ph | 81 |
a All reactions were performed using 0.2 mmol of 2a–d in dry solvent (2 mL) by first adding the nucleophile at −78 °C followed by adding BF3·OEt2 and stirring for 1 h. The mixture was then slowly warmed to r.t. over 1 h before quenching with Et3N. b Isolated yields.
Various substituents including hydrogen, alkylsilane, alkyl, and aryl groups were well tolerated. For the 7- to 9-membered cyclic N,O-acetal TMS ethers, stannylacetylenes with electron donating groups, including Me or Ph, efficiently provided the desired α-acetylenyl azacycles in high yields. The chemical yield was notably lower in reactions for the 6-membered azacycles (entries 1–4) compared to the others. Enamine formation might be attributed to the unexpected low yield in those cases, and which was supported by the isolation of N-Boc-1,2,3,4-tetrahydropyridine as a major side product. Spectral data of the isolated N-Boc-1,2,3,4-tetrahydropyridine was identical to the reported data [21].
3. Materials and Methods
3.1. General Information
Unless noted otherwise, all starting materials and solvents were used as obtained from commercial suppliers without further purification. Organic solvents used in this study were dried over appropriate drying agents and distilled prior to use. Thin layer chromatography was carried out using Merck silica gel 60 F254 plates (Merck, Kenilworth, NJ, USA), and flash chromatography was performed automatically with Isolera (Biotage, Uppsala, Sweden) or manually using Merck silica gel 60 (0.040–0.063 mm, 230–400 mesh, Merck, Kenilworth, NJ, USA). 1H- and 13C-NMR spectra were recorded using JEOL-500 (JEOL, Tokyo, Japan) and AVANCE-500 (Brucker, Billerica, MA, USA) spectrometers. 1H- and 13C-NMR chemical shifts are reported in parts per million (ppm) relative to TMS, with the residual solvent peak used as an internal reference. 1H-NMR data were reported in the order of chemical shift, multiplicity (br, broad signal; s, singlet; d, doublet; t, triplet; q, quartet; quint, quintet; m, multiplet and/or multiple resonances), number of protons, and coupling constant in Hertz (Hz). Infrared spectra were recorded on Cary 630 FT-IR spectrometer (Agilent, Santa Clara, CA, USA). Low- and high-resolution mass spectra were obtained with JMS-700 (JEOL, Tokyo, Japan) and Q TOF 6530 (Agilent, Santa Clara, CA, USA) instruments.
3.2. Synthesis
3.2.1. Representative Procedure: Synthesis of tert-Butyl 2-vinylazonane-1-carboxylate (4d)
To a solution of CuBr·Me2S (308 mg, 1.5 mmol) in THF (4 mL) was added vinylmagnesium bromide (1.0 M solution in THF, 1.5 mL) at −50 °C and the mixture was slowly warmed to −40 °C for 30 min. Then, the reaction mixture was cooled to –78 °C and neat BF3·OEt2 (185 μL, 1.5 mmol) was added. After being kept at −78 °C for 5 mins, N,O-acetal TMS ether 2d (95 mg, 0.3 mmol) in THF (5 mL) was transferred via cannula into the reaction mixture. The mixture was stirred at −78 °C for 3 h and then allowed to warm to room temperature over 2 h. The reaction was diluted with saturated aqueous NH4Cl (10 mL) and was extracted with Et2O. The organic extracts were dried over MgSO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (EtOAc:n-hexane = 1:20) to afford the desired product 4d (48 mg, 0.19 mmol). Yield 63%, colorless oil; 1H-NMR (CDCl3) δ 5.71 (m, 1H), 5.05–4.93 (m, 2H), 4.57 and 4.36 (m, 1H), 3.47 (m, 1H), 2.79 (m, 1H), 1.85 (m, 2H), 1.83–1.48 (m, 19H); 13C-NMR (CDCl3) δ 139.08, 138.96, 114.18, 79.30, 77.60, 31.95, 30.34, 29.72, 29.38, 28.58, 27.36, 27.05, 26.97, 24.25, 24.05, 22.71, 14.14; FT-IR (thin film, neat) νmax 2924, 1692, 1455, 1403 cm−1; HRMS (ESI+) found 198.1489 [calculated for C11H20NO2 ([M + H − C4H8]+): 198.1488].
The following compounds were similarly prepared (Supplementary Materials):
tert-Butyl 2-vinylazepane-1-carboxylate (4b) Yield 65%, colorless oil; 1H-NMR (CDCl3) δ 5.71 (m, 1H), 5.01–4.95 (m, 2H), 4.59 and 4.34 (m, 1H), 3.86 and 3.66 (m, 1H), 2.65 (dd, 1H, J = 14.1, 11.4 Hz), 2.04 (m, 1H), 1.79–1.20 (m, 7H), 1.45 and 1.36 (s, 9H); 13C-NMR (CDCl3) δ 155.91, 138.60, 138.31, 112.84, 112.81, 79.18, 79.11, 57.88, 56.54, 42.33, 41.79, 33.94, 33.59, 29.74, 29.71, 29.25, 29.14, 28.56, 28.50, 25.27, 24.88; FT-IR (thin film, neat) νmax 2927, 1692, 1408 cm−1; HRMS (ESI+) found 170.1176 [calculated for C9H16NO2 ([M + H − C4H8]+): 170.1174].
tert-Butyl 2-vinylazocane-1-carboxylate (4c) Yield 60%, colorless oil; 1H-NMR (500 MHz, CDCl3) δ 5.70–5.67 (m, 1H), 5.02–4.93 (m, 2H), 4.61 and 4.37 (m, 1H), 3.57–3.26 (m, 1H), 2.82 (m, 1H) 2.04–1.41 (m, 19H); 13C NMR (125 MHz, CDCl3) δ 155.98, 139.00, 138.77, 138.72, 138.58, 114.45, 114.29, 113.39, 79.18, 79.12, 78.99, 78.82, 58.27, 56.96, 41.92, 33.95, 30.56, 29.74, 29.27, 28.65, 28.62, 28.54, 28.05, 27.90, 27.16, 26.85, 26.77, 26.72, 26.48, 26.34, 26.22, 24.76, 24.44, 24.33; FT-IR (thin film, neat) νmax 2928, 1693, 1475, 1403 cm−1; MS (EI+) m/z 254 [M + H]+; HRMS (FAB+) found 240.1968 [calculated for C14H25NO2 ([M + H]+): 240.1964].
3.2.2. Representative Procedure: Synthesis of tert-Butyl 2-ethynylazonane-1-carboxylate (5d)
To a stirred solution of N,O-acetal TMS ether 2d (63 mg, 0.2 mmol) in CH2Cl2 (2 mL) was added ethynyltributylstannane (126 mg, 0.4 mmol) and BF3·OEt2 (25 μL, 0.2 mmol) at −78 °C and stirred for additional 30 min. The reaction mixture was slowly warmed to r.t. and Et3N was added to quench the reaction. The reaction mixture was filtered off insoluble precipitate and purified by flash silica gel column chromatography (EtOAc:n-hexane = 1:20) to afford the desired product 5d (29 mg, 0.115 mmol). Yield 58%, a colorless oil; 1H-NMR (CDCl3) δ 5.05 and 4.79 (m, 1H), 3.57 and 3.51 (m, 1H), 3.07 (m, 1H), 2.23 (m, 1H), 1.95–1.25 (m, 12H), 1.48 (s, 9H); 13C-NMR (CDCl3) δ 156.14, 155.10, 83.44, 83.25, 80.01, 79.92, 70.88, 70.79, 48.98, 47.93, 44.73, 43.92, 32.47, 31.58, 29.71, 28.53, 21.17, 26.62, 26.45, 25.20, 25.09, 24.92, 24.67, 24.60, 24.51; IR (ATR-IR) νmax 3300, 2919, 1687, 1399, 1162 cm−1; HRMS (ESI+) found 196.1336 [calculated C11H18NO2 ([M + H − C4H8]+): 196.1332].
The following compounds were similarly prepared (Supplementary Materials):
tert-Butyl 2-ethynylpiperidine-1-carboxylate (5a) Yield 30%, a colorless oil; 1H-NMR (CDCl3) δ 5.06 (brs, 1H), 3.90 (d, J = 9.8 Hz, 1H), 2.99 (brs, 1H), 2.24 (s, 1H), 1.79–1.70 (m, 2H), 1.65–1.59 (m, 4H), 1.44 (s, 9H); 13C-NMR (CDCl3) δ 154.64, 82.16, 80.06, 84.13, 71.90, 30.44, 28.47, 25.31, 19.90; IR (ATR-IR) νmax 3307, 2862, 2331, 1691, 1319, 1251, 1159, 921 cm−1; HRMS (ESI+) found: 154.0863 [calculated C8H12NO2 ([M + H − C4H8]+): 154.0869].
tert-Butyl 2-ethynylazepane-1-carboxylate (5b) Yield 28%, a yellow oil; 1H-NMR (500 MHz, CDCl3) δ 4.97 and 4.72 (m, 1H), 3.84 and 3.69 (d, J = 14.4 Hz, 1H), 3.00 (m, 1H), 2.22 (s, 1H), 2.17 (m, 1H), 1.66–1.24 (m, 7H), 1.46 (s, 9H); 13C-NMR (125 MHz, CDCl3) δ 155.34, 154.84, 84.22, 84.13, 79.92, 79.73, 70.34, 70.22, 47.36, 46.15, 42.54, 42.06, 35.54, 28.81, 28.75, 28.47, 28.37, 28.34, 24.81, 24.28; IR (ATR-IR) νmax 3297, 2925, 1686, 1400, 1162 cm−1; HRMS (ESI+) found 168.1020 [calculated C9H14NO2 ([M + H − C4H8]+): 168.1019].
tert-Butyl 2-ethynylazocane-1-carboxylate (5c) Yield 84%, a colorless oil; 1H-NMR (500 MHz, CDCl3) δ 5.00 and 4.74 (m, 1H), 3.62 and 3.54 (m, 1H), 3.12 (m, 1H), 2.21 and 2.19 (d, J = 2.25 and 2.30 Hz, 1H), 1.89–1.85 (m, 3H), 1.67–1.25 (m, 7H), 1.47 (s, 9H); 13C-NMR (125 MHz, CDCl3) δ 155.45, 154.59, 83.57, 83.55, 79.88, 79.78, 70.33, 47.98, 46.83, 42.50, 42.20, 31.89, 31.32, 28.52, 28.50, 27.63, 26.85, 26.59, 26.49, 25.12, 24.72, 24.17, 23.52; IR (ATR-IR) νmax 3299, 2924, 1686, 1400, 1157 cm−1; HRMS (ESI+) found: 182.1179 [calculated C10H16NO2 ([M + H − C4H8]+): 182.1176].
tert-Butyl 2-((trimethylsilyl)ethynyl)piperidine-1-carboxylate (6a) Yield 29%, a colorless oil; 1H-NMR (CDCl3) δ 5.04 (s, 1H), 3.90 (d, J = 12.1 Hz, 1H), 3.01 (m, 1H), 1.75–1.46 (m, 13H), 0.16 (s, 9H); 13C-NMR (CDCl3) δ 154.80, 104.29, 88.63, 80.00, 45.21, 40.46, 30.68, 28.56, 28.50, 25.48, 20.07, 0.19; IR (ATR-IR) νmax 2954, 2163, 1678, 1404, 1249, 1147, 834 cm−1; HRMS (ESI+) found 226.1263 [calculated for C11H20NO2Si ([M + H − C4H8]+): 226.1258].
tert-Butyl 2-((trimethylsilyl)ethynyl)azepane-1-carboxylate (6b) Yield 38%, a colorless oil; 1H-NMR (CDCl3) δ 5.01 and 4.72 (m, 1H), 3.83 and 3.68 (d, J = 14.7 and 14.8 Hz, 1H), 3.04 (m, 1H), 2.09 (m, 1H), 1.67–1.32 (m, 7H), 1.47 (s, 9H), 0.15 (s, 9H); 13C-NMR (CDCl3) δ 155.38, 155.09, 106.15, 87.04, 86.70, 79.87, 79.66, 48.44, 46.97, 42.58, 42.18, 36.00, 35.50, 28.65, 28.58, 28.12, 25.05, 24.24, 0.12; IR (ATR-IR) νmax 2931, 2174, 1689, 1161, 841 cm−1; HRMS (ESI+) found 240.1427 [calculated C12H22NO2Si ([M + H − C4H8]+): 240.1414].
tert-Butyl 2-((trimethylsilyl)ethynyl)azocane-1-carboxylate (6c) Yield 36%, a colorless oil; 1H-NMR (CDCl3) δ 5.01 and 4.70 (m, 1H), 3.55 (m, 1H), 3.14 (m, 1H), 1.89–1.81 (m, 3H), 1.73–1.44 (m, 10H), 1.47 (s, 9H), 0.14 and 0.13 (s, 9H); 13C-NMR (CDCl3) δ 155.50, 154.82, 87.05, 86.88, 79.84, 79.71, 48.92, 47.72, 42.83, 42.29, 32.33, 32.03, 31.71, 28.63, 28.61, 27.69, 27.51, 26.98, 26.86, 26.75, 25.00, 24.39, 23.53, 0.13, −0.17; IR (ATR-IR) νmax 2928, 2169, 1687, 846 cm−1; HRMS (ESI+) found 254.1573 [calculated C13H24NO2Si ([M + H − C4H8]+): 254.1571].
tert-Butyl 2-((trimethylsilyl)ethynyl)azonane-1-carboxylate (6d) Yield 64%, a colorless oil; 1H-NMR (CDCl3) δ 5.04 and 4.72 (m, 1H), 3.51 (m, 1H), 3.15 and 3.08 (m, 1H), 1.82–1.44 (m, 21H), 0.12 (s, 9H); 13C-NMR (CDCl3) δ 156.20, 155.33, 105.40, 87.46, 79.85, 49.98, 48.89, 43.94, 32.83, 31.96, 28.64, 27.28, 26.64, 26.58, 25.94, 24.95, 24.89, 24.78, 0.12; IR (ATR-IR) νmax 2921, 1689, 1162, 847 cm−1; HRMS (ESI+) found 268.1731 [calculated C14H26NO2Si ([M + H − C4H8]+): 268.1727].
tert-Butyl 2-(prop-1-yn-1-yl)piperidine-1-carboxylate (7a) Yield 35%, a colorless oil; 1H-NMR (CDCl3) δ 5.01 (s, 1H), 3.89 (d, J = 11.2 Hz, 1H), 3.03 (t, J = 12.3 Hz, 1H), 1.81 (d, J = 2.3, 3H), 1.67–1.57 (m, 6H), 1.44 (s, 9H); 13C-NMR (CDCl3) δ 154.74, 79.78, 79.61, 44.41, 40.39, 31.02, 28.57, 25.55, 20.12, 3.64; IR (ATR-IR) νmax 2932, 1689, 1401, 1258, 1155, 1028, 861 cm−1; HRMS (ESI+) found: 168.1018 [calculated for C9H14NO2 ([M + H − C4H8]+) 168.1019].
tert-Butyl 2-(prop-1-yn-1-yl)azepane-1-carboxylate (7b) Yield 89%, a yellow oil; 1H-NMR (CDCl3) δ 4.90 and 4.68 (m, 1H), 3.81 and 3.66 (d, J = 14.6 Hz, 1H), 2.99 (m, 1H), 2.09 (m, 1H), 1.78 (m, 3H), 1.62–1.27 (m, 7H), 1.45 (s, 9H); 13C-NMR (CDCl3) δ 155.49, 155.04, 79.66, 79.50, 79.44, 79.35, 78.06, 47.72, 46.53, 42.44, 42.01, 36.18, 36.13, 28.92, 28.86, 28.60, 28.40, 28.25, 24.83, 24.37, 3.72, 3.58; IR (ATR-IR) νmax 2925, 1686, 1401, 1161, 976 cm−1; HRMS (ESI+) found 182.1174 [calculated C10H16NO2 ([M + H − C4H8]+): 182.1176].
tert-Butyl 2-(prop-1-yn-1-yl)azocane-1-carboxylate (7c) Yield 82%, a colorless oil; 1H-NMR (CDCl3) δ 4.92 and 4.68 (m, 1H), 3.58 and 3.50 (m, 1H), 3.08 (m, 1H), 1.76–1.44 (m, 22H); 13C-NMR (CDCl3) δ 155.58, 154.77, 79.59, 79.52, 78.92, 78.83, 78.17, 78.03, 48.35, 47.22, 42.44, 42.13, 32.43, 31.75, 28.63, 27.78, 26.94, 26.85, 26.68, 25.03, 24.79, 24.21, 23.62, 3.63; IR (ATR-IR) νmax 2924, 1685, 1467, 1400, 1156, 934 cm−1; HRMS (ESI+) found 196.1341 [calculated C11H18NO2 ([M + H − C4H8]+): 196.1332].
tert-Butyl 2-(prop-1-yn-1-yl)azonane-1-carboxylate (7d) Yield 80%, a colorless oil; 1H-NMR (CDCl3) δ 4.97 and 4.73 (m, 1H), 3.55–3.44 (m, 1H), 3.06 (m, 1H), 1.77–1.45 (m, 24H); 13C-NMR (CDCl3) δ 156.29, 155.30, 79.73, 79.67, 78.76, 78.70, 78.62, 78.50, 49.42, 48.36, 44.68, 43.84, 39.48, 32.98, 32.05, 29.81, 29.38, 28.65, 27.51, 27.34, 26.77, 26.62, 26.04, 25.39, 25.08, 24.83, 24.76, 23.46, 14.29, 13.82, 8.86, 3.62, 3.55; IR (ATR-IR) νmax 2918, 1687, 1399, 1157, 768 cm−1; HRMS (ESI+) found 210.1493 [calculated C12H20NO2 ([M + H − C4H8]+) 210.1489].
tert-Butyl 2-(phenylethynyl)piperidine-1-carboxylate (8a) Yield 31%, a colorless oil; 1H-NMR (CDCl3) δ 7.41 (m, 2H), 7.30 (m, 3H), 5.28 (s, 1H), 3.97 (d, J = 12 Hz, 1H), 3.11 (t, J = 11.7 Hz, 1H), 1.84 (m, 2H), 1.68–1.48 (m, 13H); 13C-NMR (CDCl3) δ 154.78, 131.85, 128.36, 128.20, 123.27, 87.81, 84.22, 80.05, 44.92, 40.65, 30.89, 28.58, 25.53, 20.29; IR (ATR-IR) νmax 2932, 1681, 1401, 1149, 753 cm−1; HRMS (ESI+) found 230.1189 [calculated C14H16NO2 ([M + H − C4H8]+): 230.1176].
tert-Butyl 2-(phenylethynyl)azepane-1-carboxylate (8b) Yield 88%, a colorless oil; 1H-NMR (CDCl3) δ 7.40 (m, 2H), 7.28 (m, 3H), 5.23 and 4.96 (m, 1H), 3.90 and 3.87 (d, J = 14.4 Hz, 1H), 3.13 (m, 1H), 2.24 (m, 1H), 1.78–1.40 (m, 16H); 13C-NMR (CDCl3) δ 155.45, 155.09, 131.86, 131.72, 128.34, 128.26, 128.14. 128.09, 123.32, 123.27, 89.66, 89.59, 82.66, 82.53, 79.92, 79.71, 48.28, 46.88, 42.74, 42.32, 36.02, 35.72, 28.81, 28.61, 28.54, 28.31, 25.07, 24.40; IR (ATR-IR) νmax 2931, 1676, 1393, 1149, 764 cm−1; HRMS (ESI+) found 244.1335 [calculated C15H18NO2 ([M + H − C4H8]+): 244.1332].
tert-Butyl 2-(phenylethynyl)azocane-1-carboxylate (8c) Yield 68%, a colorless oil; 1H-NMR (CDCl3) δ 7.39 (m, 2H), 7.28 (m, 3H), 5.24–4.94 (m, 1H), 3.58 (m, 1H), 3.21 (m, 1H), 1.69–1.49 (m, 19H); 13C-NMR (CDCl3) δ 155.55, 154.79, 131.82, 131.69, 128.31, 128.24, 128.13, 128.08, 123.25, 123.17, 89.10, 82.68, 79.84, 79.72, 48.81, 47.60, 42.84, 42.39, 32.30, 31.67, 28.62, 27.75, 26.99, 26.82, 26.71, 25.12, 24.67, 24.36, 23.57; IR (ATR-IR) νmax 2925, 1685, 1400, 1156, 760 cm−1; HRMS (ESI+) found 258.1495 [calculated C16H20NO2 ([M + H − C4H8]+): 258.1489].
tert-Butyl 2-(phenylethynyl)azonane-1-carboxylate (8d) Yield 81%, a colorless oil; 1H-NMR (CDCl3) δ 7.40 (m, 2H), 7.29 (m, 3H), 5.29 and 4.99 (m, 1H), 3.59 (m, 1H), 3.17 (m, 1H), 1.93–1.50 (m, 21H); 13C-NMR (CDCl3) δ 156.35, 155.38, 131.85, 131.74, 128.40, 128.33, 128.22, 128.16, 123.31, 123.20, 88.98, 88.84, 83.18, 80.07, 79.98, 49.93, 48.81, 44.12, 32.87, 31.94, 28.70, 27.39, 26.76, 26.63, 26.09, 25.25, 25.11, 24.91, 24.83; IR (thin film, neat) νmax 2927, 1692, 1161, 772 cm−1; HRMS (ESI+) found 272.1648 [calculated C17H22NO2 ([M + H − C4H8]+): 272.1645].
4. Conclusions
In summary, we developed an efficient route for the synthesis of α-vinyl azacycles from 7- to 9-memebered lactams and α-acetylenyl azacycles from 6- to 9-membered lactams via N,O-acetal TMS ethers using organocopper and organostannane reagents, respectively. This procedure affords reasonable yields and may facilitate the concise and efficient synthesis of α-vinyl and α-acetylenyl azacycles to provide bioactive alkaloids and pharmaceuticals.
Acknowledgments
This work was supported by the Ministry of Science and ICT and the Ministry of Education through the National Research Foundation of Korea grants NRF-2015M3A9E7029176 and NRF-2017R1D1A1B03034612.
Supplementary Materials
The following are available online at http://www.mdpi.com/1420-3049/23/11/3023/s1. The 1H and 13C NMR spectra of 4b–8d can be found in the SI.
Author Contributions
S.-H.K.; Y.-G.S.; J.-W.J.; J.J. designed the experiments; M.K.; J.J.; G.C.; S.C.; J.H.; H.S.K. and C.L. performed the experiments; J.J.; Y.N.; W.S.S. and C.L. analyzed the data; Y.-G.S.; S.-H.K.; J.-W.J. and C.L. wrote the paper; all authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of compounds are available from the authors.
References
- 1.Wu P., Nielsen T.E. Scaffold diversity from N-acyliminium ions. Chem. Rev. 2017;117:7811–7856. doi: 10.1021/acs.chemrev.6b00806. [DOI] [PubMed] [Google Scholar]
- 2.Cai M., Cai C., Mayorov A.V., Xiong C., Cabello M., Soloshonok V.A., Swift J.R., Trivedi D., Hruby V.J. Biological and conformational study of beta-substituted prolines in MT-II template: Steric effects leading to human MC5 receptor selectivity. J. Pept. Res. 2004;63:116–131. doi: 10.1111/j.1399-3011.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 3.Takahata H., Takahashi K., Wang E.-C., Yamazaki T. Alkynylation of thiolactams: New synthesis of α-substituted pyrrolidine and piperidine alkaloids. J. Chem. Soc. Perkin Trans. 1989;1:1211–1214. doi: 10.1039/P19890001211. [DOI] [Google Scholar]
- 4.Cook G.R., Beholz L.G., Stille J.R. Construction of hydroxylated alkaloids (±)-mannonolactam,(±)-deoxymannojirimycin, and (±)-prosopinine through aza-annulation. J. Org. Chem. 1994;59:3575–3584. doi: 10.1021/jo00092a015. [DOI] [Google Scholar]
- 5.Schär P., Renaud P. Total synthesis of the marine alkaloid (±)-lepadiformine via a radical carboazidation. Org. Lett. 2006;8:1569–1571. doi: 10.1021/ol060083+. [DOI] [PubMed] [Google Scholar]
- 6.Neipp C.E., Martin S.F. Synthesis of bridged azabicyclic structures via ring-closing olefin metathesis. J. Org. Chem. 2003;68:8867–8878. doi: 10.1021/jo0349936. [DOI] [PubMed] [Google Scholar]
- 7.Jaekel M., Qu J., Schnitzer T., Helmchen G. Addition of Organometallic Reagents to Chiral N-methoxylactams: Enantioselective syntheses of pyrrolidines and piperidines. Chem. Eur. J. 2013;19:16746–16755. doi: 10.1002/chem.201302735. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb L., Meyers A. Electrophilic formamidines. Organometallic addition to 2-methoxy pyrrolidine or piperidine N-t-butyl formamidines. Formation of 2-substituted pyrrolidines and piperidines. Tetrahedron Lett. 1990;31:4723–4726. doi: 10.1016/S0040-4039(00)97716-5. [DOI] [Google Scholar]
- 9.Ha J.D., Cha J.K. Total synthesis of clavepictines a and b diastereoselective cyclization of δ-aminoallenes. J. Am. Chem. Soc. 1999;121:10012–10020. doi: 10.1021/ja9925958. [DOI] [Google Scholar]
- 10.Jiang J., DeVita R.J., Doss G.A., Goulet M.T., Wyvratt M.J. Asymmetric synthesis of chiral, nonracemic trifluoromethyl-substituted piperidines and decahydroquinolines. J. Am. Chem. Soc. 1999;121:593–594. doi: 10.1021/ja983389n. [DOI] [Google Scholar]
- 11.Batey R.A., MacKay D.B., Santhakumar V. Alkenyl and aryl boronates mild nucleophiles for the stereoselective formation of functionalized n-heterocycles. J. Am. Chem. Soc. 1999;121:5075–5076. doi: 10.1021/ja983801z. [DOI] [Google Scholar]
- 12.Itoh T., Yamazaki N., Kibayashi C. asymmetric synthesis of (−)-adaline. Org. Lett. 2002;4:2469–2472. doi: 10.1021/ol0200807. [DOI] [PubMed] [Google Scholar]
- 13.Collado I., Ezquerra J., Pedregal C. Stereoselective addition of Grignard-derived organocopper reagents to N-acyliminium ions: Synthesis of enantiopure 5- and 4,5-substituted prolinates. J. Org. Chem. 1995;60:5011–5015. doi: 10.1021/jo00121a020. [DOI] [Google Scholar]
- 14.Jung J.W., Kim S.H., Suh Y.G. Advances in aza-claisen-rearrangement-induced ring-expansion strategies. Asian J. Org. Chem. 2017;6:1117–1129. doi: 10.1002/ajoc.201700202. [DOI] [Google Scholar]
- 15.Campos K.R. Direct sp3 C—H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 2007;36:1069–1084. doi: 10.1039/B607547A. [DOI] [PubMed] [Google Scholar]
- 16.Noble A., MacMillan D.W. Photoredox α-vinylation of α-amino acids and N-aryl amines. J. Am. Chem. Soc. 2014;136:11602–11605. doi: 10.1021/ja506094d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh Y.-G., Kim S.-H., Jung J.-K., Shin D.-Y. The versatile conversion of lactams to the α-alkylated azacycles via cyclic N,O-acetal TMS ether. Tetrahedron Lett. 2002;43:3165–3167. doi: 10.1016/S0040-4039(02)00459-8. [DOI] [Google Scholar]
- 18.Suh Y.-G., Lee Y.-S., Kim S.-H., Jung J.-K., Yun H., Jang J., Kim N.-J., Jung J.-W. A stereo-controlled access to functionalized macrolactams via an aza-Claisen rearrangement. Org. Biomol. Chem. 2012;10:561–568. doi: 10.1039/C1OB06733H. [DOI] [PubMed] [Google Scholar]
- 19.Jung J.W., Shin D.Y., Seo S.Y., Kim S.H., Paek S.M., Jung J.K., Suh Y.G. A new entry to functionalized cycloalkylamines: Diastereoselective intramolecular amidoalkylation of N,O-acetal TMS ether possessing allylsilane. Tetrahedron Lett. 2005;46:573–575. doi: 10.1016/j.tetlet.2004.11.136. [DOI] [Google Scholar]
- 20.Meana I., Albeniz A.C., Espinet P. Selective green coupling of alkynyltins and allylic halides to trienynes via a tandem double stille reaction. Adv. Synth. Catal. 2010;352:2887–2891. doi: 10.1002/adsc.201000430. [DOI] [Google Scholar]
- 21.Wu X., Cruz F.A., Lu A., Dong V.M. Tandem catalysis: Transforming alcohols to alkenes by oxidative dehydroxymethylation. J. Am. Chem. Soc. 2018;140:10126–10130. doi: 10.1021/jacs.8b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.