Abstract
Background:
The development of long-acting injectable formulations (LAIs) of second-generation antipsychotic drugs (SGAs) has been suggested as having advantage over first-generation antipsychotic (FGA) LAIs. In this study, we investigated the hypothesis that there was a longer time to relapse in patients with schizophrenia started on SGA LAI versus FGA LAI.
Methods:
Patients with a diagnosis of schizophrenia or schizoaffective disorder who were started on an SGA LAI while on an inpatient ward were identified through searching of the anonymised historical medical records at the South London and Maudsley NHS Foundation Trust. Patients starting FGA LAIs matched for diagnosis, age and date of hospital admission were identified. Time to readmission, discontinuation of LAI or death were identified. Kaplan–Meier plots were generated for each group, and the difference between groups analysed using log-rank methods.
Results:
There were 157 patients identified in each group. There was no difference in time to readmission, medication discontinuation or death in patients on SGA LAI versus FGA LAI.
Conclusions:
We found no evidence of advantage in terms of maintaining response in SGA LAI versus FGA LAI. Prescriber choice should be guided by other factors such as side-effect profile, patient acceptability and price.
Keywords: Depot, antipsychotic, schizophrenia, efficacy
Introduction
The use of long-acting injectable antipsychotic drugs (LAIs) has been considered the gold standard in maintaining medication adherence and reducing the risk of relapse in patients with schizophrenia, although evidence for this is dependent upon the type of study performed, with clinical trials tending to show less benefit of LAI over oral antipsychotic drugs when compared with more naturalistic designs.1,2
The recent development of LAI second-generation antipsychotic drugs (SGAs) showed promise with the advantage of enhanced medication adherence compared with oral antipsychotic drugs with the potential for lower movement-related side effects to first-generation antipsychotic drugs (FGAs).3 Studies of specific SGA LAIs have shown reduced risk of hospitalization versus oral antipsychotics for paliperidone and aripiprazole, but not for risperidone depot.4–6 A large study comparing risperidone depot versus FGA LAI did not find any difference in all-cause discontinuation or hospitalization in either group.7
In this study we aimed to investigate time to relapse in patients treated with SGA LAI vs FGA LAI in patients with schizophrenia or schizoaffective disorder admitted to the South London and Maudsley Trust.
Method
We used the Clinical Record Interactive Search (CRIS), a searchable anonymised database containing the clinical records of all patients registered with the South London and Maudsley NHS Foundation Trust (SLaM). This database includes information on over 220,000 patients; while the system was set up in January 2007, some patient data have been added retroactively, with the earliest patient data being from 1996. Ethical approval for research using CRIS as a database for secondary analysis has been obtained from Oxford Research Ethics Committee C.8 Informed consent was not required for this study as deemed by the ethics committee.
We identified patients with schizophrenia or schizoaffective disorder with no previous history of LAI treatment who were initiated on SGA LAI while they were admitted to hospital or under home treatment team (index admission) up to February 2017. Search terms for SGA LAI were aripiprazole depot (Abilify Maintena®), olanzapine embonate (ZypAdhera®), paliperidone (Xeplion) and risperidone depot (Risperdal Consta®). We then identified patients matched for diagnosis, age (±5 years), sex, ethnicity and date of admission to hospital or home treatment team (±6 months), with no previous history of LAI treatment, who were started on FGA LAI during the index admission. Search terms for FGA LAI were fluphenazine decanoate (Modecate®), haloperidol depot (haloperidol decanoate), pipotiazine palmitate (Piportil Depot®) and zuclopenthixol decanoate (Clopixol®). For each group, we measured number of psychiatric inpatient or home treatment team days prior to the index admission and their total time under the care of SLaM NHS Trust. We then measured the time from discharge until they stopped or switched medication, or until their next hospital admission, admission to home treatment team, or death, which were all classed as endpoints for the purpose of the survival analysis. Lastly, we measured the number of face-to-face contacts that each patient had during the time they were on the depot medication until readmission or the end of the study.
Data were analysed using R statistical package version 3.3.2, using the ‘survival’ package. Kaplan–Meier curves for the two groups were generated, and the difference between survival curves for FGA LAI and SGA LAI was determined using the G-Rho family of tests (log rank) implemented in the ‘survdiff’ function.9 A post hoc analysis of the difference in survival curves for individual antipsychotics within the SGA and FGA LAI groups was also performed using the same statistical method.
Results
There were 157 patients identified in each group. Demographic details are detailed in Table 1. Patients in the FGA and SGA LAI groups did not differ in terms of hospitalized days prior to their index admission, but patients in the SGA LAI group had a significantly longer time under the care of SLaM NHS Trust (4.95 versus 3.97 years, p = 0.048; Table 1). In the FGA LAI group, patients were treated with zuclopenthixol (n = 77), pipotiazine (n = 14), haloperidol depot (n = 19), flupentixol (n = 43) and fluphenazine (n = 4). In the SGA LAI group, patients were treated with risperidone depot (n = 90), paliperidone (n = 49), aripiprazole depot (n = 16) and olanzapine depot (n = 2).
Table 1.
Group characteristics of patients started on first-generation antipsychotic long-acting injectable and second-generation antipsychotic long-acting injectable.
| SGA | FGA | p | |
|---|---|---|---|
| Mean (SD) age, years | 38.5 (11.7) | 38.9 (12.7) | 0.8 |
| Sex (F/M) | 65/92 | 65/92 | 1 |
| Diagnosis (F20, F22, F25) | 144, 2, 11 | 144, 2, 11 | 1 |
| Mean (SD) time under the care of SLaM NHS Trust prior to index admission, years | 4.95 (4.21) | 3.97 (4.54) | 0.048* |
| Mean (SD) number of inpatient and home treatment team days per year prior to index admission | 8.23 (21.0) | 14.46 (55.8) | 0.62 |
| Mean (SD) length of index admission, days | 127.1 (204.1) | 143.6 (213.2) | 0.5 |
| Ethnicity (African, Caribbean, Black, Mixed, British, Other White, Other) | 45, 19, 39, 1, 33, 14, 6 | 45, 19, 39, 1, 33, 14, 6 | 1 |
| Endpoint (none, medication stop or change, home treatment team, admission, death) | 68, 15, 13, 55, 6 | 63, 9, 15, 68, 3 | 0.22 |
p < 0.05.
FGA, first-generation antipsychotic; F20 - Schizophrenia; F22 - Persistent Delusional Disorder; F25 - Schizoaffective Disorder; NHS, National Health Service; SGA, second-generation antipsychotic; SD, standard deviation; SLaM, South London and Mausley.
A total of 89 patients in the SGA LAI group and 95 in the FGA LAI group reached an endpoint during the study period (Figure 1). There was no significant difference in time to endpoint in either group (p = 0.921). There was also no difference in number of face-to-face contacts with services between either group following discharge from hospital (SGA = 136.1 versus FGA = 121.6, p = 0.386). Due to the fact that risperidone depot requires more frequent dosing, we analysed the data excluding the patients on risperidone depot but found that there was still no difference in time to endpoint for the FGA versus SGA LAI groups [Chi square = 1.4, degrees of freedom (d.f.) = 3, p = 0.244]. With post hoc testing, there was no difference between any of the FGA LAI antipsychotics in terms of time to endpoint (p = 0.595). In the SGA LAI group there was a significant difference between different antipsychotics, with those treated with paliperidone and aripiprazole depots having a lower than expected number reaching endpoint during the study compared with those on risperidone and olanzapine depots (Chi square = 8.2, d.f. = 3, p = 0.04; Table 2).
Figure 1.
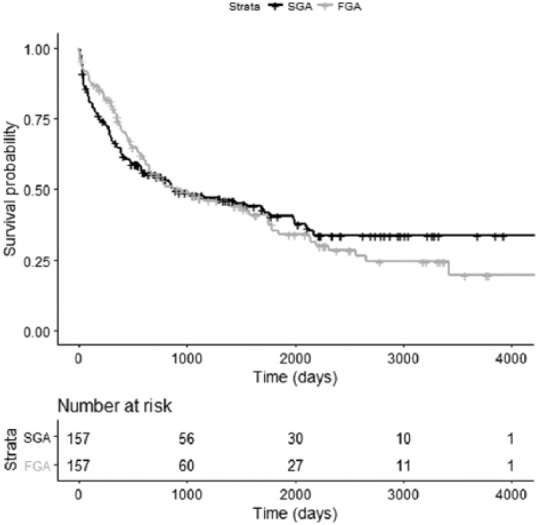
Kaplan–Meier plot of time to endpoint for patients with schizophrenia treated with first-generation antipsychotic long-acting injectable or second-generation antipsychotic long-acting injectable.
FGA, first-generation antipsychotic long-acting injectable; SGA, second-generation antipsychotic long-acting injectable.
Table 2.
Stratified log-rank analysis for second-generation antipsychotic long-acting injectable study endpoints.
| n | Observed endpoints | Expected endpoints | |
|---|---|---|---|
| Aripiprazole | 16 | 5 | 8.79 |
| Olanzapine | 2 | 2 | 0.73 |
| Paliperidone | 49 | 18 | 25.453 |
| Risperidone | 90 | 64 | 54.025 |
Observed and expected number of patients reaching study endpoints in patients on each long-acting injectable are shown.
Discussion
In this study, we did not find any evidence for a difference in medication discontinuation, readmission rates or time to readmission between patients with schizophrenia or schizoaffective disorder treated with FGA LAI and SGA LAI. This suggests that there is no advantage in terms of maintaining response in choosing either an FGA versus an SGA LAI and prescriber choice should therefore be guided by other factors such as side-effect profile, patient acceptability and price.
Post hoc testing was suggestive that paliperidone and aripiprazole depot may have a favourable profile in terms of medication discontinuation or time to readmission compared with other SGA LAIs. These findings have not been previously reported and are worthy of further research. A previous study of haloperidol depot versus paliperidone found no difference in time to relapse,10 although this study used a clinical trial methodology rather than a naturalistic design and so may not be representative of real-world experience. A recent systematic review comparing aripiprazole depot with paliperidone depot concluded that aripiprazole had advantages in terms of discontinuation and efficacy.11 Further well-designed studies will be required to investigate these hypotheses further.
Although this study has strengths in that it uses naturalistic data acquired from clinical practice, and the patients were closely matched on relevant clinical and demographic characteristics, it was dependent on the quality, detail and timing of data entry into the clinical records. While the dates and times of hard endpoints such as admission and death are likely to be accurate, discontinuation of medication is more prone to error due to differences in clinical record keeping, and so may have been underestimated. In addition, selection of LAI was down to individual clinician choice, and so may have been a source of bias, as paliperidone LAI has been found to be prescribed in patients with longer and more frequent hospital admissions.12 In the current study, we found that length of time with SLaM NHS Trust prior to the index admission was longer in patients prescribed an SGA LAI, suggesting that the SGA LAI group had a longer duration of illness, although the mean number of hospitalizations and home treatment team contacts per year prior to the index admission did not significantly differ between the two groups.
Acknowledgments
This work was supported by the NIHR Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King’s College London. The research received no specific grant from any funding agency, commercial or not for profit sectors.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: James M Stone  https://orcid.org/0000-0003-3051-0135
https://orcid.org/0000-0003-3051-0135
Contributor Information
James M. Stone, Centre for Neuroimaging Sciences, Institute of Psychiatry Psychology and Neuroscience, 16 De Crespigny Park, London SE5 8AF, UK.
Simon Roux, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
David Taylor, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Paul D. Morrison, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK
References
- 1. Fagiolini A, Rocca P, De Giorgi S, et al. Clinical trial methodology to assess the efficacy/effectiveness of long-acting antipsychotics: randomized controlled trials vs naturalistic studies. Psychiatry Res 2017; 247: 257–264. [DOI] [PubMed] [Google Scholar]
- 2. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry 2016; 77: 1–24. [DOI] [PubMed] [Google Scholar]
- 3. Rauch AS, Fleischhacker WW. Long-acting injectable formulations of new-generation antipsychotics: a review from a clinical perspective. CNS Drugs 2013; 27: 637–652. [DOI] [PubMed] [Google Scholar]
- 4. Taylor DM, Sparshatt A, O’Hagan M, et al. Effect of paliperidone palmitate on hospitalisation in a naturalistic cohort - a four-year mirror image study. Eur Psychiatry 2016; 37: 43–48. [DOI] [PubMed] [Google Scholar]
- 5. Taylor DM, Sparshatt A, Amin F, et al. Aripiprazole long-acting injection - a mirror image study of its effects on hospitalisation at one year. J Psychopharmacol 2017; 31: 1564–1569. [DOI] [PubMed] [Google Scholar]
- 6. Taylor D, Fischetti C, Sparshatt A, et al. Risperidone long-acting injection: a 6-year mirror-image study of healthcare resource use. Acta Psychiatr Scand 2009; 120: 97–101. [DOI] [PubMed] [Google Scholar]
- 7. Nielsen J, Jensen SO, Friis RB, et al. Comparative effectiveness of risperidone long-acting injectable vs first-generation antipsychotic long-acting injectables in schizophrenia: results from a nationwide, retrospective inception cohort study. Schizophr Bull 2015; 41: 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandes AC, Cloete D, Broadbent MT, et al. Development and evaluation of a de-identification procedure for a case register sourced from mental health electronic records. BMC Med Inform Decis Mak 2013; 13: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika 1982; 69: 553–566. [Google Scholar]
- 10. McEvoy JP, Byerly M, Hamer RM, et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA 2014; 311: 1978–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pae CU, Wang SM, Han C, et al. Comparison between long-acting injectable aripiprazole versus paliperidone palmitate in the treatment of schizophrenia: systematic review and indirect treatment comparison. Int Clin Psychopharmacol 2017; 32: 235–248. [DOI] [PubMed] [Google Scholar]
- 12. Patel R, Chesney E, Taylor M, et al. Is paliperidone palmitate more effective than other long-acting injectable antipsychotics? Psychol Med. Epub ahead of print 17 October 2017. DOI: 10.1017/S0033291717003051. [DOI] [PMC free article] [PubMed] [Google Scholar]


