Abstract
The Ducreyi serum resistance A (DsrA) protein of Haemophilus ducreyi belongs to a large family of multifunctional outer membrane proteins termed trimeric autotransporter adhesins responsible for resistance to the bacterial activity of human complement (serum resistance), agglutination and adhesion. The ability of DsrA to confer serum resistance and bind extracellular matrix proteins lies in its N-terminal passenger domain. We have previously reported that immunization with a recombinant form of the passenger domain of DsrA, rNT-DsrA, in complete/incomplete Freund’s adjuvant, protects against a homologous challenge in swine. We present herein the results of an immunogenicity study in mice aimed at investigating the persistence, type of immune response, and the effect of immunization route and adjuvants on surrogates of protection. Our results indicate that a 20 µg dose of rNT-DsrA administered with alum elicited antisera with comparable bacterial surface reactivity to that obtained with complete/incomplete Freund’s adjuvant. At that dose, high titers and bacterial surface reactivity persisted for 211 days after the first immunization. Administration of rNT-DsrA with CpG or Imiquimod as adjuvants elicited a humoral response with similar quantity and quality of antibodies (Abs) as seen with Freund’s adjuvant. Furthermore, intramuscular administration of rNT-DsrA elicited high-titer Abs with significantly higher reactivity to the bacterial surface than those obtained with subcutaneous immunization. All rNT-DsrA/adjuvant combinations tested, save CpG, elicited a Th2-type response. Taken together, these findings show that a 20 µg dose of rNT-DsrA administered with the adjuvants alum, CpG or Imiquimod elicits high-quality Abs with reactivity to the bacterial surface that could protect against an H. ducreyi infection.
INTRODUCTION
Haemophilus ducreyi is classically known as the etiological agent of the sexually transmitted genital ulcer disease chancroid; however, it has recently been brought to worldwide attention that H. ducreyi is also a significant cause of cutaneous ulcers in yaws-endemic regions of the world [1–6]. Moreover, mass treatment of patients with cutaneous ulcers with the antibiotic azithromycin did not affect the proportion of ulcers attributable to H. ducreyi [6, 7]. These findings suggest that a vaccine against H. ducreyi could not only target patients with genital ulcers, but also those with cutaneous lesions. One determinant of H. ducreyi shown to be a possible vaccine candidate is the multifunctional surface-exposed trimeric autotransporter adhesin DsrA, a protein involved in resistance to the bactericidal activity of complement (serum resistance) and binding to fibronectin (Fn), vitronectin (Vn) and fibrinogen (Fg) [8–12]. The N-terminal passenger domain of DsrA from class I H. ducreyi strain 35000HP, termed rNT-DsrAI, administered in complete/incomplete Freund’s adjuvant protects against a homologous challenge in the swine experimental model of chancroid [13]. Although these results proved DsrA to be a promising vaccine candidate, the experimental rNT-DsrAI vaccine was administered with Freund’s adjuvant, which cannot be safely used in humans. Furthermore, these trials did not address the persistence of the humoral immune response to rNT-DsrAI or the type of immune response generated to the vaccine. The goals of this study were therefore to measure the humoral immune response developed to different doses and routes of rNT-DsrAI administered with a variety of adjuvants, either approved or in clinical trials for human use, and to compare the responses to Freund’s adjuvant. We also measured the persistence of the humoral immune response to the rNT-DsrAI vaccine, and reactivity to homologous and heterologous H. ducreyi strains. Finally, we determined the type of humoral immune response to rNT-DsrAI administered with different adjuvants by measuring the IgG1/IgG2a subtype ratio. Although the correlates of protection of the rNT-DsrAI vaccine are currently still unknown, reactivity of vaccine-induced antibodies (Abs) to the surface of viable H. ducreyi was used as a surrogate of a protective immune response against infectious H. ducreyi challenge.
MATERIALS AND METHODS
Bacterial strains and culture conditions
H. ducreyi strains were routinely cultured and passaged on chocolate agar plates as previously described [13]. Prototypical class I strain 35000HP, a human-passage isolate [14] of strain 35000 [15], is the source of the dsrA gene used for preparation of rNT-DsrAI. 35000HP∆dsrA (FX517) is an isogenic dsrA mutant of strain 35000HP [8]. Strain HMC50 is a class I H. ducreyi strain isolated in Jackson, MS [9]. Escherichia coli strain BL21(DE3)pLys (Life Technologies, Grand Island, NY) [16], used to express rNT-DsrAI, was cultured has previously described [13].
Preparation of rNT-DsrAI and purity assessment
The nucleotide sequence encoding the passenger domain of DsrA was amplified and expressed as previously described [13, 17]. Purity and concentration of rNT-DsrA were confirmed by SDS-PAGE [17–19]. Lipopolysaccharide levels, measured using the Pyrogent 5000 LAL Assay kit (Lonza Inc., Allendale, NJ) at the Duke Human Vaccine Institute Protein Expression Facility (Durham, NC), were found to be under detectable limits. Western blotting of the rNT-DsrAI preparations using an Ab to recombinant full-length DsrAI (rFL-DsrAI) [17] was used to ensure formation of multimers by rNT-DsrAI [13].
Animal studies.
Two immunization experiments, approved by the Duke Institutional Animal Care and Use Committee (IACUC), were performed in the Regional Biocontainment Laboratory at Duke University (Durham, NC). BALB/c female mice (8-10 weeks) were administered three doses of rNT-DsrAI, ranging from 0.04 µg to 100 µg, at three-week intervals, either subcutaneously (SQ) or intramuscularly (IM), in the absence or presence of the following adjuvants: Freund’s complete/incomplete adjuvant (Sigma-Aldrich, St-Louis, MO), Alum (Alhydrogel 2%, Invivogen, San Diego, CA), synthetic monophosphoryl lipid A (MPL, cat# vac-mpls, Invivogen, San Diego, CA), CpG (ODN1826, cat# vac-1826–1, Invivogen, San Diego, CA) or Imiquimod (Imidazoquinoline, cat# vac-imq, Invivogen, San Diego, CA). Following the manufacturer’s instructions, rNT-DsrAI was administered at a 1:1 ratio for Freund’s and alum. For MPL, CpG and Imiquimod, each mouse received 10, 30 and 40 µg, respectively, of adjuvant per immunization. These adjuvants were put into solution per manufacturer’s instructions. Doses of adjuvants were chosen in the middle range recommended by the manufacturer. Mice were bled at days 0, 21, 42, and 56 days. To measure persistence of the Ab response to the immunogen, cheek and/or terminal bleeds were also performed at 122 (0.04, 0.16, 0.8 and 4 µg doses), 146 and 211 days (4, 20 and 100 µg doses) after the primary immunization.
Enzyme-linked immunosorbent assay (ELISA) assays
Anti-rNT-DsrAI endpoint binding titer
rNT-DsrAI-specific serum Ab binding titers (endpoint) were determined by standard ELISA as previously described [19], except for certain changes to secondary Abs, dilutions and substrate. HRP-conjugated mouse Ig specific Abs (Southern Biotech, Birmingham, AL) were added to plates at a 1:4,000 dilution. TMB (3,3’, 5,5”-tetramethylbenzidine; KPL, Gaithersburg, MD), used as substrate, was incubated for 10 minutes at room temperature and read at an optical density (OD) of 450 nm using a Victor3 plate reader (Perkin Elmer, Waltham, MA). The baseline was set at three times the average plate background OD, which is OD obtained with the ELISA reagents in the absence of serum. Log endpoint titer (log10) is reported as the log of the reciprocal of the highest serum dilution at which the OD value was equal to or greater than baseline.
Reactivity of anti-rNT-DsrAI to DsrA at the bacterial surface
A whole-cell binding ELISA was used to measure binding of anti-rNT-DsrAI to native DsrA at the surface of H. ducreyi [20–22].
Statistical analyses
For classical ELISAs and IgG1/IgG2a subtype ratios, a Wilcoxon rank-sum test was used to determine significant difference between adjuvant and no adjuvant samples at a given dose and route, for which we computed a time-dependent curve using the median of samples at each time point. The area under the curve (AUC) was computed using the “kulife” R extension package. Comparison of the results obtained from whole-cell binding ELISAs was analyzed using the t-test with Prism software (GraphPad Software, Inc., La Jolla, CA). A Welch correction was used for groups with unequal variances.
RESULTS
rNT-DsrAI is highly pure and forms multimers.
Preparations of rNT-DsrAI were homogeneous (Fig. 1A), save faint bands smaller than 15 kDa (Fig. 1A). To determine if those bands represented foreign proteins or degradation products of rNT-DsrAI, some of the protein preparations were subjected to Western blotting with an Ab against full-length DsrAI. Bands around the 14-kDa molecular weight marker reacted with the Ab, indicating that they were degradation products of rNT-DsrAI (Fig. 1B). Western analysis also showed that rNT-DsrAI preparations form dimers, although the major product was a monomer (Fig. 1B). Taken together, these results indicated that the immunogen rNT-DsrAI is highly pure and forms multimers.
Fig. 1. The recombinant immunogen rNT-DsrAI is devoid of contaminants.
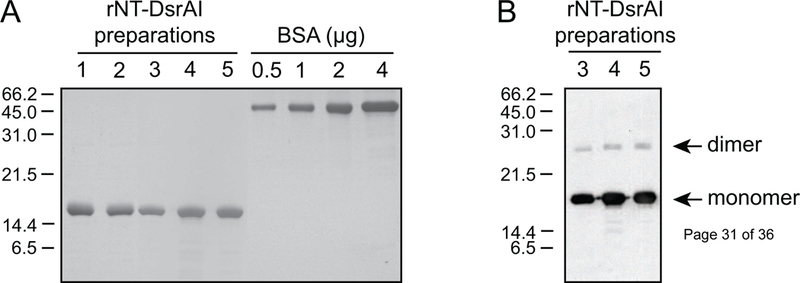
A. Preparations of rNT-DsrAI were subjected to SDS-PAGE and Coomassie Blue staining to determine the presence of foreign proteins. Bovine Serum Albumin (BSA) standards were run concurrently with the purified immunogen to confirm protein concentration previously measured using a commercial reagent. B. rNT-DsrAI preparations were subjected to Western blotting with antibodies to rFL-DsrAI [17].
A 20 µg dose of rNT-DsrAI formulated with alum elicits a qualitatively similar immune response to the one obtained with Freund’s adjuvant.
In an initial immunogenicity study, groups of five mice were immunized at three-week intervals with doses of rNT-DsrAI ranging from 0.04 µg to 100 µg, either alone, in alum, or in Freund’s adjuvant. Using ELISA (i. e. endpoint titers), immunogenicity of rNT-DsrAI alone showed a dose-dependent increase from 0.16 to 100 µg (data not shown). No humoral response was detectable to 0.04 µg rNT-DsrAI alone (Fig. 2A), but administration of rNT-DsrAI in either alum or Freund’s adjuvant improved immunogenicity of the protein at this low dose (Fig. 2A, left panel). Interestingly, only Freund’s adjuvant increased titers (i. e. quantity) of Ab elicited to 4 or 20 µg rNT-DsrAI compared to administration of immunogen alone (Fig. 2A, middle and right panels), suggesting that alum did not enhance the amount of Ab. Actually, DsrA alone, at relatively high doses, (4 and 20 µg) appears to be highly immunogenic and not enhanced by alum.
Fig. 2. Quantity and quality of the humoral immune response is enhanced in animals receiving the rNT-DsrA vaccine administered with alum or Freund’s adjuvants.
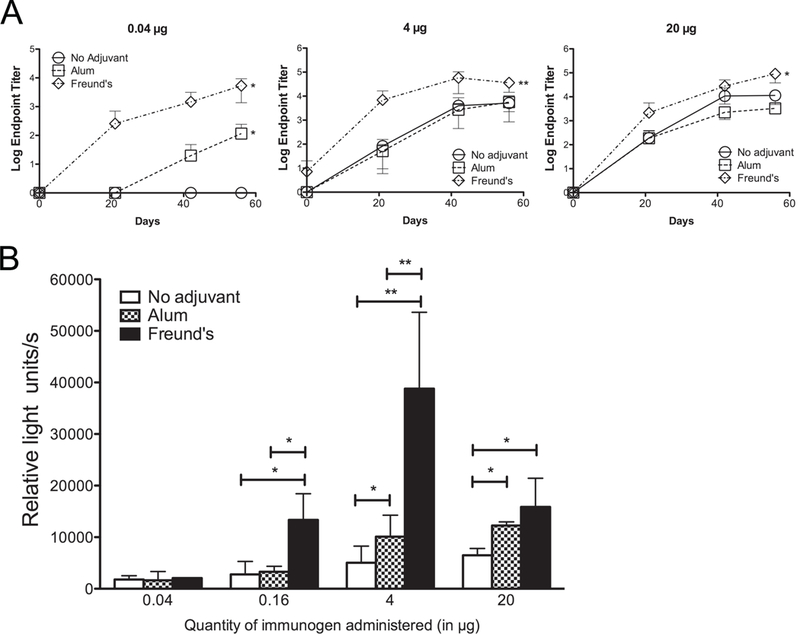
A. Log endpoint titers (means ± standard deviations) of individual rNT-DsrA antisera from mice receiving 0.04, 4, or 20 µg of rNT-DsrAI either alone, in alum or Freund’s adjuvant. B. Reactivity (means ± standard deviations of 3 independent experiments) of pooled rNT-DsrAI antisera (day 56) to the surface of viable homologous H. ducreyi. *, p<0.05; **, p<0.01 using an unpaired t-test.
The quality of the Abs elicited to different dose and adjuvant combinations was next measured by reactivity of antisera to the surface of viable homologous H. ducreyi (Fig.causing chronic skin ulceration in children 2B). Despite measurable titers to recombinant protein, none of the adjuvants increased binding of antisera to native DsrA at the surface of H. ducreyi when animals received a 0.04 µg dose (Fig. 2B). At the 0.16 µg dose, only administration with Freund’s adjuvant increased reactivity of rNT-DsrAI antisera to the bacterial surface, as compared to immunization in the absence of adjuvant (Fig. 2B). Reactivity of the rNT-DsrAI antisera to viable H. ducreyi was significantly increased using alum as the adjuvant for a 4 µg dose, while administration of the immunogen with Freund’s adjuvant increased reactivity eight times, compared to immunogen alone (Fig. 2B). When 20 µg of rNT-DsrAI was administered with either alum or Freund’s adjuvant, the quality of the humoral immune response was similar, but significantly higher than immunization with rNT-DsrAI alone (Fig. 2B). Overall, these findings demonstrate that alum elicits a humoral immune response similar to Freund’s adjuvant at a 20 µg dose.
The quantity and quality of Abs elicited to three doses of rNT-DsrAI are stable six months beyond the first immunization.
We next studied persistence of the humoral immune response elicited to rNT-DsrAI by measuring endpoint titers and bacterial surface reactivity 122, 146 and 211 days after the first immunization. For all doses and adjuvant tested, Ab titers measured at the end of the study (211 days) remained at levels similar to those measured at day 56, regardless of adjuvant (Fig. 3). At the 0.04 µg dose, titers at 122 days were highest when rNT-DsrAI was administered with Freund’s, as previously described (data not shown). Reactivity of antisera to viable bacteria from animals receiving 0.04 µg rNT-DsrAI did not increase over time, even though there was a slight increase in titers over the study period (data not shown). At the 4 µg dose, only immunization with Freund’s adjuvant elicited Abs with higher surface reactivity than Abs from mice receiving immunogen alone (Fig. 3A, bottom). At the highest dose of 20 µg, endpoint titers of antisera from animals receiving immunogen alone or in alum were not significantly different at days 146 and 211 than those from animals receiving the immunogen in Freund’s (Fig. 3B, top); however, surface reactivity of antisera from animals receiving 20 µg of rNT-DsrAI in alum, although similar at day 56, was significantly higher than those receiving this same immunogen dose in Freund’s at day 211 (Fig. 3B, bottom). In fact, surface reactivity of antisera from animals receiving 20 µg rNT-DsrAI in alum increased over time, while those from animals immunized with Freund’s decreased (Fig. 3B, bottom). These data suggest that despite equal titers, alum promoted a better quality and persistence of Abs than Freund’s adjuvant when administered with a 20 µg dose of rNT-DsrAI (Fig. 3B, bottom).
Fig. 3. rNT-DsrA elicits a persistent humoral immune response.
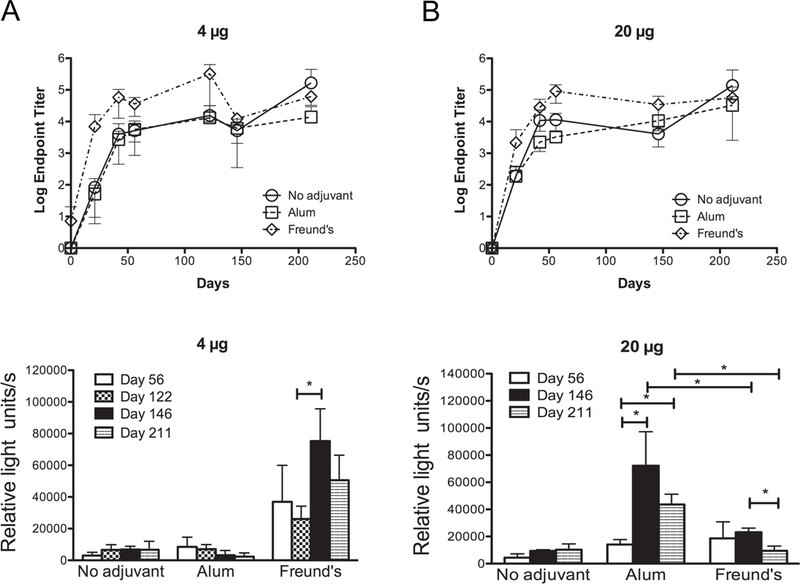
Antisera from mice immunized subcutaneously three times, three weeks apart with 4 (A) or 20 µg (B) of rNT-DsrAI administered alone or in conjunction with alum or Freund’s adjuvant were tested for reactivity to purified rNT-DsrAI (top) or to the surface of viable homologous H. ducreyi (bottom). Antisera were collected on days 0, 21, 42, 56, 122, 146 and 211, where day 0 indicates the first immunization. Shown are means ± standard deviations of individual sera (5 mice/group) for ELISA (top) or pooled sera (whole-cell binding ELISA, bottom) tested on three consecutive days. *, p<0.05 using an unpaired t-test.
Administration of rNT-DsrAI with CpG or Imiquimod elicits a qualitatively similar humoral immune response to that induced with Freund’s adjuvant.
One of the goals of the present study was to identify adjuvants safe for human use that could be administered with rNT-DsrAI to enhance a protective immune response against H. ducreyi infection. To address this question, we compared endpoint titers and surface reactivity of antisera from animals receiving rNT-DsrAI delivered with one of four adjuvants, either approved or in clinical trials for human use, to those of antisera obtained when rNT-DsrAI was administered with Freund’s adjuvant. Since alum is a poor inducer of the cellular arm of the immune response, we chose three other adjuvants reported to promote a Th1-biased immune response in addition to Abs, including monophosphoryl lipid A (MPL), CpG and Imiquimod [23]. MPL is a Toll-Like Receptor 4 (TLR-4) agonist composed of natural and synthetic lipid A from Salmonella or Escherichia coli and shown to induce a strong Th1 response [24, 25]. MPL is approved for human use when combined with alum in the HPV vaccine Cervarix [26]. The adjuvant activity of MPL has also been tested in humans when delivered intranasally with a norovirus vaccine candidate [27]. CpG is a synthetic oligodeoxynucleotide containing unmethylated CpG motifs that binds TLR-9 and induce a Th1-dominated immune response [23, 28]. The vaccine adjuvant activity of CpG has been evaluated in a number of clinical trials using infectious disease vaccines such as anthrax [29–31] and malaria [32, 33] or when administered with experimental cancer vaccines [34, 35]. Recognized by TLR7–8, Imiquimod also induces Abs and a Th1-type immune response [23]. Imiquimod is currently approved in the topically applied drug Aldara used to treat superficial basal cell carcinoma [36]. The vaccine adjuvant activity of imiquimod has also been evaluated in clinical trials using topical application combined with injection of the vaccine at the site of imiquimod application. Imiquimod has also been tested in clinical trials using an influenza vaccine [37], a hepatitis B vaccine [38] and a melanoma vaccine [39].
Most adjuvants tested enhanced reactivity of Abs to the bacterial surface at 4 and 20 µg doses, as compared to administration of rNT-DsrAI alone (Fig. 4). Although Freund’s adjuvant elicited the highest endpoint titers and reactivity to the bacterial surface (Fig. 4), both the quantity and quality of the humoral immune response to rNTDsrAI formulated with either CpG or imiquimod were similar to those obtained with Freund’s adjuvant at the 4 and 20 µg doses (Fig. 4 and data not shown for 0.04 µg dose). Taken together with findings presented above, these results suggest that administration of 4 or 20 µg doses of rNT-DsrAI with MPL, CpG, or Imiquimod mirrors the response seen in Freund’s vaccinated animals.
Fig. 4. The humoral immune response to rNT-DsrAI is similar when administered with Freund’s, MPL, CpG or imiquimod adjuvant, and improved with intramuscular compared to subcutaneous immunization route.
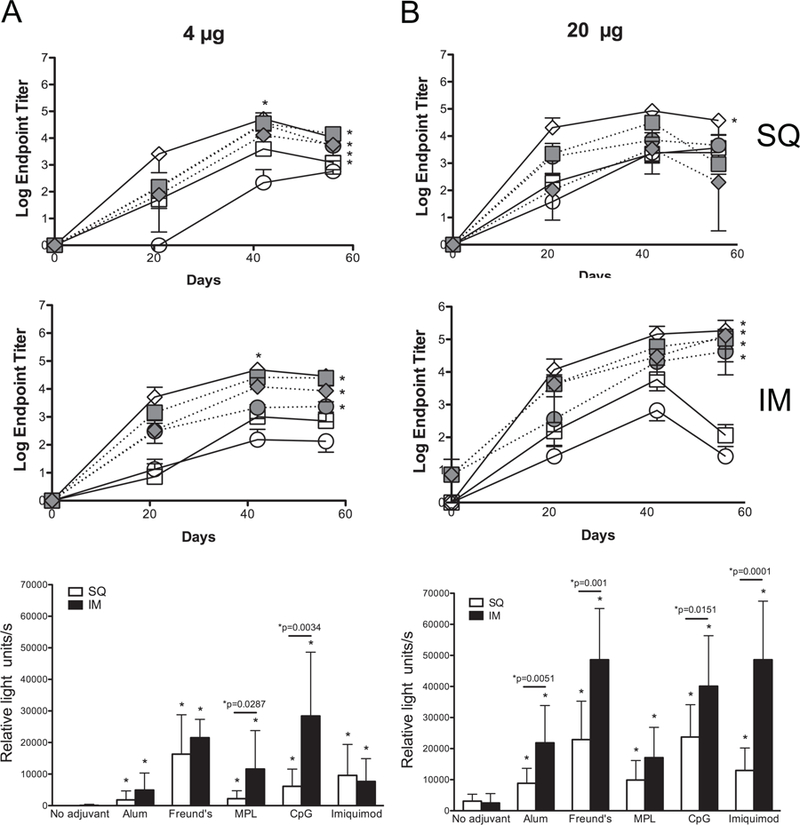
Groups of 5 mice were immunized either with 4 (A) or 20 (B) µg of rNT-DsrAI in the absence or presence of 5 different adjuvants (Alum, Freund’s, MPL, CpG and Imiquimod) following subcutaneous (SQ, top) or intramuscular (IM, middle) routes. Mice were immunized and bled three times, three weeks apart (days 0, 21, and 42), and bled at day 56. Top and middle, means ± standard deviations of endpoint titers of individual antisera after SQ or IM immunization, respectively. Bottom, reactivity of individual antisera from day 56 to viable, homologous H. ducreyi strain 35000HP (white column, SQ route; black column, IM route). * indicates p<0.05 as compared to the response obtained with “no adjuvant” at that specific dose/route for the entire time of the trial (56 days); for whole cell binding ELISAs (bottom), “*p=“ compares SQ and IM routes for pairs of doses and adjuvants using an unpaired t-test.
Intramuscular administration of rNT-DsrAI elicits a greater humoral immune response than subcutaneous immunization.
In the adjuvant comparison study, we also determined the importance of immunization route [intramuscular (IM) versus subcutaneous (SQ)] on the generation of a potentially protective immune response to rNT-DsrAI. For all three immunogen doses tested (0.04, 4 and 20 µg), SQ immunization with Freund’s adjuvant elicited the highest endpoint titers over the immunization period (56 days, p<0.05 for area under the curve) (Fig. 4, top). This was not the case using the IM route since CpG and Imiquimod, administered with 20 µg of rNT-DsrAI, both elicited antisera with similar endpoint titers and H. ducreyi reactivity to those from animals receiving the immunogen in Freund’s (Fig. 4B). By generating similar humoral immune responses to the one obtained with Freund’s adjuvant, CpG or Imiquimod could replace Freund’s adjuvant in rNT-DsrAI-containing vaccines to induce a protective humoral immune response against H. ducreyi in humans.
Antisera from mice immunized with rNT-DsrAI formulated in a wide-range of adjuvants binds equally well to homologous and heterologous H. ducreyi strains.
To determine if antisera elicited to rNT-DsrAI recognized heterologous native DsrA in the H. ducreyi membrane, we measured reactivity of rNT-DsrAI Abs to the surface of viable, heterologous H. ducreyi strain HMC50. There were no differences in the quality of the immune response to the heterologous strain compared to the homologous bacteria (Fig. 5), except when the vaccine was administered with alum or MPL using the IM route (Fig. 5B, bottom). These results suggest that immunizing mice with a 20 µg dose of rNT-DsrAI in CpG or imiquimod elicits Abs that recognize a heterologous class I H. ducreyi strain.
Fig. 5. Intramuscular administration of rNT-DsrAI using the human-approved adjuvants CpG and Imiquimod elicits a humoral immune response that recognizes equally well DsrA at the surface of both homologous and heterologous H. ducreyi strains.
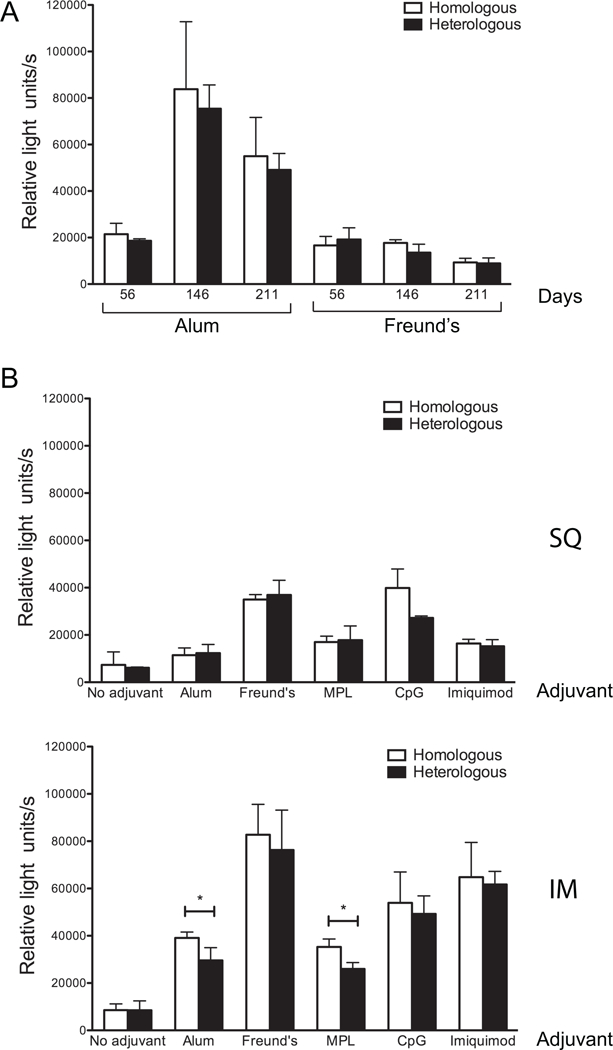
Reactivity of pooled rNT-DsrAI antisera to the surface of viable homologous (35000HP) and heterologous (HMC50) H. ducreyi strains. Shown are means ± standard deviations of three experiments conducted on three consecutive days. (A) Data from immunization trial 1 (20 µg dose, subcutaneous route only) are shown according to time point (days) and adjuvant. (B) Reactivity of antisera (day 56) from mice receiving a 20 µg dose of rNT-DsrAI alone or in combination with 5 different adjuvants, using the subcutaneous (SQ) or intramuscular (IM) route. *, p<0.05 using an unpaired t-test.
Adjuvant and route of immunization influence Ig isotype switching in response to the rNT-DsrAI vaccine.
To determine how dose, route and adjuvant affected the type of immune response elicited to the rNT-DsrAI vaccine, we determined Ig isotype switching by calculating the difference between Log2 IgG1 and Log2 IgG2a (which equals Log2(IgG1/IgG2a) in pooled antisera from mice immunized with 0.04, 4 or 20 µg of the immunogen alone, or in the presence of five different adjuvants. For the first immunogenicity study testing alum and Freund’s adjuvants only, ratios were above 1 for most animals, save Freund’s at the lowest 0.04 µg dose, suggesting an overall Th2-type response (Fig. 6A). Alum consistently provided the highest ratio compared to Freund’s or immunogen alone, while the ratios with Freund’s were similar to those for immunogen alone (Fig. 6A). Ratios remained similar for up to 211 days after primary immunization.
Fig. 6. Adjuvant, dose and route of immunization affect isotype switching of the humoral immune response to rNT-DsrAI.
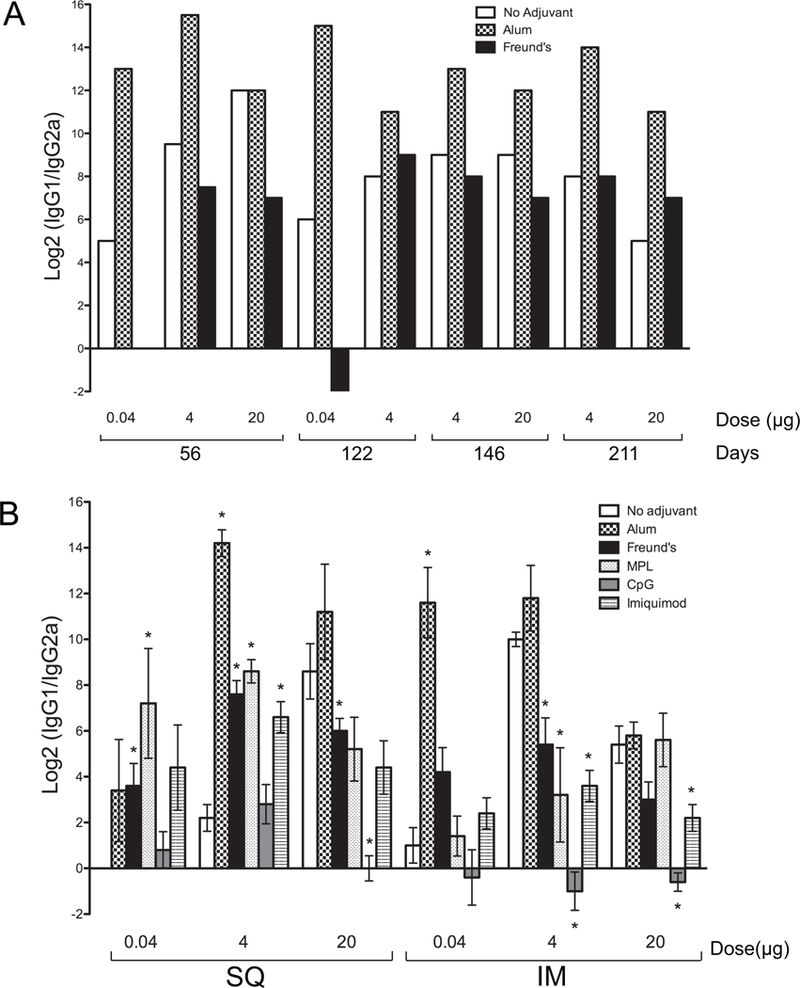
Log2 IgG1/IgG2a ratios of pooled (A) and individual (B) antisera from animals receiving 0.04, 4 and 20 µg doses administered alone, or in combination with one of 5 different adjuvants. A. Log2 IgG1/IgG2a ratios for different doses of rNT-DsrAI administered subcutaneously either alone, with Alum or Freund’s adjuvant were measured for the length of the study, up to 211 days. B. Log2 IgG1/IgG2a ratios of day 56 antisera from mice receiving the rNT-DsrAI vaccine with several different human-approved adjuvants administered subcutaneously (SQ) or intramuscularly (IM). *, p<0.05 compared to the “no adjuvant” control for each dose/route tested.
The same trend, with IgG1/IgG2a ratios equal or greater than 1, was also apparent in the second more comprehensive immunogenicity study (Fig. 6B). The exception was for the lowest dose of immunogen (0.04 µg) given alone subcutaneously, and with CpG, which resulted in IgG1/IgG2a ratios equal or lower than 1 for all doses and routes tested, save for the 4 µg dose SQ (Fig. 6B). In most cases, we again found that the IgG1/IgG2a ratios were highest in antisera from animals receiving the immunogen with alum (Fig. 6B). Ratios were significantly increased for most adjuvants when 4 µg of rNT-DsrAI was administered SQ, as compared to immunogen alone; however, this trend was reversed when the same dose of immunogen was given IM (Fig. 6B). For the 20 µg dose, SQ administration of the vaccine reduced IgG1/IgG2a ratios when the vaccine was administered with Freund’s or CpG, as compared to immunogen alone, while ratios were reduced in animals receiving CpG and Imiquimod immunized using the IM route (Fig. 6B). Overall, these results are consistent with the experimental rNT-DsrAI vaccine eliciting a Th2 rather than a Th1 mediated immune response; however, the choice of adjuvant and route of administration can significantly alter the IgG1/IgG2a ratio.
DISCUSSION
In this manuscript, we present findings from murine immunogenicity studies using a recombinant form of the passenger domain of the trimeric autotransporter adhesin DsrAI, rNT-DsrAI, as an experimental immunogen. The overarching goal of this study was to identify an adjuvant that could replace Freund’s in potency but be potentially safe for use in humans. Our first choice for a human translatable adjuvant was alum because it induces a strong humoral immune response like Freund’s [40] and is FDAapproved for human use. A 4 µg dose of immunogen was the optimal formulation for the induction of surface reactive Abs using Freund’s adjuvant (Fig. 2B). Doses of 4 or 20 µg showed the most promise with alum as the quality of the immune response, measured by reactivity of antisera to the surface of viable H. ducreyi, was significantly enhanced compared to administration of the immunogen alone (Fig. 2B). Although Ab titers from animals receiving the adjuvant in Freund’s or alum were not similar, the reactivity of Abs capable of binding the bacterial surface after immunization with 20 µg of rNT-DsrAI was comparable (Fig. 2B).
A long-term analysis of the humoral immune response to rNT-DsrAI revealed that day 56 peak titers persisted for 211 days post prime, even in the absence of adjuvant (Fig. 3). Administration of 20 µg of the immunogen elicited antisera whose surface reactivity was higher in animals receiving rNT-DsrAI in alum than when administered with Freund’s at endpoint (211 days; Fig. 3B). These findings confirmed that the quality of the humoral immune response elicited to a 20 µg dose of rNT-DsrAI immunized with alum is similar to that with Freund’s, and that it persists for long periods of time beyond the last antigenic stimulation.
In a second immunogenicity study, we investigated the humoral immune response elicited to 0.04, 4 or 20 µg of rNT-DsrAI either alone or in the presence of four different adjuvants safe for use in humans and the role of the immunization route in quantity and quality of the humoral immune response mounted against rNT-DsrAI. Most safe for human adjuvants administered with rNT-DsrAI elicited significantly higher quantity of Abs than immunogen alone at the doses tested (Fig. 4). Furthermore, some of these adjuvants generated Ab titers equal to those obtained from animals receiving rNT-DsrAI formulated with Freund’s adjuvant (Fig. 4). Endpoint titers were significantly higher when a 20 µg dose of the immunogen was administered intramuscularly, compared to subcutaneously, indicating a critical impact of route of immunization on host response (Fig. 4B). The importance of the IM route, shown for other vaccines [41], was reflected in our study of the quality (i. e. binding to viable bacteria) of the immune response after IM administration with alum, CpG and Imiquimod (Fig. 4B). Taken together, these data indicated that a 20 µg dose of rNT-DsrAI administered intramuscularly with the human-approved adjuvants alum, CpG or Imiquimod elicited a humoral immune response similar in quality to that of a known protective rNT-DsrAI vaccine formulated with Freund’s adjuvant [13].
To investigate the type (Th1 vs Th2) of immune response developed to our experimental rNT-DsrAI vaccine formulations, and the impact of adjuvant, dose and route on this immune response, we determined IgG1/IgG2a ratios. When administered alone, rNT-DsrAI elicits a Th2-type response, and the ratio increases with dose (Fig. 6). This is consistent with the nature of the protein immunogen and the BALB/c strain bias toward Th2 responses. In all combinations of dose, adjuvant and route, all IgG1/IgG2a ratios were above one, save for the adjuvant CpG (Fig. 6). Overall, our data indicate that the immunogen rNT-DsrAI elicits a Th2-type response; however, route and adjuvant affected the ratios as compared to immunogen alone.
H. ducreyi strains are grouped in classes, termed class I and II, according to polymorphisms in genome sequences [42, 43] and variant outer membrane determinants [17, 42, 44, 45], including DsrA. Although the DsrA proteins from the two classes of H. ducreyi strains share high amino acid homology in their C-terminal translocator domain, they vary greatly in their functional N-terminal passenger domain [17]. Abs directed to this domain of class I DsrA do not recognize class II DsrA, and vice versa [17, 46], which suggests that an immune response developed to rNT-DsrAI may not be protective against class II strains. Conversely, the newly described H. ducreyi strains that cause non-genital cutaneous ulcers are nearly identical to class I isolates [43], indicating that the rNT-DsrAI vaccine described herein is therefore very relevant to these strains and could be effective to prevent these infections caused by H. ducreyi.
In the research presented above, we used Ab binding at the surface of the bacteria as a correlate of protection of the rNT-DsrAI vaccine combinations tested. Surface binding of the Ab response elicited to the different vaccines tested was measured using a cell binding assay to whole, viable H. ducreyi. Since H. ducreyi has been shown to remain extracellular in both natural and experimental lesions [47, 48], vaccines that elicit Abs that bind to the surface of the bacteria could be protective through binding of complement components and/or macrophages that target H. ducreyi to the immune system. Abs that recognize DsrA at the bacterial surface could also block binding of H. ducreyi to cellular components such as fibrinogen [13]. It has yet to be determined if the rNT-DsrAI vaccine also elicits the cellular immune responses.
In conclusion, the findings from this immunogenicity study using a recombinant form of the trimeric autotransporter adhesin DsrA have informed us on several characteristics of this potential vaccine candidate for chancroid. First, the humoral immune response elicited to this vaccine is highly persistent, especially when administered with the human-approved adjuvant alum. Second, other adjuvants safe for use in humans, CpG and Imiquimod, may also be good candidate adjuvants for vaccination with rNT-DsrAI. Third, IM administration of rNT-DsrAI generated a humoral immune response with superior quantity and quality than SQ administration. Finally, except for CpG, all of the adjuvant, route and dose combinations investigated elicited a Th2-type immune response, indicative of the development of Abs specific to a major outer membrane protein of the extracellular bacteria H. ducreyi. This study has therefore identified potential adjuvants, route and dose, which may be of use in future human clinical trials involving this family of proteins.
HIGHLIGHTS.
The humoral immune response developed to rNT-DsrA is long-lasting
CpG and Imiquimod may be good adjuvants for the rNT-DsrA vaccine
IM administration of rNT-DsrA elicits an immune response superior to SQ
rNT-DsrA elicits a Th-2-type immune response
ACKNOWLEDGEMENTS
This work was supported by the Southeastern Sexually Transmitted Infections Cooperative Research Center funded by the US National Institutes of Health (U19AI031496). Research was performed in the Regional Biocontainment Laboratory at Duke, which received partial support for construction from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (UC6-AI058607). We are grateful to Dr. P. Frederick Sparling for careful review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing personal or financial interests.
REFERENCES
- [1].Ussher JE, Wilson E, Campanella S, Taylor SL, Roberts SA. Haemophilus ducreyi causing chronic skin ulceration in children visiting Samoa. Clin Infect Dis 2007;44:e85–7. [DOI] [PubMed] [Google Scholar]
- [2].McBride WJ, Hannah RC, Le Cornec GM, Bletchly C. Cutaneous chancroid in a visitor from Vanuatu. Australas J Dermatol 2008;49:98–9. [DOI] [PubMed] [Google Scholar]
- [3].Peel TN, Bhatti D, De Boer JC, Stratov I, Spelman DW. Chronic cutaneous ulcers secondary to Haemophilus ducreyi infection. Med J Aust 2010;192:348–50. [DOI] [PubMed] [Google Scholar]
- [4].Mitja O, Lukehart SA, Pokowas G, Moses P, Kapa A, Godornes C, et al. Haemophilus ducreyi as a cause of skin ulcers in children from a yaws-endemic area of Papua New Guinea: a prospective cohort study. Lancet Glob Health 2014;2:e235–41. [DOI] [PubMed] [Google Scholar]
- [5].Marks M, Chi KH, Vahi V, Pillay A, Sokana O, Pavluck A, et al. Haemophilus ducreyi associated with skin ulcers among children, Solomon Islands. Emerging infectious diseases 2014;20:1705–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ghinai R, El-Duah P, Chi KH, Pillay A, Solomon AW, Bailey RL, et al. A crosssectional study of ‘yaws’ in districts of Ghana which have previously undertaken azithromycin mass drug administration for trachoma control. PLoS neglected tropical diseases 2015;9:e0003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mitja O, Houinei W, Moses P, Kapa A, Paru R, Hays R, et al. Mass treatment with single-dose azithromycin for yaws. N Engl J Med 2015;372:703–10. [DOI] [PubMed] [Google Scholar]
- [8].Elkins C, Morrow KJ Jr., Olsen B Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect Immun 2000;68:1608–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abdullah M, Nepluev I, Afonina G, Ram S, Rice P, Cade W, et al. Killing of dsrA mutants of Haemophilus ducreyi by normal human serum occurs via the classical complement pathway and is initiated by immunoglobulin M binding. Infect Immun 2005;73:3431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cole LE, Kawula TH, Toffer KL, Elkins C. The Haemophilus ducreyi serum resistance antigen DsrA confers attachment to human keratinocytes. Infect Immun 2002;70:6158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leduc I, White CD, Nepluev I, Throm RE, Spinola SM, Elkins C. Outer membrane protein DsrA is the major fibronectin-binding determinant of Haemophilus ducreyi. Infect Immun 2008;76:1608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fusco WG, Elkins C, Leduc I. Trimeric Autotransporter DsrA Is a Major Mediator of Fibrinogen Binding in Haemophilus ducreyi. Infect Immun 2013;81:4443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fusco WG, Choudhary NR, Routh PA, Ventevogel MS, Smith VA, Koch GG, et al. The Haemophilus ducreyi trimeric autotransporter adhesin DsrA protects against an experimental infection in the swine model of chancroid. Vaccine 2014;32:3752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Al-Tawfiq JA, Thornton AC, Katz BP, Fortney KR, Todd KD, Hood AF, et al. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J Infect Dis 1998;178:1684–7. [DOI] [PubMed] [Google Scholar]
- [15].Hammond GW, Lian CJ, Wilt JC, Ronald AR. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrobial Agents & Chemotherapy 1978;13:608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Method Enzymol 1990;185:60–89. [DOI] [PubMed] [Google Scholar]
- [17].White CD, Leduc I, Olsen B, Jeter C, Harris C, Elkins C. Haemophilus ducreyi Outer membrane determinants, including DsrA, define two clonal populations. Infect Immun 2005;73:2387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Leduc I, Richards P, Davis C, Schilling B, Elkins C. A novel lectin, DltA, is required for expression of a full serum resistance phenotype in Haemophilus ducreyi. Infect Immun 2004;72:3418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fusco WG, Choudhary NR, Routh PA, Ventevogel MS, Smith VA, Koch GG, et al. The Haemophilus ducreyi trimeric autotransporter adhesin DsrA protects against an experimental infection in the swine model of chancroid. Vaccine 2014. [DOI] [PMC free article] [PubMed]
- [20].Leduc I, Olsen B, Elkins C. Localization of the domains of the Haemophilus ducreyi trimeric autotransporter DsrA involved in serum resistance and binding to the extracellular matrix proteins fibronectin and vitronectin. Infect Immun 2009;77:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fusco WG, Afonina G, Nepluev I, Cholon DM, Choudhary N, Routh PA, et al. Immunization with the Haemophilus ducreyi hemoglobin receptor HgbA with adjuvant monophosphoryl lipid A protects swine from a homologous but not a heterologous challenge. Infect Immun 2010;78:3763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Leduc I, Fusco WG, Choudhary N, Routh PA, Cholon DM, Almond GW, et al. Passive immunization with a polyclonal antiserum to the hemoglobin receptor of Haemophilus ducreyi confers protection against a homologous challenge in the experimental swine model of chancroid. Infect Immun 2011;79:3168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity 2010;33:492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fransen F, Boog CJ, van Putten JP, van der Ley P. Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B. Infect Immun 2007;75:5939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rhee EG, Kelley RP, Agarwal I, Lynch DM, La Porte A, Simmons NL, et al. TLR4 ligands augment antigen-specific CD8+ T lymphocyte responses elicited by a viral vaccine vector. Journal of virology 2010;84:10413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].FDA. FDA Approves New Vaccine for Prevention of Cervical Cancer http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm187048.htm. U.S. Food and Drug Administration; 2009. [Google Scholar]
- [27].El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis 2010;202:1649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine 2011;29:3341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Minang JT, Inglefield JR, Harris AM, Lathey JL, Alleva DG, Sweeney DL, et al. Enhanced early innate and T cell-mediated responses in subjects immunized with Anthrax Vaccine Adsorbed Plus CPG 7909 (AV7909). Vaccine 2014;32:6847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hopkins RJ, Daczkowski NF, Kaptur PE, Muse D, Sheldon E, LaForce C, et al. Randomized, double-blind, placebo-controlled, safety and immunogenicity study of 4 formulations of Anthrax Vaccine Adsorbed plus CPG 7909 (AV7909) in healthy adult volunteers. Vaccine 2013;31:3051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rynkiewicz D, Rathkopf M, Sim I, Waytes AT, Hopkins RJ, Giri L, et al. Marked enhancement of the immune response to BioThrax(R) (Anthrax Vaccine Adsorbed) by the TLR9 agonist CPG 7909 in healthy volunteers. Vaccine 2011;29:6313–20. [DOI] [PubMed] [Google Scholar]
- [32].Ellis RD, Wu Y, Martin LB, Shaffer D, Miura K, Aebig J, et al. Phase 1 study in malaria naive adults of BSAM2/Alhydrogel(R)+CPG 7909, a blood stage vaccine against P. falciparum malaria. PLoS One 2012;7:e46094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Crompton PD, Mircetic M, Weiss G, Baughman A, Huang C- Y, Topham DJ, et al. The TLR9 Ligand CpG Promotes the Acquisition of Plasmodium falciparum-Specific Memory B Cells in Malaria-Naive Individuals. J Immunol 2009;182:3318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ohno S, Okuyama R, Aruga A, Sugiyama H, Yamamoto M. Phase I trial of Wilms’ Tumor 1 (WT1) peptide vaccine with GM-CSF or CpG in patients with solid malignancy. Anticancer Res 2012;32:2263–9. [PubMed] [Google Scholar]
- [35].Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, Hammann-Haenni A, et al. Nano-particle vaccination combined with TLR-7 and −9 ligands triggers memory and effector CD8(+) T-cell responses in melanoma patients. Eur J Immunol 2012;42:3049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].FDA. FDA Approves New Use of Drug to Treat Superficial Basal Cell Carcinoma, a Type of Skin Cancer 2004. [Google Scholar]
- [37].Hung IF, Zhang AJ, To KK, Chan JF, Li C, Zhu HS, et al. Immunogenicity of intradermal trivalent influenza vaccine with topical imiquimod: a double blind randomized controlled trial. Clin Infect Dis 2014;59:1246–55. [DOI] [PubMed] [Google Scholar]
- [38].Roukens AH, Vossen AC, Boland GJ, Verduyn W, van Dissel JT, Visser LG. Intradermal hepatitis B vaccination in non-responders after topical application of imiquimod (Aldara). Vaccine 2010;28:4288–93. [DOI] [PubMed] [Google Scholar]
- [39].Adams S, O’Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol 2008;181:776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine 2009;27:3331–4. [DOI] [PubMed] [Google Scholar]
- [41].Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ 2000;321:1237–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ricotta EE, Wang N, Cutler R, Lawrence JG, Humphreys TL. Rapid divergence of two classes of Haemophilus ducreyi. J Bacteriol 2011;193:2941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gangaiah D, Webb KM, Humphreys TL, Fortney KR, Toh E, Tai A, et al. Haemophilus ducreyi Cutaneous Ulcer Strains Are Nearly Identical to Class I Genital Ulcer Strains. PLoS neglected tropical diseases 2015;9:e0003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Post DM, Gibson BW. Proposed second class of Haemophilus ducreyi strains show altered protein and lipooligosaccharide profiles. Proteomics 2007;7:3131–42. [DOI] [PubMed] [Google Scholar]
- [45].Post DM, Munson RS Jr., Baker B, Zhong H, Bozue JA, Gibson BW. Identification of genes involved in the expression of atypical lipooligosaccharide structures from a second class of Haemophilus ducreyi. Infect Immun 2007;75:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fusco WG, Choudhary NR, Stewart SM, Alam SM, Sempowski GD, Elkins C, et al. Defining Potential Vaccine Targets of Haemophilus ducreyi Trimeric Autotransporter Adhesin DsrA. Monoclonal antibodies in immunodiagnosis and immunotherapy 2015;34:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bauer ME, Goheen MP, Townsend CA, Spinola SM. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infection & Immunity 2001;69:2549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bauer ME, Townsend CA, Ronald AR, Spinola SM. Localization of Haemophilus ducreyi in naturally acquired chancroidal ulcers. Microbes Infect 2006;8:2465–8. [DOI] [PubMed] [Google Scholar]


