Recent recommendations by the Food and Drug Administration1 and the European Medicines Agency2 are to limit the clinical use of linear gadolinium-based contrast agents (GBCAs) due to convincing evidence of deposition in tissues. Macrocyclic GBCA continued to be considered safe, provided that patients have normal renal function. To date, given the low sensitivity of conventional MRI, there has been a debate about the signal increase following the injections of a macrocyclic GBCA.3,4
To overcome the relative insensitivity of MRI for gadolinium,5,6 inductively coupled plasma mass spectrometry (ICP-MS) has become a useful tool to measure gadolinium in human tissues.7,8 When coupled with a laser ablation system (LA), LA-ICP-MS can be used as a molecular microscope reaching lateral resolution in the micrometer range.9 We used LA-ICP-MS (Materials and Methods e-1, links.lww.com/NXI/A85) to retrospectively analyze human brain tissue. Exclusion criteria were reported linear GBCA application, an impaired renal function at the time point of gadolinium application, contrast enhancement in the cerebellum on MRI, and structural cerebellar changes (tumor, inflammation, edema, hypoxia, or demyelination). Inclusion criteria were a diagnostic autopsy with archived paraffin-embedded tissue in the archive of the Department of Neuropathology Charité-Universitätsmedizin Berlin and recorded application of macrocyclic GBCA for diagnostic MRI analysis before death. Autopsy tissue sections from the cerebellum of 2 patients were chosen randomly. An age-matched control was a randomly selected patient without recorded GBCA application.
The first patient (Gd1) was a woman, aged 65 years, died because of sepsis with endocarditis. The cerebral MRI with IV application of 7 mL Gadovist was performed 2 weeks before death due to cerebral ischemic infarction. The second patient (Gd2) was a man, aged 63 years, died because of pneumonia and heart failure. He underwent 4 MRI scans with IV administration of macrocyclic GBCA before death due to anti-NMDA-R encephalitis (13 mL Dotarem 4 weeks, 7 mL Gadovist 9 weeks, 15 mL Dotarem 22 weeks, and 7 mL Gadovist 25 weeks before death). The control (Co1) was a man, aged 54 years, died because of a traumatic brain injury with no history of receiving any GBCA.
The possibility of gadolinium accumulation due to environmental exposure could be excluded by the analysis of another element of the lanthanides family, europium (figure e-1 links.lww.com/NXI/A83), and an age-matched control with no reported GBCA application (figure).
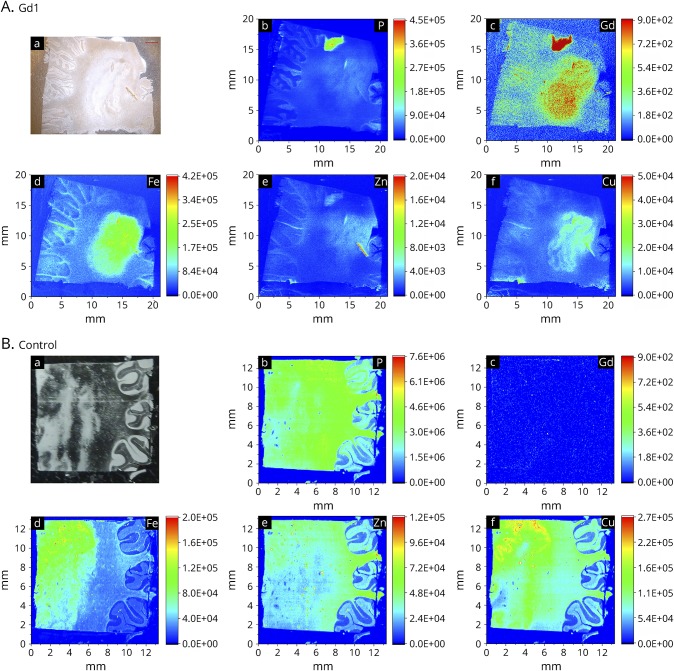
Figure. Representative images of the cerebellum of patient Gd1 who received Gadovist 2 weeks before death and the control patient.
Patient Gd1 (A) and control patient (B): (a) Light image of a paraffin-embedded section through the cerebellum. (b–f) LA-ICP-MS element distribution maps of phosphorus (P), gadolinium (Gd), iron (Fe), zinc (Zn), and copper (Cu). Although the P signal is less in the Gd1 image compared with the control, the relative intensities show clearly different concentrations of P among the section. In the control image, the signal is higher because of superior instruments settings but shows a uniform distribution of P with no “hotspot” as in Gd1.
As previous MRI studies showed high levels of GBCA-derived gadolinium depositions in the dentate nucleus,10 we analyzed the cerebellum. We could detect gadolinium signal in the dentate nucleus and cerebellar cortex of patient Gd1, who received macrocyclic GBCAs 2 times including the last application 2 weeks before death. The signal colocalized with iron, copper, zinc, and phosphorous (figure). Accumulation of gadolinium and phosphorous in this patient in the white mater could not be explained by an artifact because the amounts of iron, copper, and sulfur in that area are very low (figure).
The cerebellar section of the second patient (Gd2), who received macrocyclic GBCA 4 times, including the last application 4 weeks before death, shows substantially lower gadolinium signal in the dentate nucleus in comparison to Gd1 (figure e-2 links.lww.com/NXI/A84). The different amount of residual gadolinium could be in accordance with time-dependent excretion that remains to be studied in large patient cohorts. Quantification experiments revealed that the amount of gadolinium in patients Gd1 and Gd2 are 36 and 2 ng/g brain tissue, respectively. The amount of gadolinium after linear GBCA administration was reported to be 2,100 ng/g in human dentate nucleus after 2 weeks.7 In contrast to macrocyclic, dechelation was shown for linear GBCAs in animal studies, leading to deposits that are probably not washed out over time.6,11,12 Although we can detect possibly macrocyclic GBCA in our patients, the technique cannot provide evidence of a potential gadolinium release from the macrocyclic GBCA. The main limitations of our study are the use of heterogenic archived human material making it difficult to control the timing and quality of GBCA injections and therefore difficult to evaluate a time dependency.
To date, no signs of adverse health effects and no morphological changes have been associated with gadolinium in the brain. Nevertheless, the presence of probably macrocyclic gadolinium in human (cerebellar) tissue should stimulate further research also on other anatomical sites. This is particularly important in the context of repetitive gadolinium administration in patients with neurologic progressive diseases and an impaired blood brain barrier.
Future studies including patients with different numbers of macrocyclic GBCA applications and longer time intervals before death (up to decades) with quantitative data are necessary.
Author contributions
E. Schellenberger and N. Jakubowski conceived the project and designed experiments. A.H. El-Khatib, H. Radbruch, S. Trog, F. Paul, and E. Schellenberger wrote the manuscript. H. Radbruch and A. Koch prepared the samples and performed histologic examination. B. Neumann applied the internal standard. A.H. El-Khatib and S. Trog performed the LA-ICP-MS measurements. A.H. El-Khatib performed the LA-ICP-MS data analysis, produced the images, and performed tissue digestion and ICP-MS analysis. M.W. Linscheid, E. Schellenberger, and N. Jakubowski supervised the work.
Study funding
No targeted funding reported.
Disclosure
H. Radbruch received travel funding and/or speaker honoraria from Novartis and Sanofi and received research support from Novartis, Sanofi, and Deutsche Forschungsgemeinschaft. A.H. El-Khatib and S. Trog report no disclosures. B. Neumann is a senior scientist of Proteome Factory AG. F. Paul served on the steering committees of Novartis and MedImmune; received speaker honoraria and travel funding from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, and Shire; is an academic editor of PLoS ONE and an associate editor of Neurology: Neuroimmunology & Neuroinflammation; consulted for Sanofi Genzyme, Biogen, MedImmune, Shire, and Alexion; and received research support from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Alexion, Merck Serono, German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, EU FP7 Framework Program, Arthur Arnstein Foundation Berlin, Guthy-Jackson Charitable Foundation, and NMSS. A. Koch reports no disclosures. M. Linscheid is an editor of the Journal of Mass Spectrometry and holds a patent for metal labeling of proteins software for controlling mass spectrometric experiments capillaries for micro-ESI MS. N. Jakubowski received publishing royalties from Cambridge. E. Schellenberger received research support from the German Research Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NN.
References
- 1.FDA. FDA Drug Safety Communication: FDA identifies no harmful effects to date with brain retention of gadolinium-based contrast agents for MRIs; review to continue [online]. Available at: fda.gov/Drugs/DrugSafety/ucm559007.htm. Accessed September 22, 2017.
- 2.EMA. EMA's final opinion confirms restrictions on use of linear gadolinium agents in body scans [online]. Available at: ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Gadolinium-containing_contrast_agents/human_referral_prac_000056.jsp. Accessed September 22, 2017.
- 3.Radbruch A, Quattrocchi CC. Interpreting signal-intensity ratios without visible T1 hyperintensities in clinical gadolinium retention studies. Pediatr Radiol 2017;47:1688–1689. [DOI] [PubMed] [Google Scholar]
- 4.Espagnet MCR, Bernardi B, Pasquini L, Figa-Talamanca L, Toma P, Napolitano A. Erratum to: signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol 2017;47:1366. [DOI] [PubMed] [Google Scholar]
- 5.Jost G, Lenhard DC, Sieber MA, Lohrke J, Frenzel T, Pietsch H. Signal increase on unenhanced T1-weighted images in the rat brain after repeated, extended doses of gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Invest Radiol 2016;51:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert P, Lehericy S, Grand S, et al. . T1-Weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol 2015;50:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda T, Fukusato T, Matsuda M, et al. . Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015;276:228–232. [DOI] [PubMed] [Google Scholar]
- 8.McDonald RJ, McDonald JS, Kallmes DF, et al. . Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015;275:772–782. [DOI] [PubMed] [Google Scholar]
- 9.Becker JS, Jakubowski N. The synergy of elemental and biomolecular mass spectrometry: new analytical strategies in life sciences. Chem Soc Rev 2009;38:1969–1983. [DOI] [PubMed] [Google Scholar]
- 10.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834–841. [DOI] [PubMed] [Google Scholar]
- 11.Frenzel T, Apte C, Jost G, Schockel L, Lohrke J, Pietsch H. Quantification and assessment of the chemical form of residual gadolinium in the brain after repeated administration of gadolinium-based contrast agents: comparative study in rats. Invest Radiol 2017;52:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianolio E, Bardini P, Arena F, et al. . Gadolinium retention in the rat brain: assessment of the amounts of insoluble gadolinium-containing species and intact gadolinium complexes after repeated administration of gadolinium-based contrast agents. Radiology 2017;285:839–849. [DOI] [PubMed] [Google Scholar]