Abstract
Objective
This investigation aimed at examining whether circulating inflammatory biomarkers C-reactive protein (CRP), interleukin-6 (IL6), and alpha 1-antichymotrypsin (ACT) were related to cerebrovascular disease (CVD) assessed by MRI.
Methods
The study included nondemented elderly participants of a community-based, multiethnic cohort, who received baseline MRI scans and had CRP (n = 508), ACT (435), and IL6 (N = 357) measured by ELISA. Silent brain infarcts and white matter hyperintensities (WMH) were derived from all available MRI scans at baseline, approximately 4.4 years after blood sample collection for inflammatory biomarkers. Repeated assessments of infarcts and WMH, as well as microbleeds assessment, were performed at follow-up MRI visits around 4.5 years later. Cross-sectional and longitudinal relationship between inflammatory biomarkers and CVD were analyzed using appropriate logistic regression models, generalized linear models, or COX models.
Results
After adjusting for age, sex, ethnicity, education, APOE genotype, and intracranial volume, 1 SD increase in log10IL6 was associated with infarcts on MRI {odds ratio [OR] (95% confidence interval [CI]) = 1.28 [1.02–1.60], p = 0.033}, and 1 SD increase in log10CRP and log10ACT was associated with microbleeds (OR [95% CI] = 1.46 [1.02–2.09], p = 0.041; and 1.65 [1.11–2.46], p = 0.013; respectively). One SD increase in log10ACT was also associated with larger WMH at the follow-up MRI (b = 0.103, p = 0.012) and increased accumulation of WMH volume (b = 0.062, p = 0.041) during follow-up. The associations remained significant after additional adjustment of vascular risk factors and excluding participants with clinical stroke.
Conclusions
Among older adults, increased circulating inflammatory biomarkers were associated with the presence of infarcts and microbleeds, WMH burden, and progression of WMH.
Subclinical cerebrovascular diseases (CVDs), including primarily lacunar infarcts or silent brain infarcts, white matter hyperintensities (WMH), and cerebral microbleeds, are frequent findings on MRI scans of normal elderly people.1–3 “Silent” changes in the brain reflect individuals carrying subclinical CVD without clinical symptoms, but they are not benign because of the strong association with increased risk of recurrent strokes,4 dementia,5 and mortality.
Strong evidence suggests that chronic inflammation plays a crucial role in the development of stroke, cardiovascular disease, dementia,6 and atherosclerosis.7 Therefore, it is reasonable to hypothesize that chronic inflammation might also be involved in arteriolosclerosis damage of brain vessels, manifested radiologically as localized ischemic infarcts, diffuse WMH, or microbleeds.8 However, the association between inflammatory biomarkers and CVD remains unclear.9–27 Some existing studies were based on clinical convenience samples, some included middle-aged participants, and few studies included Hispanics or African-Americans10,11,18,19 who have a higher frequency of vascular risk factors.28 Therefore, the results may not be applied to clinically normal, ethnically diverse, elderly populations who could be the key target population for potential intervention. In addition, there are few longitudinal studies9,15,22,23 and few studies13,19–21 that explored microbleeds. Most studies measured 2 key inflammatory markers c-reactive protein (CRP) and interleukin-6 (IL6), although alpha 1-antichymotrypsin (ACT), which has long been implicated in AD,29 has never been examined.
We examined whether circulating levels of inflammatory markers (CRP, IL6, and ACT) were associated with the presence and progression of CVD (i.e., infarcts, WMH, and microbleeds) among nondemented elderly participants of a community-based, multiethnic cohort.
Methods
Study participants
The current study included participants from an ongoing prospective study of aging and dementia, the Washington Heights-Hamilton Height-Inwood and Columbia Aging Project (WHICAP), who were elders identified from Medicare beneficiaries residing in northern Manhattan.30 The original cohort for this study included 2,776 participants. At baseline, participants were asked about medical and neurologic history, received assessments of health and function, and were assessed using a neuropsychological battery. Participants were followed approximately for 18 months, repeating the baseline examinations. The diagnosis of dementia or its absence was based on standard research criteria using all available information at a consensus conference.
A total of 769 WHICAP participants received MRI scans including T1-weighted images, fluid-attenuated inversion recovery (FLAIR)-weighted images, and proton density and T2-weighted double-echo images.31 Among the 680 nondemented (n = 45 demented) participants who had baseline MRI data available and analyzed by FreeSurfer (n = 44 without FreeSurefer processing),30 a total of 508 (75%) participants had CRP, 435 (64%) had ACT, and 357 (53%) had IL6 detected (figure 1). Participants who had measured biomarkers were younger, had fewer African-Americans, had fewer vascular risk factors than those who did not have biomarker data sample.30 Repeated MRI scans were completed for 310 participants 4.5 (SD = 0.9) years after their baseline scans, and 230 of them also received gradient echo (GRE) scans for microbleeds assessment. Compared with the neuroimaging project in which participants did not have repeated scans, those with repeated scans were younger at baseline (79.3 vs 80.7, p = 0.002) and had more hypertension (90% vs 83%, p = 0.08).30
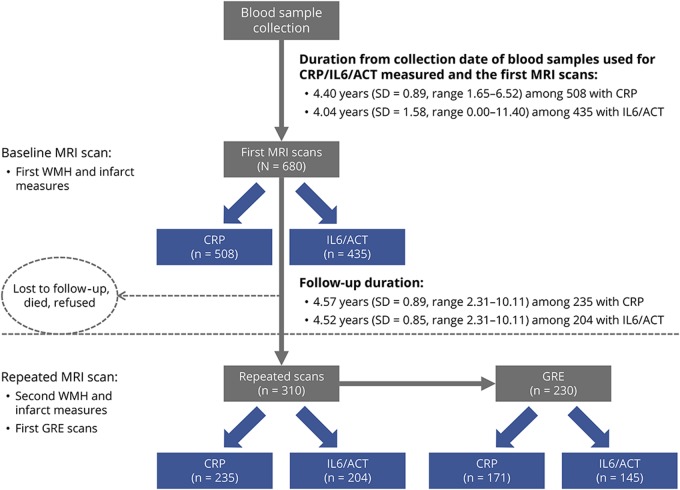
Figure 1. Selection of the study population.
Selection of study participants from the neuroimaging study of the Washington Heights/Hamilton Heights Inwood Columbia Aging Project (WHICAP). The study participants were those participants of the WHICAP imaging substudy started in 2004. A total of 769 WHICAP participants received MRI scans including T1-weighted images, FLAIR-weighted images, and proton density and T2-weighted double-echo images, which measured white matter hyperintensity (WMH) and infarcts. Among the 680 nondemented (n = 45 demented) participants who had baseline MRI data available and analyzed by FreeSurfer (n = 44 without FreeSurefer processing), a total of 508 (75%) participants had CRP and 435 (64%) had ACT/IL6 detected (figure 1). For the analysis for IL6, 357 participants were included after excluding 78 participants whose IL6 levels were out of the measurement range. A total of 310 (46% of 680) participants completed repeat MRI scans approximately 4.5 (SD = 0.9) years after their baseline scans, and 230 (74% of 310) of them also received gradient echo (GRE) scans for microbleeds assessment. In total, 235 (76%) had CRP and 204 (66%) had IL6/ACT measured among the 310 participants who received second structural MRI scans, and 171 (74%) had CRP and 145 (63%) had IL6/ACT measured among those who received GRE scans (figure 1). ACT = alpha 1-antichymotrypsin; CRP = C-reactive protein; IL6 = interleukin-6.
Standard protocol approvals, registrations, and patient consents
The Columbia University Institutional Review Board has reviewed and approved this project. All individuals provided written informed consent.
MRI protocol
All scans were acquired on a 1.5T Philips Intera scanner at Columbia University. T1-weighted images were acquired with a repetition time of 20 milliseconds (ms); echo time, 2.1 ms; field of view (FOV), 240 cm; 256 × 160–pixel matrix with 1.3-mm section thickness, and voxel size 1 × 1 × 1.3 mm. Freesurfer (V.5.1) (surfer.nmr.mgh.harvard.edu/) was used to analyze T1 images with visual quality control and manual correction. WMH quantification was based on T2-weighted FLAIR MRI scans (repetition time, 11,000 ms; echo time, 144 ms; inversion time, 2,800; and FOV 25 cm, 2 nex, 256 × 192 matrix with 3-mm slice thickness). An optimized, high-resolution three-dimensional T2*-weighted GRE image (repetition time, 45 ms; echo time, 31 ms; flip angle, 13; and slice thickness, 2 mm) was acquired at the follow-up MRI visit for microbleeds visualization and quantification. The imaging processing was blinded to any information about participants.
We used all the available images to determine whether a participant had brain infarction on MRI, which was defined as lesions 3 mm or larger. Signal void seen on the T2-weighted images was used to indicate a vessel. We also consider other necessary imaging characteristics such as CSF density on the T1-weighted image and distinct separation from the circle of Willis vessels and perivascular spaces.
Total WMH volume was determined following the procedures as previously described.32 First, FLAIR images were skull stripped, and then a threshold and seed-growing algorithm was applied to identify voxels that fell within an a priori–determined distribution of hyperintense signal. Total WMH volume was then calculated by multiplying labeled voxels by voxel dimensions. We calculated the ratio of WMH over total intracranial volume (ICV) to adjust the head size differences. We log-transformed WMH/ICV ratio value in the analyses.
Microbleeds were visually inspected and rated according to the research criteria.2 We classified microbleeds into “lobar” (in the frontal, temporal, parietal, and occipital lobes) and “deep” (in basal ganglia, thalamus, and infratentorial regions). The number of microbleeds was also recorded. A dichotomous microbleeds status, presence (at least one microbleeds) vs absence (as reference group), was used in the main analysis.
Biomarker measurement
The circulating levels of CRP, IL6, and ACT were measured in previous studies of WHICAP. Nonfasting plasma (CRP) and serum (IL6 and ACT) samples, collected 4.4 years before the first MRI scan (figure 1), were used in all analyses. Briefly, blood samples were collected in BD Vacutainer Ethylenediaminetetraacetic acid (EDTA) tubes and centrifuged at 2,000g for 15 minutes at room temperature and then stored at −80°C in polypropylene cryotubes until analysis. For serum samples, collected blood samples were allowed to sit for 30 minutes before they were centrifuged, aliquoted, and stored at −80°C. The CRP levels were measured using a high-sensitivity (1.6 ng/mL) ELISA kit (Diagnostic systems laboratories, Inc., Webster, TX, with the intra-assay and interassay coefficient of variations (CVs) of 4.6% and 11.7%, respectively. The IL-6 levels were measured using a high-sensitivity (0.11 pg/mL) ELISA kit (R&D Systems, Minneapolis, MN; intra-assay CV = 7.4% and inter-assay CV = 7.8%). The ACT levels were measured using an immunoperoxidase assay kit (Immunology Consultants Laboratory, Inc., Newberg; sensitivity of 2.518 ng/mL; both intra-assay and interassay CVs <10%). Laboratory personnel were blinded as to the demographic and clinical status of the study participants.
Covariates
Ethnicity, including Hispanic, non-Hispanic black, non-Hispanic white, or Other, based on self-report using the format of the 2000 US census, was used as a dummy variable with non-Hispanic white or other as the reference. Sex (male as the reference), smoking status (nonsmoker [as the reference] or ever smoked [including current smokers and past smokers who had ≥1 cigarette/day for ≥1 year]), and apolipoprotein (APOE) status (presence of either 1 or 2 vs absence of ε4 alleles [as the reference]), were used as dichotomous variables. Body mass index (BMI; kg/m2) was calculated as weight over height squared. The presence of depression was defined as ≥4 on the 10-item version of the Center for Epidemiologic Studies Depression scale. Presence or absence of heart disease, diabetes mellitus, and hypertension, as well as the use of medication for the conditions, were based on self-reported information, and clinical stroke was determined by self-report, neurologic examination, or a review of medical records. A vascular comorbidity burden score (range 0–4) was calculated by summing these 4 dichotomized vascular comorbidity variables. Fasting plasma lipids were determined on baseline blood samples using standard techniques.
Statistical analyses
Characteristic variables of the participants
Circulating levels of inflammatory biomarkers were transformed using logarithm and then standardized ([log10Biomarker − mean of log10Biomarker]/SD of log10Biomarker). The resulting standardized values were approximately normally distributed. Demographics, clinical characteristics, vascular and lifestyle factors, and brain imaging findings of participants by biomarker tertiles were compared using analysis of variance for continuous variables and the χ2 test for categorical variables.
Cross-sectional associations between the inflammatory biomarkers and cerebrovascular disease
Generalized linear models were used to test the associations between the inflammatory biomarkers and baseline WMH, and logistic regression models were used for the inflammatory biomarkers and infarcts or microbleeds status. All the models were adjusted for age at time of scan, sex, education, ethnicity, and APOE ε4 status. In the fully adjusted models, we additionally adjusted for health and vascular risk factors, including BMI, depression, plasma lipids level, smoking status, and vascular comorbidity burden. All covariates were treated as time-independent variables.
Longitudinal data analysis
The changes of WMH were calculated as [log10(follow-up WMH/ICV) − log10(baseline WMH/ICV)]. Multiple regression models were used to test whether inflammatory biomarkers were associated with differential WMH change, adjusted for time from baseline scan to the follow-up scan in addition to all the other covariates. Cox proportional hazard models were used to examine whether baseline inflammatory biomarkers were associated with the risk of developing incident infarcts among those without infarcts at baseline.
Supplementary analyses
We excluded 102 (20.1% of 508) participants with clinical stroke and repeated the analyses only among participants without clinical stroke. We also excluded 24 participants who developed incident dementia during follow-up and repeated the analysis for microbleeds and infarcts at follow-up. In addition, we examined whether certain regions were particularly vulnerable to inflammatory effects by examining WMH in the frontal, parietal, occipital, and temporal regions separately and by examining lobar and deep microbleeds separately. Finally, we examined the relationship between inflammatory biomarkers and CVD by race/ethnic groups, sex, and APOE status.
All analyses were performed using PASW Statistics 17.0 (formerly SPSS Inc., Chicago, IL). All p values were based on two-sided tests with the statistical significance level set at 0.05.
Data availability
The data that support the findings of this study are available on request from the corresponding author, yg2121@columbia.edu. Some access restrictions apply to the data used for the current study because of privacy concerns including the following: (1) the data contain elements that are considered Protected Health Information under HIPAA regulations; and (2) there is a potential risk for linkage of data made publicly available in conjunction with multiple studies based on the same cohort. Although the data on which the manuscript is based are not publicly available, a limited data set is available under a standard HIPAA Data Use Agreement, subject to review and approval by the Columbia University Privacy Officer.
Results
Demographic and cerebrovascular disease characteristics according to levels of inflammatory biomarkers
The 3 inflammatory markers positively correlated with each other,30 with correlation coefficients between CRP and IL6, between CRP and ACT, and between IL6 and ACT being 0.51, 0.39, and 0.21, respectively, all p < 0.0001.
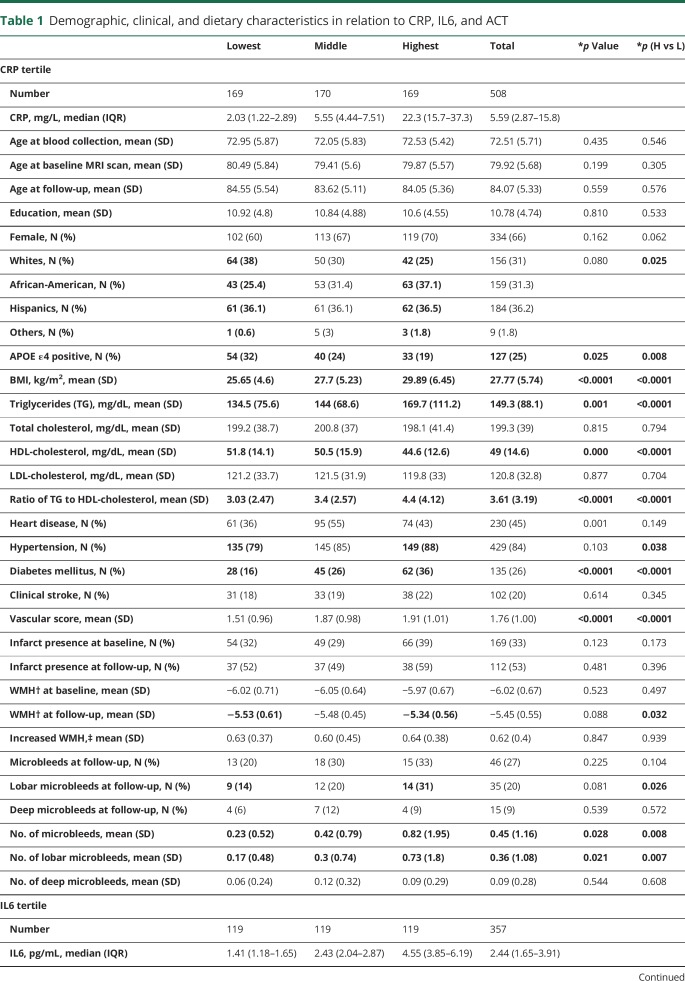
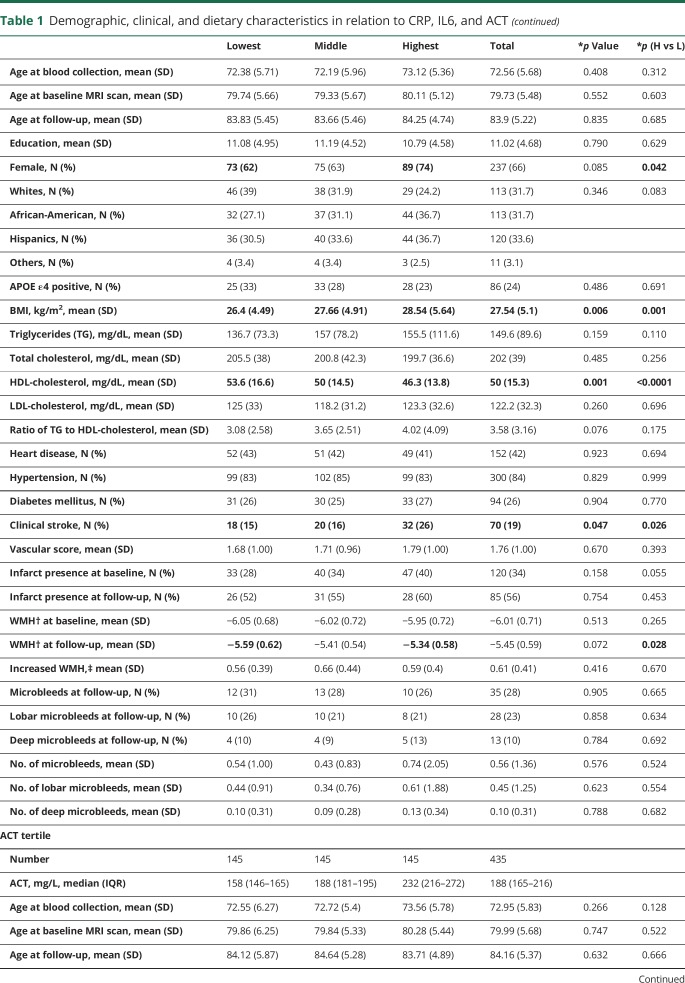
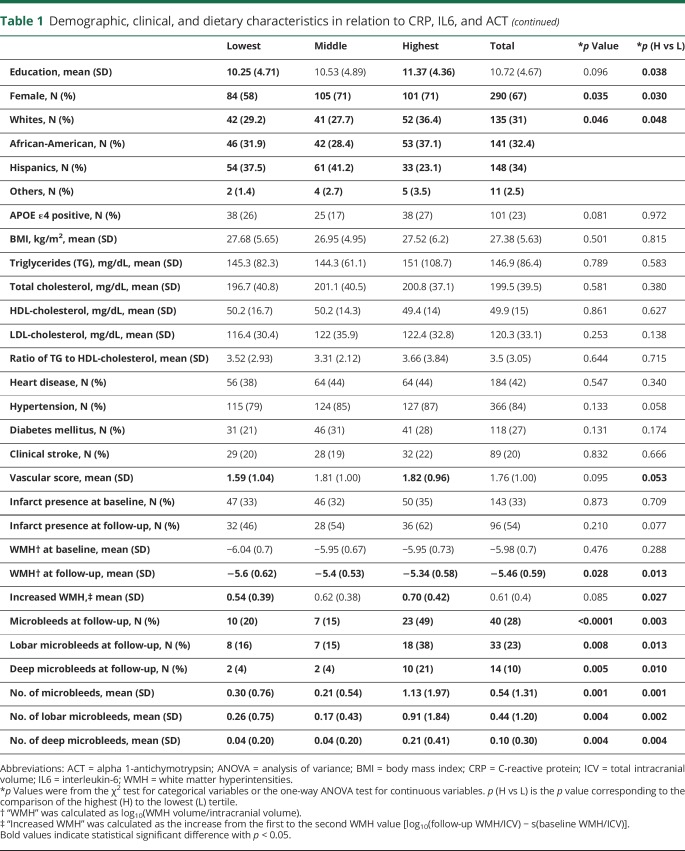
Those with a higher level of inflammatory biomarkers are more likely to be women and more likely to be African-Americans (table 1). Those in the highest tertiles were less likely to be APOE ε4 positive (CRP), had higher BMI (CRP and IL6), had worse lipids profiles (CRP and IL6), had more vascular comorbidities (CRP), and had higher education (ACT) compared with those in the lowest tertile of the corresponding biomarker (table 1).
Table 1.
Demographic, clinical, and dietary characteristics in relation to CRP, IL6, and ACT
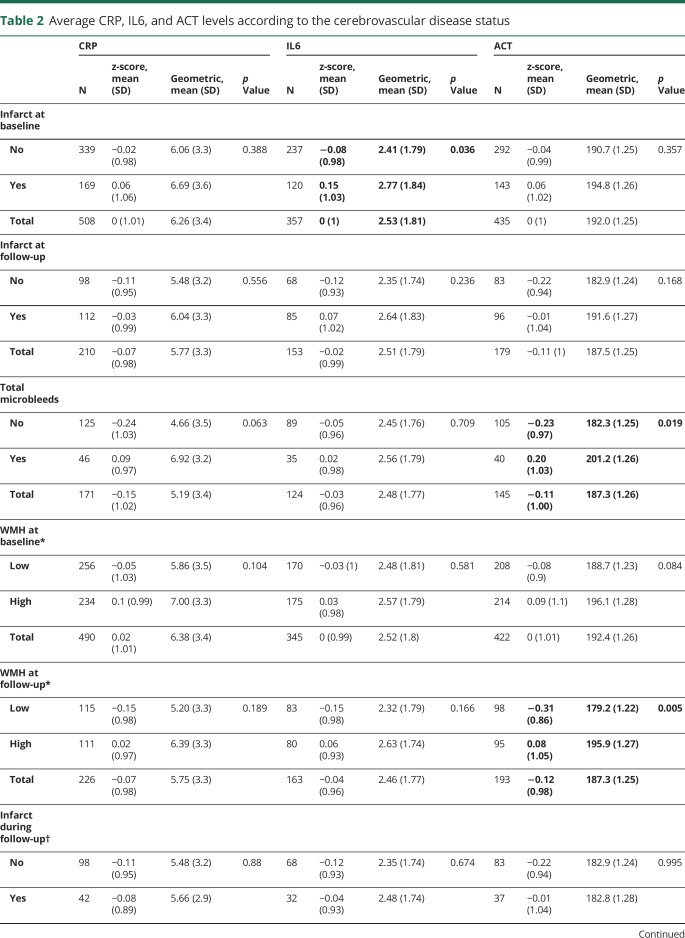
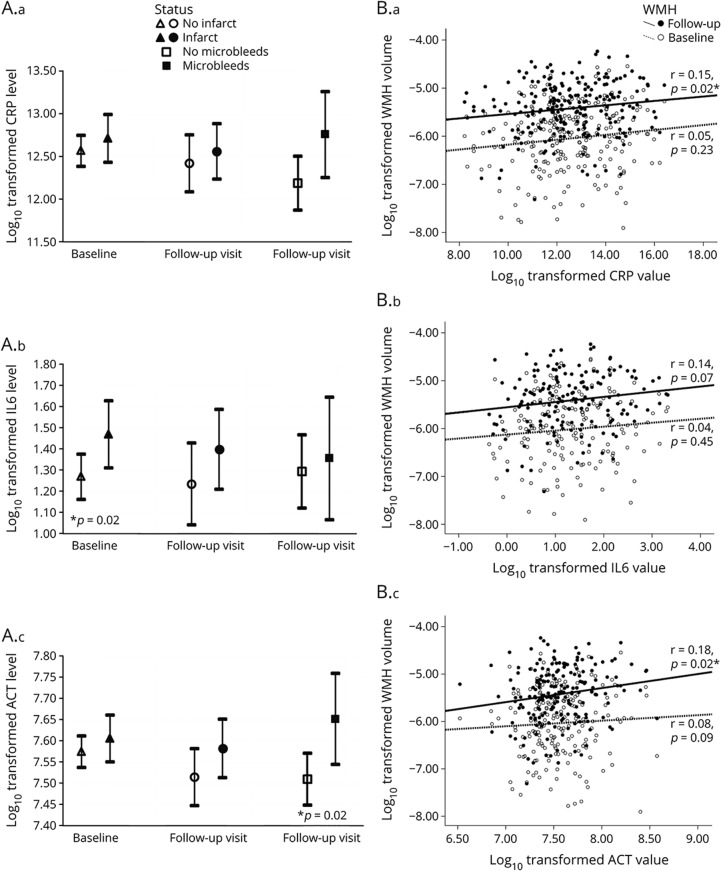
Those with a higher tertile of any of the inflammatory biomarker tended to have more CVD (table 1). Comparing the inflammatory marker levels according to the CVD status revealed a similar relationship, i.e., participants with infarcts at baseline had higher IL6 levels than those without, those with higher WMH at follow-up had higher ACT levels than those with lower WMH, and those with microbleeds had higher ACT levels than those without microbleeds (table 2 and figure 2).
Table 2.
Average CRP, IL6, and ACT levels according to the cerebrovascular disease status
Figure 2. Association of circulating concentrations of inflammatory biomarkers and cerebrovascular disease.
Association of circulating concentrations of C-reactive protein (CRP), interleukin-6 (IL6), and alpha 1-antichymotrypsin (ACT) with infarct and microbleeds status (A.a–A.c) and white matter hyperintensity (WMH) level (B.a–B.c). p Values in the figure were from unadjusted t-test (for infarcts and microbleeds) or from Pearson correlation (for WMH). WMH = white matter hyperintensity. *p < 0.05.
Cross-sectional association between inflammatory biomarkers and cerebrovascular disease
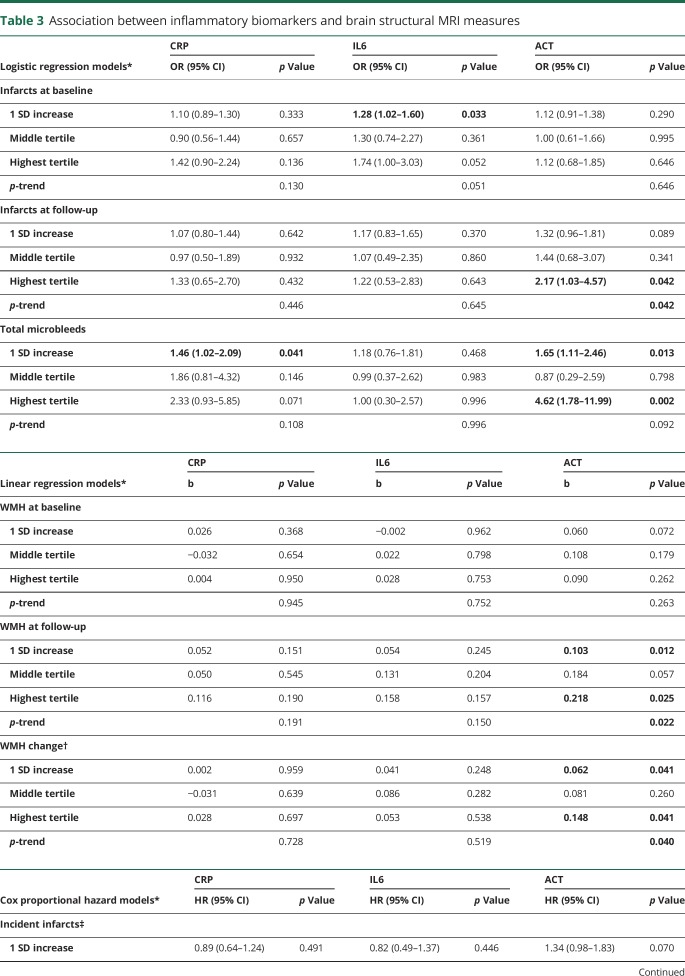
In adjusted models, 1 SD increase in circulating log-transformed CRP level was associated with increased odds of having microbleeds (table 3), and the association remained significant in the fully adjusted model (odds ratio [OR] = 1.80, 95% confidence interval [CI] = 1.07–3.03, p = 0.026).
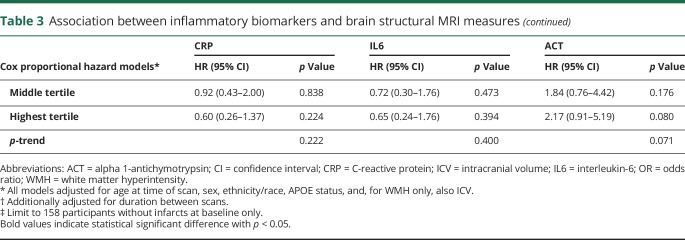
Table 3.
Association between inflammatory biomarkers and brain structural MRI measures
One SD increase in circulating log-transformed IL6 level was associated with increased odds of having infarcts at initial (baseline) scan visit (table 3), and the association remained significant in the fully adjusted model (OR = 1.30, 95% CI = 1.01–1.66, p = 0.039).
ACT seemed to be associated with increased odds of infarcts at the follow-up visit (table 3), but the association was no longer significant in the fully adjusted model. An increased ACT level was associated with increased odds of having microbleeds (table 3) and remained so in the fully adjusted model (OR comparing 3rd to 1st tertile = 3.74, 95% CI = 1.28–10.90, p = 0.016; p-trend = 0.011). ACT was also associated with larger WMH at the follow-up visit (table 3) and remained associated in the fully adjusted model (b = 0.087, p = 0.041).
Longitudinal association between inflammatory biomarkers and cerebrovascular disease
A higher ACT level was associated with greater accumulation of WMH during follow-up (table 3). In the fully adjusted model, a 1-SD increase in log10aACT was associated with 0.083 (p = 0.011) increase in log10WMH during follow-up (i.e., approximately 1.21 times increase in WMH volume). None of the inflammatory biomarkers was associated with risk of incident infarcts (table 3).
Supplementary analyses
When participants with clinically diagnosed stroke were excluded, log10CRP remained associated with microbleeds (OR = 1.77, 95% CI = 1.08–2.92, p = 0.024), log10IL6 with baseline infarcts (OR = 1.40, 95% CI = 1.05–1.87, p = 0.022), and log10ACT with microbleeds (OR = 2.01, 95% CI = 1.15–3.50, p = 0.015), larger WMH at follow-up visit (b = 0.101, p = 0.044), and greater accumulation of WMH (b = 0.119, p = 0.001), all in the fully adjusted models.
Excluding 24 participants who developed dementia during the follow-up, adjusted models showed that higher log10CRP and log10ACT remained significantly associated with increased odds of microbleeds, with OR (95% CI) = 1.49 (1.03–2.14), p = 0.03, and 1.62 (1.08–2.42), p = 0.02, respectively, and log10ACT was also associated with WMH burden at follow-up (b = 0.116, p = 0.006) and accumulation (b = 0.063, p = 0.048).
In the fully adjusted model, higher log10ACT was associated with larger WMH at the follow-up visit in the frontal (b = 0.114, p = 0.017), parietal (b = 0.127, p = 0.028), and temporal (b = 0.140, p = 0.029) regions and was associated with greater accumulation of WMH during follow-up in the frontal (b = 0.102, p = 0.037), parietal (b = 0.117, p = 0.052), and temporal (b = 0.133, p = 0.047) regions.
One SD increase in log10CRP level was associated with increased odds of lobar microbleeds (OR = 1.81, 95% CI = 1.12–2.94, p = 0.016; p-trend = 0.019 for tertiles), whereas ACT was associated with deep microbleeds (OR = 2.72, 95% CI = 1.11–6.66, p = 0.028; p-trend = 0.007 for tertiles) in the fully adjusted model.
The associations of inflammatory biomarkers with CVD in general did not vary by ethnic groups, sex, or APOE status. The only exception was that the association between ACT and WMH accumulation during the follow-up (b for interaction between ACT and Hispanic ethnicity = −0.143, p = 0.068) was significant for whites (b = 0.124, p = 0.042) and African-Americans (b = 0.131, p = 0.037), but not for Hispanics (b = −0.018, p = 0.669).
Discussion
In this multiethnic, nondemented, elderly population, we found that higher circulating proinflammatory biomarkers were associated with more CVD. Our finding that IL6 was associated with the presence of silent brain infarcts is consistent with some previous studies,10,12 but not others.14,22 Although some studies found CRP to be associated with infarcts,10,12,13 our study, along with a few other studies,9,14–16,19 did not replicate this finding. Studies also found that CRP or intercellular adhesion molecule-1 (sICAM-1) levels were associated with incident lacunar infarcts.9,23 Overall, there is some evidence showing a cross-sectional relationship between inflammatory biomarkers and CVD, but longitudinal evidence is limited. The potential role of inflammatory markers other than CRP or IL6 in CVD is worth further exploration.
A few studies found that higher CRP,9,10,19 IL6,10,26 or both22 were associated with more white matter lesions. Two other studies found that the association between CRP and WMH was attenuated by the adjustment of cardiovascular13 or other factors.11 Our study and several others did not find CRP15,16,24,27 or other cytokines14,25,27 to be associated with the severity of WMH volume. One previous study9 found that CRP was associated with more progression of white matter lesions. However, other studies15,22,23 did not find such a relationship. We found that ACT, but not CRP or IL6, was associated with WMH severity cross-sectionally at the follow-up MRI scan visit and with progression of WMH longitudinally. Two recent studies found that sICAM-1, but not CRP, was associated with the presence21 or progression of WMH.23 Therefore, inflammatory biomarkers other than CRP or IL6 may be relevant for WMH. Interestingly, both ACT and sICAM-1 play a role in cell adhesion and endothelial dysfunction, which may contribute to the pathogenesis of WMH.33 Similar to our study, other biracial studies10,19 found that there was no evidence for effect of inflammatory biomarkers on WMH to be different between white and black adults. More studies are needed to examine the effect of inflammatory biomarkers on WMH among Hispanics.
Only a few studies have examined the relationship between circulating inflammatory biomarkers with microbleeds,13,19–21 but the results are inconsistent. Two studies found that CRP was not associated with microbleeds,13,19 but a Japanese study found that CRP, IL6, and IL18 were all associated with the presence of microbleeds.20 The Framingham offspring study found that tumor necrosis factor receptor 2 and myeloperoxidase were associated with increased odds of having microbleeds, especially deep microbleeds.21 We found that CRP was associated with increased odds of lobar but not deep microbleeds, whereas ACT was associated with increased odds of deep but not lobar microbleeds. The distribution of microbleeds is believed to reflect 2 distinct underlying types of microangiopathy34: lobar microbleeds are considered likely to be attributable to cerebral amyloid angiopathy (CAA), whereas deep microbleeds are considered to be due to hypertensive arteriopathy. Therefore, CRP and ACT may represent 2 different pathways toward small hemorrhagic lesions in the brain. This is in line with our previous findings that increased CRP, but not ACT, was associated with smaller gray matter volume,30 as it has been shown that CAA-related, but not CAA-unrelated, microbleeds are associated gray matter atrophy.35 Nevertheless, such differential roles of CRP, ACT, or other inflammatory biomarkers in microbleeds need to be confirmed in future studies.
Growing evidence suggest that inflammatory cytokines are associated with increased risk of developing stroke, cardiovascular disease, and dementia.6 In addition, our study and other longitudinal studies9,23 found inflammatory biomarkers were associated with progression of CVD (particularly WMH), suggesting cytokines, acute-phase proteins, endothelial cell adhesive molecules, and other immune-related proteins may play an active role in building up or contributing to the vascular injuries in the brain. However, current evidence has been inconsistent. The exact reason for this is unknown, but it might be due to differences in age, prevalence of vascular risk factors, ethnicity, MRI acquisition techniques, and analytic strategies. In addition, there are few longitudinal studies and some existing ones that failed to establish this longitudinal relationship. Therefore, an alternative explanation of the results might be that elevated inflammatory biomarkers may be a marker of the inflammation as a result of vascular or other pathologic injury. Generation of cytokines can be upregulated in the brains of patients with stroke36 and CAA,37 probably representing an immune response stimulated by the vascular deposits of β-amyloid and other injuries. In addition, increased circulating levels of peripheral proinflammatory cytokines are found in patients with AD compared with controls.38 Overall, it is possible that both directions exist and create a vicious circle for a progressive accumulation of vascular and neurodegenerative damages, as well as elevated inflammatory responses. Either way, further understanding the role of inflammatory biomarkers in the presence and progression of CVD is important because it may help elucidate the pathogenesis of CVD and allow the development of immune-modulating intervention measures for CVD and related neurologic outcomes or it may help determine how inflammatory markers can be used to monitor disease progression.
Although we adjusted for several key factors, we cannot completely exclude the possibility that our results are subject to residual confounding due to unmeasured factors. We only examined 3 of the many inflammatory markers in our study, whereas the role of many other inflammatory markers is also worthy of examination. We only measured the inflammatory markers once and they may not represent the long-term average level of the subject. Although WMH overall is strongly associated with vascular factors and viewed as a reflection of small vessel CVD,39 the pathogenesis of WMHs is not fully understood and could be multifactorial.40 Thus, another limitation of our study is that we did not subclassify WMHs by their putative etiologies. Our study might be underpowered to examine interactions, stratified analyses in subgroups, and the longitudinal relationship. We did not correct for multiple comparison as we consider our study to be exploratory in nature and need replication in an external sample.
Our study has many strengths. We added new evidence to this line of research by including several key inflammatory markers, both cross-sectional and longitudinal analyses, an elderly population with advanced age, and different cerebrovascular features. In addition, our study population comprised multiple ethnic groups, making the results more generalizable to the increasingly diverse US population. Finally, several key potential confounding factors were adjusted in the analyses.
Among the old participants of our study, higher levels of peripheral CRP and IL6 were associated with increased odds of microbleeds and infarcts, respectively, and increased ACT was associated with cerebrovascular events including increased odds of infarcts and microbleeds and larger burden and more accumulation of WMH. More studies, especially longitudinal studies with a wide range of inflammatory biomarkers, are needed to clarify whether inflammatory biomarkers play an etiologic role in CVD or represent an epiphenomenon of CVD.
Acknowledgment
This manuscript has been reviewed by WHICAP investigators for scientific content and consistency of data interpretation with previous WHICAP Study publications. The authors acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study.
Glossary
- ACT
alpha 1-antichymotrypsin
- APOE
apolipoprotein
- BMI
body mass index
- CAA
cerebral amyloid angiopathy
- CVD
cerebrovascular disease
- CRP
C-reactive protein
- CV
coefficient of variation
- FOV
field of view
- GRE
gradient echo
- ICV
intracranial volume
- IL6
interleukin-6
- WHICAP
Washington Heights/Hamilton Heights Inwood Columbia Aging Project
- WMH
white matter hyperintensity
- sICAM-1
intercellular adhesion molecule-1
Author contributions
Y. Gu: design and conceptualized the study; analysis and interpretation; and drafted the manuscript for intellectual content. J. Gutierrez-Contreras, I.B. Meier, V.A. Guzman, J.J. Manly, and N. Schupf: acquisition of data and revised the manuscript for intellectual content. A.M. Brickman: major role in the acquisition of data; interpreted the data; and revised the manuscript for intellectual content. R. Mayeux: design and conceptualized the study; acquisition of data; study supervision; and revised the manuscript for intellectual content.
Study funding
Supported by NIH PO1AG007232, R01AG037212, RF1AG054023, UL1TR001873, AG042483 and AG034189.
Disclosure
Y. Gu received travel funding from California Walnut Commission and received research support from NIH. J. Gutierrez served as a review editor for PLoS One and Frontiers in Neurology; provided medico-legal consultancies; and received research support from the NIH and NINDS. I.B. Meier received research support from the Synapsis Foundation and VELUX Stiftung. V.A. Guzman received research support from the NIH and NIMHD. J.J. Manly received travel funding from the Alzheimer's Association; served as an associate editor for the Journal of the International Neuropsychological Society; consulted for National Academies of Medicine and National Academy of Sciences; and received research support from NIA, NIDDK, and NINDS. N. Schupf received research support from the NIH and Alzheimer's Association. A.M. Brickman served on the scientific advisory board of Keystone Heart, an NIA-supported study; served on the editorial board of the Journal of the International Neuropsychological Society and Neuropsychology Review; served as an associate editor of Neurodegenerative Diseases; holds a patent for technologies for white matter hyperintensity quantification; consulted for ProPhase, Keystone Heart, and Cognition Therapeutics; and received research support from NIH, Columbia University, Alzheimer's Association, and Mary E. Groff Surgical Medical Research and Education Charitable Trust. R. Mayeux received research support from NIA. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NN.
References
- 1.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001;70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology 1965;15:774–784. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003;34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 5.Brickman AM, Zahodne LB, Guzman VA, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol Aging 2015;36:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes C. Review: systemic inflammation and Alzheimer's disease. Neuropathol Appl Neurobiol 2013;39:51–68. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 8.Saji N, Toba K, Sakurai T. Cerebral small vessel disease and arterial stiffness: tsunami effect in the brain? Pulse (Basel) 2016;3:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dijk EJ, Prins ND, Vermeer SE, et al. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation 2005;112:900–905. [DOI] [PubMed] [Google Scholar]
- 10.Fornage M, Chiang YA, O'Meara ES, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: the cardiovascular health study. Stroke 2008;39:1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright CB, Moon Y, Paik MC, et al. Inflammatory biomarkers of vascular risk as correlates of leukoaraiosis. Stroke 2009;40:3466–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M. Relations of serum high-sensitivity C-reactive protein and interleukin-6 levels with silent brain infarction. Stroke 2005;36:768–772. [DOI] [PubMed] [Google Scholar]
- 13.Mitaki S, Nagai A, Oguro H, Yamaguchi S. C-reactive protein levels are associated with cerebral small vessel-related lesions. Acta Neurol Scand 2016;133:68–74. [DOI] [PubMed] [Google Scholar]
- 14.Baune BT, Ponath G, Rothermundt M, Roesler A, Berger K. Association between cytokines and cerebral MRI changes in the aging brain. J Geriatr Psychiatry Neurol 2009;22:23–34. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt R, Schmidt H, Pichler M, et al. C-reactive protein, carotid atherosclerosis, and cerebral small-vessel disease: results of the Austrian Stroke Prevention Study. Stroke 2006;37:2910–2916. [DOI] [PubMed] [Google Scholar]
- 16.Wada M, Nagasawa H, Kurita K, et al. Cerebral small vessel disease and C-reactive protein: results of a cross-sectional study in community-based Japanese elderly. J Neurol Sci 2008;264:43–49. [DOI] [PubMed] [Google Scholar]
- 17.Jefferson AL, Massaro JM, Wolf PA, et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology 2007;68:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Windham BG, Wilkening SR, Lirette ST, et al. Associations between inflammation and physical function in African Americans and European Americans with prevalent cardiovascular risk factors. J Am Geriatr Soc 2016;64:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker KA, Power MC, Hoogeveen RC, et al. Midlife systemic inflammation, late-life white matter integrity, and cerebral small vessel disease: the atherosclerosis risk in communities study. Stroke 2017;48:3196–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miwa K, Tanaka M, Okazaki S, Furukado S, Sakaguchi M, Kitagawa K. Relations of blood inflammatory marker levels with cerebral microbleeds. Stroke 2011;42:3202–3206. [DOI] [PubMed] [Google Scholar]
- 21.Shoamanesh A, Preis SR, Beiser AS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology 2015;84:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology 2012;78:720–727. [DOI] [PubMed] [Google Scholar]
- 23.Umemura T, Kawamura T, Umegaki H, et al. Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small-vessel disease and cognitive impairment: a 6-year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry 2011;82:1186–1194. [DOI] [PubMed] [Google Scholar]
- 24.Avci AY, Lakadamyali H, Arikan S, Benli US, Kilinc M. High sensitivity C-reactive protein and cerebral white matter hyperintensities on magnetic resonance imaging in migraine patients. J Headache Pain 2015;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aribisala BS, Wiseman S, Morris Z, et al. Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke 2014;45:605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai K, Kozaki K, Sonohara K, Akishita M, Toba K. Relationship between interleukin-6 and cerebral deep white matter and periventricular hyperintensity in elderly women. Geriatr Gerontol Int 2011;11:328–332. [DOI] [PubMed] [Google Scholar]
- 27.Silbert LC, Lahna D, Promjunyakul NO, et al. Risk factors associated with cortical thickness and white matter hyperintensities in dementia free Okinawan elderly. J Alzheimers Dis 2018;63:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 1998;147:259–268. [DOI] [PubMed] [Google Scholar]
- 29.Licastro F, Parnetti L, Morini MC, et al. Acute phase reactant alpha 1-antichymotrypsin is increased in cerebrospinal fluid and serum of patients with probable Alzheimer disease. Alzheimer Dis Assoc Disord 1995;9:112–118. [DOI] [PubMed] [Google Scholar]
- 30.Gu Y, Vorburger R, Scarmeas N, et al. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain Behav Immun 2017;65:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and Whites from northern Manhattan. Arch Neurol 2008;65:1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brickman AM, Sneed JR, Provenzano FA, et al. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry Res 2011;193:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Zhang W, Lin L, et al. Is endothelial dysfunction of cerebral small vessel responsible for white matter lesions after chronic cerebral hypoperfusion in rats? J Neurol Sci 2010;299:72–80. [DOI] [PubMed] [Google Scholar]
- 34.Kakar P, Charidimou A, Werring DJ. Cerebral microbleeds: a new dilemma in stroke medicine. JRSM Cardiovasc Dis 2012;1:2048004012474754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuraki M, Matsunari I, Yoshita M, et al. Cerebral amyloid angiopathy-related microbleeds correlate with glucose Metabolism and brain volume in Alzheimer's disease. J Alzheimers Dis 2015;48:517–528. [DOI] [PubMed] [Google Scholar]
- 36.Tarkowski E, Rosengren L, Blomstrand C, et al. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin Exp Immunol 1997;110:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eng JA, Frosch MP, Choi K, Rebeck GW, Greenberg SM. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann Neurol 2004;55:250–256. [DOI] [PubMed] [Google Scholar]
- 38.Fillit H, Ding WH, Buee L, et al. Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neurosci Lett 1991;129:318–320. [DOI] [PubMed] [Google Scholar]
- 39.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gouw AA, Seewann A, van der Flier WM, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011;82:126–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, yg2121@columbia.edu. Some access restrictions apply to the data used for the current study because of privacy concerns including the following: (1) the data contain elements that are considered Protected Health Information under HIPAA regulations; and (2) there is a potential risk for linkage of data made publicly available in conjunction with multiple studies based on the same cohort. Although the data on which the manuscript is based are not publicly available, a limited data set is available under a standard HIPAA Data Use Agreement, subject to review and approval by the Columbia University Privacy Officer.