Abstract
Background
A recent large-scale mega genome-wide association study identified, for the first time, genetic variants at 12 loci significantly associated with attention-deficit/hyperactivity disorder (ADHD). In this study we use a powerful polygenic approach, with polygenic scores derived from the genome-wide association study, to investigate the etiological overlap between ADHD and frequently co-occurring traits and disorders.
Methods
Polygenic risk scores for ADHD derived from the mega genome-wide association study (20,183 cases and 35,191 control subjects) were computed in a large-scale adult population sample (N = 135,726) recruited by the UK Biobank. Regression analyses were conducted to investigate whether polygenic risk for ADHD is associated with related traits and disorders in this population sample. The effects of sex were investigated via inclusion of an interaction term in the models.
Results
Polygenic risk for ADHD significantly and positively predicted body mass index (R2 = .45%; p = 5 × 10−129), neuroticism (R2 = .09%; p = 2 × 10−24), depression (R2 = .11%; p = 2 × 10−13), anxiety (R2 = .06%; p = 3 × 10−4), risk taking (R2 = .12%; p = 9 × 10−25), alcohol intake (R2 = .09%; p = 8 × 10−29), smoking (R2 = .33%; p = 4 × 10−21), alcohol dependency (R2 = .21%; p = 5 × 10−6), and negatively predicted verbal-numerical reasoning (R2 = .38%; p = 5 × 10−36). Polygenic risk scores did not significantly predict schizophrenia or bipolar disorder, although this may be because of the small number of diagnostic cases. We found no interaction effects between polygenic risk for ADHD and sex on any phenotypes.
Conclusions
Our findings suggest that common genetic variation underlying risk for clinically diagnosed ADHD also contributes to higher body mass index, neuroticism, anxiety and depressive disorders, alcohol and nicotine use, risk taking, and lower general cognitive ability in the general population. These findings suggest that the co-occurrence of several traits with ADHD is partly explained by the same common genetic variants.
Keywords: ADHD, Comorbidity, Co-occurring disorders, Genetics, Pleiotropy, Polygenic risk
SEE COMMENTARY ON PAGE 577
A recent mega genome-wide association study (GWAS) was the first to identify 12 loci significantly associated with attention-deficit/hyperactivity disorder (ADHD) (1). The statistical power of this GWAS allows the investigation of aspects of the genetic etiology of ADHD and its co-occurring features through polygenic approaches. Typically in polygenic risk analyses, composite scores, known as polygenic risk scores (PRSs), are created for individuals based on the sum of their risk alleles across the genome, weighted by GWAS-derived effect sizes. These PRSs optimize the genetic signal underlying complex traits and disorders and have been widely used to investigate shared genetic etiology between phenotypes 2, 3, 4.
Previous GWAS and candidate studies failed to identify rare and common genetic variants underlying ADHD that explain more than a small fraction of its heritability 5, 6, 7, despite the high heritability of ADHD estimated at 0.76 from twin studies (8) and estimated at 0.22 to 0.32 based on single nucleotide polymorphisms (SNPs) 1, 9. The difficulty in identifying genetic variants has likely been because of low statistical power and the polygenic nature of ADHD, i.e., that risk is a consequence of many small genetic effects. This has been supported by recent polygenic studies that show that significant associations emerge when a high number of genetic variants are considered en masse 1, 10.
ADHD has a prevalence rate of around 5.3% in childhood and 2.5% to 2.9% in adulthood 11, 12, 13. While the diagnosis of ADHD is based on inattentive and hyperactive-impulsive symptoms, affected individuals often also experience other adverse conditions. Individuals with ADHD are more likely than the general population to present with higher body mass index (BMI) 14, 15, neurotic (16) and risk-taking 17, 18, 19 behavior, lower IQ scores, and conditions such as bipolar disorder (BD), depression, anxiety 20, 21, 22, 23, 24, schizophrenia 24, 25, and substance abuse 20, 21, 23, 26, 27.
Family and twin studies suggest that several of these associations between ADHD and co-occurring traits and disorders are moderately to substantially explained by genetic influences 24, 25, 28, 29, 30, 31, 32, 33, 34. Until recently, the genetic overlap between ADHD and associated traits and disorders had not been studied using genome-wide approaches; however, limited recent and yet unpublished studies using linkage disequilibrium score regression (LDSR) report significant genetic correlations between ADHD and BMI (rg = 0.21–0.26), educational and cognitive measures (rg = −0.25 to 0.54), depression (rg = 0.48), BD (rg = 0.25), schizophrenia (rg = 0.22), and smoking (rg = 0.38–0.48), but not neuroticism and obsessive-compulsive disorder 1, 35. No genome-wide studies have yet investigated the genetic association between ADHD and risk taking, or alcohol and drug use.
While associations between ADHD and co-occurring impairments are well documented, our knowledge of the shared etiological influences underlying these co-occurrences is still limited with regard to the magnitude and type of genetic variants implicated in the genetic associations. The advantage of using a polygenic approach to study the genetic associations between phenotypes is that 1) we use molecular genetic data that do not rely on assumptions of relatedness, as in twin studies; 2) the design captures the polygenic nature of complex traits and disorders; and this design in turn 3) increases power to detect significant effects in studies compared with those considering only the most associated variants or candidate genes. In contrast to LDSR, the polygenic scoring method uses individual-level SNP, resulting in greater statistical power data and allowing for direct testing of interaction effects.
In this study, we use a powerful polygenic approach exploiting PRSs derived from the recently published mega GWAS on ADHD to test whether genetic variants that contribute to ADHD also influence frequently co-occurring traits and disorders in a large-scale adult population sample. A greater understanding of why ADHD often co-occurs with other impairing conditions may in turn improve preventative strategies and treatment for affected individuals. We further investigate whether the genetic overlap between ADHD and co-occurring features varies as a function of sex. Although a recent study suggested a near complete overlap of common genetic variants associated with ADHD between males and females (36), there may be sex differences in the genetic overlap between ADHD and comorbid features.
Methods and Materials
Discovery Sample
We used the recently published mega GWAS on ADHD as the discovery dataset (1). Summary results were downloaded from the PGC website (https://www.med.unc.edu/pgc/results-and-downloads). This GWAS contains data from 55,374 children and adults (20,183 ADHD cases and 35,191 control subjects), and 8,047,421 SNPs. Twelve independent loci were significantly associated with ADHD, and polygenic risk calculated from the GWAS explained on average up to 5.5% variance in ADHD case-control status, when using five different sets of discovery and independent target samples. The SNP-based heritability was calculated as 0.22 (1).
Target Sample
Participants
We used baseline data from the UK Biobank Study (http://www.ukbiobank.ac.uk) (41). A total of 502,655 community-dwelling participants between 37 and 73 years of age were recruited between 2006 and 2010 through the United Kingdom National Health Service patient registers (response rate = 5.47%) and underwent extensive cognitive and physical assessments. We analyzed data on 135,726 individuals (71,874 females) between 40 and 73 years of age (mean ± standard deviation [SD], 56.79 ± 7.96 years) who had available genotyping data after quality control (detailed below). UK Biobank received ethical approval from the Research Ethics Committee (reference 11/NW/0382).
Genotyping and Quality Control
A total of 152,729 blood samples were genotyped using either the UK Biobank Lung Exome Variant Evaluation array (N = 49,979) or the UK Biobank axiom array (N = 102,750). Details on genotyping, quality control, and imputation procedures can be found on the UK Biobank website (http://www.ukbiobank.ac.uk/scientists-3/genetic-data/) and Sudlow et al. (37). We further excluded SNPs based on minor allele frequency (<0.01), Hardy-Weinberg equilibrium (p < 10–8), and missingness (>0.02), and removed participants based on missingness (>0.01), relatedness (>0.088 [r∼ = .25]), gender mismatch, and non-Caucasian ancestry. Table 1 shows the sample sizes after quality control for each phenotype. The resulting dataset had 512,536 SNPs and 135,726 samples available for analysis.
Table 1.
Rates of Diagnoses and Mean Scores on Target Phenotypes
| Target Phenotypes | Value | Total, n |
|---|---|---|
| Continuous Phenotypes, Mean ± SD | ||
| Verbal-numerical reasoning | 6.11 ± 2.11 | 43,637 |
| Neuroticism | 4.11 ± 3.27 | 110,213 |
| Alcohol intake frequency | 2.89 ± 1.50 | 135,586 |
| Body mass index, kg/cm2 | 27.52 ± 4.84 | 135,348 |
| Binary Phenotypes, n (%) | ||
| Anxiety disorder | 2575 (2.14) | 120,362 |
| Depressive disorder | 8818 (6.96) | 126,605 |
| Bipolar disorder | 2232 (1.86) | 120,019 |
| Schizophrenia | 288 (0.24) | 118,075 |
| Alcohol dependency | 988 (0.83) | 118,775 |
| Risk-taking | 39,245 (29.00) | 135,348 |
| Tobacco use | 2911 (2.15) | 135,348 |
Verbal-numerical reasoning score was assessed as the number of correctly answered multiple choice questions (range, 0–13). Neuroticism was assessed as the number of neurotic traits present (range, 0–12). Alcohol intake frequency was scored as follows: 5 = daily or almost daily; 4 = 3 or 4 times a week; 3 = 1 or 2 times a week; 2 = 1 to 2 times a month; and 1 = special occasions only.
Phenotypes: BMI
BMI, which is constructed from weight and height (kg/cm2), was measured during the initial assessment. BMI values were excluded if data on either height or weight were missing.
Phenotypes: General Cognitive Ability
Participants completed a verbal-numerical reasoning test, consisting of 13 multiple choice questions (6 verbal/7 numerical) answered within a 2-minute time period (Supplemental Table S1). The test has shown a satisfactory level of test–retest reliability (r = .65) and a high genetic correlation with a general factor of cognitive ability (rg = .81, p = 6.2 × 10−18) 38, 39.
Phenotypes: Internalizing Traits and Psychiatric Disorders
Neuroticism was measured using 12 items (Supplemental Table S2) from the Eysenck Personality Inventory Neuroticism Scale–Revised (40). The score of each individual corresponds to the number of neurotic traits present, each coded as a binary variable (1 = yes, 0 = no).
Primary (the most resource-intensive condition) or secondary ICD-10 diagnoses (accessed through hospital records) and self-report measures (reports of having experienced a disorder during an interview with a nurse) were used to identify individuals who had experienced instances of anxiety and depressive disorders, BD, and schizophrenia (ICD-10 codes can be found in Supplemental Table S3). Individuals were indexed as having experienced a psychiatric disorder if they met criteria either through self-report or an ICD-10 diagnosis (any ICD subtype as seen in Supplemental Table S3).
Phenotypes: Substance Use and Risk-Taking
Alcohol intake frequency was measured by asking participants “About how often do you drink alcohol?” and was coded on a 5-point scale (Supplemental Table S4). Primary or secondary ICD-10 diagnoses (accessed through hospital records) and self-report measures (reports of having experienced a disorder during an interview with a nurse) were used to identify individuals that had ever experienced alcohol dependency or a mental/behavioral disorder owing to alcohol use (Supplemental Table S3). Information on smoking (ICD-10 code Z72.0) was accessed through hospital records. Risk-taking was measured by asking participants “Would you describe yourself as someone who takes risks?” and was coded as a binary variable (1 = yes, 0 = no).
The control group used for comparisons with the diagnostic groups consisted of individuals that did not have any ICD-10 or self-reported diagnosis of alcohol dependency, anxiety disorder, depressive disorder, BD, or schizophrenia and did not take lithium, antidepressants, or antipsychotics.
We did not investigate participants with ADHD because only 7 individuals had an ICD-10 diagnosis (secondary) for ADHD or were taking stimulant medications (methylphenidate or Ritalin) in our genotyped sample. There were also few participants (n < 25) diagnosed with oppositional defiant disorder, conduct disorder, or autism spectrum disorder. The low prevalence rate of ADHD and these other disorders in the UK Biobank is likely related to the older age of the sample (40–73 years of age), as they are most often diagnosed in childhood but were not as commonly recognized when participants were school-aged children.
Phenotypes: Control Traits
We also investigated eight “control” phenotypes that we did not expect to be significantly associated with PRS ADHD, in order to confirm that any reported significant results were not caused by the inflation of type I errors. These control traits were height, age, year of initial assessment, menstruation during initial assessment, number of self-reported cancers, hand grip strength, visual acuity, and sex of baby (Supplemental Table S5).
PRS Analyses
PRSs were computed for each UK Biobank participant using PRSice software (http://www.prsice.info/) (41), with the mega GWAS summary statistics as the discovery dataset. PRSice computes scores by calculating the sum of trait-associated alleles, weighted by the odds ratio generated from a GWAS in an independent sample. An r2 ≥ .1 (250-kb window) was used for clumping to remove SNPs in linkage disequilibrium. Logistic and linear regression models were used to estimate associations between PRSs and phenotypes in the UK Biobank. PRSs were calculated at a large number of p value thresholds for SNP inclusion (“high resolution scoring”) (41) to provide the most predictive PRS. p Value thresholds were between pT = 0 and pT = 0.5 at increments of .001. Results are presented where the most predictive PRS is identified for each phenotype. We set a conservative significance threshold of p < 2.1 × 10−4 for the main analyses on traits of interest and “control” traits, based on testing the most predictive PRS across 19 phenotypes (see Supplemental Methods).
We controlled for population stratification by conducting analyses with imputed markers and 15 principal components as covariates. We included birthplace, age, and sex as covariates in all analyses, and also batch, in order to control for any genetic differences associated with the batches that samples were analyses in or the genotyping platforms. The R2 values we report are adjusted from a baseline model including the covariates. In addition, we ran secondary analyses where we explored the effect of sex by including PRS by sex interaction effects. For these analyses, we set a stringent significance threshold of p < 4.5 × 10−4 (see Supplemental Methods). In the prediction model for height, we added BMI as a covariate because of the significant phenotypic association between BMI and height (r = −.0145, p = 1.07 × 10−24).
Results
Table 1 summarizes the number of individuals included in analyses for each target phenotype and presents mean values and standard deviations for the continuous phenotypes and the number of “cases” for the binary phenotypes.
Body Mass Index
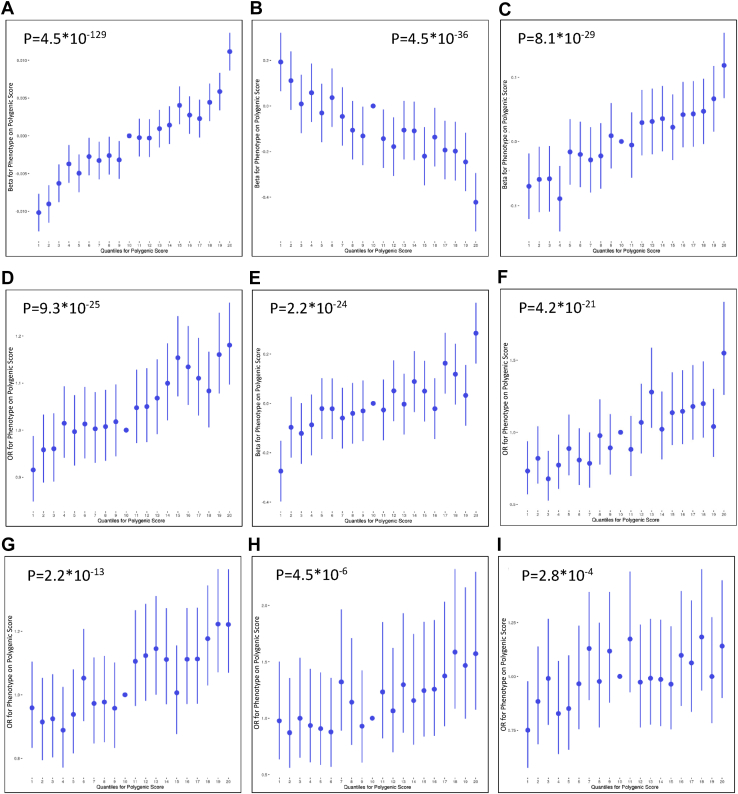
PRS for ADHD significantly (p = 4.5 × 10−129) predicted BMI (R2 = .45%, pT = .44) (Figure 1), and the quantile plot demonstrates the positive nature of this relationship as BMI increases with greater polygenic load for ADHD (Figure 2). Mean BMI was significantly higher in males (mean ± SD, 27.95 ± 4.31) than in females (27.14 ± 5.23).
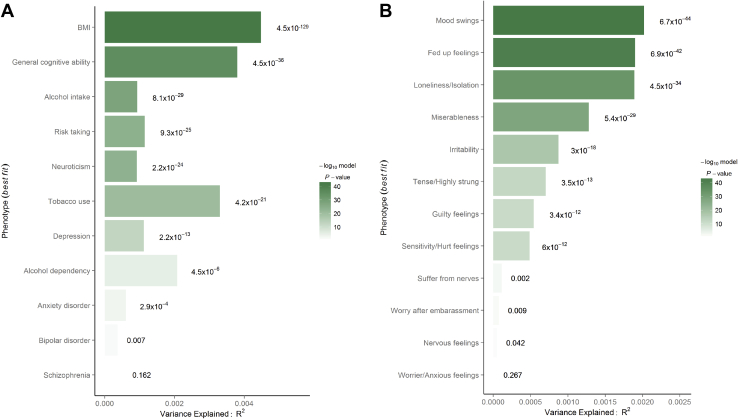
Figure 1.
Association between polygenic risk scores for attention-deficit/hyperactivity disorder and (A) target phenotypes and (B) items on the neuroticism scale. Values displayed next to each bar represent the p value for significance for the most predictive models. The significance threshold was set to p < 2.1 × 10−4. BMI, body mass index.
Figure 2.
Quantiles of polygenic risk scores plotted against effects on phenotypes. (A) Body mass index; (B) verbal-numerical reasoning; (C) alcohol intake; (D) risk-taking; (E) neuroticism; (F) tobacco use; (G) depression; (H) alcohol dependency; and (I) anxiety disorder. A regression is performed with phenotype as outcome and each 5% quantile separately, whereby the effect size of each quantile is compared to the central quantile as reference, such that each polygenic score in the quantile in question is coded 1 and each polygenic score in the reference quantile is coded 0. In each regression, the covariates used in the main analyses are included. OR, odds ratio.
General Cognitive Ability
PRS for ADHD significantly (p = 4.5 × 10−36) predicted verbal-numerical reasoning scores (R2 = .38%, pT = .42) (Figure 1), and the quantile plot shows that verbal-numerical reasoning scores decreased with increasing polygenic load for ADHD (Figure 2). Verbal-numerical reasoning test scores were significantly higher in males (6.22 ± 2.18) than in females (6.01 ± 2.05).
Internalizing Traits and Psychiatric Disorders
PRS for ADHD significantly (p = 2.2 × 10−24) predicted neuroticism (R2 = .09%, pT = .14) and the quantile plot demonstrates that neuroticism scores increase with higher polygenic load for ADHD (Figure 2). Females showed significantly higher neuroticism levels (4.60 ± 3.26) than males (3.60 ± 3.20). We further investigated the separate 12 neuroticism items (Figure 1). PRS for ADHD significantly and positively predicted mood swings (R2 = .002%), fed-up feelings (R2 = .20%), feelings of loneliness and isolation (R2 = .19%), miserableness (R2 = .13%), irritability (R2 = .09%), being tense/highly strung (R2 = .07%), guilty feelings (R2 = .05%), and having easily hurt feelings (R2 = .05%). The PRS did not predict suffering from nerves, often worrying after embarrassment, or being a nervous person or a worrier.
PRS for ADHD also significantly (p = 2.2 × 10−13) predicted depressive disorder (R2 = .11%, pT = .03) and suggestively (p = 2.8 × 10−4) predicted anxiety (R2 = .06%, pT = .12) but not BD or schizophrenia (Figure 1). Quantile plots (Figure 2) show that the significant associations were positive. A significantly higher proportion of females than males presented with anxiety (2.6% vs. 1.6%), depression (8.5% vs. 5.2%), and BD (2.3% vs. 1.4%), but the opposite trend was observed for schizophrenia (0.2% vs. 0.3%).
Substance Use and Risk-Taking
PRS for ADHD significantly (p < 2.1 × 10−4) predicted risk-taking (R2 = .12%, pT = .29), alcohol intake frequency (R2 = .09%, pT = .23) and dependency (R2 = .21%, pT = .18), and smoking (R2 = .33%, pT = .49). Quantile plots suggest that all of these relationships were positive in nature (Figure 2). A significantly higher proportion of males than females were risk-takers (36.3% vs. 22.3%), alcohol dependent (1.2% vs. 0.4%), and smokers (2.5% vs. 1.8%). Females showed significantly higher alcohol intake frequency (3.14 ± 1.53) than males (2.60 ± 1.42).
We found no significant PRS by sex interaction effects for any of the target phenotypes (Table 2).
Table 2.
Polygenic Risk Score by Sex Interaction and Main Effects of Sex on Target Phenotypes
| Target Phenotype | PRSMβ | PRSFβ | pinteraction | t/z Score | Sexβ | psex | t/z Score |
|---|---|---|---|---|---|---|---|
| Body Mass Index | 0.07 | 0.07 | .03 | −2.23 | 0.10 | 1.55 × 10−725 | 35.36 |
| Verbal-Numerical Reasoning | −0.05 | −0.07 | .12 | 1.55 | 0.05 | 4.82 × 10−25 | 10.34 |
| Alcohol Intake | 0.03 | 0.04 | .11 | −1.62 | −0.18 | <2 × 10−285 | −66.46 |
| Risk-Taking | 0.15 | 0.14 | .26 | 1.13 | 0.81 | <2 × 10−285 | 57.70 |
| Neuroticism | 0.03 | 0.03 | .52 | −0.64 | −0.15 | <2 × 10−285 | −49.19 |
| Tobacco Use | 1.10 | 1.33 | .57 | −0.56 | 1.02 | 5.79 × 10−14 | 7.51 |
| Depressive Disorders | 0.45 | 0.27 | .36 | 0.91 | −1.07 | 2.55 × 10−116 | −22.93 |
| Alcohol Dependency | 1.28 | 2.93 | .36 | −0.92 | 5.45 | 1.79 × 10−39 | 13.15 |
| Anxiety Disorders | 0.37 | 0.57 | .37 | −0.89 | −1.62 | 1.69 × 10−28 | −11.07 |
| Bipolar Disorder | 0.21 | 0.53 | .29 | −1.06 | −1.79 | 1.73 × 10−26 | −10.65 |
| Schizophrenia | 2.56 | 0.40 | .29 | 1.06 | 4.35 | .00071 | 3.39 |
Significance threshold set at p < 4.5 × 10−4.
PRSM/F, prediction of polygenic risk score on target phenotype for males and females.
Control Phenotypes
PRS for ADHD significantly (p < 2.1 × 10−4) and negatively predicted height (R2 = .03%, pT = .08) and age (R2 = .03%, pT = .18), but not any of the remaining six control phenotypes (Table 3). After controlling for educational achievement (detailed in Supplemental Table S6), which has been found to be genetically associated with height (42), the significant association between PRS for ADHD and height was no longer significant (R2 = .005%, p = .0001); however, the association between PRS and age remained and was significant in both males (R2 = .021%, p = 3 × 10−5) and females (R2 = .029%, p = 8 × 10−6). When we reran all the main analyses controlling for educational achievement and BMI, which were the two additional covariates in the PRS–height model, the overall pattern of results remained the same, although effect sizes decreased for most traits (Supplemental Table S7).
Table 3.
Prediction of Polygenic Risk Score for Attention-Deficit/Hyperactivity Disorder on Target and Control Phenotypes
| Target or Control Phenotype | p | pT | R2 (%) | SNPs, n |
|---|---|---|---|---|
| Body Mass Index | 4.5 × 10−129 | .440 | .448 | 69,995 |
| Verbal-Numerical Reasoning | 4.5 × 10−36 | .418 | .379 | 67,558 |
| Alcohol Intake Frequency | 8.1 × 10−29 | .231 | .093 | 44,307 |
| Risk-Taking | 9.3 × 10−25 | .291 | .115 | 52,388 |
| Neuroticism | 2.2 × 10−24 | .139 | .092 | 30,306 |
| Tobacco Use | 4.2 × 10−21 | .485 | .333 | 74,809 |
| Height | 8.7 × 10−20 | .081 | .030 | 20,147 |
| Depressive Disorder | 2.2 × 10−13 | .033 | .112 | 10,158 |
| Age, Years | 5.8 × 10−9 | .177 | .026 | 36,443 |
| Alcohol Dependency | 4.5 × 10−6 | .175 | .208 | 36,101 |
| Anxiety Disorder | 2.8 × 10−4 | .116 | .062 | 26,355 |
| Visual Acuity | .005 | .001 | .029 | 792 |
| Bipolar Disorder | .007 | .117 | .037 | 26,551 |
| Hand Grip Strength | .024 | .494 | .002 | 75,689 |
| Menstruation at Assessment | .115 | .051 | .025 | 14,128 |
| No. of Cancers | .127 | .131 | .002 | 28,929 |
| Schizophrenia | .162 | .257 | .053 | 47,870 |
| Year of Assessment | .159 | .036 | .001 | 10,871 |
| Sex of Child | .234 | .010 | .062 | 4085 |
Significance threshold set at p < 2.1 × 10−4.
SNP, single nucleotide polymorphism.
Table 3 and Supplemental Figures S1 to S11 provide more detailed information and plots for the PRS prediction models.
Discussion
Using PRSs derived from the recently published mega GWAS (1), we found that polygenic risk for clinically diagnosed ADHD predicts higher BMI, neuroticism, risk-taking, tobacco and alcohol use, and anxiety and depressive disorders, and lower general cognitive ability in an adult population sample. These are the first reports of significant genetic associations between ADHD and neuroticism traits, risk-taking, and alcohol use based on genome-wide data. The remaining associations are consistent with a relatively limited literature of studies demonstrating pleiotropy of the genetic variants underlying ADHD. No sex-specific effects were observed in relation to the association between PRS for ADHD and co-occurring features.
Individuals with many risk alleles for ADHD were more likely to have higher BMI than those with few risk alleles. There is limited research investigating why ADHD and high BMI often co-occur, but our findings, together with recent findings using LDSR 1, 35, suggest that they have an overlapping genetic basis. Further research is needed to identify genetic pathways and neurobiological mechanisms relating to this genetic overlap, which could prove vital for improving prevention and treatment interventions for individuals with ADHD who are at risk of obesity. One possibility is that dopaminergic pathways and pathways implicated in eating patterns (e.g., binge- and emotional-eating), sleeping patterns, and sedentary behavior explain the association between ADHD and BMI, which would be in line with initial evidence 42, 43, 44, 45, 46. The common mechanisms underlying both ADHD and BMI could either reflect biological pleiotropy, where similar mechanisms influence both traits, or mediated pleiotropy, where certain mechanisms influences one of the traits, which in turn influences the other.
Polygenic risk for ADHD was significantly associated with lower cognitive ability, which is in line with previous twin and molecular genetic studies 2, 29, 30, 35. The association between ADHD and general cognitive ability is thought to be mainly driven by ADHD symptoms that influence IQ, at least in adolescence (47). It may therefore be possible that there are common biological mechanisms underlying both ADHD and IQ, but perhaps also certain biological mechanisms underlie ADHD, which in turn influences IQ, possibly through poor educational achievement owing to difficulties concentrating in school 47, 48.
Polygenic risk for ADHD significantly and positively predicted neuroticism, including individual items such as mood swings and irritability. Two recent studies failed to find any genetic correlation between ADHD and neuroticism using LDSR 35, 49. The discrepancy in findings may be due to the previous studies having smaller sample sizes or the use of LDSR rather than polygenic scoring, potentially resulting in insufficient statistical power to detect effects.
PRSs for ADHD also predicted depression, and anxiety at a suggestive level, which is in line with findings from twin and genome-wide studies 9, 31, 32, 33, 35. The ADHD PRSs did not predict BD or schizophrenia; however, these results should be considered with caution because previous family-based and genome-wide studies using other statistical methods have reported significant genetic associations between these disorders 34, 35. The discrepancy in findings may be related to the older age of our sample, the use of a population cohort rather than clear case-control groups, or insufficient power to detect effects, in particular for schizophrenia (288 cases) based on power calculations using Avengeme R package (power for analyses: BD = 0.99, schizophrenia = 0.22). Further polygenic studies are needed to investigate the association of ADHD with BD and schizophrenia across different study populations to clarify the true etiological relationship between the disorders.
Individuals with many risk alleles for ADHD were more likely to display alcohol dependency, have higher alcohol intake frequency, and be smokers and risk-takers compared with those with few risk alleles. Previous genome-wide studies reported significant genetic associations between ADHD and smoking 35, 50, 51 but not between ADHD and alcohol use (52), and no studies to our knowledge have investigated the genetic association between ADHD and risk-taking. The shared genetic risk between ADHD and these risk-taking and health-related outcomes may be explained by common neurobiological mechanisms involved in self-regulation and inhibitory control. Further research targeting relevant genes and pathways is needed to test such hypotheses.
Overall, our findings lend support for the continuous nature of ADHD across the entire population. We find that common risk alleles that contribute to clinically diagnosed ADHD also influence common traits and disorders in the general population, across ages, which suggests that ADHD symptoms represent continuous traits and that similar genetic influences may be present in younger and older individuals. This fits well with the current understanding of ADHD based on evidence from behavioral, family-based, and genetic studies 53, 54, 55.
To investigate if our significant results could be the result of type I errors, we examined if PRSs for ADHD significantly predicted several “control” phenotypes that were not expected to be associated with polygenic risk for ADHD. Out of the eight “control” traits, only age was significantly predicted by ADHD PRS. It is possible that this association is caused by some real effect, such as genetic influences on ADHD being stronger during certain developmental periods, for example in childhood, when the prevalence of ADHD is the highest. Twin studies suggest that the heritability of childhood ADHD is stronger than in adult ADHD, but this may also be due to rater effects (56). Hypothetically, this would then have been captured in the discovery GWAS, where genetic effect sizes in children would be larger than in adults and in turn lead to PRS associations with younger age in the UK Biobank. However, we cannot rule out the possibility that the “age” result reflects a false positive or is related to the overlap between UK Biobank participants and those of the Psychiatric Genomics Consortium/iPSYCH ADHD GWAS, which may cause slight inflation in results. It is reassuring, however, that seven of eight control traits showed nonsignificant results and that the relative strength of the significant results are in line with other preliminary genetic findings.
An advantage of using a large dataset and the PRS approach is that we could directly investigate sex differences in the relationship between PRSs and the target phenotypes. A recent study based on the ADHD mega GWAS data found a strong genetic correlation for ADHD across sex and no difference in polygenic load across sex (36), and we extend these findings to show that the polygenic influences underlying the relationship between ADHD and co-occurring features are similar across men and women.
Limitations and Future Directions
One should interpret our findings in light of the study limitations. Our study participants were between 40 and 73 years of age, had a lower prevalence of mental health disorders, and were recruited within the United Kingdom. It would be informative to investigate the generalizability of our findings by replicating the analyses using participants of different age groups and from different populations. Selection bias of the sample could also have influenced the associations we report (57); however, we controlled for several important measures, including age and birthplace, to minimize the chance for bias. In addition, several of the significant genetic associations that we identified confirm previous statistical genetic findings (35), offering some validation of our results. PRSs explain only a tiny fraction of the variance in the target phenotypes, and obtaining a complete picture of the etiological overlap between ADHD and co-occurring features will require larger sample sizes and inclusion of other genetic factors, such as copy number and rare variants.
In conclusion, higher polygenic load for clinical ADHD was associated with higher BMI, neurotic and risk-taking behavior, anxiety and depressive disorders and substance use, and lower general cognitive ability in the general population. These findings suggest that the co-occurrence of several traits and disorders with ADHD are partly explained by the same common genetic factors. Further investigations are needed to determine the specific neurobiological mechanisms associated with the shared genetic etiology between ADHD and co-occurring features.
Acknowledgments and Disclosures
This research has been conducted using the UK Biobank Resource under Application Number 18177. EDR is supported by a doctoral studentship from the UK Medical Research Council. This independent research was funded in part by the National Institute for Health Research Biomedical Research Centre at South London, the Maudsley National Health Service Foundation Trust, and King’s College London. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health. High-performance computing facilities were funded with capital equipment grants from the GSTT Charity (TR130505) and Maudsley Charity (980). POR receives funding from the UK Medical Research Council (MR/N015746/1), the Wellcome Trust (109863/Z/15/Z), and the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. JK’s research on ADHD and comorbidities is supported by the European Commission’s H2020 Programme under Grant Agreement No. 667302 (Comorbid Conditions of Attention deficit/hyperactivity disorder).
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2017.11.013.
Supplementary Material
References
- 1.Demontis D., Walters R.K., Martin J., Mattheisen M., Als T.D., Agerbo E. Discovery of the first genome-wide significant risk loci for ADHD. BioRxiv. 2017 doi: 10.1038/s41588-018-0269-7. [published online ahead of print Jun 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stergiakouli E., Martin J., Hamshere M.L., Heron J., St Pourcain B., Timpson N.J. Association between polygenic risk scores for attention-deficit hyperactivity disorder and educational and cognitive outcomes in the general population. Int J Epidemiol. 2016;46:421–428. doi: 10.1093/ije/dyw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke T.K., Lupton M.K., Fernandez-Pujals A.M., Starr J., Davies G., Cox S. Common polygenic risk for autism spectrum disorder (ASD) is associated with cognitive ability in the general population. Mol Psychiatr. 2016;21:419–425. doi: 10.1038/mp.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reginsson G.W., Ingason A., Euesden J., Bjornsdottir G., Olafsson S., Sigurdsson E. Polygenic risk scores for schizophrenia and bipolar disorder associate with addiction. Addict Biol. 2018;23:485–492. doi: 10.1111/adb.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akutagava-Martins G.C., Rohde L.A., Hutz M.H. Genetics of attention-deficit/hyperactivity disorder: An update. Expert Rev Neurother. 2016;16:145–156. doi: 10.1586/14737175.2016.1130626. [DOI] [PubMed] [Google Scholar]
- 6.Neale B.M., Medland S., Ripke S., Anney R.J., Asherson P., Buitelaar J. Case-control genome-wide association study of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:906–920. doi: 10.1016/j.jaac.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams N.M., Franke B., Mick E., Anney R.J., Freitag C.M., Gill M. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: The role of rare variants and duplications at 15q13.3. Am J Psychiatry. 2012;169:195–204. doi: 10.1176/appi.ajp.2011.11060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faraone S.V., Perlis R.H., Doyle A.E., Smoller J.W., Goralnick J.J., Holmgren M.A., Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Cross-Disorder Group of the Psychiatric Genomics Consortium. Lee S.H., Ripke S., Neale B.M., Faraone S.V., Purcell S.M. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamshere M.L., Langley K., Martin J., Agha S.S., Stergiakouli E., Anney R.J. High loading of polygenic risk for ADHD in children with comorbid aggression. Am J Psychiatry. 2013;170:909–916. doi: 10.1176/appi.ajp.2013.12081129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polanczyk G., De Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 12.Faraone S.V., Biederman J., Mick E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychol Med. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 13.Simon V., Czobor P., Balint S., Meszaros A., Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: Meta-analysis. Br J Psychiatry. 2009;194:204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- 14.Nigg J.T., Johnstone J.M., Musser E.D., Long H.G., Willoughby M.T., Shannon J. Attention-deficit/hyperactivity disorder (ADHD) and being overweight/obesity: New data and meta-analysis. Clin Psychol Rev. 2016;43:67–79. doi: 10.1016/j.cpr.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortese S., Moreira-Maia C.R., St Fleur D., Morcillo-Penalver C., Rohde L.A., Faraone S.V. Association between ADHD and obesity: A systematic review and meta-analysis. Am J Psychiatry. 2016;173:34–43. doi: 10.1176/appi.ajp.2015.15020266. [DOI] [PubMed] [Google Scholar]
- 16.Gomez R., Corr P.J. ADHD and personality: A meta-analytic review. Clin Psychol Rev. 2014;34:376–388. doi: 10.1016/j.cpr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Du Rietz E., Kuja-Halkola R., Brikell I., Jangmo A., Sariaslan A., Lichtenstein P. Predictive validity pf parent- and self-rated ADHD symptoms in adolescence on adverse socioeconomic and health outcomes. Eur Child Adolesc Psychiatry. 2017;26:857–867. doi: 10.1007/s00787-017-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkley R.A., Cox D. A review of driving risks and impairments associated with attention-deficit/hyperactivity disorder and the effects of stimulant medication on driving performance. J Safety Res. 2007;38:113–128. doi: 10.1016/j.jsr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Sarver D.E., McCart M.R., Sheidow A.J., Letourneau E.J. ADHD and risky sexual behavior in adolescents: Conduct problems and substance use as mediators of risk. J Child Psychol Psychiatry. 2014;55:1345–1353. doi: 10.1111/jcpp.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler R.C., Adler L., Barkley R., Biederman J., Conners C.K., Demler O. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faraone S.V., Biederman J., Spencer T., Mick E., Murray K., Petty C. Diagnosing adult attention deficit hyperactivity disorder: Are late onset and subthreshold diagnoses valid? Am J Psychiatry. 2006;163:1720–1729. doi: 10.1176/ajp.2006.163.10.1720. [DOI] [PubMed] [Google Scholar]
- 22.Wilens T.E., Biederman J., Brown S., Tanguay S., Monuteaux M.C., Blake C., Spencer T.J. Psychiatric comorbidity and functioning in clinically referred preschool children and school-age youths with ADHD. J Am Acad Child Adolesc Psychiatry. 2002;41:262–268. doi: 10.1097/00004583-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Kessler R.C., Chiu W.T., Demler O., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson H., Ryden E., Boman M., Langstrom N., Lichtenstein P., Landen M. Risk of bipolar disorder and schizophrenia in relatives of people with attention-deficit hyperactivity disorder. Br J Psychiatry. 2013;203:103–106. doi: 10.1192/bjp.bp.112.120808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath J., Saha S., Chant D., Welham J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- 26.Frei A., Hornung R., Eich D. Tobacco consumption of adults diagnosed with ADHD. Nervenarzt. 2010;81:860–866. doi: 10.1007/s00115-009-2922-y. [DOI] [PubMed] [Google Scholar]
- 27.Pomerleau O.F., Downey K.K., Stelson F.W., Pomerleau C.S. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 28.Quen Q., Kuja-Halkola R., Sjolander A., Serlachius E., Cortese S., Faraone S.V. Shared familial risk factor between ADHD and overweight/obesity – a population-based familial coaggregation study in Sweden. J Child Psychol Psychiatry. 2017;58:711–718. doi: 10.1111/jcpp.12686. [DOI] [PubMed] [Google Scholar]
- 29.Kuntsi J., Eley T.C., Taylor A., Hughes C., Asherson P., Caspi A., Moffitt T.E. Co-occurrence of ADHD and low IQ has genetic origins. Am J Med Genet B Neuropsychiatr Genet. 2004;124B:41–47. doi: 10.1002/ajmg.b.20076. [DOI] [PubMed] [Google Scholar]
- 30.Wood A.C., Asherson P., Van der Meere J.J., Kuntsi J. Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on IQ. Psychol Med. 2010;40:1027–1037. doi: 10.1017/S003329170999119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole J., Ball H.A., Martin N.C., Scourfield J., McGuffin P. Genetic overlap between measures of hyperactivity/inattention and mood in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:1094–1101. doi: 10.1097/CHI.0b013e3181b7666e. [DOI] [PubMed] [Google Scholar]
- 32.Michelini G., Eley T.C., Gregory A.M., McAdams T.A. Aetiological overlap between anxiety and attention deficit hyperactivity symptom dimensions in adolescence. J Child Psychol Psychiatry. 2015;56:423–431. doi: 10.1111/jcpp.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz S., Mrazek D.A. Genetic and environmental influences on the associations between attention problems and other problem behaviors. Twin Res. 2001;4:453–458. doi: 10.1375/1369052012786. [DOI] [PubMed] [Google Scholar]
- 34.Faraone S.C., Biederman J., Wozniak J. Examining the comorbidity between attention deficit hyperactivity disorder and bipolar I disorder: A meta-analysis of family genetic studies. Am J Psychiatry. 2012;169:1256–1266. doi: 10.1176/appi.ajp.2012.12010087. [DOI] [PubMed] [Google Scholar]
- 35.Anttila V., Bulik-Sullivan B., Finucane H.K., Bras J., Duncan L., Escott-Price V. Analysis of shared heritability in common disorders of the brain. BioRxiv. 2016 doi: 10.1126/science.aap8757. [published online ahead of print Apr 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin J., Walters R.K., Demontis D., Mattheisen M., Lee S.H., Robinson E. A genetic investigation of sex bias in the prevalence of attention deficit hyperactivity disorder. Biol Psychiatry. 2018;83:1044–1053. doi: 10.1016/j.biopsych.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagenaars S.P., Harris S.E., Davies G., Hill W.D., Liewald D.C., Ritchie S.J. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112,151) and 24 GWAS consortia. Mol Psychiatry. 2016;21:1624–1632. doi: 10.1038/mp.2015.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies G., Marioni R.E., Liewald D.C., Hill W.D., Hagenaars S.P., Harris S.E. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N=112151) Mol Psychiatry. 2016;21:758–767. doi: 10.1038/mp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eysenck H.J., Eysenck S.B.G. Hodder & Stoughton; London: 1994. Manual for the Eysenck Personality Questionnaire (EPQ-R Adult) [Google Scholar]
- 41.Euesden J., Lewis C.M., O’Reilly P.F. PRSice: Polygenic risk score software. Bioinformatics. 2015;31:1466–1468. doi: 10.1093/bioinformatics/btu848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bulik-Sullivan B., Finucane H.K., Anttila V., Gusev A., Day F.R., Loh P.R. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patte K.A., Davis C.A., Levitan R.D., Kaplan A.S., Carter-Major J., Kennedy J.L. A behavioural genetic model of the mechanisms underlying the link between obesity and symptoms of ADHD. J Attention Disord. 2016 doi: 10.1177/1087054715618793. [published online ahead of print Jan 21] [DOI] [PubMed] [Google Scholar]
- 44.Docet M.F., Larranaga A., Perez Mendez L.F., Garcia-Mayor R.V. Attention deficit hyperactivity disorder increases the risk of having abnormal eating behaviours in obese adults. Eat Weight Disord. 2012;17:e132–e136. doi: 10.1007/BF03325337. [DOI] [PubMed] [Google Scholar]
- 45.Nazar B.P., de Sousa Pinna C.M., Suwwan R., Duchesne M., Freitas S.R., Sergeant J., Mattos P. ADHD rate in obese women with binge eating and bulimic behaviors from a weight-loss clinic. J Attention Disord. 2016;20:610–616. doi: 10.1177/1087054712455503. [DOI] [PubMed] [Google Scholar]
- 46.Vogen S.W.N., Bijlenga D., Tanke M., Bron T.I., van der Heijden K.B., Swaab H. Circadian rhythm disruption as a link between attention-deficit/hyperactivity disorder and obesity? J Psychosom Res. 2015;79:443–450. doi: 10.1016/j.jpsychores.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Rommel A.S., Rijsdijk F., Greven C.U., Asherson P., Kuntsi J. A longitudinal twin study of the direction of effects between ADHD symptoms and IQ. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker A.J., Batchelor J., Shores A. Effects of education and cultural background on performance on WAIS-III, WMS-III, WAIS-R and WMS-R measures: Systematic review. Aust Psychol. 2009;44:216–223. [Google Scholar]
- 49.Lo M.T., Hinds D.A., Tung J.Y., Franz C., Fan C.C., Wang Y. Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nat Genet. 2017;49:152–156. doi: 10.1038/ng.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClernon F.J., Kolins S.H. ADHD and smoking: From genes to brain to behaviour. Ann N Y Acad Sci. 2008;1141:131–147. doi: 10.1196/annals.1441.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munafo M., Clark T., Johnstone E., Murphy M., Walton R. The genetic basis for smoking behaviour: A systematic review and meta-analysis. Nicotine Tob Res. 2004;6:583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- 52.Carey C.E., Agrawal A., Bucholz K.K., Hartz S.M., Lynskey M.T., Nelson E.C. Associations between polygenic risk for psychiatric disorders and substance involvement. Front Genet. 2016;7:149. doi: 10.3389/fgene.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsson H., Anckarsater H., Rastam M., Chang Z., Lichtenstein P. Childhood attention-deficit hyperactivity disorder as an extreme of a continuous trait: A quantitative genetic study of 8,500 twin pairs. J Child Psychol Psychiatry. 2012;53:73–80. doi: 10.1111/j.1469-7610.2011.02467.x. [DOI] [PubMed] [Google Scholar]
- 54.Lubke G.H., Hudziak J.J., Derks E.M., Van Bijsterveldt T.C., Boomsma D.I. Maternal ratings of attention problems in ADHD: Evidence for the existence of a continuum. J Am Acad Child Adolesc Psychiatry. 2009;48:1085–1093. doi: 10.1097/CHI.0b013e3181ba3dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stergiakouli E., Martin J., Hamshere M.L., Langley K., Evans D.M., St Pourcain B. Shared genetic influences between attention-deficit/hyperactivity disorder (ADHD) traits in children and clinical ADHD. J Am Acad Child Adolesc Psychiatry. 2015;54:322–327. doi: 10.1016/j.jaac.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brikell I., Kuja-Halkola R., Larsson H. Heritability of attention-deficit hyperactivity disorder in adults. Am J Med Genet B Neuropsychiatr Genet. 2015;168:406–413. doi: 10.1002/ajmg.b.32335. [DOI] [PubMed] [Google Scholar]
- 57.Munafò M.R., Tilling K., Taylor A.E., Evans D.M., Davey Smith G. Collider scope: When selection bias can substantially influence observed associations. Int J Epidemiol. 2018;47:226–235. doi: 10.1093/ije/dyx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.