Abstract
Objectives
The goal of this study was to explore sex differences in myocardial remodeling in aortic stenosis (AS) by using echocardiography, cardiac magnetic resonance (CMR), and biomarkers.
Background
AS is a disease of both valve and left ventricle (LV). Sex differences in LV remodeling are reported in AS and may play a role in disease phenotyping.
Methods
This study was a prospective assessment of patients awaiting surgical valve replacement for severe AS using echocardiography, the 6-min walking test, biomarkers (high-sensitivity troponin T and N-terminal pro–brain natriuretic peptide), and CMR with late gadolinium enhancement and extracellular volume fraction, which dichotomizes the myocardium into matrix and cell volumes. LV remodeling was categorized into normal geometry, concentric remodeling, concentric hypertrophy, and eccentric hypertrophy.
Results
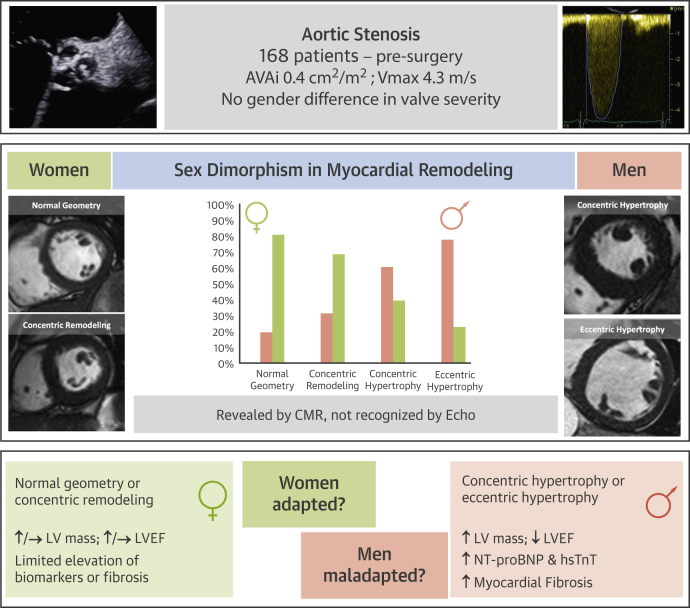
In 168 patients (age 70 ± 10 years, 55% male, indexed aortic valve area 0.40 ± 0.13 cm2/m2, mean gradient 47 ± 4 mm Hg), no sex or age differences in AS severity or functional capacity (6-min walking test) were found. CMR captured sex dimorphism in LV remodeling not apparent by using 2-dimensional echocardiography. Normal geometry (82% female) and concentric remodeling (60% female) dominated in women; concentric hypertrophy (71% male) and eccentric hypertrophy (76% male) dominated in men. Men also had more evidence of LV decompensation (pleural effusions), lower left ventricular ejection fraction (67 ± 16% vs. 74 ± 13%; p < 0.001), and higher levels of N-terminal pro–brain natriuretic peptide (p = 0.04) and high-sensitivity troponin T (p = 0.01). Myocardial fibrosis was higher in men, with higher focal fibrosis (late gadolinium enhancement 16.5 ± 11.2 g vs. 10.5 ± 8.9 g; p < 0.001) and extracellular expansion (matrix volume 28.5 ± 8.8 ml/m2 vs. 21.4 ± 6.3 ml/m2; p < 0.001).
Conclusions
CMR revealed sex differences in associations between AS and myocardial remodeling not evident from echocardiography. Given equal valve severity, the myocardial response to AS seems more maladaptive in men than previously reported. (Regression of Myocardial Fibrosis After Aortic Valve Replacement [RELIEF-AS]; NCT02174471)
Key Words: aortic stenosis, fibrosis, left ventricular hypertrophy
Abbreviations and Acronyms: AS, aortic stenosis; AVR, aortic valve replacement; BSA, body surface area; CMR, cardiac magnetic resonance; ECV, extracellular volume fraction; EDVi, indexed end-diastolic volume; ESVi, indexed end-systolic volume; hsTnT, high-sensitivity troponin T; IQR, interquartile range; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVMi, indexed left ventricular mass; NT-proBNP, N-terminal pro–brain natriuretic peptide
Graphical abstract
In aortic stenosis (AS), narrowing of the aortic valve is the hallmark of disease progression, but symptom onset and patient outcome are also determined by the left ventricular (LV) response to increasing afterload (1), which remodels in an attempt to maintain normal wall stress. This scenario is highlighted by the limited performance of markers of valve stenosis in predicting symptom onset (2). In contrast, left ventricular ejection fraction (LVEF), left ventricular hypertrophy (LVH), and myocardial fibrosis have all been shown to predict outcomes in AS 3, 4, 5, 6, 7, 8, 9, 10. However, LV remodeling is heterogeneous 11, 12, 13. Four main geometric patterns have been defined: normal geometry, concentric remodeling, concentric hypertrophy, and eccentric hypertrophy. These patterns are based on LV mass, cavity size, and the ratio of these 2 factors 14, 15. Functionally, the spectrum of LV responses ranges from hypercontractile to “myopathic” states. Echocardiography and cardiac magnetic resonance (CMR) are the gold standards for the assessment of valve severity and LV geometry/function, respectively. CMR is also able to quantify focal myocardial fibrosis 4, 5, 6, 7, 8 and extracellular expansion 16, 17, 18, whereas blood biomarkers (high sensitivity troponin T [hsTnT] and N-terminal pro–brain natriuretic peptide [NT-proBNP]) reflect whole heart myocyte death and increased wall stress.
Sex appears to exert an important influence on LV remodeling 11, 19, 20. Previous research has shown that men are more likely to have higher indexed LV mass, lower LVEF, and increased diastolic myocardial stiffness 21, 22, whereas women have more concentric remodeling with higher relative wall thickness and LVEF. To date, however, most studies have relied on echocardiography alone, with only limited combined echocardiography and CMR data available (22). The goal of the present study was to understand the influence of sex on AS remodeling by using all available modalities to investigate patterns of remodeling at macroscopic and tissue levels.
Methods
This study was a prospective observational cohort analysis of patients with severe, symptomatic AS undergoing aortic valve replacement (AVR) in a single tertiary referral cardiac center, University College London Hospital NHS Trust, between January 2012 and January 2015. The study was approved by the ethical committee of UK National Research Ethics Service (07/H0715/101) and was registered on ClinicalTrials.gov (NCT02174471). The study conformed to the principles of the Declaration of Helsinki, and all subjects provided written informed consent.
Patients were recruited before pre-operative evaluation, which included a comprehensive assessment with clinical history, resting blood pressure, 6-min walk test (23), blood sampling (for hsTnT and NT-proBNP), electrocardiogram, transthoracic 2-dimensional echocardiogram, and CMR (further details are given in the Online Appendix). Patients met the inclusion criteria if they were >18 years of age with severe AS (≥2 of aortic valve area <1 cm2, peak pressure gradient >64 mm Hg, mean pressure gradient >40 mm Hg, and aortic valve velocity ratio <0.25) undergoing AVR ± coronary artery bypass grafting. Exclusion criteria were pregnancy/breastfeeding, an estimated glomerular filtration rate <30 ml/min/1.73 m2, CMR-incompatible devices, inability to complete the protocol, previous valve surgery, or severe valve disease other than AS. Overall, 48% of patients undergoing surgical AVR for severe AS at our institution during the study period were recruited.
Cardiac imaging
Echocardiography assessed diastolic function and valve area/velocities (with CMR for regurgitant volumes if needed). CMR cine imaging assessed LV structure and function, as well as late gadolinium enhancement (LGE), T1 mapping, and extracellular volume fraction (ECV) for myocardial tissue characterization.
Echocardiography
Clinical transthoracic echocardiography was performed using a GE Vivid E9 system (GE Healthcare, Wauwatosa, Wisconsin) with a 4-MHz transducer, following the guidelines for assessment of AS severity and diastolic function as recommended by the American and European Societies of Echocardiography (24). Parameters of AS severity (energy loss index), myocardial work (myocardial contraction fraction) (25), end-diastolic wall stress (26), and vascular afterload (systemic arterial compliance, systemic vascular resistance, and valvuloarterial impedance) (27) are detailed in the Online Appendix.
CMR
CMR was performed at 1.5-T (Magnetom Avanto, Siemens Medical Solutions, Malvern, Pennsylvania) using a standard clinical scan protocol with LGE imaging and T1 mapping (modified Look-Locker inversion-recovery) before and after bolus gadolinium contrast (0.1 mmol/kg of gadoterate meglumine [gadolinium-DOTA, marketed as Dotarem, Guerbet S.A., Paris, France]). Post-contrast imaging was performed at 10 min (LGE) and 15 min (T1 mapping). CMR image analysis was performed by using CVI42 software version 5.1.2 [303] (Circle Cardiovascular Imaging, Calgary, Alberta, Canada) by operators blinded to clinical parameters.
LGE was quantified in grams and as a percentage of the LV using a signal intensity threshold of 3 SDs above the mean remote myocardium. ECV was calculated as: ECV = (1– hematocrit) × [ΔR1myocardium]/[ΔR1bloodpool] (28), where ΔR1 is the difference in relaxation rates pre- and post-contrast. ECV divides the myocardium into its cell and matrix compartments, giving insights into tissue-level pattern of LV remodeling. Total LV matrix and cell volumes were calculated from the product of LV myocardial volume and ECV or (1 − ECV), respectively (Online Appendix).
Patterns of LV remodeling
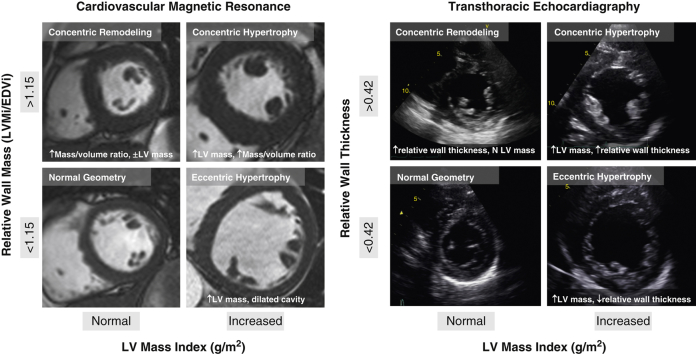
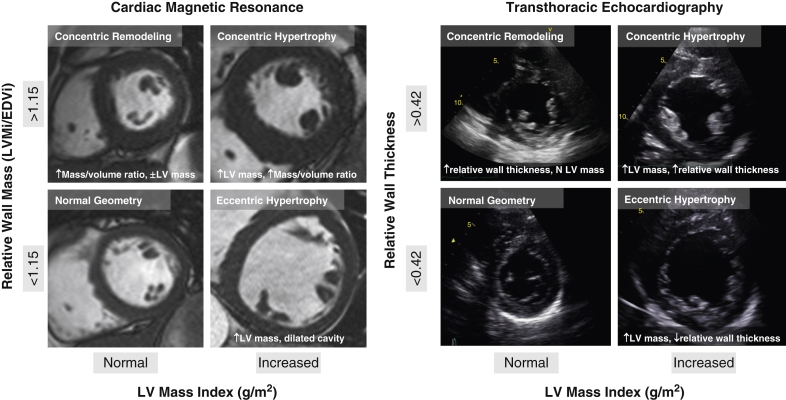
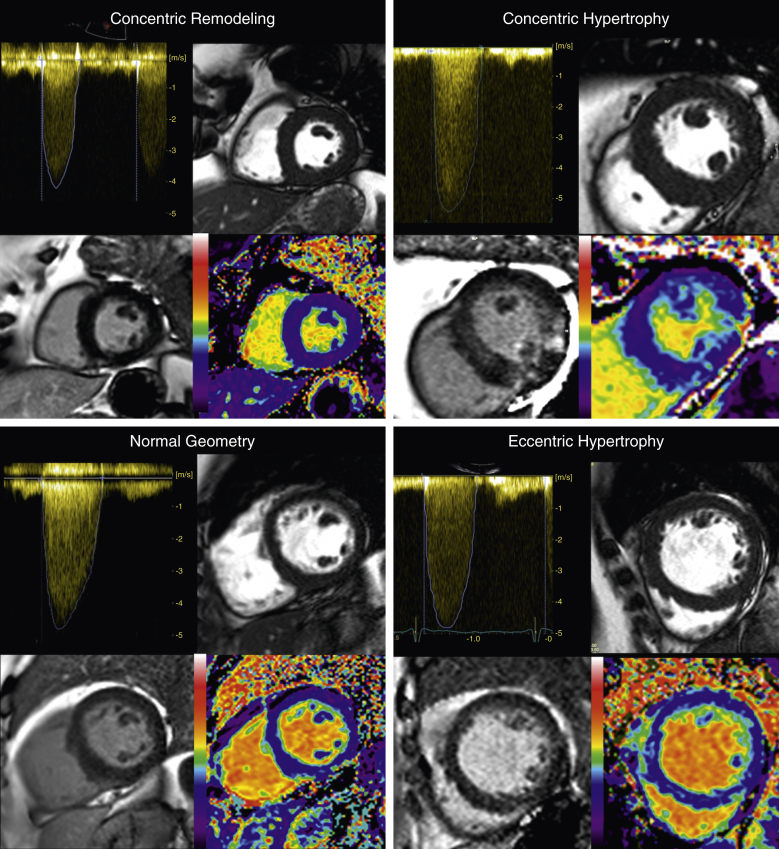
Patients with AS were categorized into 4 patterns of LV geometric adaption (Figure 1): normal geometry, concentric remodeling, concentric hypertrophy, and eccentric hypertrophy. For CMR, categories were defined by body surface area (BSA)-indexed left ventricular mass (LVMi), indexed left ventricular end-diastolic volume, and mass/volume ratio (14). For echocardiography, categories were defined by using BSA-indexed LVMi, end-diastolic cavity dimension, and relative wall thickness, as previously described (15) (Online Appendix).
Figure 1.
Remodeling by Cardiac Magnetic Resonance and Echocardiography
Patients were categorized into 4 patterns of left ventricular (LV) geometric adaption: normal geometry, concentric remodeling, concentric hypertrophy, and eccentric hypertrophy. For cardiac magnetic resonance, categories were defined by using body surface area–indexed LV mass (LVMi), indexed LV end-diastolic volume (EDVi), and mass/volume ratio. For 2-dimensional echocardiography, categories were defined by using body surface area–LVMi, end-diastolic cavity dimension, and relative wall thickness.
Statistical analysis
Statistical analyses were conducted by using SPSS version 22 (IBM SPSS Statistics, IBM Corporation, Armonk, New York). All continuous variables are expressed as mean ± SD or median (interquartile range [IQR]) for skewed data. Categorical variables are expressed as percentages. Normality was checked by using the Shapiro-Wilk test. Groups were compared by using independent-sample Student t tests for normally distributed continuous variables, the Mann-Whitney U test for non-normally distributed variables, and the Fisher exact test or a chi-square test for categorical variables. A 2-sided p value <0.05 was considered significant.
Results
Study population
There were 181 patients with severe, symptomatic AS recruited (age 69 ± 10 years; 56% male) representing 48% of all surgical AVRs at the study institution. Thirteen patients were excluded: claustrophobia (n = 2), hemodynamic instability (n = 1), pseudo-severe AS (n = 1), severe mitral regurgitation (n = 2), and significant myocardial bystander disease (cardiac amyloidosis, n = 6; Fabry disease, n = 1) (29).
Characteristics of the remaining 168 patients (age 70 ± 10 years; 55% male; 70% trileaflet AS) are summarized in Tables 1 and 2. All but 7 patients were symptomatic (96%) with dyspnea (82%), chest pain (32%), and/or syncope (8%). CMR identified pericardial effusions (>5 mm) in 47 patients and pleural effusions (>1 cm) in 36 patients (22 with both).
Table 1.
Baseline Characteristics
| Total (N = 168, 100%) | Men (n = 92, 55%) | Women (n = 76, 45%) | p Value | |
|---|---|---|---|---|
| Age, yrs | 70 ± 10 | 70 ± 10 | 70 ± 10 | 0.90 |
| Trileaflet∗ | 118 (100) | 61 (66) | 57 (76) | 0.20 |
| Bicuspid∗ | 49 (100) | 31 (34) | 18 (24) | 0.20 |
| BSA, m2 | 1.88 ± 0.21 | 1.98 ± 0.19 | 1.76 ± 0.17 | <0.001 |
| Comorbidities | ||||
| Hypertension, % | 77 | 81 | 73 | 0.40 |
| SBP, mm Hg | 133 ± 18 | 130 ± 18 | 137 ± 18 | 0.01 |
| DBP, mm Hg | 75 ± 11 | 74 ± 10 | 77 ± 13 | 0.10 |
| Diabetes, % | 26 | 22 | 29 | 0.50 |
| Coronary artery disease, % | 30 | 37 | 21 | 0.03 |
| Atrial fibrillation, % | 14 | 16 | 14 | 0.70 |
| Smoker, current/ex/never | 50/21/97 | 28/17/46 | 22/04/51 | 0.20 |
| Risk scores | ||||
| STS, % | 1.43 (0.98–2.37) | 1.31 (0.88–2.32) | 1.62 (1.04–2.39) | 0.30 |
| EuroSCORE II, % | 1.49 (1.01–2.44) | 1.42 (0.98–2.47) | 1.54 (1.02–2.40) | 0.60 |
| Drug history | ||||
| ACE inhibitor/ARB, % | 43 | 53 | 31 | 0.006 |
| Beta-blocker, % | 34 | 32 | 56 | 0.50 |
| Statin, % | 61 | 63 | 59 | 0.80 |
| Aspirin, % | 44 | 47 | 41 | 0.40 |
| Symptomatic (yes/no) | 161/7 | 87/5 | 74/2 | 0.30 |
| NYHA functional class | 2.3 ± 0.7 | 2.2 ± 0.8 | 2.4 ± 0.6 | 0.10 |
| I | 30 | 23 | 10 | |
| II | 79 | 40 | 39 | |
| III | 54 | 26 | 28 | |
| IV | 5 | 4 | 1 | |
| Chest pain by CCS | 0.90 | |||
| 0 | 115 | 60 | 55 | |
| 1 | 14 | 12 | 2 | |
| 2 | 29 | 9 | 20 | |
| 3 | 10 | 8 | 2 | |
| Syncope | 14 (8) | 7 (8) | 7 (9) | 0.70 |
| Six-min walk test, m | 480 (338–600) | 510 (360–630) | 420 (300–510) | 0.02 |
| ECG | ||||
| LVH by Cornell criteria | 43 (26) | 25 (27) | 18 (24) | 0.30 |
| ECG strain | 29 (17) | 17 (19) | 12 (16) | 0.50 |
| Blood | ||||
| NT-proBNP, ng/l | 71 (29–238) | 94 (36–304) | 50 (28–143) | 0.04 |
| NT-proBNP ratio | 0.18 (0.08–0.69) | 0.33 (0.09–1.12) | 0.11 (0.05–0.35) | 0.04 |
| hsTnT, pmol/l | 14 (9–20) | 15 (11–25) | 12 (7–16) | 0.02 |
| Creatinine, μmol/l | 81 (70–98) | 90 (77–103) | 74 (63–86) | <0.001 |
| eGFR, ml/min/1.73 m2 | 74 (63–92) | 75 (64–95) | 72 (61–86) | 0.30 |
| Hematocrit, % | 40 ± 4 | 41 ± 5 | 39 ± 4 | 0.01 |
Values are mean ± SD, n (%), n, or median (interquartile range).
ACE = angiotensin-converting enzyme; ARB = angiotensin-receptor blocker; BSA = body surface area; CCS = Canadian Cardiovascular Society Grading System; DBP = diastolic blood pressure; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; EuroSCORE II = European System for Cardiac Operative Risk Evaluation II score; hsTnT = high-sensitivity troponin T; IQR = interquartile range; LVH = left ventricular hypertrophy; NT-proBNP = N-terminal pro–brain natriuretic peptide; NYHA = New York Heart Association; SBP = systolic blood pressure; STS = Society of Thoracic Surgeons' risk model score.
1 patient had unicuspid AS (female). Bold values indicates p < 0.05.
Table 2.
Imaging Parameters (Echocardiography and CMR)
| Total | Men | Women | p Value | |
|---|---|---|---|---|
| Echocardiography | ||||
| Vmax, m/s | 4.33 ± 0.59 | 4.38 ± 0.59 | 4.27 ± 0.59 | 0.30 |
| Peak gradient, mm Hg | 76 ± 20 | 78 ± 21 | 75 ± 19 | 0.40 |
| Mean gradient, mm Hg | 47 ± 14 | 49 ± 15 | 46 ± 13 | 0.30 |
| AVA, cm2 | 0.76 ± 0.27 | 0.78 ± 0.27 | 0.74 ± 0.26 | 0.10 |
| AVAi, cm2/m2 | 0.40 ± 0.13 | 0.39 ± 0.13 | 0.41 ± 0.13 | 0.30 |
| VTI ratio | 0.23 ± 0.08 | 0.22 ± 0.07 | 0.24 ± 0.08 | 0.10 |
| Energy loss index, cm2/m2 | 0.48 ± 0.19 | 0.46 ± 0.17 | 0.53 ± 0.30 | 0.06 |
| Systemic vascular resistance, dyne*s/cm5 | 1,237 (1,038–1,550) | 1,167 (1,010–1,400) | 1,338 (1,142–1,647) | 0.001 |
| Systemic arterial compliance, ml/mm Hg*m2 | 1.35 ± 0.47 | 1.28 ± 0.49 | 1.42 ± 0.43 | 0.06 |
| Zva, mm Hg/ml*m2 | 4.2 ± 1.2 | 4.1 ± 1.3 | 4.4 ± 1.0 | 0.20 |
| E wave | 0.85 ± 0.30 | 0.83 ± 0.30 | 0.87 ± 0.29 | 0.40 |
| E/A ratio | 0.97 ± 0.49 | 1.03 ± 0.59 | 0.89 ± 0.32 | 0.10 |
| E deceleration time, ms | 237 ± 75 | 236 ± 82 | 238 ± 66 | 0.90 |
| E/e' ratio | 13.6 ± 5.9 | 13.5 ± 6.2 | 13.8 ± 5.6 | 0.80 |
| PASP, mm Hg | 30 (25–25) | 30 (25–25) | 30 (26–35) | 0.50 |
| CMR parameters | ||||
| EDVi, ml/m2 | 67 ± 22 | 73 ± 23 | 61 ± 19 | 0.001 |
| ESVi, ml/m2 | 23 ± 20 | 27 ± 22 | 18 ± 16 | 0.001 |
| LVMi, g/m2 | 88 ± 25 | 98 ± 23 | 75 ± 20 | 0.001 |
| Septal wall thickness, mm | 14 ± 3 | 15 ± 2 | 13 ± 2 | <0.001 |
| Left ventricular diameter, mm | 50 ± 7 | 52 ± 7 | 47 ± 6 | <0.001 |
| Mass/volume ratio | 1.37 ± 0.35 | 1.44 ± 0.39 | 1.30 ± 0.28 | 0.001 |
| LAAi, pre-operative, cm2/m2 | 13.5 ± 3.7 | 13.6 ± 3.3 | 13.4 ± 4.1 | 0.80 |
| LVEF, % | 70 ± 15 | 67 ± 16 | 74 ± 13 | 0.001 |
| SVi, ml/m2 | 45 ± 10 | 46 ± 12 | 43 ± 8 | 0.30 |
| Myocardial contraction fraction, % | 0.53 ± 0.15 | 0.48 ± 0.13 | 0.59 ± 0.14 | 0.001 |
| Wall stress index, kPa | 1.40 ± 0.29 | 1.35 ± 0.29 | 1.46 ± 0.27 | 0.008 |
| Pattern of remodeling by CMR | ||||
| Normal geometry | 28 (17) | 5 (18) | 23 (82) | Chi-square test = 34; p < 0.001 |
| Concentric remodeling | 45 (27) | 18 (40) | 27 (60) | |
| Concentric hypertrophy | 70 (41) | 50 (71) | 20 (29) | |
| Eccentric hypertrophy | 25 (15) | 19 (76) | 6 (24) | |
| CMR flow | ||||
| Aortic regurgitant fraction, % | 12 (4–35) | 14 (6–47) | 10 (3–24) | 0.10 |
| Mitral regurgitant fraction, % | 5 (1–23) | 3 (0–24) | 6 (1–22) | 0.40 |
| Late gadolinium enhancement | ||||
| 3 SDs method, g | 9.6 (5.0–22.4) | 14.8 (8.4–26.9) | 6.0 (4.0–17.4) | <0.001 |
| T1 mapping (MOLLI) | ||||
| T1 myocardium (native, in ms) | 1,045 ± 45 | 1,041 ± 42 | 1,051 ± 47 | 0.20 |
| ECV, % | 28.6 ± 2.9 | 28.7 ± 3.1 | 28.6 ± 2.5 | 0.20 |
| Cell volume, indexed, ml/m2 | 65 ± 18 | 73 ± 17 | 55 ± 13 | <0.001 |
| Matrix volume, indexed, ml/m2 | 25 ± 9 | 29 ± 9 | 21 ± 6 | <0.001 |
Values are mean ± SD, median (interquartile range), or n (%). Bold values indicates p < 0.05.
AVA = aortic valve area; AVAi = aortic valve area index; CMR = cardiac magnetic resonance; E = peak early velocity of the transmitral flow; e’ = peak early diastolic velocity of the mitral annulus displacement; E/A ratio = ratio of peak velocity flow in early diastole (E wave) to peak velocity flow in late diastole (A wave); ECV = extracellular volume; EDVi = end-diastolic volume index; ESVi = end-systolic volume index; IQR = interquartile range; LAAi = left atrial area index; LVMi = left ventricular mass index; LVEF = left ventricular ejection fraction; MOLLI = modified Look-Locker inversion-recovery; PASP = pulmonary artery systolic pressure measured by echocardiography; SVi = stroke volume index; Vmax = peak velocity through the aortic valve; VTI = velocity-time-integral; Zva = valvuloarterial impedance.
There were no sex differences in the aortic valve regurgitant fraction (14% vs. 10%; p = 0.10), or mitral valve regurgitant fraction (3% vs. 6%; p = 0.40). Furthermore, there were no sex differences in age, smoking status, diabetes, or hypertension prevalence, although office systolic blood pressure (130 ± 18 mm Hg vs. 137 ± 18 mm Hg; p = 0.01) and glycosylated hemoglobin levels (38% [IQR: 35% to 41%] vs. 42% [IQR: 39% to 46%]; p = 0.003) were higher in women. Coronary artery disease (stenosis >50%) was more prevalent in men (37% vs. 21%; p = 0.03).
AS severity and sex
There were no sex differences in standard echocardiographic parameters of AS severity (valve area, gradient, or velocity ratios) (Table 2). Advanced echocardiographic parameters revealed subtle sex differences in AS severity and vascular load: men had a trend toward lower energy recovery measured by using the energy loss index (0.46 ± 0.17 cm2/m2 vs. 0.53 ± 0.30 cm2/m2; p = 0.06) (30) with larger aortic dimensions (6.4 ± 1.7 cm2 vs. 4.6 ± 1.6 cm2; p < 0.001). Furthermore, men had lower mean arterial pressure (93 ± 11 mm Hg vs. 97 ± 12 mm Hg; p = 0.02) and systemic vascular resistance (1,167 dyne·s·cm−5 [IQR, 1,010 to 1,400 dyne·s·cm−5] vs. 1,338 dyne·s·cm−5 [IQR: 1,142 to 1,647 dyne·s·cm−5]; p = 0.001), although global afterload assessed according to valvulo-arterial impedance (p = 0.2) did not differ.
Pattern of remodeling and sex
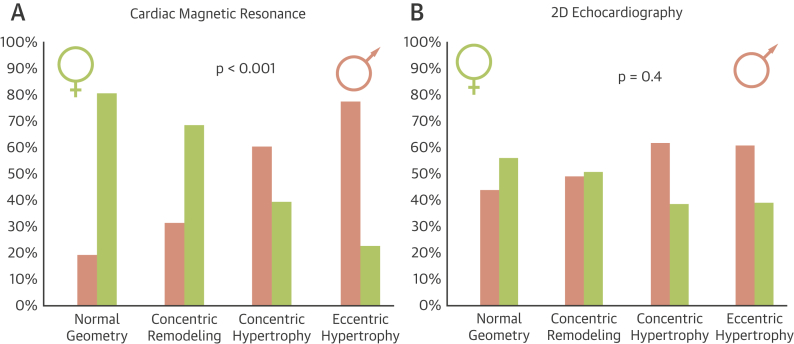
The geometry and function according to CMR (Table 2) differed according to sex. Men had larger LV dimensions, even when indexed (EDVi: 73 ± 23 ml vs. 61 ± 19 ml [p < 0.001]; ESVi: 27 ± 22 g vs. 18 ± 16 g [p = 0.004]) and greater LVMi (98 ± 23 g/m2 vs. 75 ± 20 g/m2; p < 0.001) and mass/volume ratio (1.44 ± 0.39 vs. 1.30 ± 0.28; p < 0.001). There were also marked sex differences in remodeling (chi-square test = 34; p < 0.001): normal geometry (82% female) and concentric remodeling (60% female) were predominantly seen in women, whereas concentric hypertrophy (71% male) and eccentric hypertrophy (76% male) dominated in men. This outcome was not apparent according to echocardiography (p = 0.40; female: normal geometry 56%, concentric remodeling 51%, concentric hypertrophy 38%, and eccentric hypertrophy 39%) (Figure 2, Online Figure 1).
Figure 2.
Sex Differences in Left Ventricular Pattern of Remodeling in Aortic Stenosis
(A) Cardiac magnetic resonance found marked sex differences in left ventricular remodeling (chi-square test = 34; p < 0.001). Normal geometry (82% female) and concentric remodeling (60% female) were predominantly seen in women, whereas concentric hypertrophy (71% male) and eccentric hypertrophy (76% male) in men. (B) This outcome was not apparent by 2-dimensional (2D) echocardiography (female: normal geometry 56%, concentric remodeling 51%, concentric hypertrophy 38%, and eccentric hypertrophy 39% [chi-square test = 2.7; p = 0.40]). Percentages are expressed as male/female split per remodeling category.
Symptoms and myocardial response
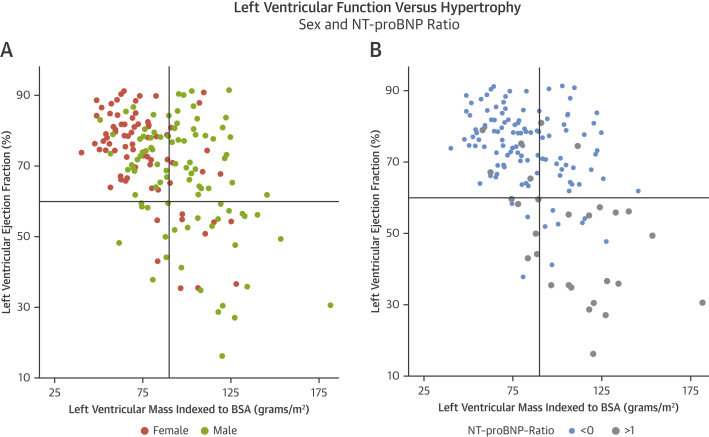
No sex differences in New York Heart Association functional class were found (p = 0.20). Although men were able to walk farther than women on the 6-min walk test assessment (510 m [IQR: 360 to 630 m] vs. 420 m [IQR: 300 to 510 m]; p = 0.02), the percentage-predicted 6-min walk test distance (31) did not significantly differ between men and women (97 ± 34% vs. 96 ± 40%; p = 0.60). LVEF was lower in men than in women (67 ± 16% vs. 74 ± 13%; p < 0.001) (Table 2). Men had lower minute work (15.6 ± 4.7 ml·mm Hg/min vs. 17.7 ± 4.5 ml·mm Hg/min; p = 0.005) and myocardial contraction fraction (48 ± 13% vs. 59 ± 14%; p < 0.001). Furthermore, both NT-proBNP and hsTnT were higher in men (NT-proBNP: 94 pmol/l [IQR: 36 to 304 pmol/l] vs. 50 pmol/l [IQR: 28 to 143 pmol/l] [p = 0.04]; hsTnT: 15 pg/l [IQR: 11 to 25 pg/l] vs. 12 pg/l [IQR: 7 to 16 pg/l] [p = 0.01]). Figure 3 displays the distribution of LVEF versus indexed LV mass according to sex and according to BNP clinical activation, defined as a NT-proBNP ratio >1 (absolute NT-proBNP concentration indexed for the 95th centile of normal range for age and sex [32]).
Figure 3.
Sex, Left Ventricular Hypertrophy, and Decompensation
(A) Indexed left ventricular mass (LVMi) and left ventricular ejection fraction (LVEF) by sex. Men had greater LVMi (98 ± 23 g/m2 vs. 75 ± 20 g/m2; p < 0.001) and lower LVEF than women (67 ± 16% vs. 74 ± 13%; p < 0.001). (B) LVMi and LVEF by N-terminal pro–brain natriuretic peptide (NT-proBNP) ratio greater (gray dots) or less than 1 (blue dots), which were higher in men than women (0.33 [interquartile range: 0.09 to 1.12] vs. 0.11 [interquartile range: 0.05 to 0.35]; p = 0.04). BSA = body surface area.
Focal fibrosis, extracellular expansion, and sex
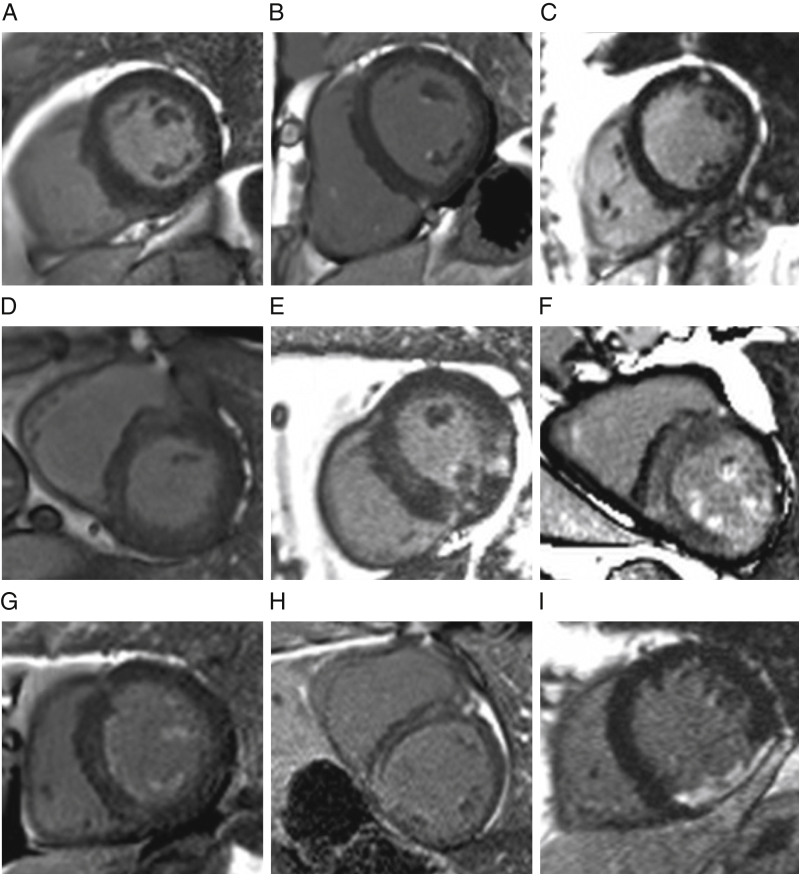
Examples of LGE patterns are shown in Figure 4. There was more LGE in men according to both overall prevalence (71% vs. 46%; p < 0.01) and extent (14.8 g [IQR: 8.4 to 26.9 g] vs. 6.0 g [IQR: 4.0 to 17.4 g]; p < 0.001), although these differences were not statistically significant when expressed as a percentage of the LV mass (8.6 ± 5.6% vs. 7.7 ± 5.9%; p = 0.10). Whereas prevalence of infarct pattern LGE was the same (men 16% vs. women 17%), noninfarct pattern LGE was more common in men (59% vs. 37%). No sex differences in native myocardial T1 or ECV (T1: 1,041 ± 42 ms vs. 1,051 ± 47 ms [p = 0.20]; ECV: 28.6 ± 3.1% vs. 28.2 ± 2.7% [p = 0.20]) were observed. However, using the ECV to dichotomize the LVMi into matrix and cell compartments, both indexed matrix (28.5 ± 8.8 ml/m2 vs. 21.4 ± 6.3 ml/m2; p < 0.001) and cell volumes (72.7 ± 16.7 ml/m2 vs. 54.7 ± 13.0 ml/m2; p < 0.001) were higher in men.
Figure 4.
LGE Pattern in Severe Aortic Stenosis
(A) No late gadolinium enhancement (LGE). (B) Focal papillary muscle and right ventricular (RV) insertion point LGE. (C) Focal mid-wall LGE in the anterolateral wall. Diffuse, patchy myocardial LGE ranging from (D) mild to (E) moderate to (F) severe LGE burden, associated with papillary muscle RV insertion and RV free wall LGE. (G) Noninfarct, subendocardial, and papillary muscle LGE. (H) Dilated cardiomyopathy pattern LGE. (I) Full thickness infarct in the thinned inferior wall.
Discussion
In this prospective multimodality study of 168 patients with symptomatic severe AS referred for surgical AVR, despite the same referral age, valve severity, and functional status, there were major sex differences in myocardial remodeling, fibrosis, and resultant LV function. Our data highlight the importance of the myocardial response in AS encompassing a wide geometric and functional range (Figure 5), which is neither associated with the hemodynamic severity of the aortic valve stenosis nor observed by using conventional echocardiography. Men predominantly had concentric or eccentric LVH as well as a less favorable, maladaptive ventricular phenotype (lower LVEF, higher NT-proBNP and hsTnT, and more focal fibrosis and extracellular expansion). In contrast, women exhibited a possibly more favorable phenotype with less hypertrophy, less focal fibrosis and extracellular expansion, and a higher prevalence of normal geometry or concentric remodeling with higher LVEF. Although the higher levels of NT-proBNP and hsTnT could be partially explained by more LVH and larger hearts in men, functional and fibrosis parameters were consistent with a worse myocardial remodeling in men.
Figure 5.
Left Ventricular Remodeling in Aortic Stenosis by Multimodality Imaging
There are 4 images each for all 4 patterns of remodeling: continuous-wave Doppler assessment of aortic stenosis severity (top left); Steady State Free Precession short-axis cine clip demonstrating the pattern of remodeling (as described in Figure 1) (top right); phase-sensitive inversion recovery late gadolinium enhancement image for focal fibrosis (bottom left); extracellular volume fraction map for diffuse fibrosis (bottom right).
These findings raise a few key issues. First, given the stark differences in myocardial remodeling, how do these affect the interpretation of the hemodynamic severity of the valve stenosis? Second, these changes may be adaptive or maladaptive: can LVEF, NT-proBNP, and hsTnT adequately highlight the transition into maladaptation, or are other biomarkers needed? In addition, are blood biomarkers more informative than imaging? Finally, what are the mechanisms driving the sex differences in remodeling?
Sex dimorphism in myocardial response
In this study, women seemed to tolerate a similar level of valve-related afterload better (women even had higher blood pressures and fewer cardioprotective drugs), with better-preserved wall stress and better systolic pump performance (LVEF and myocardial contraction fraction) than men. Sex-related differences in myocardial remodeling have been reported in the elderly with or without AS 11, 19, 33, 34, 35. In animal models, sex dimorphism exists in the baseline findings of the heart (difference in size, physiology, gene profiles, and contractile properties), response to pressure or volume overload (more hypertrophy and dilatation, respectively), and cardiomyocyte response to aging and modification of cardiac gene expression (36). Cellular, molecular, and neurohormonal mechanisms for the differential response in men have been proposed, including increased interstitial fibrosis, greater activation of profibrotic and inflammatory pathways, and differential expression of androgen and estrogen receptors 21, 37, 38, 39. Although the interplay of protective effects of estrogens and deleterious effects of androgens may play a key role in the sex dimorphism, the majority of female patients in our study were post-menopausal, and none were receiving hormone replacement therapy. Sex differences in the renin-angiotensin system, nitric oxide activity, and norepinephrine release may contribute to differences in LV remodeling (40); similar differences in cardiac function and arterial hemodynamic variables to those observed here have also been seen in community-based samples of older men and women (41). A less explored possibility is that the myocardium could have been sex-patterned during cardiac fetal formation to adapt differently during adult life.
Discordance with previous echocardiographic data
Sex dimorphism in cardiac remodeling in AS is present in the literature but has not been emphasized. For example, in an echocardiographic study of 2,017 patients (36% female) awaiting AVR (42), LV impairment had a 3.5 to 1 male-to-female ratio and LVEF >70% had a 1:1 male-to-female ratio. Given the study entry sex ratios, if there had been no sex dimorphism, both of these ratios should have been 1.7 to 1.0. However, the sex dimorphism of cardiac remodeling according to CMR was much more extreme than that according to echocardiography (Figure 6). There are modality-specific differences in ascertainment that could explain this: cross-sectional echocardiography uses derived wall thickness to cavity width ratios, whereas CMR uses a 3-dimensional–derived mass to volume ratio 14, 22. Each technique also has indexed sex-specific reference ranges and cut-points (Online Appendix), which could be inaccurate and magnify differences. These may be differently sensitive to sex-influenced confounders (e.g., a basal septal bulge). Such explanations seem inadequate, however, and the impression is that an echocardiography-based approach to cardiac remodeling has induced an underestimation of biological sex dimorphism in cardiac remodeling in AS.
Figure 6.
Sex Dimorphism in Myocardial Response to AS
Aortic stenosis (AS) is a disease of both valve and LV. Sex differences may play a role in disease phenotyping. The present study investigated 168 patients with severe symptomatic AS by using echocardiography (echo), cardiac magnetic resonance (CMR), and biomarkers. There were no sex differences in AS severity or functional capacity, but CMR captured a sex dimorphism in the LV remodeling pattern, missed by 2-dimensional echocardiography and more adverse in men with more LV dysfunction (by LVEF, N-terminal pro–brain natriuretic peptide [NT-proBNP], high-sensitivity troponin T [hsTnT]) and myocardial fibrosis (focal and diffuse). Given equal valve severity, LV associations with AS appear more maladaptive in men, with more extreme sex differences than previously reported. AVAi = indexed aortic valve area; Vmax = peak velocity; other abbreviations as in Figures 1 and 3.
Perspective: Do we need sex-specific thresholds for AVR?
Timing of aortic valve intervention is one of the greatest challenges in AS, particularly in asymptomatic patients. Recent focus has turned toward the complex interplay between aortic valve stenosis, vascular load, and myocardial response (inappropriate hypertrophy, myocardial stress [NT-proBNP], fibrosis [troponin, LGE, and ECV], and myocardial perfusion reserve). Our data support the notion that we may need to treat men and women differently because they experience a different cardiac “milieu,” different combined (valve and vasculature) afterload, and display a different myocardial response. Crucially, data showing reverse remodeling after valve replacement and its impact on outcome are required and pending.
Study limitations
Only patients with severe symptomatic AS, and specifically those referred for surgery at a specialist center, were included. The study is therefore not representative of patients treated medically or by transcatheter AVR, or patients with milder disease. Other factors, including hypertension duration and control, duration of severe AS, and coronary artery disease, may in part account for the sex dimorphism in LV remodeling. CMR inclusion criteria excluded patients with pacemakers and an estimated glomerular filtration rate <30 ml/min/1.73 m2; this approach only excluded 7% of patients and is unlikely to have biased our findings. No invasive LV pressure data were obtained; due to stroke risk associated with crossing the aortic valve, this measurement is not routinely performed in our institution. Imprecision in T1 mapping may have been introduced due to partial-voluming of blood (although this possibility was minimized by using a 10% offset) (Online Appendix) and in those patients with atrial fibrillation (n = 24; 14%). Furthermore, reduced capillary density (lower ECV) or compensatory vasodilatation (higher ECV) may confound ECV measurements, which capture all extracellular space including the intravascular plasma 43, 44. Finally, no data were available on the duration of AS.
Conclusions
CMR revealed sex differences in associations between AS and myocardial remodeling that were not evident from conventional echocardiography. Given equal valve severity, the myocardial response to AS seems more maladaptive in men than previously reported. These data suggest that more detailed phenotyping of patients with AS is required; the resultant uncovering of a maladaptive ventricular response may be influential in the current debate regarding immediate or deferred intervention for severe AS.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: AS is a disease of both valve and left ventricle. Sex difference may play a role in disease phenotyping. This study found sex differences in the associations between AS and myocardial remodeling that were more adverse in men, including more LV decompensation and myocardial fibrosis (focal and diffuse) despite similar valve severity.
TRANSLATIONAL OUTLOOK: Timing of aortic valve intervention is one of the greatest challenges in AS. Recent focus has turned toward the complex interplay between valve stenosis, vascular load, and myocardial response. Sex differences in the myocardial response suggest that men and women may need to be managed differently. Crucially, outcome data and reverse remodeling after valve replacement and its impact on outcome are required and pending.
Footnotes
This research was undertaken at University College London Hospital, which received a proportion of funding from the UK Department of Health National Institute for Health Research Biomedical Research Centers funding scheme. Dr. Treibel was supported by Doctoral Research Fellowships from the National Institute for Health Research, United Kingdom (NIHR-DRF-2013-06-102). Dr. Fontana was supported by a Clinical Research Training Fellowship from the British Heart Foundation (grants FS/12/56/29723). Dr. Moon has received grant funding from GlaxoSmithKline. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
References
- 1.Dweck M.R., Boon N.A., Newby D.E. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A., Alfieri O., Andreotti F. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2012;42:S1–S44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 3.Cioffi G., Faggiano P., Vizzardi E. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97:301–307. doi: 10.1136/hrt.2010.192997. [DOI] [PubMed] [Google Scholar]
- 4.Azevedo C.F., Nigri M., Higuchi M.L. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 5.Dweck M.R., Joshi S., Murigu T. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 6.Barone-Rochette G., Pierard S., De Meester de Ravenstein C. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol. 2014;64:144–154. doi: 10.1016/j.jacc.2014.02.612. [DOI] [PubMed] [Google Scholar]
- 7.Weidemann F., Herrmann S., Stork S. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 8.Herrmann S., Stork S., Niemann M. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58:402–412. doi: 10.1016/j.jacc.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 9.Chin C.W., Shah A.S., McAllister D.A. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosjo H., Andreassen J., Edvardsen T., Omland T. Prognostic usefulness of circulating high-sensitivity troponin T in aortic stenosis and relation to echocardiographic indexes of cardiac function and anatomy. Am J Cardiol. 2011;108:88–91. doi: 10.1016/j.amjcard.2011.02.346. [DOI] [PubMed] [Google Scholar]
- 11.Carroll J.D., Carroll E.P., Feldman T. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86:1099–1107. doi: 10.1161/01.cir.86.4.1099. [DOI] [PubMed] [Google Scholar]
- 12.Page A., Dumesnil J.G., Clavel M.A. Metabolic syndrome is associated with more pronounced impairment of left ventricle geometry and function in patients with calcific aortic stenosis: a substudy of the ASTRONOMER (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin) J Am Coll Cardiol. 2010;55:1867–1874. doi: 10.1016/j.jacc.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 13.Lund B.P., Gohlke-Barwolf C., Cramariuc D., Rossebo A.B., Rieck A.E., Gerdts E. Effect of obesity on left ventricular mass and systolic function in patients with asymptomatic aortic stenosis (a Simvastatin Ezetimibe in Aortic Stenosis [SEAS] substudy) Am J Cardiol. 2010;105:1456–1460. doi: 10.1016/j.amjcard.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 14.Dweck M.R., Joshi S., Murigu T. Left ventricular remodeling and hypertrophy in patients with aortic stenosis: insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:50. doi: 10.1186/1532-429X-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganau A., Devereux R.B., Roman M.J. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 16.Flett A.S., Hayward M.P., Ashworth M.T. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 17.White S.K., Sado D.M., Fontana M. T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. J Am Coll Cardiol Img. 2013;6:955–962. doi: 10.1016/j.jcmg.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.P., Lee W., Lee J.M. Assessment of diffuse myocardial fibrosis by using MR imaging in asymptomatic patients with aortic stenosis. Radiology. 2015;274:359–369. doi: 10.1148/radiol.14141120. [DOI] [PubMed] [Google Scholar]
- 19.Douglas P.S., Otto C.M., Mickel M.C., Labovitz A., Reid C.L., Davis K.B. Gender differences in left ventricle geometry and function in patients undergoing balloon dilatation of the aortic valve for isolated aortic stenosis. NHLBI Balloon Valvuloplasty Registry. Br Heart J. 1995;73:548–554. doi: 10.1136/hrt.73.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aurigemma G.P., Gaasch W.H. Gender differences in older patients with pressure-overload hypertrophy of the left ventricle. Cardiology. 1995;86:310–317. doi: 10.1159/000176895. [DOI] [PubMed] [Google Scholar]
- 21.Villari B., Campbell S.E., Schneider J., Vassalli G., Chiariello M., Hess O.M. Sex-dependent differences in left ventricular function and structure in chronic pressure overload. Eur Heart J. 1995;16:1410–1419. doi: 10.1093/oxfordjournals.eurheartj.a060749. [DOI] [PubMed] [Google Scholar]
- 22.Dobson L.E., Fairbairn T.A., Musa T.A. Sex-related differences in left ventricular remodeling in severe aortic stenosis and reverse remodeling after aortic valve replacement: a cardiovascular magnetic resonance study. Am Heart J. 2016;175:101–111. doi: 10.1016/j.ahj.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Castillo-Moreno J.A., Garcia-Escribano I.A., Martinez-Pascual-de-Riquelme M. Prognostic usefulness of the 6-minute walk test in patients with severe aortic stenosis. Am J Cardiol. 2016;118:1239–1243. doi: 10.1016/j.amjcard.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner H., Hung J., Bermejo J. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]
- 25.King D.L., El-Khoury Coffin L., Maurer M.S. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40:325–329. doi: 10.1016/s0735-1097(02)01944-7. [DOI] [PubMed] [Google Scholar]
- 26.Alter P., Rupp H., Stoll F. Increased end diastolic wall stress precedes left ventricular hypertrophy in dilative heart failure—use of the volume-based wall stress index. Int J Cardiol. 2012;157:233–238. doi: 10.1016/j.ijcard.2011.07.092. [DOI] [PubMed] [Google Scholar]
- 27.Pibarot P., Dumesnil J.G. Improving assessment of aortic stenosis. J Am Coll Cardiol. 2012;60:169–180. doi: 10.1016/j.jacc.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 28.Moon J.C., Messroghli D.R., Kellman P. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treibel T.A., Fontana M., Gilbertson J.A. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging. 2016:9. doi: 10.1161/CIRCIMAGING.116.005066. pii: e005066. [DOI] [PubMed] [Google Scholar]
- 30.Garcia D., Pibarot P., Dumesnil J.G., Sakr F., Durand L.G. Assessment of aortic valve stenosis severity: a new index based on the energy loss concept. Circulation. 2000;101:765–771. doi: 10.1161/01.cir.101.7.765. [DOI] [PubMed] [Google Scholar]
- 31.Enright P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 32.Clavel M.A., Malouf J., Michelena H.I. B-type natriuretic peptide clinical activation in aortic stenosis: impact on long-term survival. J Am Coll Cardiol. 2014;63:2016–2025. doi: 10.1016/j.jacc.2014.02.581. [DOI] [PubMed] [Google Scholar]
- 33.Kostkiewicz M., Tracz W., Olszowska M., Podolec P., Drop D. Left ventricular geometry and function in patients with aortic stenosis: gender differences. Int J Cardiol. 1999;71:57–61. doi: 10.1016/s0167-5273(99)00114-x. [DOI] [PubMed] [Google Scholar]
- 34.Legget M.E., Kuusisto J., Healy N.L., Fujioka M., Schwaegler R.G., Otto C.M. Gender differences in left ventricular function at rest and with exercise in asymptomatic aortic stenosis. Am Heart J. 1996;131:94–100. doi: 10.1016/s0002-8703(96)90056-3. [DOI] [PubMed] [Google Scholar]
- 35.Piro M., Della Bona R., Abbate A., Biasucci L.M., Crea F. Sex-related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55:1057–1065. doi: 10.1016/j.jacc.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 36.Deschepper C.F., Llamas B. Hypertensive cardiac remodeling in males and females: from the bench to the bedside. Hypertension. 2007;49:401–407. doi: 10.1161/01.HYP.0000256279.49882.d8. [DOI] [PubMed] [Google Scholar]
- 37.Petrov G., Regitz-Zagrosek V., Lehmkuhl E. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation. 2010;122:S23–S28. doi: 10.1161/CIRCULATIONAHA.109.927764. [DOI] [PubMed] [Google Scholar]
- 38.Kararigas G., Dworatzek E., Petrov G. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. 2014;16:1160–1167. doi: 10.1002/ejhf.171. [DOI] [PubMed] [Google Scholar]
- 39.Marsh J.D., Lehmann M.H., Ritchie R.H., Gwathmey J.K., Green G.E., Schiebinger R.J. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation. 1998;98:256–261. doi: 10.1161/01.cir.98.3.256. [DOI] [PubMed] [Google Scholar]
- 40.Orlowska-Baranowska E., Placha G., Gaciong Z. Influence of ACE I/D genotypes on left ventricular hypertrophy in aortic stenosis: gender-related differences. J Heart Valve Dis. 2004;13:574–581. [PubMed] [Google Scholar]
- 41.Mitchell G.F., Gudnason V., Launer L.J., Aspelund T., Harris T.B. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension. 2008;51:1123–1128. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dahl J.S., Eleid M.F., Michelena H.I. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002917. pii: e002917. [DOI] [PubMed] [Google Scholar]
- 43.Rakusan K., Flanagan M.F., Geva T., Southern J., Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992;86:38–46. doi: 10.1161/01.cir.86.1.38. [DOI] [PubMed] [Google Scholar]
- 44.Mahmod M., Piechnik S.K., Levelt E. Adenosine stress native T1 mapping in severe aortic stenosis: evidence for a role of the intravascular compartment on myocardial T1 values. J Cardiovasc Magn Reson. 2014;16:92. doi: 10.1186/s12968-014-0092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.