Abstract
We evaluated the ability of monosodium glutamate (MSG) to reduce salivary and kidney uptake of a prostate-specific membrane antigen (PSMA) radioligand without affecting tumor uptake. Methods: LNCaP tumor–bearing mice were intraperitoneally injected with MSG (657, 329, or 164 mg/kg) or phosphate-buffered saline (PBS). Fifteen minutes later, the mice were intravenously administered 68Ga-PSMA-11. PET/CT imaging and biodistribution studies were performed 1 h after administration. Results: Tumor uptake (percentage injected dose per gram [%ID]) was not statistically different between groups, at 8.42 ± 1.40 %ID in the 657 mg/kg group, 7.19 ± 0.86 %ID in the 329 mg/kg group, 8.20 ± 2.44 %ID in the 164 mg/kg group, and 8.67 ± 1.97 %ID in the PBS group. Kidney uptake was significantly lower in the 657 mg/kg group (85.8 ± 24.2 %ID) than in the 329 mg/kg (159 ± 26.2 %ID), 164 mg/kg (211 ± 27.4 %ID), and PBS groups (182 ± 33.5 %ID) (P < 0.001). Salivary gland uptake was lower in the 657 mg/kg (3.72 ± 2.12 %ID) and 329 mg/kg (5.74 ± 0.62 %ID) groups than in the PBS group (10.04 ± 2.52 %ID) (P < 0.01). Conclusion: MSG decreased salivary and kidney uptake of 68Ga-PSMA-11 in a dose-dependent manner, whereas tumor uptake was unaffected.
Keywords: prostate-specific membrane antigen, 68Ga-PSMA-11, monosodium glutamate, salivary glands, kidney
Prostate-specific membrane antigen (PSMA) is an excellent prostate cancer target for theranostic applications. Many imaging agents showing high sensitivity or specificity for PSMA-expressing tissues have been developed (1). Some have been labeled with therapeutic radionuclides (i.e., 177Lu and 225Ac) and have had success in treating castration-resistant metastatic prostate cancer (2,3). The activity administered to patients is limited by toxicity to normal organs; high uptake is observed in the lacrimal glands, parotid glands, submandibular glands, and renal cortex (4). The potential side effects of higher doses include hematotoxicity, xerostomia, and renal dysfunction (2,3). In particular, xerostomia with α-emitters is so severe that patients have discontinued treatment (5). A means of decreasing this toxicity without affecting tumor uptake would allow administration of greater activity with presumably greater tumoricidal effect.
Different pharmaceuticals, including 2-(phosphonomethyl)pentanedioic acid (PMPA), a PSMA inhibitor, have been explored for nephroprotection (6,7). PMPA displaced renal activity of a PSMA radiotherapeutic in cancer models, but this was generally accompanied by a reduction in tumor uptake (6,7). Mannitol infusion reduced renal uptake of 68Ga-PSMA-11 (8), but its effect on tumor uptake requires further investigations. Botulinum toxin was administered to the parotid gland of a patient and significantly decreased PSMA-ligand uptake (9). Although this procedure is promising, it is invasive and costly and may affect salivary gland function for weeks. In this study, we investigated monosodium glutamate (MSG) for reducing uptake of 68Ga-PSMA-11 in the salivary glands and kidneys in LNCaP tumor–bearing mice. MSG is a well-studied food additive and can stimulate salivary flow (10,11). Although PSMA is expressed in the salivary glands and kidneys, part of PSMA-ligand uptake in salivary glands may be due to off-target binding, as uptake is not observed in human studies with the radiolabeled J591 monoclonal antibody (12–14). Because many PSMA ligands integrate glutamate for binding to PSMA, we hypothesized that MSG could reduce nonspecific accumulation in noncancerous tissues.
MATERIALS AND METHODS
68Ga-PSMA-11 Radiolabeling
The standard and radiolabeling precursor were purchased from ABX Advanced Biochemical Compounds. Radiolabeling was performed as previously published (15).
Cell Culture
LNCaP prostate adenocarcinoma cells (ATCC) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Sigma-Aldrich), penicillin (100 IU/mL), and streptomycin (100 μg/m) (Life Technologies) in a humidified incubator (37°C, 5% CO2).
Competition Binding Assay
LNCaP cells were washed with phosphate-buffered saline (PBS) and harvested by trypsinization. Cells (200,000/well) were seeded onto a 24-well poly-d-lysine–coated plate for 48 h. Growth medium was replaced with PBS buffer (with 20 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, 2% bovine serum albumin, pH 7.5) 1 h before the study. 18F-DCFPyL (0.1 nM), synthesized according to published procedures (16), was added to wells containing MSG (1 × 10−2 to 1.3 × 10−7 M), in triplicate. The assays were incubated for 1 h at 37°C with agitation. After aspiration, cells were washed twice with cold 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid-buffered saline. To harvest cells, 400 μL of trypsin were added to each well. Samples were counted using a Wizard2 2480 (PerkinElmer) automatic γ-counter. Binding affinity (Ki) was calculated using nonlinear regression in Prism 7 (GraphPad Software) with a dissociation constant of 0.49 nM for 18F-DCFPyL.
PET Imaging and Biodistribution
Animal experiments were approved by the Animal Ethics Committee of the University of British Columbia. Immunodeficient NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ male mice were obtained in-house. Mice were subcutaneously inoculated with 5 × 106 LNCaP cells (100 μL; 1:1 PBS/Matrigel [Corning]), with tumors grown for 4–6 wk. Mice were injected intraperitoneally with MSG (657, 329, or 164 mg/kg) or PBS. After 15 min, mice were anesthetized with isoflurane (2.5% in a 2.0 L/min flow of O2) and injected intravenously with 68Ga-PSMA-11 (5.34 ± 0.95 MBq for PET/CT, 1.86 ± 0.71 MBq for biodistribution studies).
PET/CT imaging was conducted on a Siemens Inveon small-animal PET/CT device. After a 45-min uptake period, the mice were sedated with isoflurane and placed on the scanner. Body temperature was maintained by a heating pad. A CT scan was obtained for localization and attenuation correction (80-kV tube voltage, 500-μA current, 3 bed positions, 34% overlap, and 220° of continuous rotation). This scan was followed by a 10-min PET acquisition 1 h after injection of 68Ga-PSMA-11. PET imaging data were acquired in list mode, reconstructed using 3-dimensional ordered-subsets expectation maximization (2 iterations) followed by a fast maximum a priori algorithm (18 iterations) with CT-based attenuation correction. Images were analyzed using Inveon Research Workplace software (Siemens Healthineers).
For biodistribution studies, 1 h after radiopharmaceutical injection, the mice were anesthetized with isoflurane and euthanized by CO2 inhalation. Blood was collected via cardiac puncture. Organs and tissues were harvested, rinsed with PBS, blotted dry, and weighed. The radioactivity in tissues was assayed by γ-counter and expressed as percentage injected dose per gram of tissue (%ID/g).
Statistical Analysis
Analysis was performed with R, version 3.4.0 (R Foundation for Statistical Computing). Outliers identified by one round of the Grubb test (α < 0.01) were removed. When data distribution was normal according to the Shapiro–Wilk test (α < 0.05), groups were compared using the Welch t test or the Wilcoxon rank sum test. The Holm correction was used for multiple comparisons. The significance level was an α value of less than 0.05. PBS controls repeated for each experiment (different batches of radiotracer) were aggregated in a single group for analysis.
RESULTS
68Ga-PSMA-11 was synthesized in 66.3% ± 8.8% decay-corrected radiochemical yields with more than 99% radiochemical purity and 82.6 ± 44.3 GBq/μmol molar activity (n = 6).
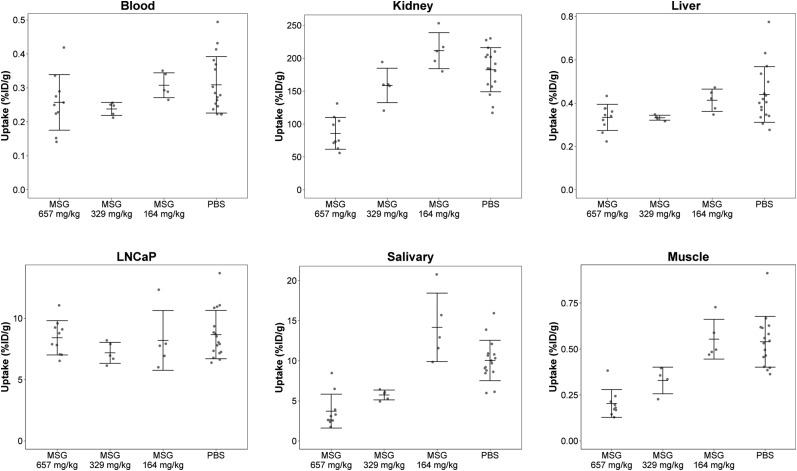
Biodistribution results are shown in Figure 1 and Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org). There were no statistical differences for tumor uptake between the 4 groups, at 8.42 ± 1.40 %ID/g in the 657 mg/kg group, 7.19 ± 0.86 %ID/g in the 329 mg/kg group, 8.20 ± 2.44 %ID/g in the 164 mg/kg group, and 8.67 ± 1.97 %ID/g in the PBS group. Kidney uptake was significantly lower in the 657 mg/kg group (85.8 ± 24.2 %ID/g) than in the groups given 329 mg/kg (159 ± 26.2 %ID/g), 164 mg/kg (211 ± 27.4 %ID/g), and PBS (182 ± 33.5 %ID/g) (P < 0.001). The difference between the 329 and 164 mg/kg groups was significant (P < 0.05), but not when they were, respectively, compared with PBS. Salivary gland uptake was lower in the 657 mg/kg (3.72 ± 2.12 %ID/g) and 329 mg/kg (5.74 ± 0.62 %ID/g) groups than in the PBS group (10.04 ± 2.52 %ID/g); P < 0.01. There were no significant differences between the 164 mg/kg (14.2 ± 4.27 %ID/g) and PBS groups, or between the 657 and 329 mg/kg groups. Muscle uptake was generally lower in MSG-treated groups.
FIGURE 1.
Biodistribution of 68Ga-PSMA-11 in selected organs at 1 h after injection. Mice received MSG (657, 329, or 164 mg/kg) or PBS intraperitoneally 15 min before tracer administration.
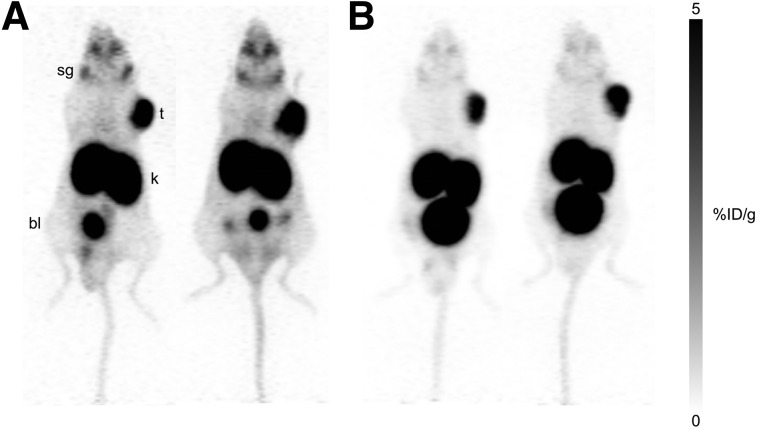
PET imaging studies were performed on mice preinjected with PBS or a 657 mg/kg dose of MSG (Fig. 2). High uptake in LNCaP tumors was observed, with the remainder of the radioactivity cleared through the renal pathway. Compared with the PBS group, MSG-treated mice showed lower uptake in the salivary glands and faster excretion (more urinary output). The difference in clearance rates led to higher-contrast images for MSG-treated mice.
FIGURE 2.
PET maximum-intensity projections of 4 mice with 68Ga-PSMA-11 at 1 h after injection. LNCaP tumor–bearing mice received PBS (A) or MSG (B) (657 mg/kg) intraperitoneally 15 min before tracer administration. bl = bladder; k = kidney; sg = salivary glands; t = tumor.
The binding affinity (Ki value) of MSG to PSMA was 0.90 ± 0.58 mM (n = 3), as measured using a cell-based competition assay with 18F-DCFPyL (Fig. 3).
FIGURE 3.
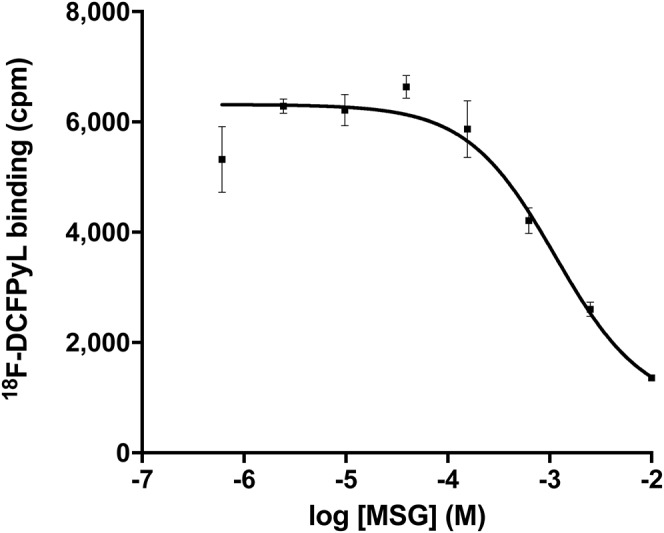
Representative competition binding assay for MSG against 18F-DCFPyL in LNCaP cells. Ki value for this assay was 0.95 mM.
DISCUSSION
The development of PSMA radiotheranostic agents has had a significant impact on prostate cancer management (17). Small-molecule inhibitors were developed as alternatives to monoclonal antibodies, primarily for their fast targeting properties and clearance profiles (17). However, radiolabeled PSMA inhibitors show undesired uptake in salivary glands (17). Although this does not hinder diagnostic interpretation, it imposes a limit on the maximum-tolerable dose for therapy.
We investigated the effect of MSG on 68Ga-PSMA-11 salivary uptake. The doses of MSG chosen for this study corresponded to one tenth, twentieth, and fortieth of the median lethal dose for mice (18).
We observed a significant decrease in kidney uptake (50% for 657 mg/kg) and salivary uptake (43%–53% for 329 and 657 mg/kg) of 68Ga-PSMA-11 for MSG-treated mice compared with control. Uptake in kidney and salivary glands was higher for the 164 mg/kg group than for the PBS group, but this difference was not statistically significant. Notably, tumor uptake did not significantly differ among the 4 groups. At more than 329 mg/kg, MSG blocked renal and salivary uptake without affecting tumor uptake. PET imaging corroborated the biodistribution results, notwithstanding enhanced contrast for MSG-treated mice, which can be explained by accelerated clearance of 68Ga-PSMA-11 or reduced nonspecific accumulation.
Kratochwil et al. demonstrated that PMPA can reduce renal uptake of 125I-MIP-1095 without affecting LNCaP tumor uptake; however, a 16-h latency period was required for tracer binding and clearance before PMPA administration (6). Chatalic et al. showed that coinjection of PMPA with 111In/177Lu-PSMA I&T could improve the tumor-to-kidney absorbed dose ratio, but this improvement was accompanied by a reduction in tumor uptake (7). The high affinity of PMPA to PSMA (reported Ki, 0.28 nM) may complicate dose optimization (19). Conversely, MSG has poor binding affinity to PSMA (Ki, 0.90 ± 0.58 mM), which may benefit this application.
Although MSG has been linked to headaches and “Chinese restaurant syndrome,” human studies have shown MSG to be safe; based on a no-adverse-event limit of 3,200 mg/kg/d for neurodevelopmental toxicity, the European Food Safety Authority advised that a daily intake of 30 mg/kg is acceptable (18,20). Fernstrom administered a single 12.7-g dose of MSG orally to humans without side effects, with a return of plasma glutamate levels to baseline after 3 h (21). The MSG quantity required to achieve effective off-target blocking of 68Ga-PSMA-11 in humans is not known, as human physiology may vary. Whether sufficient quantities can be practically administered to human subjects will require further investigation.
CONCLUSION
MSG can decrease salivary and renal uptake of 68Ga-PSMA-11 without affecting tumor uptake in mice, presumably by competing with off-target binding sites. This effect could potentially be used to increase the therapeutic index of glutamate-based radioligands. Further work is needed to assess whether this blocking effect is sufficiently durable to protect these organs from longer-lived radioisotopes, to understand the mechanism of action and to assess if the same effect can be translated to humans.
DISCLOSURE
Financial support was provided by the Canadian Institutes of Health Research (FDN-148465), the BC Cancer Foundation, and the BC Leading Edge Endowment Fund. No other potential conflict of interest relevant to this article was reported.
Supplementary Material
REFERENCES
- 1.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–235. [DOI] [PubMed] [Google Scholar]
- 2.Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. [DOI] [PubMed] [Google Scholar]
- 3.Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–1631. [DOI] [PubMed] [Google Scholar]
- 4.Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44:2117–2136. [DOI] [PubMed] [Google Scholar]
- 5.Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802. [DOI] [PubMed] [Google Scholar]
- 6.Kratochwil C, Giesel FL, Leotta K, et al. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J Nucl Med. 2015;56:293–298. [DOI] [PubMed] [Google Scholar]
- 7.Chatalic KL, Heskamp S, Konijnenberg M, et al. Towards personalized treatment of prostate cancer: PSMA I&T, a promising prostate-specific membrane antigen-targeted theranostic agent. Theranostics. 2016;6:849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matteucci F, Mezzenga E, Caroli P, et al. Reduction of 68Ga-PSMA renal uptake with mannitol infusion: preliminary results. Eur J Nucl Med Mol Imaging. 2017;44:2189–2194. [DOI] [PubMed] [Google Scholar]
- 9.Baum RP, Langbein T, Singh A, et al. Injection of botulinum toxin for preventing salivary gland toxicity after PSMA radioligand therapy: an empirical proof of a promising concept. Nucl Med Mol Imaging. 2018;52:80–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maluly HDB, Arisseto-Bragotto AP, Reyes FGR. Monosodium glutamate as a tool to reduce sodium in foodstuffs: technological and safety aspects. Food Sci Nutr. 2017;5:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodson NA, Linden RW. The effect of monosodium glutamate on parotid salivary flow in comparison to the response to representatives of the other four basic tastes. Physiol Behav. 2006;89:711–717. [DOI] [PubMed] [Google Scholar]
- 12.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51:1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807–1811. [PubMed] [Google Scholar]
- 14.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 15.Kuo HT, Pan J, Zhang Z, et al. Effects of linker modification on tumor-to-kidney contrast of 68Ga-Labeled PSMA-targeted imaging probes. Mol Pharm. 2018;15:3502–3511. [DOI] [PubMed] [Google Scholar]
- 16.Bouvet V, Wuest M, Jans HS, et al. Automated synthesis of [18F]DCFPyL via direct radiofluorination and validation in preclinical prostate cancer models. EJNMMI Res. 2016;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Keefe DS, Bacich DJ, Huang SS, Heston WD. A perspective on the evolving story of PSMA biology and PSMA based imaging and endoradiotherapeutic strategies. J Nucl Med. 2018;59:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monosodium glutamate. National Institutes of Health website. https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+580. Published October 14, 1986. Updated October 11, 2007. Accessed October 4, 2018.
- 19.Jackson PF, Cole DC, Slusher BS, et al. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated alpha-linked acidic dipeptidase. J Med Chem. 1996;39:619–622. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen A, Aguilar F, Crebelli R, et al. Re‐evaluation of glutamic acid (E 620), sodium glutamate (E 621), potassium glutamate (E 622), calcium glutamate (E 623), ammonium glutamate (E 624) and magnesium glutamate (E 625) as food additives. EFSA J. 2017;15:e04910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernstrom JD, Cameron JL, Fernstrom MH, McConaha C, Weltzin TE, Kaye WH. Short-term neuroendocrine effects of a large oral dose of monosodium glutamate in fasting male subjects. J Clin Endocrinol Metab. 1996;81:184–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.