Abstract
Background
The relationship among chronic inflammation, innate immunity, and cancer is well established. Mannose-binding lectin (MBL) is a key player in innate immunity. Five polymorphisms in the promoter and first exon of the MBL2 gene alter the expression and function of MBL in humans and are associated with inflammation-related disease susceptibility. These five polymorphisms create six well-characterized haplotypes that result in lower (i.e., LYB, LYC, HYD, and LXA) or higher (i.e., HYA and LYA) serum MBL concentrations. We investigated whether survival of patients with lung cancer was associated with these polymorphisms.
Methods
We used a multivariable Cox proportional hazards model to study the association between MBL2 polymorphisms and their haplotypes and diplotypes in 558 white and 173 African American patients with non–small-cell lung cancer in the Baltimore, MD, area and lung cancer mortality. Smoking history and race were obtained from interviews, tumor stage was obtained from medical records, and cause of death was obtained from the National Death Index. All statistical tests were two-sided.
Results
We found a statistically significant association between the X allele of the promoter Y/X polymorphism (which results in a lower serum MBL concentration) and improved lung cancer survival among white patients (risk ratio [RR] of death from lung cancer with X/X or X/Y genotype compared with Y/Y genotype = 0.61, 95% confidence interval [CI] = 0.46 to 0.81) but not among African American patients (RR = 1.11, 95% CI = 0.69 to 1.77). The associations among white patients were strongest in heavy smokers and were independent of stage. We also found a statistically significant interaction between the Y/X polymorphism and race for lung cancer survival (Pinteraction = .019). The MBL2 LXA haplotype and XA/B diplotype, which are also associated with low serum MBL levels, were statistically significantly associated with improved lung cancer survival among white patients.
Conclusion
The functional Y/X polymorphism of the innate-immunity gene MBL2 and MBL2 haplotypes and diplotypes appear to be associated with lung cancer survival among white patients.
Lung cancer, predominantly non–small-cell lung cancer, is the leading cause of cancer mortality worldwide. There continues to be a wide variation in survival within any given stage, and the 5-year overall survival rates remain less than 20% (1, 2). Familial aggregation and molecular epidemiologic studies have found that genetic factors are associated with lung cancer survival (3–5). Additional survival-associated genetic markers could improve the stratification of lung cancer patients into more precise risk groups, enhance prognosis models, and help understand the biology of the disease.
Genetic variations in innate-immunity genes may be associated with lung cancer mortality. Mannose-binding lectin (MBL) is encoded by the MBL2 gene and is a central component of the innate immune system. MBL binds to specific carbohydrate structures on the surface of microorganisms and activates both the lectin complement system and complement-independent opsonization by monocytes (6). Variations in MBL levels or activity are caused, in part, by two single-nucleotide polymorphisms (SNPs) in the promoter region, known as L/H and Y/X, and three SNPs in the first exon, known as the D, B, and C variant alleles; the A allele for the SNPs in the first exon is the wild-type allele. We examined the SNPs L/H, position −618G>G; Y/X, position −289G>C; A/D, position Ex1-34C>T; A/B, position Ex1-27G>A; and A/C, position Ex1-18G>A. These SNPs are components of what is termed the secretor haplotype block and create six haplotypes—four of which (i.e., LYB, LYC, HYD, and LXA) result in lower serum MBL concentrations and two of which (i.e., HYA and LYA) result in higher MBL concentrations (7). The haplotype pairs, or diplotypes, are described by a simplified scheme (7, 8), in which the HYA and LYA haplotypes are in the same category (YA), the diplotypes that are exon 1 variants are noted by a single-letter notation (B, C or D), and the haplotype pairs containing homozygous or compound heterozygous variant alleles are represented by the letter O. From this scheme, 10 different diplotypes are distinguished.
Serum levels of MBL range more than 1000-fold in humans, and there is no consensus about the range of serum MBL concentrations that constitutes normal, healthy levels. Several studies (8–14) have reported associations between secretor haplotypes that result in low levels of serum MBL and susceptibility to repeated serious infections, autoimmune disorders, stomach cancer, and pediatric acute lymphoblastic leukemia. Low levels of serum MBL have also been associated with a decreased risk of certain mycobacterial infections (15), and high levels of serum MBL have been associated with inflammation-related disorders, such as childhood asthma, primary biliary cirrhosis, tissue damage from excessive complement activity, and reperfusion injury (16–20). Thus, both high and low serum MBL levels can be disadvantageous.
Chronic inflammation and innate immunity are associated with cancer development and progression, by generating DNA-damaging reactive oxygen and nitrogen species (21). In this study, we examined five MBL2 secretor haplotype polymorphisms from 558 white and 173 African American patients with lung cancer who were recruited from the metropolitan area of Baltimore, MD. We evaluated whether MBL2 polymorphisms were associated with lung cancer–specific survival.
Materials and Methods
Study Subjects
Patients with histologically confirmed non–small-cell lung cancer were prospectively recruited from the metropolitan area of Baltimore, MD, through two separate study protocols, a case-only series (22) and a case–control study (23, 24). Patients from the case-only series were recruited from the following hospitals: University of Maryland, Baltimore Veteran’s Administration, St Agnes Hospital, Northwest Hospital Center, Sinai Hospital, Mercy Medical Center, and Union Memorial Hospital. Three hundred nine patients with available tissue samples from surgeries that were performed between January 1, 1984, and December 31, 1999, were eligible; inclusion and exclusion criteria have been described previously (22). Suitable genomic DNA was available for 231 of the 309 case patients. Patients from the case–control study were residents of the Baltimore metropolitan area or any of the Maryland counties east of the Chesapeake Bay. Five hundred sixty-eight patients diagnosed between January 1, 1998, and December 31, 2004, were recruited; the inclusion and exclusion criteria have been described previously (23, 24). Suitable genomic DNA was available for 500 of the 568 case patients. There were no statistically significant differences in current smoking status, stage, age, sex, or race between those who had suitable amounts and quality of DNA and those who did not for either protocol. Patients were combined for this study, for a total of 558 white and 173 African American patients. Written informed consent was obtained from all participants. This study was approved by the Institutional Review Boards of the participating institutions.
Genotyping
Genomic DNA was isolated from 500 buffy coat samples containing white blood cells from patients in the case–control study and from 181 frozen normal lung tissue samples and 50 buffy coat samples from patients in the case series. Five SNPs located within the secretor haplotype region of the MBL2 gene were genotyped at the National Cancer Institute Core Genotyping Facility by use of a Taqman assay (Applied Biosystems, Foster City, CA). The assay conditions for each genotype (L/H, rs11003125; Y/X, rs7096206; A/D, rs5030737; A/B, rs1800450; and A/C rs1800451) are detailed on the SNP500 Web site (http://snp500cancer.nci.nih.gov). The case, control, and 10% duplicate samples were randomly distributed for order of processing, and samples were assigned a unique identification number that lacked identifiers. Staff at the Core Genotyping Facility were blinded to the case, control, and duplicate statuses. The genotype concordance for each SNP was at least 99% among duplicates.
Statistical Analysis
Linkage disequilibrium of the secretor haplotype SNPs has been previously demonstrated (25). The secretor haplotype block was inferred separately for white and African American patients. Linkage disequilibrium was verified by use of the default modus for a haplotype block definition as implemented in the Haploview software (http://www.broad.mit.edu/mpg/haploview/index.php). Estimated haplotypes were obtained from Phase software, version 2.1 (http://www.stat.washington.edu/stephens/software.html), which uses a Bayesian method for haplotype reconstruction. The best-pairs diplotypes were estimated for each individual, and the diplotypes for each individual were split into individual haplotypes.
Patients were followed prospectively from the time of enrollment. For the case–control and case-only series studies, survival was calculated from the date of lung cancer diagnosis and the date of surgery, respectively, to the last reported search for death entries (December 31, 2004) in the National Death Index (http://www.cdc.gov/nchs/ndi.htm). The cumulative follow-up was 20 years (from date of first lung cancer diagnosis to date of the National Death Index search). We obtained death certificates for the deceased patients and censored all patients whose cause of death was not related to lung cancer. Deaths were attributed to lung cancer if the death certificate listed lung cancer as a cause or contributory factor of death or if any other solid-tumor cancer was listed as a cause of death within 2 years after diagnosis.
A multivariable Cox proportional hazards model was used to compute risk ratios (RRs) and 95% confidence intervals (CIs) of lung cancer–specific death by genotypes, haplotypes, and diplotypes. All analyses were stratified by race. The following covariates were included in the analyses: sex, age at diagnosis (as a continuous variable), tumor–node–metastasis stage (categorized as stages III–IV versus stages I–II), current smoking status (categorized as never and former smokers versus current smokers), and pack-years of smoking (as a continuous variable). Proportional hazards assumptions were verified by visual inspection of log–log plots and with a nonzero slope test of the Schoenfeld residuals (26). A Bonferroni-adjusted P value was calculated by multiplying the actual P value by the 10 analyses performed (i.e., five secretor SNPs and each race). A statistical test for interaction between MBL2 genotypes and covariates was performed by using a likelihood ratio test to calculate P values by comparing main effects models with main effects models plus an interaction term. This comparison was done by inclusion of a dichotomous indicator for the covariate and genotype (homozygous wild type versus heterozygous and homozygous variant). A power analysis to detect associations between the Y/X MBL2 SNP and lung cancer–specific survival was conducted with the Sample Size software, version 2.1.31 (27) (http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/PowerSampleSize). We had 100% and 86% power to detect a twofold relative risk of death among the white patients and the African American patients, respectively, at an α value of .05, if we assumed a dominant effect for the variant allele. Smoking levels were categorized by using the 25th and 75th percentile pack-year values of the white patients as the cut points (i.e., 0.1 to <28.6 pack-years, 28.6 to 64.8 pack-years, and >64.8 pack-years) (28).
Calculations were performed by use of STATA version 9 software (STATA Corp, College Station, TX). A P value of less than .05 was used as the criterion of statistical significance, and all statistical tests were two-sided.
Results
MBL2 Secretor Genotypes and Lung Cancer–Specific Survival
We investigated the relationship between five MBL2 polymorphisms and lung cancer–specific survival among 558 white and 173 African American patients. The relevant demographic and clinicopathologic features are given in Table 1. Among the 558 white patients, 244 (49.0%) were current smokers and 365 (67.3%) had stage I–II lung cancer. Among the 173 African American patients, 102 (58.9%) were current smokers and 89 (56.0%) had stage I–II lung cancer. The 20-year cumulative lung cancer–specific survival for white patients was 27.3% (95% CI = 15.0% to 41.3%) and for African American patients was 17.2% (95% CI = 7.1% to 30.9%). Allele frequencies of MBL2 genotypes are shown in Table 2. There was a statistically significant difference in allele frequencies between white patients and African American patients (two-sided chi-square test, for L/H, A/D, A/B, and A/C, P <.001, and for Y/X, P = .02).
Table 1.
Distribution of selected characteristics and clinical data for study patients with non–small-cell lung cancer*
| Characteristic | White patients (N = 558) | African American patients (N = 173) |
|---|---|---|
| Age, y (mean ± SD) | 66.4 ± 9.9 | 63.2 ± 9.2 |
| Male | 66.3 ± 9.8 | 64.0 ± 8.3 |
| Female | 66.6 ± 10.1 | 62.5 ± 10.1 |
| Sex, No. (%) | ||
| Male | 317 (56.8) | 88 (50.9) |
| Female | 241 (43.2) | 85 (49.1) |
| Smoking status, No. (%)† | ||
| Never | 36 (7.2) | 11 (6.4) |
| Former | 218 (43.8) | 60 (34.7) |
| Current | 244 (49.0) | 102 (58.9) |
| Pack-years‡ | ||
| <28.6, No. of patients (<25th percentile) | 138 (24.7) | 66 (38.2) |
| 28.6–64.8, No. of patients (25th–75th percentile) | 276 (49.5) | 75 (43.4) |
| >64.8, No. of patients (>75th percentile) | 144 (25.8) | 32 (18.5) |
| Tumor type, No. | ||
| Adenocarcinoma | 217 | 57 |
| Squamous cell carcinoma | 93 | 40 |
| NSCLC§ | 172 | 61 |
| Stage,† No. (%) | ||
| I–II | 365 (67.3) | 89 (56.0) |
| III–IV | 177 (32.7) | 70 (44.0) |
| Follow-up | ||
| Cumulative survival rate, % (95% CI)‖ | 27.3 (15.0 to 41.3) | 17.2 (7.1 to 30.9) |
| Median follow-up, mo (95% CI)¶ | 45.0 (40.8 to 48.9) | 43.9 (34.8 to 51.5) |
SD = standard deviation; NSCLC = non–small-cell lung cancer; CI = confidence interval.
Patients with missing data were not included.
Smokers were categorized by using the 25th and 75th percentile pack-year values of the white patients.
Histologic subtype data were not available for all NSCLC tumors.
The follow-up period was 20 years.
Median survival months of patients who were alive at the end of follow-up.
Table 2.
Association between secretor MBL2 polymorphisms and disease-specific survival*
| White patients | African American patients | |||||
|---|---|---|---|---|---|---|
| Genotype | No. (%) | RR of death† (95 % CI) | P‡ | No. (%) | RR of death† (95 % CI) | P‡ |
| Promoter SNPs | ||||||
| L/H (mbl2_11; rs11003125) | ||||||
| C/C (L/L) | 215 (41.1) | 1.0 (referent) | 120 (71.4) | 1.0 (referent) | ||
| C/G (L/H) | 238 (45.5) | 0.94 (0.70 to 1.26) | .67 | 44 (26.2) | 1.02 (0.62 to 1.69) | .93 |
| G/G (H/H) | 70 (13.4) | 1.29 (0.86 to 1.92) | .22 | 4 (2.4) | 2.27 (0.70 to 7.41) | .14 |
| Y/X (mbl2_12; rs7096206) | ||||||
| G/G (Y/Y) | 329 (61.7) | 1.0 (referent) | 124 (73.4) | 1.0 (referent) | ||
| G/C (Y/X) | 180 (33.8) | 0.61 (0.46 to 0.82) | .001 | 41 (24.3) | 1.02 (0.63 to 1.66) | .96 |
| C/C (X/X) | 24 (4.5) | 0.62 (0.31 to 1.27) | .20 | 4 (2.3) | 2.76 (0.81 to 9.40) | .11 |
| G/C + C/C§ | 204 (38.3) | 0.61 (0.46 to 0.81) | .001 | 45 (26.6) | 1.11 (0.69 to 1.77) | .69 |
| Exon 1 SNPs | ||||||
| A/D (mbl2_03; rs5030737) | ||||||
| C/C (non-D/non-D) | 447 (85.0) | 1.0 (referent) | 160 (96.4) | 1.0 (referent) | ||
| C/T (non-D/D) | 75 (14.2) | 0.97 (0.68 to 1.40) | .89 | 6 (3.6) | 0.48 (0.11 to 1.98) | .30 |
| T/T (D/D) | 4 (0.8) | 3.32 (0.68 to 13.64) | .10 | 0 | – | – |
| A/B (mbl2_01; rs1800450) | ||||||
| G/G (non-B/non-B) | 391 (75.5) | 1.0 (referent) | 152 (91.6) | 1.0 (referent) | ||
| G/A (non-B/B) | 120 (23.2) | 0.86 (0.63 to 1.17) | .34 | 13 (7.8) | 1.63 (0.67 to 3.95) | .28 |
| A/A (B/B) | 7 (1.3) | 1.91 (0.60 to 6.07) | .28 | 1 (0.6) | – | – |
| A/C (mbl2_02; rs1800451) | ||||||
| G/G (non-C/non-C) | 479 (97.0) | 1.0 (referent) | 91 (57.2) | 1.0 (referent) | ||
| G/A (non-C/C) | 14 (2.8) | 1.09 (0.44 to 2.67) | .18 | 58 (36.5) | 0.84 (0.53 to 1.33) | .42 |
| A/A (C/C) | 1 (0.2) | – | – | 10 (6.3) | 1.09 (0.42 to 2.83) | .88 |
RR = risk ratio; CI = confidence interval; SNP = single-nucleotide polymorphism; – = insufficient data.
Risk ratio of lung cancer death was adjusted for sex, stage (III–IV versus I–II), age at diagnosis, current smoking status, and pack-years of smoking.
A Cox proportional hazards test was used. All statistical tests were two-sided.
A dominant model was assumed.
The Y/X promoter SNP was statistically significantly associated with survival among white patients, as shown by a Kaplan–Meier analysis (Fig. 1) and multivariable analyses (Table 2). A total of 329 (61.7%) of the 533 genotyped white patients had the common Y/Y genotype, 180 (33.8%) had one variant allele (Y/X), and 24 (4.5%) had both variant alleles (X/X). Among white patients, a heterozygous or double-variant genotype, corresponding to lower levels of serum MBL, was associated with lower risk of death (RR = 0.61, 95% CI = 0.46 to 0.81, P = .001) than the Y/Y genotype (Table 2). In a univariate analysis, among white patients but not among African American patients, the X allele was also statistically significantly associated with improved survival compared with the Y/Y genotype (Table 3). None of the remaining four MBL2 SNPs were associated with survival in either multivariable (Table 2) or univariate (data not shown) analyses. The association between the Y/X MBL2 SNP and lung cancer–specific survival remained statistically significant after a Bonferroni correction (P = .01).
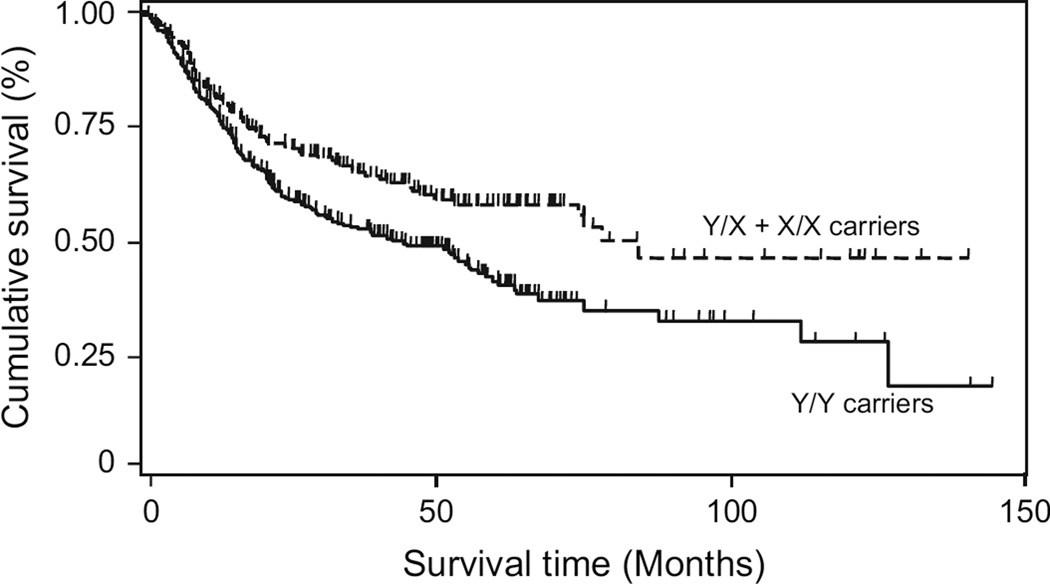
Fig. 1.
MBL2 Y/X promoter single-nucleotide polymorphism (SNP) genotype in relation to lung cancer survival among white patients: Kaplan–Meier survival analysis. Cumulative disease-specific survival of white patients with lung cancer is presented by MBL2 Y/X SNP status (patients at risk at time zero = 533). Survival of patients who carried the variant X allele (patients at risk at time zero = 204) was statistically significantly better than that of patients with the common Y/Y genotype (patients at risk at time zero = 329; two-sided log-rank test: P<.001). The 5-year cumulative survival rate was 41.5% (95% CI = 35.0% to 47.9%) for patients with the Y/Y genotype (patients at risk at 5 years = 53) and 58.2% (CI = 50.0% to 65.4%) for patients with at least one X allele (patients at risk at 5 years = 43). At the end of the follow-up period (12 years), the cumulative survival rate was 18.9% (95% CI = 5.8% to 37.7%) for patients with the Y/Y genotype and 46.6% (CI = 32.2% to 58.0%) for patients with at least one X allele.
Table 3.
Disease-specific survival of lung cancer patients in relation to clinicopathologic characteristics and the MBL2 Y/X polymorphism*
| Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
| Variable | Category | RR of death (95% CI) | P† | RR of death (95% CI) | P† |
| White patients | |||||
| Age | Continuous | 1.00 (0.99 to 1.01) | .92 | 1.01 (1.00 to 1.02) | .19 |
| Sex | Female vs male | 0.88 (0.66 to 1.09) | .21 | 0.79 (0.60 to 1.04) | .09 |
| Stage | Stages II–IV vs I | 3.53 (2.70 to 4.61) | <.001 | 3.83 (2.89 to 5.07) | <.001 |
| Smoking pack-years | Continuous | 1.00 (0.99 to 1.00) | .30 | 1.00 (0.99 to 1.00) | .12 |
| Y/X polymorphism | C/C + C/G vs G/G | 0.66 (0.51 to 0.86) | .003 | 0.55 (0.42 to 0.73) | <.001 |
| African American patients | |||||
| Age | Continuous | 1.00 (0.98 to 1.03) | .74 | 1.02 (0.99 to 1.04) | .14 |
| Sex | Female vs male | 0.82 (0.55 to 1.21) | .31 | 0.70 (0.46 to 1.07) | .10 |
| Stage | Stages II–IV vs I | 4.32 (2.74 to 6.80) | <.001 | 4.71 (2.87 to 7.75) | <.001 |
| Smoking pack-years | Continuous | 0.99 (0.99 to 1.00) | .15 | 1.00 (0.99 to 1.01) | .88 |
| Y/X polymorphism | C/C + C/G vs G/G | 1.19 (0.77 to 1.85) | .43 | 1.01 (0.63 to 1.60) | .98 |
RR = risk ratio; CI = confidence interval.
A Cox proportional hazards test was used. All statistical tests were two-sided.
We found a statistically significant interaction between the Y/X SNP status and race for lung cancer–specific survival (Pinteraction = .019). No association was found between the Y/X polymorphism and lung cancer–specific survival among the African American patients in a multivariable analysis (RR for lung cancer death in patients with at least one variant X allele compared with those with a Y/Y genotype = 1.11, 95% CI = 0.69 to 1.77) (Table 2).
Among white patients, the association between the X allele and lung cancer–specific survival was independent of the clinicopathologic factors: stage, age, sex, weight loss, histologic subtype (adenocarcinoma or squamous cell carcinoma), tumor grade, and chronic obstructive pulmonary disorder. We found no statistical interaction between the Y/X SNP and any of these factors, and in all stratified analyses, the 95% confidence intervals overlapped (data not shown). As expected, there was also no association between the Y/X SNP and chronic obstructive pulmonary disorder (chi-square test, P = .69).
We performed survival analyses for all causes of death. As shown in Supplementary Table 1 (available online), white patients who carried the X allele had better 5-year overall survival than those in the reference group who carried the Y/Y genotype (RR = 0.62, 95% CI = 0.47 to 0.81). We observed no association between any of the MBL2 polymorphisms and overall survival among the African American patients (data not shown).
When the analysis was stratified by the two study protocols from which the patients were recruited, white patients carrying at least one X allele had better lung cancer–specific survival than white patients carrying the wild-type Y/Y genotype in the case–control (RR = 0.65, 95% CI = 0.45 to 0.93) and case-series (RR = 0.56, 95% CI = 0.34 to 0.92) studies (Supplementary Table 2, available online). No association between any polymorphism and lung cancer–specific survival was observed in either study among the African American patients (Supplementary Table 2, available online).
Because smoking and MBL levels are both associated with inflammation and because smoking was recently associated with increased serum MBL levels (29), we hypothesized that smoking may modify the association between high MBL serum levels and lung cancer survival. The variant X allele was statistically significantly associated with improved survival among white patients who smoked more than 64.8 pack-years (the >75th percentile; RR = 0.33, 95% CI = 0.17 to 0.61, P = .001). When the smoking cut points were moved up and down by 5 pack-years, the results were similar (data not shown). Smoking did not modify the association between the X allele and lung cancer–specific survival among African American patients (Table 4).
Table 4.
Disease-specific survival in relationship to promoter Y/X single-nucleotide polymorphism genotypes and smoking*
| No. of patients | ||||
|---|---|---|---|---|
| Smoking level† | G/G | C/C + C/G | RR of death‡ (95% CI) | P§ |
| White patients | ||||
| <28.6 pack-years | 82 | 51 | 0.94 (0.54 to 1.65) | .84 |
| 28.6–64.8 pack-years | 159 | 104 | 0.70 (0.47 to 1.03) | .07 |
| >64.8 pack-years | 88 | 49 | 0.33 (0.17 to 0.61) | .001 |
| African American patients | ||||
| <28.6 pack-years | 45 | 20 | 1.95 (0.94 to 4.05) | .07 |
| 28.6–64.8 pack-years | 55 | 18 | 0.51 (0.22 to 1.18) | .11 |
| >64.8 pack-years | 24 | 7 | 0.92 (0.24 to 3.48) | .91 |
RR = risk ratio; CI = confidence interval.
Smoking levels were categorized by using the 25th and 75th percentile pack-year values of the white patient subjects (<25th percentile, 25th–75th percentile, and >75th percentile).
Risk ratio of death of C/C (X/X) plus C/G (X/Y) versus G/G (Y/Y) genotypes was adjusted for sex, stage (III–IV/I–II), current smoking status, and age. A dominant model was assumed.
A multivariable Cox proportional hazards model was used. All statistical tests were two-sided.
Cause of Death Among Lung Cancer Patients
High and low serum MBL levels, respectively, have been associated with elevated inflammation during myocardial ischemia and reperfusion injury (17) and with serious infections in chemotherapy patients (30). We examined whether the association between the Y/X SNP and lung cancer–specific survival among white patients in our study was partially driven by cardiac- or sepsis-related causes of death. A total of 43 and 39 white patients, respectively, had a cardiac- or infection-related cause of death in addition to a lung cancer cause of death. The X allele was not associated with risk of cardiac-related (RR = 1.25, 95% CI = 0.55 to 2.92) or sepsis-related (RR = 0.94, 95% CI = 0.48 to 1.61) death, compared with the Y/Y genotype.
MBL2 Secretor Haplotypes or Diplotypes and Lung Cancer Survival
The six haplotypes that were estimated from the five secretor SNPs and examined in this study were the same as those analyzed in previous studies (7, 8, 31). The HYA haplotype was chosen as the referent because it is the most common among white patients and was previously used as the reference in association studies (6, 8). The LXA haplotype, which causes reduced serum MBL levels (7), compared with the HYA haplotype, was associated with improved lung cancer–specific survival (RR = 0.70, 95% CI = 0.53 to 0.93, P = .01) and improved 5-year overall survival (RR = 0.71, 95% CI = 0.54 to 0.92) among white patients (Table 5; Supplementary Table 1, available online). There was no association between survival and the LXA haplotype among African American patients (RR = 0.75, 95% CI = 0.47 to 1.19) (Table 5).
Table 5.
Association between secretor MBL2 secretor haplotypes and disease-specific survival by smoking amount*
| Smoking status and haplotype† |
White patients | African American patients | ||
|---|---|---|---|---|
| No. (%) | RR of death‡ (95% CI) | No. (%) | RR of death‡ (95% CI) | |
| Overall | ||||
| GGCGG (HYA) | 294 (28.6) | 1.0 (referent) | 46 (14.2) | 1.0 (referent) |
| CGCGG (LYA) | 280 (27.3) | 1.14 (0.89 to 1.46) | 135 (41.7) | 0.85 (0.50 to 1.47) |
| CCCGG (LXA) | 220 (21.4) | 0.70 (0.53 to 0.93) | 46 (14.2) | 0.75 (0.47 to 1.19) |
| CGCAG (LYB) | 135 (13.2) | 0.94 (0.69 to 1.28) | 15 (4.6) | 0.98 (0.39 to 2.46) |
| CGCGA (LYC) | 16 (1.6) | 0.91 (0.37 to 2.24) | 76 (23.4) | 0.69 (0.42 to 1.14) |
| GGTGG (HYD) | 81 (7.9) | 1.03 (0.72 to 1.47) | 6 (1.9) | 0.40 (0.09 to 1.69) |
| Low vs high MBL§ | 0.78 (0.65 to 0.95) | 0.93 (0.69 to 1.26) | ||
| <28.6 pack-years‖ | ||||
| GGCGG (HYA) | 84 (32.5) | 1.0 (referent) | 17 (13.3) | 1.0 (referent) |
| CGCGG (LYA) | 68 (26.4) | 1.11 (0.68 to 1.83) | 52 (40.6) | 1.00 (0.47 to 2.15) |
| CCCGG (LXA) | 55 (21.3) | 1.01 (0.59 to 1.74) | 21 (16.4) | 1.77 (0.77 to 4.02) |
| CGCAG (LYB) | 32 (12.4) | 1.32 (0.76 to 2.30) | 7 (5.5) | 1.52 (0.40 to 5.83) |
| CGCGA (LYC) | 2 (0.8) | 3.09 (0.69 to 13.93) | 28 (21.9) | 0.88 (0.39 to 1.99) |
| GGTGG (HYD) | 17 (6.6) | 0.80 (0.38 to 1.69) | 3 (2.3) | 1.81 (0.36 to 9.16) |
| Low vs high MBL§ | 1.04 (0.72 to 1.51) | 1.23 (0.76 to 1.98) | ||
| 28.6–64.8 pack-years‖ | ||||
| GGCGG (HYA) | 136 (27.3) | 1.0 (referent) | 22 (15.9) | 1.0 (referent) |
| CGCGG (LYA) | 140 (28.1) | 1.22 (0.86 to 1.73) | 55 (39.8) | 0.65 (0.13 to 0.84) |
| CCCGG (LXA) | 111 (22.3) | 0.81 (0.55 to 1.19) | 19 (13.8) | 0.33 (0.13 to 0.84) |
| CGCAG (LYB) | 61 (12.3) | 1.00 (0.64 to 1.58) | 3 (2.2) | 0.86 (0.11 to 6.83) |
| CGCGA (LYC) | 7 (1.4) | 0.40 (0.06 to 2.91) | 36 (26.1) | 0.45 (0.20 to 0.97) |
| GGTGG (HYD) | 43 (8.6) | 1.29 (0.78 to 2.12) | 3 (2.2) | – |
| Low vs high MBL§ | 0.84 (0.64 to 1.10) | 0.54 (0.33 to 0.88) | ||
| >64.8 pack-years‖ | ||||
| GGCGG (HYA) | 74 (27.4) | 1.0 (referent) | 7 (12.1) | 1.0 (referent) |
| CGCGG (LYA) | 72 (26.7) | 0.90 (0.56 to 1.45) | 28 (48.3) | 0.57 (0.16 to 1.97) |
| CCCGG (LXA) | 54 (20.0) | 0.34 (0.18 to 0.63) | 6 (10.3) | 0.57 (0.12 to 2.62) |
| CGCAG (LYB) | 42 (15.5) | 0.47 (0.25 to 0.89) | 5 (8.6) | 0.39 (0.04 to 4.04) |
| CGCGA (LYC) | 7 (2.6) | 0.71 (0.36 to 1.60) | 12 (20.7) | 0.62 (0.17 to 2.30) |
| GGTGG (HYD) | 21 (7.8) | 0.76 (0.36 to 1.60) | 0 | – |
| Low vs high MBL§ | 0.48 (0.32 to 0.72) | 0.90 (0.42 to 1.92) | ||
RR = risk ratio; CI = confidence interval; MBL = mannose-binding lectin;– = insufficient data.
Single-nucleotide polymorphisms are ordered in the haplotypes by location on the gene, as L/H, Y/X, A/D, A/B, and A/C.
Risk ratio of death was adjusted for sex, stage (III–IV versus I–II), current smoking status, and age at diagnosis.
Haplotypes were categorized according to their association with low (LXA, LYB, LYC, and LYD) or high (HYA and LYA) serum MBL levels.
Smoking levels were categorized by using the 25th and 75th percentile pack-year values of the white patients (<25th percentile, 25th–75th percentile, and >75th percentile).
When the patients were stratified by study protocol, a statistically significant association between the LXA haplotype and lung cancer–specific survival was observed among the white patients in the case–control study, but not in the case-only series (Supplementary Table 2, available online), although the magnitudes of the associations were similar. When the white patients were stratified by smoking, the LXA haplotype was associated with improved survival among those who smoked more than 64.8 pack-years (RR = 0.34, 95% CI = 0.18 to 0.63, P<.001) (Table 5). Among African American patients who smoked between 28.6 and 64.8 pack-years, improved survival was associated with the LXA (RR = 0.33, 95% CI = 0.13 to 0.84, P = .02) and LYC (RR = 0.45, 95% CI = 0.20 to 0.97, P = .04) haplotypes, compared with the HYA haplotype (Table 5).
The haplotypes were dichotomized as previously described by Garred et al. (7) into high MBL (HYA and LYA) and low MBL (LXA, LYB, LYC, and HYD) haplotypes. Among white patients, the low MBL haplotypes were associated with better lung cancer–specific survival overall than high MBL haplotypes (RR = 0.78, 95% CI = 0.65 to 0.95, P = .01), and this association was even stronger in the subgroup of patients who had smoked more than 64.8 pack-years (RR = 0.48, 95% CI = 0.32 to 0.72, P<.001) (Table 5). Among African American patients who smoked between 28.6 and 64.8 pack-years, low MBL haplotypes were associated with improved lung cancer–specific survival (RR = 0.54, 95% CI = 0.33 to 0.88, P = .01) (Table 5). To determine whether the association of the low MBL haplotypes with survival was driven mainly by the Y/X SNP, patients with the LXA haplotype were removed from the analysis. Among white patients, low MBL haplotypes (LYB, LYC, and HYD) were associated with better lung cancer–specific survival than high MBL haplotypes (HYA and LYA) (RR = 0.82, 95% CI = 0.73 to 0.93, P = .002). Thus, the association between the low MBL haplotypes and improved lung cancer–specific survival was not dependent on the Y/X SNP.
Diplotypes were categorized by use of a common scheme (7, 8), in which the HYA and LYA haplotypes, representing the highest MBL serum levels, were designated group Y and the exon 1 variants were designated by a single letter (A for wild type; D, B, or C for variants). Among white patients, the XA/B diplotype was associated with better survival than the YA/YA diplotype (RR = 0.30, 95% CI = 0.14 to 0.67, P = .003), consistent with the genotype and haplotype data (Table 6). The YA/XA and XA/B diplotypes were also statistically significantly associated with 5-year overall survival (Supplementary Table 1, available online). When the analyses were stratified by study protocol, the association between the XA/B diplotype and lung cancer–specific survival remained statistically significant among white patients in the case–control study, but not among those in the case-only series (Supplementary Table 2, available online), although the magnitudes of the associations were similar.
Table 6.
Association between MBL2 diplotypes and disease-specific survival*
| White patients | African American patients | |||
|---|---|---|---|---|
| Diplotype | No. (%) | RR of death† (95% CI) | No. (%) | RR of death† (95% CI) |
| YA/YA | 159 (31.0) | 1.0 (referent) | 46 (28.4) | 1.0 (referent) |
| YA/XA | 124 (24.2) | 0.71 (0.49 to 1.04) | 31 (19.1) | 1.36 (0.73 to 2.53) |
| XA/XA | 23 (4.5) | 0.65 (0.46 to 1.39) | 3 (1.9) | 5.29 (1.10 to 25.51) |
| YA/B | 78 (15.2) | 1.01 (0.68 to 1.49) | 7 (4.3) | 1.82 (0.50 to 6.58) |
| XA/B | 30 (5.8) | 0.30 (0.14 to 0.67) | 2 (1.2) | 2.33 (0.29 to 18.71) |
| YA/C | 8 (1.5) | 1.38 (0.50 to 3.80) | 47 (29.0) | 1.10 (0.62 to 1.96) |
| YA/D | 46 (9.0) | 0.91 (0.56 to 1.48) | 4 (2.5) | 0.30 (0.04 to 2.28) |
| XA/D | 17 (3.3) | 0.58 (0.27 to 1.27) | 0 | – |
| XA/C | 3 (0.6) | – | 7 (4.3) | 0.24 (0.05 to 1.03) |
| O/O‡ | 25 (4.9) | 1.13 (0.61 to 2.08) | 15 (9.3) | 1.03 (0.40 to 2.62) |
| Low vs high§ | 0.63 (0.44 to 0.92) | 0.67 (0.35 to 1.28) | ||
RR = risk ratio; CI = confidence interval; – = insufficient data.
Risk ratio of death was adjusted for sex, stage (III–IV versus I–II), age at diagnosis, current smoking status, and pack-years of smoking.
O/O indicates carriers of two exon 1 variants.
Diplotypes were categorized according to their association with low (XA/XA, XA/B, XA/C, XA/D, and O/O) or high (YA/YA, YA/XA, YA/B, YA/C, and YA/D) serum levels of mannose-binding lectin.
We next dichotomized the patients into high (YA/YA, YA/XA, YA/B, YA/C, and YA/D) and low (XA/XA, XA/B, XA/C, XA/D, and O/O) serum MBL diplotypes, according to serum MBL concentration, as described by Garred et al (7). Among white patients, low serum MBL diplotypes, compared with high serum MBL diplotypes, were associated with increased survival (RR = 0.63, 95% CI = 0.44 to 0.92, P = .016) (Table 6).
Discussion
Our investigation indicates that genetic variants in the innate-immunity gene MBL2 are associated with lung cancer–specific survival in white patients. The association was strongest among heavy smokers and was independent of other prognostic and demographic factors. The genetic variants are functional in that they alter serum MBL levels or oligomeric structures.
The X allele, which is associated with lower MBL levels, was statistically significantly associated with improved lung cancer–specific survival among white patients. In other words, the wild-type Y/Y genotype, and thus high MBL levels, was associated with poorer survival than a genotype with at least one X allele. Consistent with the genotype data, the MBL2 secretor LXA haplotype and XA/B diplotype, which result in low serum MBL levels, were associated with improved outcome among the white patients. In addition, the combined groups of haplotypes or diplotypes with low MBL levels were associated with better survival than the combined groups of haplotypes or diplotypes with high MBL levels, respectively.
The mechanism through which the MBL2 genetic variants are associated with lung cancer–specific survival is unclear. High levels of MBL, which result from certain polymorphisms within the MBL2 secretor haplotype, are associated with inflammation-related disorders (16–20). The MBL-mediated complement cascade leads to the activation of inflammatory cells and the liberation of proinflammatory factors, such as interleukins 1β and 6 and tumor necrosis factor-α. Chronic inflammation and exposure to cytokines have been shown to potentiate tumor progression in animal tumor models (32, 33) and to augment invasiveness of human lung tumors (34). Mannose-binding lectin may also contribute to tumor aggressiveness by enhancing the DNA-damaging environment. Mannose-binding lectin, through interleukin 1β and tumor necrosis factor-α, causes increased expression of the inducible form of nitric oxide synthase, which increases the production of DNA-damaging reactive nitrogen and oxygen species (35). Because smoking causes chronic inflammation of the lung, the combination of high MBL levels and heavy smoking would be expected to be associated with further decreased survival. Indeed, we found that the association between MBL2 variants related to high MBL levels and poor survival were strongest among patients who were heavy smokers. Furthermore, smoking was recently shown to be associated with increased MBL levels (29), which further supports the association between the MBL2 genetic variants and lung cancer–specific survival among the heavy smokers.
This study may provide insight into the continued disparity in lung cancer survival between African American and white patients. Low serum MBL levels are more prevalent among those of African descent (36), and the selection process directing MBL levels may also be affected by how patients respond to the disease. It has been suggested that, because low MBL levels are protective against opsonization of parasites and mycobacteria (36), which are more prevalent in African countries, there is selective pressure for decreased MBL levels. An interaction between the promoter Y/X SNP and race raises questions about the driving factor behind the racial differences. Additional components of the innate-immunity pathways could influence the aggressiveness of disease or response to treatment. One candidate, MBL serine protease 2 (MASP-2), which activates complement after forming a complex with MBL, also displays wide variability between interethnic/racial groups (36–38). Expression of MASP-2 in esophageal tumors was shown to be associated with aggressive tumor behavior (39). Thus, a comprehensive investigation of the relationship of MBL2 and MASP-2 to lung cancer–specific survival may lead to important insights about why African American and white patients with lung cancer have disparate survivals.
A limitation of this study was that only one of the five secretor haplotype SNPs was associated with survival, even though all five SNPs are considered to be functionally related to reduced serum MBL levels or structure. Changes in MBL levels are more dependent on the variant promoter than on structural alleles (7). Perhaps lung cancer survival is more dependent on total MBL serum levels than fully assembled MBL subunits. Functional promoter analyses have demonstrated that the Y/X SNP is much more sensitive to change of promoter activity than the L/H SNP (20), which suggests that the Y/X SNP might have a stronger impact on affecting disease related to abnormal serum MBL levels. A second limitation of the study was the low frequency of the variant allele for the Y/X SNP and the associated haplotypes and diplotypes, and so the results from the population examined in this study should be treated with caution until the associations can be validated in an independent study. Another limitation of the study was that there were fewer African American patients than white patients. The association between the low MBL LXA haplotype and survival among African American patients who were also heavy smokers raises the possibility that MBL might also be associated with lung cancer survival among African American patients. Lastly, the study was limited to smoking status at the time of interview for the case–control study, and patients might have quit smoking between the time of diagnosis and the interview. Smoking status was considered to be current if the patient quit smoking within 6 months before the interview, and thus, the smoking status was accurate for the majority of patients. Because of this limitation, we were unable to stratify patients by smoking status and, instead, relied on total smoking amount. This result attenuated our ability to determine whether current and/or former smoking is statistically significantly associated with poor lung cancer survival among white patients who have MBL variants related to high MBL serum levels.
In summary, genetic variations in the innate-immunity gene MBL2 may be associated with lung cancer–specific survival. The improved survival associated with low MBL haplotypes indicated that, in a reduced inflammatory environment, the tumor may be less aggressive. The clinical meaning of a decreased innate immune response must be taken into consideration and the prognostic importance of modulation by smoking should be examined in more detail in future studies, as well as the role of MBL within the lung mucosa.
Supplementary Material
CONTEXT AND CAVEATS.
Prior knowledge
The innate-immunity MBL2 gene encodes the mannose-binding lectin (MBL) protein, which activates the lectin pathway of the complement system, enhances opsonophagocytosis, and modulates the cytokine response to inflammation. Altered function or expression of MBL caused by polymorphisms within the secretor haplotype block of the MBL2 gene has been associated with increased susceptibility to inflammation-related disorders and certain cancers.
Study design
A prospective study to examine the association between five polymorphisms within the MBL2 secretor haplotype block and disease-specific survival among 558 white patients and 173 African American patients with non–small-cell lung cancer in the metropolitan area of Baltimore, MD.
Contribution
The functional MBL2 Y/X promoter polymorphism and the secretor haplotypes and diplotypes associated with low serum MBL concentrations were associated with improved lung cancer–specific survival among white patients but not among African American patients.
Implications
Genetic polymorphisms in genes in the innate immune response pathway that modulate inflammation could be a prognostic factor in lung cancer patients.
Limitations
This study had fewer African American patients than white patients and thus had reduced power to assess associations among African American patients. Stage and histotype information was not available on all patients. Clinical follow-up information was restricted to death certificate data.
Acknowledgments
Funding
Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
We thank Dorothea Dudek-Creaven for editorial assistance and Karen MacPherson for bibliographic assistance. We also thank Dirk Petersen for providing the Tab2Phase and Phase2Tab software program to format our data for haplotype analysis. We are grateful to Donna Perlmutter, Anthony Alberg, Raymond Jones, Leoni Leondaridis, Glennwood Trivers, Bonnie Cooper-Remmell, Audrey Salabes, Lauren Richey, John Cottrell, Rex Yung, Mark J. Krasna, and the Surgery and Pathology Departments at University of Maryland Hospital, Johns Hopkins Hospital, Baltimore Veteran’s Administration Medical Center, Sinai Hospital, Union Memorial Hospital, St Agnes Hospital, Northwest Hospital Center, and Mercy Medical Center for contributions to this study.
The authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
References
- 1.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer, 1975– 2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Liu G, Zhou W, Christiani DC. Molecular epidemiology of non-small cell lung cancer. Semin Respir Crit Care Med. 2005;26:265–272. doi: 10.1055/s-2005-871983. [DOI] [PubMed] [Google Scholar]
- 4.Yoon SM, Hong YC, Park HJ, Lee JE, Kim SY, Kim JH, et al. The polymorphism and haplotypes of XRCC1 and survival of non-small-cell lung cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:885–891. doi: 10.1016/j.ijrobp.2005.07.951. [DOI] [PubMed] [Google Scholar]
- 5.Isla D, Sarries C, Rosell R, Alonso G, Domine M, Taron M, et al. Single nucleotide polymorphisms and outcome in docetaxel-cisplatin-treated advanced non-small-cell lung cancer. Ann Oncol. 2004;15:1194–1203. doi: 10.1093/annonc/mdh319. [DOI] [PubMed] [Google Scholar]
- 6.Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40:423–429. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- 7.Garred P, Larsen F, Madsen HO, Koch C. Mannose-binding lectin deficiency — revisited. Mol Immunol. 2003;40:73–84. doi: 10.1016/s0161-5890(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 8.Baccarelli A, Hou L, Chen J, Lissowska J, El Omar EM, Grillo P, et al. Mannose-binding lectin-2 genetic variation and stomach cancer risk. Int J Cancer. 2006;119:1970–1975. doi: 10.1002/ijc.22075. [DOI] [PubMed] [Google Scholar]
- 9.Davies EJ, Snowden N, Hillarby MC, Carthy D, Grennan DM, Thomson W, et al. Mannose-binding protein gene polymorphism in systemic lupus erythematosus. Arthritis Rheum. 1995;38:110–114. doi: 10.1002/art.1780380117. [DOI] [PubMed] [Google Scholar]
- 10.Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ. 1997;314:1229–1232. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch A, Melbye M, Sorensen P, Homoe P, Madsen HO, Molbak K, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 2001;285:1316–1321. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- 12.Boniotto M, Braida L, Baldas V, Not T, Ventura A, Vatta S, et al. Evidence of a correlation between mannose binding lectin and celiac disease: a model for other autoimmune diseases. J Mol Med. 2005;83:308–315. doi: 10.1007/s00109-004-0623-3. [DOI] [PubMed] [Google Scholar]
- 13.Scudiero O, Nardone G, Omodei D, Tatangelo F, Vitale DF, Salvatore F, et al. A mannose-binding lectin-defective haplotype is a risk factor for gastric cancer. Clin Chem. 2006;52:1625–1627. doi: 10.1373/clinchem.2006.071696. [DOI] [PubMed] [Google Scholar]
- 14.Schmiegelow K, Garred P, Lausen B, Andreassen B, Petersen BL, Madsen HO. Increased frequency of mannose-binding lectin insufficiency among children with acute lymphoblastic leukemia. Blood. 2002;100:3757–3760. doi: 10.1182/blood-2002-06-1627. [DOI] [PubMed] [Google Scholar]
- 15.Hoal-Van Helden EG, Epstein J, Victor TC, Hon D, Lewis LA, Beyers N, et al. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatr Res. 1999;45:459–464. doi: 10.1203/00006450-199904010-00002. [DOI] [PubMed] [Google Scholar]
- 16.Uguz A, Berber Z, Coskun M, Halide AS, Yegin O. Mannose-binding lectin levels in children with asthma. Pediatr Allergy Immunol. 2005;16:231–235. doi: 10.1111/j.1399-3038.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 17.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, et al. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175:541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita M, Miyakawa H, Tanaka A, Hijikata M, Kikuchi K, Fujikawa H, et al. Single nucleotide polymorphisms of the mannose-binding lectin are associated with susceptibility to primary biliary cirrhosis. J Autoimmun. 2001;17:251–257. doi: 10.1006/jaut.2001.0538. [DOI] [PubMed] [Google Scholar]
- 19.Garred P, Harboe M, Oettinger T, Koch C, Svejgaard A. Dual role of mannan-binding protein in infections: another case of heterosis? Eur J Immunogenet. 1994;21:125–131. doi: 10.1111/j.1744-313x.1994.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 20.Naito H, Ikeda A, Hasegawa K, Oka S, Uemura K, Kawasaki N, et al. Characterization of human serum mannan-binding protein promoter. J Biochem (Tokyo) 1999;126:1004–1012. doi: 10.1093/oxfordjournals.jbchem.a022543. [DOI] [PubMed] [Google Scholar]
- 21.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19(2):209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Marrogi AJ, Mechanic LE, Welsh JA, Bowman ED, Khan MA, Enewold L, et al. TP53 mutation spectrum in lung cancer is not different in women and men. Cancer Epidemiol Biomarkers Prev. 2005;14:1031–1033. doi: 10.1158/1055-9965.EPI-04-0640. [DOI] [PubMed] [Google Scholar]
- 23.Zheng YL, Loffredo CA, Yu Z, Jones RT, Krasna MJ, Alberg AJ, et al. Bleomycin-induced chromosome breaks as a risk marker for lung cancer: a case-control study with population and hospital controls. Carcinogenesis. 2003;24:269–274. doi: 10.1093/carcin/24.2.269. [DOI] [PubMed] [Google Scholar]
- 24.Zheng YL, Loffredo CA, Alberg AJ, Yu Z, Jones RT, Perlmutter D, et al. Less efficient g2-m checkpoint is associated with an increased risk of lung cancer in African Americans. Cancer Res. 2005;65:9566–9573. doi: 10.1158/0008-5472.CAN-05-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernig T, Boersma BJ, Howe TM, Welch R, Yadavalli S, Staats B, et al. The mannose-binding lectin (MBL2) haplotype and breast cancer: an association study in African American and Caucasian women. Carcinogenesis. 2007;28:828–836. doi: 10.1093/carcin/bgl198. [DOI] [PubMed] [Google Scholar]
- 26.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 27.Dupont WD, Plummer WD., Jr Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589–601. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 28.Sun T, Miao X, Zhang X, Tan W, Xiong P, Lin D. Polymorphisms of death pathway genes FAS and FASL in esophageal squamous-cell carcinoma. J Natl Cancer Inst. 2004;96:1030–1036. doi: 10.1093/jnci/djh187. [DOI] [PubMed] [Google Scholar]
- 29.Maffei G, Brouwer N, Dolman KM, van der Velden U, Roos D, Loos BG. Plasma levels of mannan-binding lectin in relation to periodontitis and smoking. J Periodontol. 2005;76:1881–1889. doi: 10.1902/jop.2005.76.11.1881. [DOI] [PubMed] [Google Scholar]
- 30.Schlapbach LJ, Aebi C, Otth M, Luethy AR, Leibundgut K, Hirt A, et al. Serum levels of mannose-binding lectin and the risk of fever in neutropenia pediatric cancer patients. Pediatr Blood Cancer. 2007;49:11–16. doi: 10.1002/pbc.21097. [DOI] [PubMed] [Google Scholar]
- 31.Larsen F, Madsen HO, Sim RB, Koch C, Garred P. Disease-associated mutations in human mannose-binding lectin compromise oligomerization and activity of the final protein. J Biol Chem. 2004;279:21302–21311. doi: 10.1074/jbc.M400520200. [DOI] [PubMed] [Google Scholar]
- 32.Abdalla SI, Sanderson IR, Fitzgerald RC. Effect of inflammation on cyclooxygenase (COX)-2 expression in benign and malignant oesophageal cells. Carcinogenesis. 2005;26:1627–1633. doi: 10.1093/carcin/bgi114. [DOI] [PubMed] [Google Scholar]
- 33.Chen T, Nines RG, Peschke SM, Kresty LA, Stoner GD. Chemopreventive effects of a selective nitric oxide synthase inhibitor on carcinogen-induced rat esophageal tumorigenesis. Cancer Res. 2004;64:3714–3717. doi: 10.1158/0008-5472.CAN-04-0302. [DOI] [PubMed] [Google Scholar]
- 34.Marrogi AJ, Travis WD, Welsh JA, Khan MA, Rahim H, Tazelaar H, et al. Nitric oxide synthase, cyclooxygenase 2, and vascular endothelial growth factor in the angiogenesis of non-small cell lung carcinoma. Clin Cancer Res. 2000;6:4739–4744. [PubMed] [Google Scholar]
- 35.Hussain SP, Trivers GE, Hofseth LJ, He P, Shaikh I, Mechanic LE, et al. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res. 2004;64:6849–6853. doi: 10.1158/0008-5472.CAN-04-2201. [DOI] [PubMed] [Google Scholar]
- 36.Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998;161:3169–3175. [PubMed] [Google Scholar]
- 37.Mayilyan KR, Presanis JS, Arnold JN, Sim RB. Discrete MBL-MASP complexes show wide inter-individual variability in concentration: data from UK vs Armenian populations. Int J Immunopathol Pharmacol. 2006;19:567–580. doi: 10.1177/039463200601900313. [DOI] [PubMed] [Google Scholar]
- 38.Thiel S, Steffensen R, Christensen IJ, Ip WK, Lau YL, Reason IJ, et al. Deficiency of mannan-binding lectin associated serine protease-2 due to missense polymorphisms. Genes Immun. 2007;8:154–163. doi: 10.1038/sj.gene.6364373. [DOI] [PubMed] [Google Scholar]
- 39.Verma A, Matta A, Shukla NK, Deo SV, Gupta SD, Ralhan R. Clinical significance of mannose-binding lectin-associated serine protease-2 expression in esophageal squamous cell carcinoma. Int J Cancer. 2006;118:2930–2935. doi: 10.1002/ijc.21721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.