Abstract
Combination immunotherapy approaches involving radiation, chemotherapy, androgen manipulation and T-cell modulation have been studied extensively in animal models, setting the stage for clinical trials. Radiation therapy, in particular, is an interesting modality in this regard, leading to synergistic efficacy when used in combination with immunotherapies in several models. Chemotherapy, the foundation of treatment of metastatic disease, may also augment the immune response to cancer; however, the potential immunosuppressive effects of chemotherapy render issues of dosing and timing critical. Perhaps, the most exciting combinatorial approach may be the co-administration of multiple immunological treatments. For example, in preclinical investigations, combined blockade of programmed death-1 (PD1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), which have key roles in the negative regulation of T-cell activation, has been shown to enhance antitumour immune responses compared with either agent alone. Taken together, the available data provide a strong rationale for initiating combination clinical trials, but lend a note of caution in that issues of dosing and timing likely require careful exploration in a phase II setting.
Keywords: cancer vaccines, chemotherapy, combination, immunotherapy, radiotherapy, T-cell modulation
introduction
The field of immuno-oncology includes the development of therapies that harness the immune system to provide durable and adaptable cancer control. Recently, phase III clinical trials of ipilimumab, an antibody against cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), and sipuleucel-T, an autologous cellular vaccine, provided proof-of-principle that immunotherapies could prolong survival in patients with metastatic melanoma and prostate cancer, respectively [1–3]. These agents are the first among the increasing armamentarium of immunotherapeutic anticancer treatments. However, understanding the ultimate clinical value of immuno-oncology will likely require further research into optimal dosing and treatment schedules, including the combination of immunotherapies with other agents, to broaden application to other tumour types. Traditional or conventional treatment options for patients with advanced cancer include surgery, radiation, chemotherapy, hormone therapy and immunotherapy, all of which have differing mechanisms of action. Combining immunotherapies either with each other or with other modalities used in the treatment of cancer could potentially lead to enhanced efficacy with diminished toxic effect. Before immunotherapy-based combination treatment can be integrated into clinical practice, a better understanding of the interaction between modalities and potential synergistic behaviour is required. The aim of this review is to discuss the rationale and data supporting the selected immunotherapeutic combinations, primarily based on preclinical data.
the rationale for immunotherapy-based combination treatment
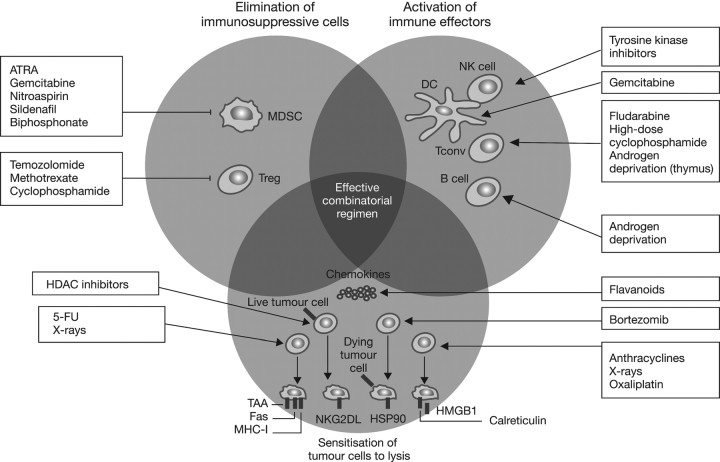
To understand the rationale for combining immunotherapy with other modalities, it is first important to understand the role of the immune system in cancer. The immune response to cancer includes three key phases: elimination, equilibrium and escape [4]. First, recognition of tumour-specific antigens can initiate an immune response, resulting in the elimination of early lesions by components of the innate and adaptive immune systems. In the second phase, tumour cells that are not eliminated are maintained in a state of equilibrium, whereby the tumour is not eradicated, but does not progress. However, disruption of the equilibrium between the immune system and the tumour can result in the growth of tumour cells that are able to avoid, resist or suppress the natural immune response [5, 6]. Many factors in the tumour microenvironment contribute to the escape of tumour cells from immune surveillance [6]. For example, myeloid-derived suppressive cells (MDSC) and their derived cytokines, interleukin-6 (IL-6), tumour necrosis factor-α and IL-23, have dominant tumour-promoting activity. These factors and others in the tumour microenvironment can lead to the induction and/or recruitment of regulatory T cells (Tregs) that use multiple mechanisms, including both IL-10 and TGF-β, to down-regulate an antitumour immune response [6–8]. Against this backdrop of immunosuppression, the goal of cancer immunotherapy is to enhance the inherent antitumour capabilities of the immune system. It is noteworthy, though, that many conventional cancer treatments such as chemotherapy and radiotherapy also have immune potentiating mechanisms of action (Figure 1) [9]. For example, anticancer therapies can deplete immunosuppressive cells such as Tregs and MDSC to enhance a latent antitumour immune response, and treatment-induced cell death may release tumour antigens that are taken up by antigen-presenting cells, processed and presented to naïve T cells, thereby making them sensitive to lysis [9–11].
Figure 1.
Immune potentiating mechanisms of action of conventional chemotherapies and radiotherapy. Republished with permission of the American Society for Clinical Investigation, from ‘The anticancer immune response: indispensable for therapeutic success?’ by Zitvogel et al. [9]; permission conveyed through Copyright Clearance Center, Inc. 5-FU, 5-fluorouracil; ATRA, all-trans-retinoic acid; DC, dendritic cell; HDAC, histone deacetylases; HSP90, heat shock protein 90; MDSC, myeloid-derived suppressor cell; MHC-I, major histocompatibility complex class I; HMGB1, high mobility group box 1 protein; NK, natural killer cell; NKG2DL, NK cell group 2D ligands; Tconv, conventional effectors; Treg, regulatory-T cell.
One mechanism of potentiating an immune response that is often overlooked in this context is the physical removal of tumour burden. In mice, for example, persistent presentation of a tumour antigen causes cytotoxic T cells that were once active against the antigen to become tolerant, resulting in tumour out-growth. Interestingly, functional capacities are regained when T cells are transferred to an antigen-free environment [12]. These results demonstrate that elimination of tumour antigen may play a role in generating an effective immune response, and further suggest that cancer treatments that are effective in reducing a primary tumour burden, such as androgen ablation for prostate cancer [13], may be important modalities to combine with immunotherapy.
combining immunotherapies with chemotherapy
vaccine-based combination therapy
Cancer vaccines present single or multiple tumour antigens to the immune system in a pro-inflammatory context in order to generate new antitumour immune responses. Despite the poor performance of first-generation cancer vaccines, durable, nontoxic antitumour responses have been observed, maintaining interest in improving this immunotherapeutic approach to treatment. Vaccine treatment alone, however, is usually not sufficient to generate objective tumour regression, especially in the advanced disease setting in which most clinical trials have been conducted. Combining cancer vaccines with chemotherapy is complicated by the notion that most chemotherapy regimens are profoundly immunosuppressive at standard doses. This is true for the alkylating agent cyclophosphamide (CY), which is commonly used in the treatment of several malignancies, including breast cancer and lymphoma. However, early work with CY showed that low doses of this agent could profoundly augment a vaccine response [14]. Further studies extended these earlier results, showing that the augmentation of antitumour vaccination by low-dose CY was particularly impressive when administered with cell-based, granulocyte–macrophage colony-stimulating factor (GM-CSF) secreting vaccines [15]. Recently, this combination approach was tested in an autochthonous model of prostate cancer, using a murine version of prostate GVAX [16]. When prostate/prostate cancer-specific CD8+ T cells were transferred to tumour-bearing mice, treatment with prostate GVAX alone was not sufficient to expand or induce an effector phenotype, suggesting T-cell tolerance. However, when given 1 day before immunotherapy administration, low-dose CY significantly augmented GVAX-mediated cytotoxic T-cell expansion, resulting in a substantial reduction in tumour burden (Figure 2) [17]. In these studies, the dosage and timing of CY were absolutely critical, with additive effects observed only when CY was given 1 day before vaccination, and only with doses that did not result in T-cell depletion. Perhaps most significantly, these results have been confirmed in early phase human studies, in the context of an important dose-escalation combination study with breast GVAX. Here, doses of CY in the range of 200 mg/m2 significantly augmented an anti-Her-2 antibody response, whereas larger doses seemed to suppress both delayed type hypersensitivity and antibody responses. Taken together these data clarify the importance of considering dose, timing and mechanism of action when taking combinatorial cancer treatment strategies to the clinic. It should also be noted that various chemotherapy agents differ widely in their ability to augment an antitumour immune response [9], another important consideration in such approaches.
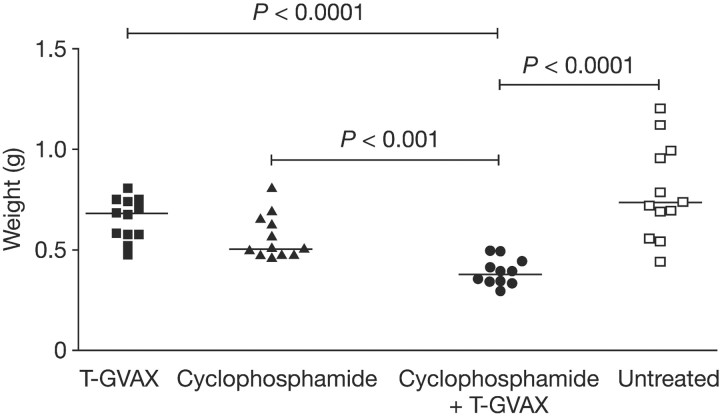
Figure 2.
In an autochthonous, murine model of prostate cancer, the wet weight of the urogenital tract, a gross surrogate for tumour burden, was significantly decreased in the T-GVAX plus cyclophosphamide (CY) treatment group compared with either agent alone. As monotherapy, T-GVAX immunotherapy had no treatment effect, whereas cyclophosphamide showed a nonsignificant trend toward efficacy compared with untreated control animals. Reprinted by permission from the American Association for Cancer Research: Wada et al. [17].
T-cell modulation plus chemotherapy
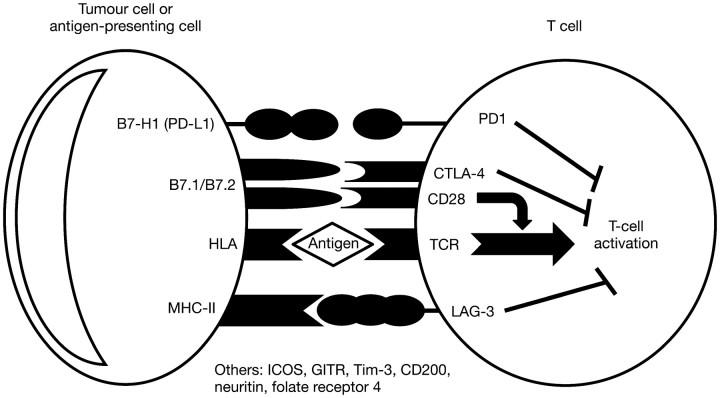
Treatments designed to modulate T-cell responses against tumours have broad therapeutic potential, targeting processes involved in T-cell survival, activation, proliferation, migration and tumour destruction [18]. Negative regulators of T-cell function include (among others) molecules such as CTLA-4, a key negative regulatory molecule that down-regulates pathways of T-cell activation; programmed death-1 (PD1), a transmembrane receptor up-regulated on activated T cells that when bound to its ligand (programmed death ligand-1, PD-L1) leads to decreased cytokine production and proliferation of T cells, and lymphocyte activation gene-3 (LAG-3), the expression of which is increased on activated antigen-specific cytotoxic T cells (Figure 3) [19–24]. In vivo blockade of LAG-3 results in the increased accumulation and activity of cytotoxic T cells within organs and tumours that express the related antigen. All three molecules are involved in the regulation of peripheral tolerance [20, 25–27].
Figure 3.
Examples of molecular interactions and signalling at the antigen-presenting cell/T-cell immune synapse that inhibit T-cell activation and contribute to negative regulation of the immune response. CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; GITR, glucocorticoid-induced TNFR-related protein; HLA, human leukocyte antigen; ICOS, inducible T-cell costimulator; LAG-3, lymphocyte-activation gene-3; MHC-II, major histocompatibility complex class II; PD1, programmed death 1; PD-L1, programmed death ligand-1.
Ipilimumab, a fully human monoclonal antibody against CTLA-4, has been clinically evaluated in combination with chemotherapy with interesting results. In a recent phase III trial, patients with metastatic melanoma receiving ipilimumab in combination with dacarbazine had significantly improved overall survival compared with patients receiving dacarbazine alone (11.2 versus 9.1 months; hazard ratio: 0.72, P < 0.001) [3]. Additionally, a recent and important phase II trial in patients with stage IIIb/IV non-small-cell lung cancer or extensive-disease small-cell lung cancer investigated whether ipilimumab could be given safely in combination with standard chemotherapy (carboplatin–paclitaxel (Taxol) [CP]) as well as whether it would be optimal to initiate ipilimumab at the same time as chemotherapy, or after two cycles of treatment. The results from this phase II trial were interesting, showing that the combination was reasonably well-tolerated, and that a ‘phased regimen’ in which immunotherapy began after chemotherapy resulted in substantially improved progression-free survival (PFS) compared with CP alone [28, 29]. While this study did not actively investigate dosing effects, the data clearly show that the clinical effects of administering immunotherapy in combination with chemotherapy are strongly dependent on the sequencing of treatment.
radiation therapy plus immunotherapy
Evidence suggests that the combination of radiation and immunotherapy can prevent cancer cells from evading an immune response via several mechanisms [30]. First, radiation-induced tumour-cell death increases the supply of tumour-specific antigens for presentation and cross-presentation, thereby activating tumour-specific immune responses [31]. Radiation-induced damage of cancer cells, for example, leads to the release of signal molecules such as high mobility group box 1 (HMGB1) protein that attracts immune cells to the tumour microenvironment. Additionally, the interaction of HMGB1 with toll-like receptor 4 signalling on dendritic cells (DC) results in the efficient processing and cross-presentation of antigens from dying tumour cells [32, 33]. Second, the phenotype of tumour cells is modulated following radiation treatment, making them more susceptible to immune-mediated killing [31]. Ionizing radiation, for example, increases the expression of major histocompatibility complex (MHC) class I molecules, Fas and intracellular adhesion molecule-1, among other cell surface proteins, leading to enhanced susceptibility of tumour cells to lysis by cytotoxic T cells [34, 35]. Finally, effective radiotherapy can dramatically reduce tumour burden, leading to the elimination (or down-modulation) of persistent antigen which contributed to ongoing T-cell tolerance. This synergistic effect of radiotherapy and immunotherapy may also explain the abscopal effect, whereby ionizing radiation can inhibit distant tumours after local radiation therapy [35, 36].
Although these data suggest that radiotherapy may be an important supplement to anticancer immunotherapy, only a small number of early phase trials have investigated the combination in a clinical setting. One of these was an important randomised phase II clinical trial in which 30 patients with clinically localised prostate cancer were treated with a poxviral vaccine encoding prostate-specific antigen (PSA) plus radiotherapy, or radiotherapy alone. Among 17 patients in the combination arm who completed all their vaccinations, 13 had increases in PSA-specific T cells of at least three-fold compared with no detectable increases in the radiotherapy-only arm (P < 0.0005). Evidence of T cells with reactivity to prostate-associated antigens not found in the vaccine provided indirect evidence of immune-mediated tumour killing [37]. Similarly, in a phase I study of 14 patients with advanced/metastatic stage hepatoma, radiation therapy followed by localised vaccination with autologous immature DC resulted in tumour-specific immune responses in 7 out of 10 assessable patients and several partial tumour responses [38]. Taken together, these data suggest that radiation therapy in combination with immunotherapeutic approaches may increase tumour-cell killing compared with either modality alone; a concept that requires further assessment in well-designed clinical trials.
combining different immunotherapeutic approaches
Given that multiple immunosuppressive mechanisms may conspire to restrain an antitumour immune response, it is feasible that immunotherapeutic agents, with differing mechanisms of action, could be combined as a means of further enhancing immune responses against tumours. Recently, the results from a phase III trial of gp100 peptide vaccine plus high-dose IL-2 in patients with stage III/IV melanoma showed significantly improved response rates and PFS compared with IL-2 alone [16% versus 6% (P = 0.03) and 2.2 versus 1.6 months (P = 0.008), respectively] [39], suggesting that vaccines can enhance cytokine therapy in patients with metastatic melanoma.
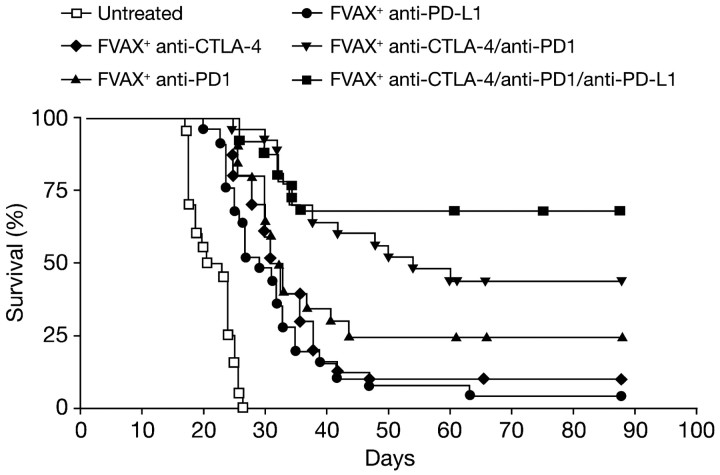
Perhaps most exciting in this regard are recent data suggesting that antitumour T cells may express multiple inhibitory receptors, a finding mirrored in murine models of chronic viral infection [40]. In a relevant mouse model of melanoma, single blockade of either CTLA-4 or PD1 enhanced the infiltration of activated T cells into tumours, but the T cells accumulated high levels of unblocked negative coreceptors that eventually limited their expansion. Blocking CTLA-4, PD1 and PD-L1 simultaneously allowed T cells to continue to survive, proliferate and resulted in enhanced infiltration, activation and cytokine production, thereby reducing tumour-induced immune suppression and promoting tumour rejection (Figure 4) [41]. Similar results were obtained in a mouse model of metastatic colon carcinoma evaluating the combination of IL-15 with antibodies against CTLA-4 and PD-L1. In this study, although IL-15 significantly prolonged survival in mice with metastatic tumours, it also increased the expression of PD1 and the secretion of the immunosuppressive cytokine, IL-10. Combining the immune stimulatory properties of IL-15 with the simultaneous removal of two critical immune system inhibitory checkpoints significantly increased antitumour activity compared with IL-15 alone or combined with either anti-PD-L1 or anti-CTLA-4 [42]. These data support the idea that the synergistic blockade of multiple checkpoints can enhance immune responses; however, clinical development is restricted by the paucity of licensed immunotherapeutic agents. Nevertheless, a phase I clinical trial investigating the safety and efficacy of ipilimumab plus an antibody against PD1 in patients with metastatic melanoma is underway (NCT01024231) with more trials likely as further immunotherapies are developed.
Figure 4.
Combination blockade of the PD1, CTLA-4 and PD-L1 coinhibitory molecules coupled with Fvax vaccination increased survival of mice challenged with antigen-presenting melanoma cells. Lack of survival was defined as death or tumour size >1000 mm3. CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; PD1, programmed death 1; PDL-1, programmed death ligand-1.
conclusions
Increased understanding of the role of the immune system in recognising and responding to cancer, together with recent phase III trials supporting the efficacy of ipilimumab in patients with metastatic melanoma and sipuleucel-T in patients with prostate cancer, has augmented enthusiasm for combination treatment approaches involving immunotherapy. Preliminary evidence from preclinical and early phase clinical trials indicates promising activity when conventional anticancer therapies, such as chemotherapy and radiation therapy, are used in combination with immunotherapy. However, the immune-related effects of these agents, including the depletion of suppressive cell populations and induction of immunogenic cell death, are not well understood and require further investigation. In addition, defining the optimum dose and schedule of combination therapies remains a major challenge, and early phase clinical investigations to optimise dose and schedule in patients are required. Combining immunotherapies, particularly agents that target different immune checkpoints, is a promising approach, with preclinical studies suggesting potential for synergistic effects on tumour response and overall survival.
disclosure
CGD has served as a consultant for Bristol-Myers Squibb, Dendreon and Amplimmune. He holds patents with Bristol-Myers Squibb and Amplimmune and has received royalties from Bristol-Myers Squibb.
references
- 1.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 6.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 9.Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menard C, Martin F, Apetoh L, et al. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57:1579–1587. doi: 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.den Boer AT, van Mierlo GJ, Fransen MF, et al. The tumoricidal activity of memory CD8+ T cells is hampered by persistent systemic antigen, but full functional capacity is regained in an antigen-free environment. J Immunol. 2004;172:6074–6079. doi: 10.4049/jimmunol.172.10.6074. [DOI] [PubMed] [Google Scholar]
- 13.Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 16.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada S, Yoshimura K, Hipkiss EL, et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardoll D. T cells take aim at cancer. Proc Natl Acad Sci USA. 2002;99:15840–15842. doi: 10.1073/pnas.262669499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peggs KS, Quezada SA, Allison JP. Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists. Clin Exp Immunol. 2009;157:9–19. doi: 10.1111/j.1365-2249.2009.03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosso JF, Kelleher CC, Harris TJ, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi T, Hirota K, Nagahama K, et al. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Barbi JJ, Yu H, Pan F, et al. The role of neuritin in regulating the immune response. Int Immunol Meeting. 2010;22(Suppl 1 Pt 3):iii8–iii9. abstr WS/PP-045a-04. [Google Scholar]
- 23.Siva A, Xin H, Qin F, et al. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2008;57:987–996. doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyers JH, Sabatos CA, Chakravarti S, et al. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med. 2005;11:362–369. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keilholz U. CTLA-4: negative regulator of the immune response and a target for cancer therapy. J Immunother. 2008;31:431–439. doi: 10.1097/CJI.0b013e318174a4fe. [DOI] [PubMed] [Google Scholar]
- 27.Sharpe AH, Abbas AK. T-cell costimulation––biology, therapeutic potential, and challenges. N Engl J Med. 2006;355:973–975. doi: 10.1056/NEJMp068087. [DOI] [PubMed] [Google Scholar]
- 28.Lynch T, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-Line treatment in non-small cell lung cancer: analysis by baseline histology in a phase 2 trial. Presented at World Conference on Lung Cancer (WCLC); 2011. Abstr 701. [Google Scholar]
- 29.Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in extensive disease-small cell lung cancer (ED-SCLC): results from a phase 2 trial. Presented at World Conference on Lung Cancer (WCLC); 2011. Abstr 1365. [Google Scholar]
- 30.Drake CG. Radiation-induced immune modulation. In: DeWeese TH, Laiho M, editors. Molecular Determinants of Radiation Response. Springer; 2011. pp. 251–264. [Google Scholar]
- 31.Higgins JP, Bernstein MB, Hodge JW. Enhancing immune responses to tumor-associated antigens. Cancer Biol Ther. 2009;8:1440–1449. doi: 10.4161/cbt.8.15.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 33.Hagemann T, Balkwill F, Lawrence T. Inflammation and cancer: a double-edged sword. Cancer Cell. 2007;12:300–301. doi: 10.1016/j.ccr.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 35.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 38.Chi KH, Liu SJ, Li CP, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005;28:129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 39.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu P, Steel JC, Zhang M, et al. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16:6019–6028. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]