Abstract
The purpose of this trial was to evaluate the efficacy of 2-year consolidation therapy with nilotinib, at a dose of 300 mg twice daily, for achieving treatment-free remission in chronic myeloid leukemia patients with a deep molecular response (BCR-ABL1IS ≤0.0032%). Successful treatment-free remission was defined as no confirmed loss of deep molecular response. We recruited 96 Japanese patients, of whom 78 sustained a deep molecular response during the consolidation phase and were therefore eligible to discontinue nilotinib in the treatment-free remission phase; of these, 53 patients (67.9%; 95% confidence interval: 56.4–78.1%) remained free from molecular recurrence in the first 12 months. The estimated 3-year treatment-free survival was 62.8%. Nilotinib was readministered to all patients (n=29) who experienced a molecular recurrence during the treatment-free remission phase. After restarting treatment, rapid deep molecular response returned in 25 patients (86.2%), with 50% of patients achieving a deep molecular response within 3.5 months. Tyrosine kinase inhibitor withdrawal syndrome was reported in 11/78 patients during the early treatment-free remission phase. The treatment-free survival curve was significantly better in patients with undetectable molecular residual disease than in patients without (3-year treatment-free survival, 75.6 versus 48.6%, respectively; P=0.0126 by the log-rank test). There were no significant differences in treatment-free survival between subgroups based on tyrosine kinase inhibitor treatment before the nilotinib consolidation phase, tyrosine kinase inhibitor-withdrawal syndrome, or absolute number of natural killer cells. The results of this study indicate that it is safe and feasible to stop tyrosine kinase inhibitor therapy in patients with chronic myeloid leukemia who have achieved a sustained deep molecular response with 2 years of treatment with nilotinib. This study was registered with UMIN-CTR (UMIN000005904).
Introduction
Nilotinib is a second-generation tyrosine kinase inhibitor (TKI) that has been shown to be highly efficacious as a first- or second-line treatment for patients with Philadelphia chromosome-positive chronic myeloid leukemia (CML) in chronic phase. In patients newly diagnosed with CML in chronic phase superior rates of deep molecular response (DMR) were achieved with nilotinib in comparison with imatinib, which is a first-generation TKI currently used as the standard treatment for this disease.1–3 In addition, switching to nilotinib after a minimum of 2 years on imatinib led to increased DMR rates compared to remaining on imatinib.4
Recently, treatment-free remission (TFR) has been proposed as a goal for CML treatment.5–7 Indeed, prospective trials have indicated that imatinib therapy can be successfully discontinued in CML patients who have maintained a DMR for at least 2 years.8–10 In these prospective trials, the TFR rate was 43% [95% confidence interval (CI): 33–52%] at 6 months9 and 41% (95% CI: 29–52%) at 12 months in the STIM1 trial,8 while the TWISTER study revealed a TFR rate of 47.1% (95% CI: 31.5–62.7%) at 24 months.10 Moreover, the first TFR study of second-generation TKI, the DADI trial reported by Imagawa et al., showed that second-generation TKI therapy can be successfully discontinued.11 In this trial, all patients received dasatinib consolidation therapy for at least 1 year. The estimated TFR rate was 49% (95% CI: 36–61%) at 6 months.11 On the other hand, the ENESTfreedom study, which is a TFR study following frontline nilotinib treatment, required that all patients sustained DMR during the consolidation phase with nilotinib for 1 year. The TFR rate at 48 weeks was 51.6% (95% CI: 44.2–58.9%).12 Although the DMR in the consolidation phase with a second-generation TKI was sustained in both the DADI trial and the ENESTfreedom study, the TFR rate was not superior to those in the previously reported imatinib TFR studies.8–10 Most relapses occurred within 6 months of discontinuing second-generation TKI or imatinib therapy, and there was no disease progression in patients with molecular relapse after discontinuation.8–12 All patients who relapsed remained sensitive to TKI re-treatment in these TFR studies.8–12
Compared to imatinib, nilotinib may enable a greater proportion of patients with CML in chronic phase to achieve successful TFR if they receive nilotinib consolidation therapy for 2 years to sustain DMR; this is the same length of time required to achieve TFR in imatinib studies.8,9 The aim of this STAT2 trial (Stop Tasigna® Trial) was to evaluate the efficacy of 2-year consolidation treatment with nilotinib for achieving successful TFR in patients with chronic phase CML.
Methods
Patients and study design
The eligibility criteria for this multicenter, phase II, single-treatment arm, open-label clinical trial included: patients with CML in chronic phase, age ≥16 years, an Eastern Cooperative Oncology Group performance status of 0–2, and no severe primary organ dysfunction. Patients who had accelerated phase or blast crisis CML, a T315I mutation, or who had received allogeneic hematopoietic stem-cell transplantation were excluded from this study. Patients with a DMR (BCR-ABL1IS ≤0.0032% or a molecular response, MR4.5, defined as a 4.5-log reduction in BCR-ABL1 transcripts according to the international scale], assessed by real-time quantitative polymerase chain reaction (RQ-PCR), under treatment with imatinib or a second-generation TKI following imatinib were eligible for the STAT2 trial. Nilotinib (300 mg) was administered twice daily (600 mg/day) for 2 years in the consolidation phase. Patients who maintained a MR4.5 during the 2-year consolidation phase were eligible to enter the TFR phase and cease nilotinib treatment. Molecular recurrence was defined as the loss of a major molecular response (MMR: BCR-ABL1IS ≤0.1%) or confirmed loss of MR4.5 (at two consecutive assessments within 4 weeks) after discontinuing nilotinib, based on criteria used both in the STIM1 trial8 and the TWISTER study.9 Patients with molecular recurrence during the TFR phase restarted nilotinib 300 mg twice daily, thus entering the re-treatment phase.
Endpoints and assessments
The primary endpoint of the STAT2 trial was the 12-month TFR rate after discontinuing nilotinib treatment; secondary endpoints were the 24-month TFR rate after discontinuing nilotinib treatment, the 3-year treatment-free survival, and the MR4.5 rate and time to MR4.5 achieved by nilotinib in the re-treatment phase. Safety profiles, especially vascular adverse events in the consolidation phase or symptoms related to TKI withdrawal syndrome in the TFR phase, were evaluated. Adverse events were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
MR was evaluated by BCR-ABL1IS RQ-PCR analysis upon study entry and every 3 months thereafter in the consolidation phase. After discontinuing nilotinib in the TFR phase, molecular recurrence was monitored by monthly BCR-ABL1IS RQ-PCR testing in the first year, bi-monthly testing in the second year, then every 3 months thereafter. In the re-treatment phase, BCR-ABL1IS was monitored by monthly RQ-PCR testing. The study protocol was terminated when MR4.5 was re-achieved, or when BCR-ABL1IS increased twice consecutively in the re-treatment phase.
BCR-ABL1IS RQ-PCR was performed using a Molecular MD One-Step qRT-PCR BCR-ABL kit (BML Inc., Kawagoe, Japan). To validate BCR-ABL1 amplification, ABL1 was used as an internal control. A MMR was defined as a 3-log reduction in the BCR-ABL1 transcript according to the international scale (BCR-ABL1IS ≤0.1%), MR4.5 was defined as a 4.5-log reduction in the BCR-ABL1 transcript (BCR-ABL1IS ≤0.0032%), and MR5 was defined as a 5-log reduction in the BCR-ABL1 transcript (BCR-ABL1IS ≤0.001%), as described above. Undetectable molecular residual disease was defined as undetectable BCR-ABL1 transcript with MR5 (UMRD with MR5). At least 100,000 control genes (ABL1) were required for a sample to be considered as adequate.
Ethics
Forty-six institutions participated in this study. The study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from each par ticipant before enrollment. The study was approved by the Ethics Committee of Akita University (N. 786) and by all institutional ethic committees that participated in this study. The study was registered with UMIN-CTR (UMIN000005904).
Results
Patients and treatment
Between July 2011 and December 2012, 96 patients who achieved MR4.5 were enrolled in the STAT2 trial. These patients started treatment in the consolidation phase and were defined as the safety analysis set. Seventy-eight patients entered the TFR phase and were analyzed as the full analysis set for TFR. The baseline demographics of the safety and full analysis sets are shown in Table 1.
Table 1.
Baseline demographics of all patients included in the study.
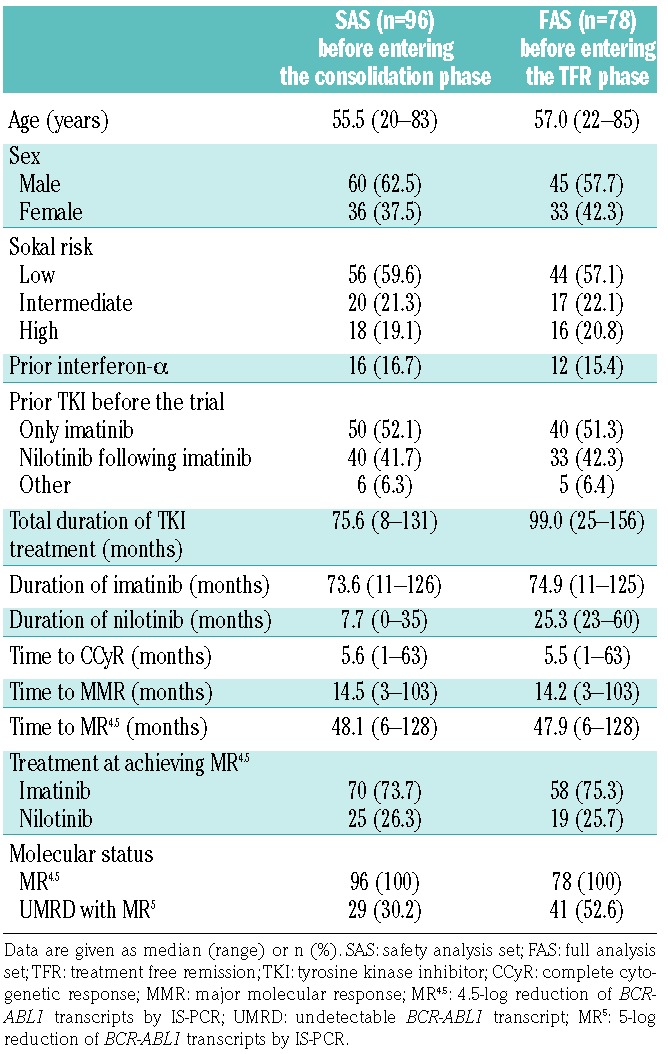
The median age in the safety analysis set was 55.5 years (range, 20–83). Thirty-six (37.5%) patients were female and 16 (16.7%) patients had been administered interferon α before TKI treatment. Based on TKI therapy prior to entry into the current study, patients were classified into three groups: 50 patients (52.1%) who had received only imatinib (‘imatinib only’), 40 patients (41.7%) who had received nilotinib following imatinib (‘nilotinib following imatinib’, including patients from the STAT1 study), and six patients (6.3%) who had received other therapy (‘other’). The STAT1 study (Switch to Tasigna® Trial) is a clinical trial to evaluate the efficacy of 2-year consolidation treatment with nilotinib for achieving DMR in chronic phase CML patients with MMR, recently reported by our study group.13 Among 40 patients in STAT2 treated by nilotinib following imatinib, 21 patients joined this study from STAT1 since they achieved MR4.5 in the STAT1 study. The reasons for switching from imatinib to nilotinib in the 40 patients included imatinib resistance in 7.5%, imatinib intolerance in 20.0%, and upon patients’ request in 72.5% (Online Supplementary Table S1). The median duration of imatinib or nilotinib treatment was 73.6 months (range, 11–126) or 7.7 months (range, 0–35), respectively. All patients showed MR4.5 at the time of entry into the study, and the median time to MR4.5 on TKI therapy was 48.1 months (range, 6–128). Among 96 patients in the safety analysis set, 70 (73.7%) achieved the MR4.5 by prior imatinib treatment and 25 (26.3%) by prior nilotinib treatment. Comparing patients in different subgroups based on prior TKI therapy (‘imatinib only’ versus ‘nilotinib following imatinib’), there were no significant differences except in the duration of imatinib therapy and time to MR4.5 (Online Supplementary Table S1). Of these, 40 patients in the ‘imatinib only’ group, 33 patients in the ‘nilotinib following imatinib’ group, and five patients in the ‘other’ group entered the TFR phase.
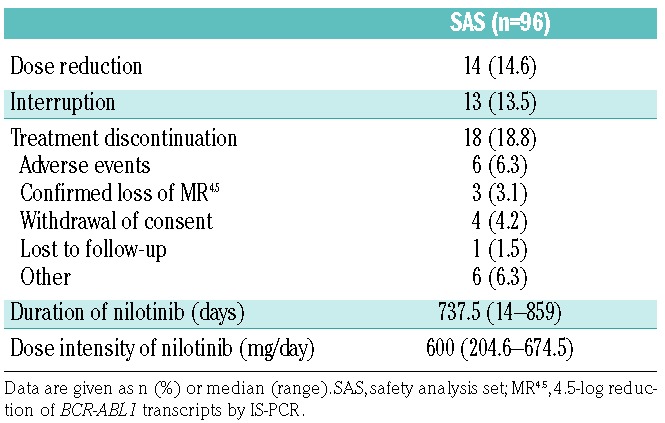
Nilotinib was taken twice daily (600 mg/day) for 2 years in the consolidation phase (median dose intensity, 600 mg/day). Patients’ outcomes at the end of the consolidation phase at 24 months are summarized in Table 2. Among the 96 patients in the safety analysis set, 18 (18.8%) discontinued the study treatment. The most frequent reason for discontinuation was adverse events; disease progression was not observed in any of the patients.
Table 2.
Patients’ outcomes and dose intensity at the end of the 2-year nilotinib consolidation phase.
Treatment-free remission after nilotinib discontinuation
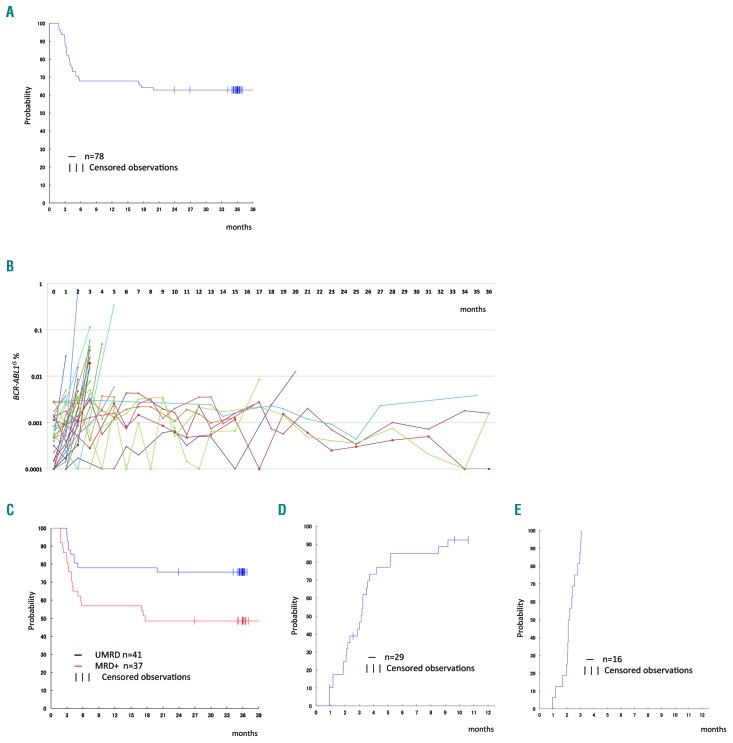
Among the 96 patients in the safety analysis set, 78 with sustained MR4.5 during the consolidation phase were eligible for nilotinib discontinuation in the TFR phase. The median follow-up of patients in the TFR phase was 35.4 months (range, 1.8–44.2). Among the 78 patients, 53 remained in the TFR phase without a confirmed loss of MR4.5 in the first 12 months; the 12-month TFR primary endpoint was 67.9% (95% CI: 56.4–78.1%), exceeding the targeted 40% success rate. The 24-month TFR rate was 62.8% (95% CI: 51.1–73.5%). The Kaplan-Meier curve for treatment-free survival is shown in Figure 1A. The estimated 3-year treatment-free survival was 62.8%. Among patients with a confirmed loss of MR4.5, 25 patients lost the MR4.5 within the first 6 months after discontinuing nilotinib (median, 3.4 months; range, 1.8–5.8 months), and the remaining four patients lost the MR4.5 between 16 and 20 months within the TFR phase (Figure 1B).
Figure 1.
Treatment-free remission after 2-year consolidation with nilotinib. (A) Kaplan-Meier estimates of treatment-free survival after discontinuation of nilotinib (n=78). (B) The kinetics of BCR-ABL1 transcripts in the treatment-free remission (TFR) phase. Twenty-five patients lost MR4.5 within 12 months and eight patients showed fluctuations in the amounts of BCR-ABL1 transcript around the MR4.5 level. Among the eight patients with fluctuations, four lost MR4.5 after 16, 16, 17, and 20 months. (C) Kaplan-Meier estimates of treatment-free survival after discontinuation of nilotinib according to the molecular response [molecular residual disease (MRD) positive or undetectable MRD (UMRD with MR5)] at enrollment in the TFR phase. UMRD with MR5 was defined as undetectable BCR-ABL1 transcripts by IS-PCR in which at least 100,000 control genes (ABL1) were required for the sensitivity of MR5. (D) Cumulative incidence of MR4.5 in patients who lost MR4.5 during the TFR phase and were subsequently readministered nilotinib (n=29). (E) Cumulative incidence of reacquisition of major molecular response (MMR) in patients who lost MMR and were readministered nilotinib (n=16).
In a subanalysis of groups based on prior TKI before entry into the STAT2 trial, the 12-month TFR rate was 62.5% (95% CI: 45.8–77.3%) in the ‘imatinib only’ group and 69.7% (95% CI: 51.3–84.4%) in the ‘nilotinib following imatinib’ group. In another subanalysis based on positivity of molecular residual disease (MRD) before the TFR phase, the 12-month TFR rate was 56.8% (95% CI: 39.5–72.9%) in the MRD group and 78.0% (95% CI: 62.4–89.4%) in the UMRD with MR5 group; the corresponding 24-month TFR rates were 48.6% (95% CI: 31.9–65.6%) and 75.6% (95% CI: 59.7–87.6%).
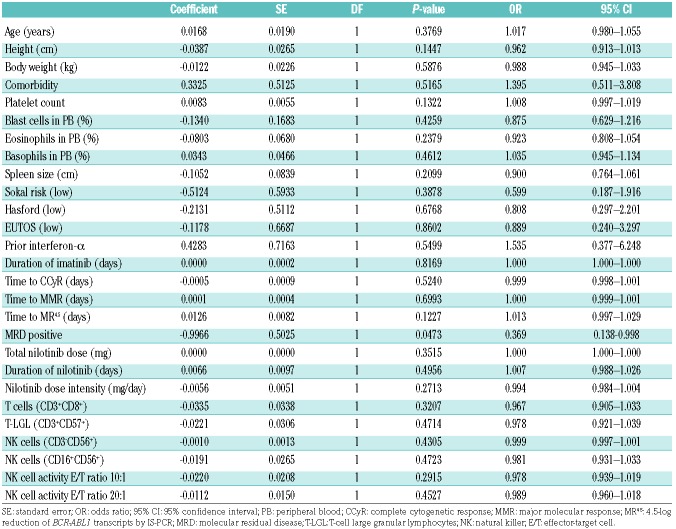
An analysis of baseline factors as predictors of TFR at 12 months was conducted. With the exception of detectable MRD before entering the TFR phase (odds ratio, 0.369; 95% CI: 0.138–0.988; P=0.0473), there were no significant predictors in the univariate logistic regression analysis, including the absolute number of natural killer cells and natural killer cell cytotoxicity (Table 3). Multivariate analysis was not, therefore, performed. On the other hand, the treatment-free survival curve was significantly better in the UMRD with MR5 group than in the MRD group (estimated 3-year treatment-free survival, 75.6 versus 48.6%; P=0.0126 by the log-rank test) (Figure 1C). There were no significant differences in the treatment-free survival curves between subgroups based on TKI treatment prior to the consolidation phase by nilotinib (‘imatinib only’ versus ‘nilotinib following imatinib’ group, P=0.9508 by the log-rank test), presence of TKI withdrawal syndrome (P=0.4096 by the log-rank test), or absolute number of natural killer cells (≥ median versus < median, P=0.4527 by the log-rank test).
Table 3.
Univariate analysis of predictive factors for treatment-free remission at 12 months.
Response to nilotinib treatment re-initiation
Nilotinib was readministered to all 29 patients with a molecular recurrence during the TFR phase. After recommencing treatment, MR4.5 rapidly returned in 25 patients (86.2%), with 50% of patients achieving MR4.5 within 3.5 months (Figure 1D). Four out of 29 patients discontinued treatment: one patient discontinued at 1 month because of a nilotinib-associated rash, one at 2.5 months following the patient’s request, and two at 10 and 11 months because of increasing BCR-ABL1IS. Despite discontinuation of the study, all patients, including the two patients with increasing BCR-ABL1IS during the re-treatment phase, achieved DMR. The clinical course details of these four patients are shown in Online Supplementary Figure S1. Among 29 patients with a molecular recurrence, 16 had lost their MMR at the first or second assessment of BCR-ABL1IS using RQ-PCR during the TFR phase. After starting treatment again, a MMR rapidly returned in all 16 patients (100%) in 3 months and 50% of patients achieved the MMR within 2 months (Figure 1E).
Safety, vascular adverse events, and tyrosine kinase inhibitor withdrawal syndrome
No patients progressed to accelerated phase or blast crisis CML, or died during this study. Adverse events (all grades) were reported in 55 patients (57.3%) in the safety analysis set in the consolidation phase, and 30 patients (38.7%) in the full analysis set in the TFR phase; the incidence of grade 3/4 adverse events was 14.6%, and 2.6% in the safety analysis set and full analysis set, respectively (Online Supplementary Table S2).
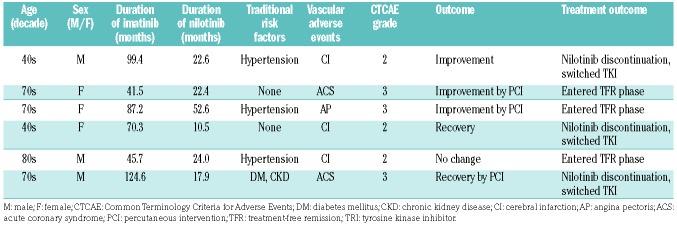
Vascular adverse events of any grade were reported in six patients (6.2%) during the nilotinib consolidation phase (Table 4). Ischemic heart disease (acute coronary syndrome or angina pectoris) was reported in three patients and cerebral infarctions in three patients, but peripheral arterial occlusive disease was not reported in any patient. Among the six patients with vascular adverse events, four had at least one traditional risk factor for such events (e.g., hypertension, hyperlipidemia, diabetes mellitus, smoking, or chronic kidney disease). Percutaneous intervention was performed in three patients with ischemic heart disease. All patients, except one, with vascular adverse events recovered or improved. Although three patients with vascular adverse events stopped nilotinib treatment and switched to another TKI, three patients continued the study treatment and entered the TFR phase, eventually achieving successful TFR. No patients developed new vascular adverse events during the TFR phase.
Table 4.
Treatment-related vascular adverse events in the consolidation phase.
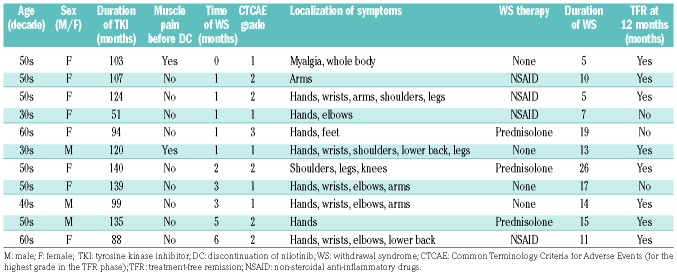
Arthralgia was reported only in the TFR phase (Online Supplementary Table S2). Eleven patients reported musculoskeletal pain events during the early phase of the TFR; these events were categorized as TKI withdrawal syndrome. The characteristics of the patients with TKI withdrawal syndrome are described in Table 5. The median time of onset of the TKI withdrawal syndrome in the TFR phase was 1 month (range, 0–6). All patients recovered completely with or without treatment. Of the 11 patients with the syndrome, eight (73%) maintained TFR at 12 months and remained in remission throughout the 36-month follow-up period. There were no significant differences in the TFR survival curves between subgroups based on TKI withdrawal syndrome.
Table 5.
Tyrosine kinase inhibitor withdrawal syndrome in the treatment-free remission phase.
Discussion
The design of the STAT2 trial resembled that of the STIM18,9 and TWISTER trials,10 with the aim of administering a TKI during a 2-year consolidation phase to obtain a sustained DMR before TKI discontinuation. In this trial, in contrast to the aforementioned studies, imatinib was replaced with nilotinib as the consolidation TKI therapy. However, the assessment of BCR-ABL1 and the definition of molecular recurrence in this trial are identical to those in the aforementioned studies.8–10 Nilotinib was administered instead of imatinib because previous studies had indicated that nilotinib could induce DMR in a greater number of patients than imatinib,1–3 thereby potentially increasing the number of patients who achieve successful TFR. The 2-year period of consolidation therapy in this study was selected as the STIM18,9 and TWISTER10 trials required 2 years of sustained DMR before stopping imatinib therapy. Additionally, our retrospective study revealed that a DMR duration of at least 2 years was a significant predictive factor for successful TFR in Japanese CML patients.14 Therefore, the treatment duration for this trial involved a consolidation phase of 2 years, and sustained DMR without loss of MR4.5 was confirmed by regular BCR-ABL1IS RQ-PCR testing for 2 years.
Among 78 patients who entered the TFR phase, 53 remained in TFR in the first 12 months; the 12-month TFR primary endpoint was, therefore, 67.9% (95% CI: 56.4–78.1%). Although the TFR rate is higher than that in the STIM1 trial (41%; 95% CI: 29–52%), it is difficult to compare this result with those in other TFR studies with varying designs. Among 29 patients with a molecular recurrence, MR4.5 was rapidly regained in 25 patients who were readministered nilotinib, and no patients progressed to accelerated phase or blast crisis CML in this study. Thus, our findings suggest that nilotinib therapy may allow the majority of patients to achieve successful TFR after discontinuation of nilotinib; this result is comparable to those of previous TFR studies with imatinib.8–10
In this study, the identified predictive factor for successful 12-month TFR was UMRD with MR5 before discontinuation of nilotinib. In the EURO-SKI trial, there were no differences in MMR status at 6 months after treatment stop between depths of molecular response (MR4.5 versus no MR4.5).15 However, our finding suggests that a deeper MR favors successful achievement of TFR in CML patients, which is consistent with data from previous studies.16,17 Among four patients without TFR in the late TFR phase, in whom MR4.5 loss occurred at 16, 16, 17, and 20 months, three patients had MRD before entering the TFR phase. It is difficult to determine the quantity of BCR-ABL1 mRNA below MR4.5/MR5 because of the sensitivity of IS-RQ-PCR.18 However, UMRD with MR5 before TFR is one of the minimum requirements for TFR and the sustained duration of DMR might be a surrogate marker for the magnitude of the MR or the eradication of MRD during TKI treatment.19,20 Meanwhile, 1-year consolidation with either dasatinib or nilotinib, which are both second-generation TKI, was proposed in the DADI trial11 and the ENEStop study,21 respectively. Although the 1-year consolidation enabled identification of enrolled patients who had sustained MR4.5 and were eligible to stop treatment,21 it is still unknown whether 1 year of consolidation with a second-generation TKI is sufficient to achieve DMR/UMRD below MR4.5/MR5 for TFR.
Of the 96 patients in the safety analysis set, 78 achieved a sustained MR4.5 on nilotinib consolidation and entered the TFR phase, including 33 patients (42.3%) who were treated with nilotinib following imatinib prior to enrollment in STAT2. Most patients (n=29, 72.5%) were switched from imatinib to nilotinib at the patients’ request prior to the trial, primarily to obtain a deeper and more sustained MR, despite not having imatinib resistance or intolerance. Similarly, the ENESTcmr study showed that a significant minority of patients who continued with imatinib therapy did later achieve DMR.4 In the subanalysis evaluating prior TKI exposure, the 12-month TFR was almost identical between patients treated with only imatinib (62.5%) and those treated with nilotinib following imatinib (69.7%). This suggests that, regardless of the specific TKI, DMR is the first step for achieving successful TFR in patients with CML.
The proportion of natural killer cells in peripheral blood has been reported as a predictive marker, which might be related to a previously reported immuno-oncological effect.22,23 However, it was beyond the remit of this trial to identify the significance of either the activity or the proportion or natural killer cells in peripheral blood.
TKI withdrawal syndrome is the most common musculoskeletal pain-related adverse event in imatinib TFR studies, being first reported in the EURO-SKI trial.24 TKI withdrawal syndrome was detected in 11 patients in the TFR phase in this study. Rousselot et al. suggested that prolonged inhibition of c-Kit signaling by imatinib may modulate nociceptive sensitivity, and that the sudden discontinuation of imatinib may reverse this phenomenon.25 As nilotinib targets the same tyrosine kinases as imatinib, including BCR-ABL kinase and c-Kit, albeit with differing potencies, nilotinib may also result in TKI withdrawal syndrome via the same mechanisms. Although TKI withdrawal syndrome was reported as an independent predictive factor for successful TFR by a Korean group,26 there was no significant relationship between TKI withdrawal syndrome and TFR identified in this nilotinib TFR study. However, because of the limited number of events during the TFR phase, univariate analysis is definitely limited in identifying a significant relationship between TKI withdrawal syndrome and TFR. Further examination with larger numbers of patients will be necessary to identify biomarkers for successful TFR.
Although the safety of nilotinib is generally regarded as being acceptable, vascular adverse events are an important concern in nilotinib therapy. The frequency of such events in this study was similar to the frequency in the nilotinib 300 mg twice daily arm in the ENESTnd trial.3 The incidence of vascular adverse events in patients treated with nilotinib 300 mg twice daily was estimated to be 2.8 per 100 patient-years in a meta-analysis.27 In the STAT2 trial, all patients with vascular adverse events, except one, either recovered or improved with intervention or supportive care after nilotinib discontinuation. Moreover, three patients achieved TFR after the occurrence of vascular adverse events during the TFR phase of STAT2. Since four of the six patients had at least one traditional risk factor for vascular adverse events, patients should be carefully screened for risk factors prior to nilotinib administration, with appropriate treatment or supportive care of comorbidities to avoid the development of vascular adverse events. After considering the risk of vascular adverse events in patients given consolidation with nilotinib, we conclude that this therapeutic agent can be safely administered to achieve a successful TFR in CML patients.
In conclusion, although previous evidence regarding TFR after discontinuation of second-line nilotinib therapy is limited, our study suggests that a 2-year consolidation period of nilotinib therapy can safely induce higher TFR rates in patients with MR4.5. Thus, 2-year consolidation therapy with this agent may be an effective strategy for achieving TFR in large numbers of CML patients.
Supplementary Material
Acknowledgments
This study was supported by research funding from Novartis Pharmaceuticals to NT. The authors would like to thank all study participants and their families, and the study investigators at participating study sites. We also thank Professor Takuhiro Yamaguchi for some advice as a biostatician, the STAT data center (EPS, Co.) for monitoring the clinical trial, and Dr. Toshihiro Miyamoto, Dr. Yosuke Minami, and Dr. Hidetaka Niitsu for their cooperation as members of the data and safety monitoring committee.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/11/1835
References
- 1.Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9):841–851. [DOI] [PubMed] [Google Scholar]
- 2.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. [DOI] [PubMed] [Google Scholar]
- 3.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes TP, Lipton JH, Spector N, et al. Deep molecular responses achieved in patients with CML-CP who are switched to nilotinib after long-term imatinib. Blood. 2014;124(5):729–736. [DOI] [PubMed] [Google Scholar]
- 5.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128(1):17–23. [DOI] [PubMed] [Google Scholar]
- 6.Dulucq S, Mahon FX. Deep molecular responses for treatment-free remission in chronic myeloid leukemia. Cancer Med. 2016;5(9):2398–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rea D, Cayuela JM. Treatment-free remission in patients with chronic myeloid leukemia. Int J Hematol 2017. July 8 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035. [DOI] [PubMed] [Google Scholar]
- 9.Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515–522. [DOI] [PubMed] [Google Scholar]
- 10.Etienne G, Guilhot J, Rea D, et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017; 35(3):298–305. [DOI] [PubMed] [Google Scholar]
- 11.Imagawa J, Tanaka H, Okada M, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2(12):e528–535. [DOI] [PubMed] [Google Scholar]
- 12.Hochhaus A, Masszi T, Giles FJ, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7):1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguchi S, Nakaseko C, Nishiwaki K, et al. Switching to nilotinib is associated with deeper molecular responses in chronic myeloid leukemia chronic phase with major molecular responses to imatinib: STAT1 trial in Japan. Int J Hematol. 2018;108(2):176–183. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi N, Kyo T, Maeda Y, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. 2012;97(6):903–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahon F, Richter J, Guilhot J, et al. Cessation of tyrosine kinase inhibitors treatment in chronic myeloid leukemia patients with deep molecular response: results of the Euro-Ski trial. Blood. 2016;128(22):787. [Google Scholar]
- 16.Mori S, Vegge E, le Coutre P, et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am J Hematol. 2015;90(10):910–914. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi N, Tauchi T, Kitamura K, et al. Deeper molecular response is a predictive factor for treatment-free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: the JALSG-STIM213 study. Int J Hematol. 2018;107(2):185–193. [DOI] [PubMed] [Google Scholar]
- 18.Deininger M. Hematology: curing CML with imatinib–a dream come true¿ Nat Rev Clin Oncol. 2011;8(3):127–128. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi N. Predictive factors of successful treatment-free remission for patients with chronic myeloid leukemia. Rinsho Ketsueki. 2014;55(5):489–496. [PubMed] [Google Scholar]
- 20.Saussele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30(8):1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahon FX, Boquimpani C, Kim DW, et al. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann Intern Med. 2018;168(7):461–470. [DOI] [PubMed] [Google Scholar]
- 22.Mizoguchi I, Yoshimoto T, Katagiri S, et al. Sustained upregulation of effector natural killer cells in chronic myeloid leukemia after discontinuation of imatinib. Cancer Sci. 2013;104(9):1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilander M, Olsson-Strömberg U, Schlums H, et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia. 2017;31(5):1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter J, Soderlund S, Lubking A, et al. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome? J Clin Oncol. 2014;32(25):2821–2823. [DOI] [PubMed] [Google Scholar]
- 25.Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Reply to J. Richter et al. J Clin Oncol. 2014;32(25):2823–2825. [DOI] [PubMed] [Google Scholar]
- 26.Lee SE, Choi SY, Song HY, et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101(6):717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chai-Adisaksopha C, Lam W, Hillis C. Major arterial events in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a meta-analysis. Leuk Lymphoma. 2016;57(6):1300–1310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.