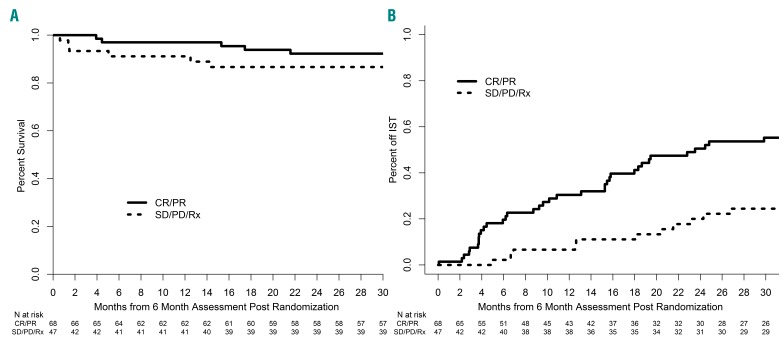
Figure 5.
Six-month landmark analysis. (A) Overall survival after the 6-month landmark with a median follow up for survivors of 30 months. The mortality rates after the 6-month landmark were similar in the complete or partial response group and stable or progressive disease group (hazard ratio, 0.71; 95% confidence interval: 0.17–2.96; P=0.63) or secondary treatment groups (hazard ratio, 0.54; 95% confidence interval: 0.13–2.26; P=0.39), respectively. (B) Cumulative incidence of discontinuation of immunosuppressive therapy after the 6-month landmark. Patients who died or were lost to follow up or experienced relapse before the landmark were excluded (n=19) and no patients ended immunosuppressive therapy before the landmark. CR/PR: patients with complete or partial response without relapse and without secondary therapy at the time of assessment (n=68). SD/PD/Rx: patients not in complete or partial response and alive without relapse and without secondary therapy at the time of assessment (n=31), and patients who had secondary therapy without relapse regardless of response before the landmark (n=16); IST: immunosuppressive therapy.