The Philadelphia chromosome (Ph; t(9;22)(q34;q11)) and the associated BCR-ABL1 gene are unique markers for chronic myeloid leukemia (CML). The chimeric gene is remarkably homogenous in CML, as nearly all patients express the P210BCRABL1 protein originated, in the majority of cases, by the e14a2 or e13a2 fusion transcripts. Nevertheless, rarer isoforms of the transcript can be found in 5% of CML cases: P190 (e1a2); P195 (e6a2); P200 (e8a2); P225 (e18a2); P230 (e19a2).1 All BCR-ABL1 isoforms encode for a constitutively active tyrosine kinase that disturbs cell proliferation, differentiation, and survival. This aberrant kinase is the target for effective therapy against CML. After the introduction of tyrosine kinase inhibitors (TKIs), such as imatinib, the annual mortality of CML has reduced significantly to less than 3% per year.2 Several reports state that different breakpoints might be associated to different prognosis, response to treatment and/or survival. For example, P190BCR-ABL1 is more common in acute lymphoblastic leukemia and results in a greater malignant potential than P210BCR-ABL1. P195BCR-ABL1 has been described in a few CML patients and linked to a poor clinical outcome due to the aggressive progression.3–7
The genetic instability of BCR-ABL1 expressing blast cells may lead to additional cytogenetic aberrations (ACAs), such as double Ph, trisomy 8, and i(17)(q10). These are known to reduce the response to imatinib and are detected in less than 5% of CML patients at diagnosis. Cytogenetic monitoring for ACAs detection during TKI treatment is mandatory due to the clonal evolution of the disease. It is hypothesized that the presence of a double BCR-ABL1 chimeric gene induces stronger kinase activity and a more active blast cells proliferation.8,9
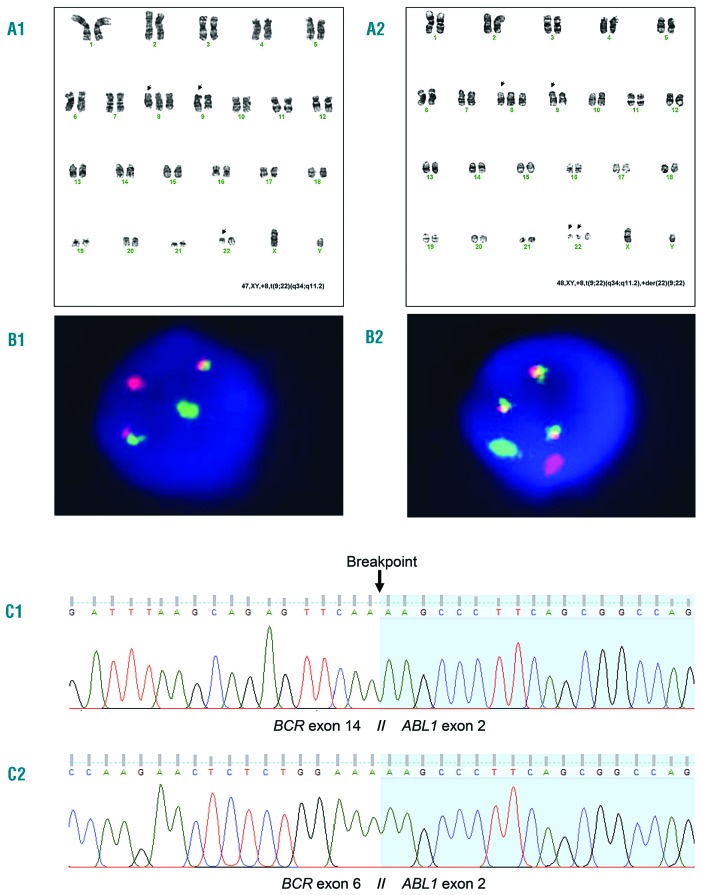
Here, we present a 36-year-old male patient without any relevant past diseases but with a one-month history of fever and night sweats. On physical examination, no hepatosplenomegaly or lymphadenopathy were found. Hemoglobin was 12.9 g/dL, white blood cell count was 58300/pL (90.8% neutrophils, 1.4% eosinophils, 0% basophils, 6.5% lymphocytes, 1.3% monocytes) and platelet count was 507 000/pL Bone marrow aspiration showed 1% blasts, and fluorescence in situ hybridization (FISH) for BCR-ABL1 was 98% positive (Table 1). Cytogenetic analysis of 20 mitotic bone marrow cells showed: four nuclei with one Ph chromosome; seven nuclei harboring one Ph chromosome and trisomy 8; and nine double Ph+ nuclei and trisomy 8 (Figure 1a and 1b). A diagnosis of chronic phase-CML was made, with a Sokal score of 0.57, Hasford 58 and EUTOS 0.
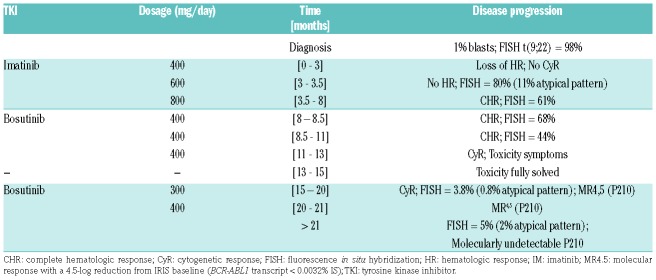
Table 1.
CML patient disease progression chart, including TKI treatment regimen, hematologic, cytogenetic and molecular levels.
Figure 1.
Cytogenetic and molecular analysis of patient’s bone marrow and/or peripheral blood. (A) Karyotype banding and (B) fluorescence in situ hybridization (FISH): chromosome G-banding showed 4 nuclei 46,XY,t(9;22)(q34.1;q11.2); 7 nuclei 47,XY,+8,t(9;22)(q34.1;q11.2) [A1, with corresponding interphase FISH image – B1]; 9 nuclei 48,XY,+8,t(9;22)(q34.1;q11.2), +der(22)t(9;22)(q34.1;q11.2) [A2, with corresponding interphase FISH image – B2]. FISH was performed using LSI BCR/ABL1 Dual Color, Dual Fusion translocation probe (Abbott Molecular, Des Plaines, IL, USA). (C) Sequence analysis of BCR-ABL1: C1) Sanger sequencing of PCR product represented in Online Supplementary Data - Figure S1 (patient). C2) Sanger sequencing of PCR product represented in Online Supplementary Data - Figure S1 (patient).
The patient initiated imatinib at 400 mg/day. Cytogenetic response (CyR) was not achieved at three months of treatment, despite an increase of the imatinib dosage to 600 mg/day for two weeks (FISH=80%) and 800 mg/day for four and half months (FISH=61%) (Table 1). Due to incomplete CyR, molecular analysis of BCR-ABL1 was performed, which linked the double Ph+ cells to two different isoforms of the gene: the typical P210 (e14a2) and the rare P195 (e6a2) (Figure 1c; Online Supplementary Data - Figures S1, S2 and S5). Except for two well-characterized SNPs, both transcripts exhibited no point mutations in the ABL1 domain (see Online Supplementary Data – Table S3 and Figures S3, S4, S6, and S7). Clinical studies have shown CML cases expressing e6a2 isoform where imatinib failed to induce a response;7 others describe the ability of first-line TKIs (imatinib, dasatinib or nilotinib) to tackle the disease in these patients.5,10–12 Even though all e6a2-expressing cases exhibited an aggressive disease course, a direct comparison between them is not always straightforward since they present distinct clinical, hematological or molecular features, such as accelerated phase disease; marked thrombocytosis; basophilia, leukopenia or eosinophilic dysplasia; der(9) deletions; or other atypical BCR-ABL1 rearrangements (e.g., ins (22;9)).5,7,10–12 Also, quantitative monitoring of rare BCR-ABL1 transcripts is not standard practice among laboratories making it more difficult to correlate all trials.5 At month eight after diagnosis, treatment was exchanged to a second-line TKI, bosutinib, whose side effects (diarrhea grade I, elevation of liver enzymes grade III and rash grade I) required dose adjustments. CyR was accomplished at month 13. At a later point, deep molecular response was achieved for P210BCR-ABL1 but the patient remained positive at cytogenetic level (FISH = 5%). Even though this patient initially presented in a chronic phase, the disease progressed to a lymphoid blastic crisis and the patient was ultimately proposed for allogeneic stem cell transplant (Table 1).
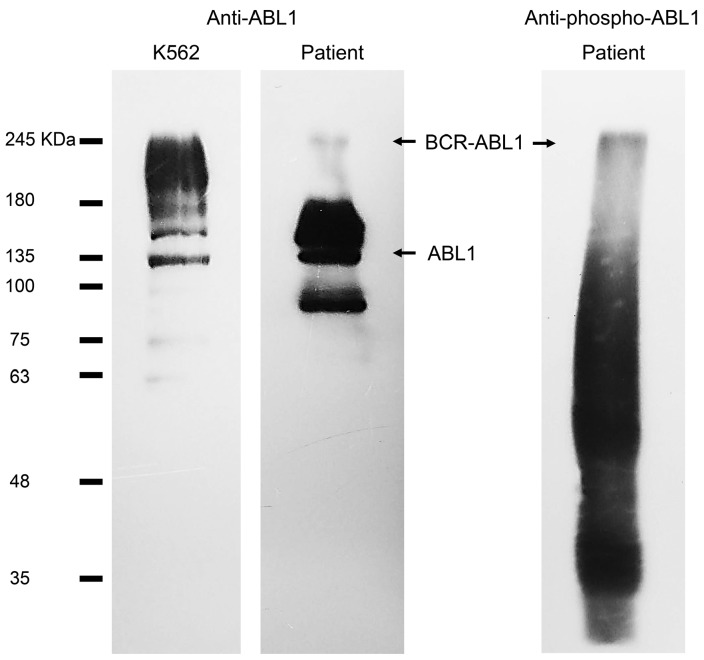
Ph duplication is usually associated with an overexpression of BCR-ABL1, and therefore associated with an aggressive clinical outcome, and regarded as a resistance factor to imatinib therapy.8,9 This chromosomal aberration, allied to the presence of an atypical short isoform of BCR-ABL1, might explain the progression of the disease, the lack of CyR to imatinib and the need for a third-generation TKI targeting the BCR-ABL1 protein.12 Indeed, shorter BCR-ABL1 transcripts have been reported to induce an aggressive clinical phenotype due to the lack of important regulatory BCR sequences. In fact, the guanine exchange factor/dbl-like domain, which mediates the communication with several Ras-proteins is crucial for the regulation of signaling pathways and processes, such as proliferation, differentiation, adhesion, apoptosis, and migration.3,13–15 Moreover, immunoblot analysis of a peripheral blood sample collected after patient deep molecular response to P210 and stable CyR to P195 under treatment with bosutinib, shows BCR-ABL1 expression at protein level and phosphorylation of ABL1 kinase domain (Figure 3), which is associated to the pathogenesis of CML. It is difficult to characterize kinase response drug treatment because regulatory phosphorylation events are largely transient changes affecting low abundance proteins, however, immunoblot results clearly indicate that bosutinib (or imatinib for that matter) was not able to abolish phosphorylation of Tyr245 on the ABL1 kinase domain, a commonly used BCR-ABL1 activation marker.
Figure 2.
Immunoblot analysis of patient’s white blood cells after attaining deep molecular response for P210 and stable CyR for P195 under treatment with bosutinib, using antibodies against ABL1 (second lane) and phosphorylated Tyr245 on ABL1 kinase domain (third lane). K562 cell lysates were used as control (first lane). Full images of each immunoblot are provided in Online Supplementary Data (Figure S8), as well as the required methodology. BCR-ABL1 = 195–210 KDa; ABL1 = 135 KDa.
This is the first report of a patient attaining deep molecular response for P210 and stable CyR for P195 under treatment with bosutinib. We conclude that this CML scenario is associated with an inferior outcome to therapy with first-generation TKI and that this subset of patients need to be identified as high-risk patients and monitored closely for efficacy during chemotherapy. This study highlights the importance of transcript type identification, particularly in those cases where more than one transcript co-exists, to further select the more TKI regimen for improvement of response and management in CML patients.
Supplementary Material
Footnotes
Funding: this work was supported by the Unidade de Ciências Biomoleculares Aplicadas-UCIBIO which is financed by national funds from FCT/MEC (UID/Multi/04378/2013) and co-financed by the ERDF under the PT2020 Partnership Agreement (POCI-01-0145-FEDER-007728). RV acknowledges PD/BD/52211/2013 and Inn-INDIGO/0002/2015.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Vinhas R, Cordeiro M, Pedrosa P, Fernandes AR, Baptista PV. Current trends in molecular diagnostics of chronic myeloid leukemia. Leuk Lymphoma. 2017;58(8):1791–1804. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H, O’Brien S, Jabbour E, et al. Improved survival in chron ic myeloid leukemia since the introduction of imatinib therapy: A single-institution historical experience. Blood. 2012;119(9):1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres F, Ivanova-Dragoeva A, Pereira M, et al. An e6a2 BCR-ABL fusion transcript in a CML patient having an iliac chloroma at initial presentation. Leuk Lymphoma. 2007;48(5):1034–1037. [DOI] [PubMed] [Google Scholar]
- 4.Beel KA, Lemmens J, Vranckx H, Maertens J, Vandenberghe P. CML with e6a2 BCR-ABL1 transcript: An aggressive entity¿ Ann Hematol. 2011;90(10):1241–1243. [DOI] [PubMed] [Google Scholar]
- 5.Zagaria A, Anelli L, Coccaro N, et al. BCR–ABL1 e6a2 transcript in chronic myeloid leukemia: biological features and molecular monitoring by droplet digital PCR. Virchows Arch. 2015;467(3):357–363. [DOI] [PubMed] [Google Scholar]
- 6.Colla S, Sammarelli G, Voltolini S, Crugnola M, Sebastio P, Giuliani N. e6a2 BCR-ABL transcript in chronic myeloid leukemia: Is it associated with aggressive disease? Haematologica. 2004;89(5):611–613. [PubMed] [Google Scholar]
- 7.Vefring HK, Gruber FXE, Wee L, et al. Chronic myelogenous leukemia with the e6a2 bcr-abl and lacking imatinib response: Presentation of two cases. Acta Haematol. 2009;122(1):11–16. [DOI] [PubMed] [Google Scholar]
- 8.Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: Long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118(26):6760–6768. [DOI] [PubMed] [Google Scholar]
- 9.Otero L, Ornellas MH, Dobbin J, De Souza Fernandez T. Double Philadelphia-chromosome: a resistance factor on the imatinib mesylate therapy for chronic myeloid leukemia. Int J Lab Hematol. 2008;30(4):346–348. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie DS, McBean M, Westerman DA, Kovalenko S, Seymour JF, Dobrovic A. Complete molecular response of e6a2 BCR-ABL positive acute myeloid leukemia to imatinib then dasatinib. Blood. 2008;111(5):2896–2898. [DOI] [PubMed] [Google Scholar]
- 11.Breccia M, Cannella L, Diverio D, et al. Isolated thrombocytosis as first sign of chronic myeloid leukemia with e6a2 BCR/ABL fusion transcript, JAK2 negativity and complete response to imatinib. Leuk Res. 2008;32(1):177–180. [DOI] [PubMed] [Google Scholar]
- 12.Langabeer SE, Crampe M, Kelly J, Fadalla K, Connaghan G, Conneally E. Nilotinib and allogeneic stem cell transplantation in a chronic myeloid leukemia patient with e6a2 and e1a2 BCR-ABL transcripts. Leuk Res. 2010;34(8):e204–5. [DOI] [PubMed] [Google Scholar]
- 13.Rohon P, Divoka M, Calabkova L, et al. Identification of e6a2 BCR-ABL fusion in a philadelphia-positive CML with marked basophilia: Implications for treatment strategy. Biomed Pap. 2011;155(2):187–190. [DOI] [PubMed] [Google Scholar]
- 14.Schultheis B, Wang L, Clark RE, Melo JV. BCR-ABL with an e6a2 fusion in a CML patient diagnosed in blast crisis. Leukemia. 2003;17(10):2054–5. [DOI] [PubMed] [Google Scholar]
- 15.Verma D, Kantarjian HM, Jones D, et al. Chronic myeloid leukemia (CML) with P190BCR-ABL: Analysis of characteristics, outcomes, and prognostic significance. Blood. 2009;114(11):2232–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.