Cancer origin and development is associated not only with genetic alterations, but also with the disturbance of epigenetic profiles.1 In this regard, the tumoral epigenome is characterized by both specific and general shifts in the DNA methylation and histone-modification landscapes.1 However, in contrast to genetic disruption, the effect of epigenetic modifications or marks may potentially be reversed by the use of drugs that target enzymes involved in adding, removing or signaling DNA methylation and histone modifications.1 This basic knowledge has been adopted into clinical practice, and inhibitors of histone deacetylases and DNA demethylating agents have been approved for use in the therapy of hematologic malignancies, such as cutaneous T-cell lymphoma and myelodysplastic syndrome, respectively.2 Other promising epigenetic drugs include inhibitors of histone methyltransferases,2 histone demethylases,2 histone kinases,3 and bromodomain proteins that interfere with the ‘reading’ of acetylated histone residues.4,5
Histone deacetylase 6 (HDAC6) is a protein modifier that is an increasingly attractive pharmacological target. It is a member of class IIb of the histone deacetylase family, together with HDAC10.6 Unlike most HDACs, HDAC6 is expressed primarily in the cytoplasm and its deacetylase activity involves mainly non-histone substrates, such as the cytoskeletal protein α-tubulin and the heatshock protein Hsp90. In this way, it plays an important role in microtubule dynamics and chaperone signaling.6 HDAC6 is involved in various human diseases, and in cancer promotes tumor initiation, development, and metastasis, having been shown to be over-expressed in various tumor types. Interestingly, the observation that the HDAC6 knock-out mouse is not lethal7 in contrast to those undergoing complete loss of class I, II and III HDACs, suggests that specific HDAC6 inhibitors may be better tolerated than pan-HDAC inhibitors or drugs that target the other HDAC classes. In this regard, the compounds tubacin, its derivative tubastatin A and ACY-1215 (Ricolinostat) are selective HDAC6 inhibitors.8 The latter agent is undergoing clinical trials as a single agent or in combination for the treatment of multiple myeloma and other tumors (clinicaltrials.gov identifiers: 01997840, 01323751, 02189343, 01583283). Given the interest in HDAC6 inhibition, we decided to design, synthesize, and test the activity of new small-molecule inhibitors of HDAC6 that could have a potential antitumor impact.
Almost all HDAC inhibitors, including those of HDAC6, share a typical structure comprising three primary regions: i) a zinc-chelating group that binds the zinc ion present at the active site, preventing activation of the enzyme (typically, a hydroxamic acid in HDAC6 inhibitors); ii) a hydrophobic linker region that mimics the 1,4-butylene alkyl chain of the lysine residue present in the natural substrates of HDACs; and iii) a filling-cap group, usually an aromatic or heteroaromatic ring, that binds to the substrate-binding region of the enzyme and fills the entrance to the hydrophobic channel. Variations in one or more of these regions may result in greater selectivity to one isoform than the others. Thus, the HDAC6 hydrophobic channel appears wider than that of other HDAC subtypes, suggesting that replacement of the traditional alkyl chain linker with bulkier and shorter aromatic moieties might enhance HDAC6 selectivity. Taking into account these HDAC6 inhibitor structures, the structural differences between HDAC6 and other HDAC isoforms (and also the structural information of our previously developed HDAC inhibitors9), a new potential HDAC6 selective inhibitor was designed and synthesized: QTX125 [3-(3-furyl)-N-{4-[(hydroxyamino) carbonyl]benzyl}-5-(4-hydroxyphenyl)- 1H-pyrrole-2-carboxamide] (Figure 1A). QTX125 was synthesized by means of a 7-step synthetic procedure, starting with an aldol condensation reaction of 4-hydrox-yacetophenone with 3-furaldehyde to obtain the corresponding α,β-unsaturated ketone. This ketone was then treated with ethyl nitroacetate and triethylamine, the mixture obtained being oxidized by means of the Nef reaction with sodium ethoxide to yield the corresponding γ-ketoester. Through a cyclization reaction of this ester in the presence of ammonium salts,10 good yields of the ethyl 3-(furan-3-yl)-5-(4-hydroxyphenyl)-1H-pyrrole-2-carboxylate were obtained. Deprotection of the methyl ester group of the pyrrole, and coupling with the methyl 4-(aminomethyl)benzoate yielded the corresponding amide. Further elaboration produced the final hydroxamic acid. It is noteworthy that, as designed, the synthesis is well-suited to kilogram-scale production.
Figure 1.
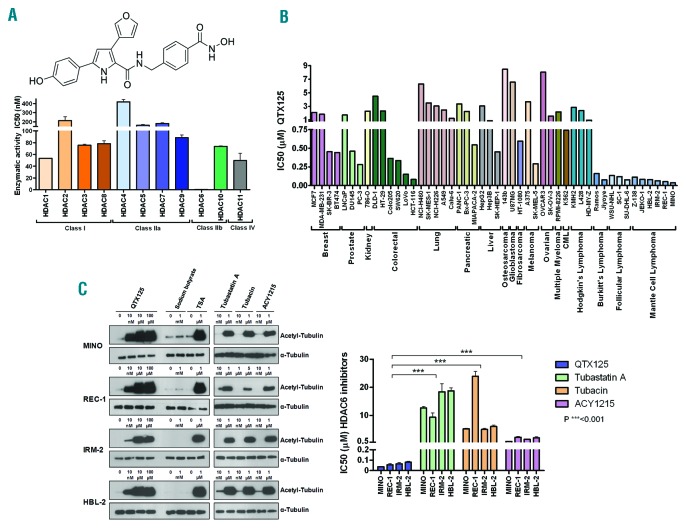
Chemical structure and HDAC specificity of QTX125 and its effect on α-tubulin acetylation and cell growth. (A) (Top) Chemical structure of QTX125. (Below) In vitro enzymatic activity of 11 HDACs upon QTX125 use. (B) Growth-inhibitory effect of QTX125 in cancer cell lines determined by the MTS assay. (C) (Left) Western-blot assays in MINO, REC-1, IRM-2 and HBL-2 cells show the induction of α-tubulin hyperacetylation upon QTX125 administration. Sodium butyrate is shown as an HDAC inhibitor that does not affect HDAC6 (negative control). TSA is shown as an HDAC inhibitor that affects all HDAC classes, including HDAC6 (positive control). The effect of the three available specific HDAC6 inhibitors (tubastatin A, tubacin, and ACY1215) is also shown. Total α-tubulin is used as a loading control. (Right) Growth-inhibitory effect of QTX125 in the mantle cell lymphoma cell lines determined by the MTS assay in comparison to the other, previously described HDAC6 inhibitors. CML: chronic myelogenous leukemia.
The selectivity of QTX125 for inhibiting HDAC6 function in comparison with all the other HDACs was measured by determining the in vitro enzymatic activity of 50 μM of each acetylated AMC-labeled peptide substrate and an optimal concentration of the corresponding enzyme (HDAC1, HDAC2, HDAC3, HDAC4, HDAC5, HDAC6, HDAC7, HDAC8, HDAC9, HDAC10 and HDAC11) (Online Supplementary Appendix). QTX125 demonstrated an exceptional specificity for inhibiting HDAC6 enzymatic activity (Figure 1A). To test the capacity of QTX125 as an antitumor agent, we first determined the 72-hour IC50 values using the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay in a panel of 48 human cancer cell lines, including solid tumors (breast, prostate, kidney, colorectal, lung, pancreas, liver, osteosarcoma, glioblastoma, fibrosarcoma, melanoma, and ovarian cancer), and hematologic malignancies [chronic myelogenous leukemia, multiple myeloma, Hodgkin lymphoma, Burkitt cell lymphoma, follicular lymphoma, and mantle cell lymphoma (MCL)] (Online Supplementary Appendix). We observed that QTX125 had the strongest growth-inhibitory effect in Burkitt cell lymphoma, follicular lymphoma, and MCL (Figure 1B).
Mantle cell lymphoma therapy constitutes an unmet medical need because there is no single accepted treatment approach for the disease.11 R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) is a common first-line therapeutic approach, but is of limited duration.12 Due to the antitumoral effect of QTX125 in MCL cell lines and its specificity in blocking HDAC6 activity that we observed here, we decided to characterize in detail QTX125 in MCL, further encouraged by the finding that HDAC6 activity is essential for MCL growth.13 The growth-inhibitory effect of QTX125 at nanomolar levels in the aforementioned MCL cell lines was confirmed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and the cell count of alive cells by trypan blue staining and by flow cytometry of AnnexinV/PI (Online Supplementary Figure S1). A further analysis of the MCL cell lines MINO, REC-1, IRM-2 and HBL-2 also showed that the use of QTX125 induced dose-dependent hyperacetylation of α-tubulin (Figure 1C), the best known target of HDAC6, thus providing further validation that the drug targets this particular enzyme. Trichostatin A was used as a general inhibitor for HDAC classes I, IIa, IIB (including HDAC6) and IV, whereas sodium butyrate was used as an HDAC inhibitor that does not affect HDAC6 (Figure 1C).14 The three available HDAC6 inhibitors (tubastatin A, tubacin, and ACY1215) were also used as specific positive controls for HDAC6-inhibition-mediated hyperacetylation of α-tubulin (Figure 1C). Importantly, QTX125 was able to induce α-tubulin acetylation even at 10 nM concentration, whereas the other HDAC6 inhibitors did not (Figure 1C). In our MCL cell lines, and with QTX125, we did not observe any increase in CD20 expression as occurs with other HDAC6 inhibitors in Burkitt and diffuse large B-cell lymphoma cell lines15 (Online Supplementary Figure S2). Strikingly, QTX125 was the most powerful inhibitor of growth in the MCL cell lines compared with the other three HDAC6 inhibitors reported (Figure 1C).
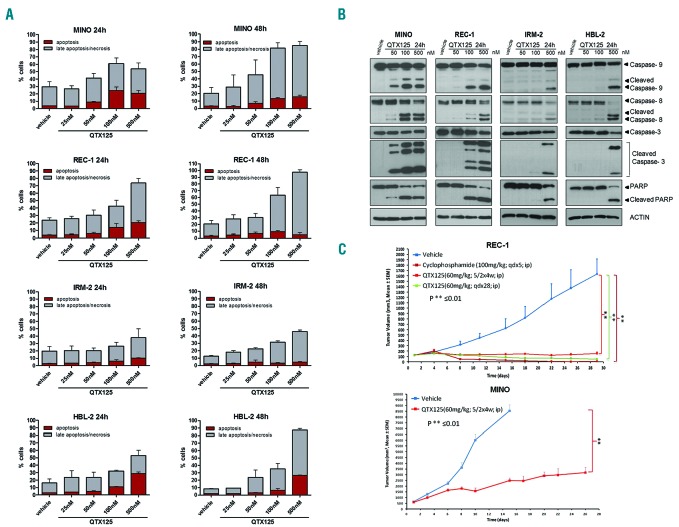
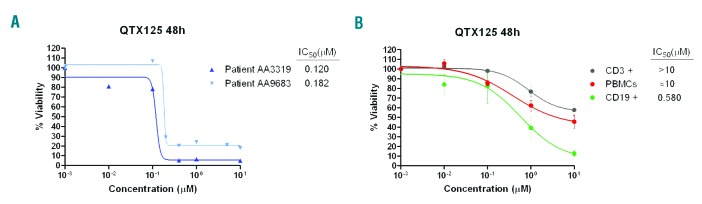
We also showed that the observed inhibition of cell proliferation upon QTX125 administration was associated with the induction of subsequent apoptosis demonstrated by annexin V/propidium iodide double staining (Figure 2A) and the cleavage of caspase-9, caspase-8, caspase-3, and PARP (Figure 2B) (Online Supplementary Appendix). Finally, we translated our experiments from the in vitro assays described above to the in vivo setting of a mouse model. The antitumor activity of QTX125 was evaluated using REC-1 cells xenografted in nude mice (Online Supplementary Appendix). We randomly selected 8 mice as the control group treated with vehicle and another 8 for QTX125 treatments (intraperitoneal administration of 60 mg/kg in two different regimens). Tumor volume was monitored every two days. The use of the HDAC6 inhibitor QTX125 was significantly associated with the inhibition of lymphoma growth in comparison to the control group (Figure 2C). The extent of blockage of tumor growth was similar to that observed in xenografted lymphomas treated with cyclophosphamide, a DNA alkylating drug commonly used in MCL therapy.11,12 Use of QTX125 was also associated with the growth inhibition of a second xenografted MCL cell line, MINO (Figure 2C). The cytotoxicity of QTX125 was also evaluated in 2 primary samples obtained from patients with MCL (see Online Supplementary Table S1 for details of patients’ samples). Incubation with QTX125 strongly reduced cell viability, with IC50 values of 0.120 and 0.182 μM. (Figure 3A). Non-malignant lymphocytes, such as peripheral blood mononuclear cells (PBMCs), CD3+ cells (T cells) and CD19+ (B cells) were more resistant to QTX125–mediated growth inhibition (Figure 3B) than MCL cell lines (Online Supplementary Figure S1) or MCL primary samples (Figure 3A).
Figure 2.
QTX125 use in mantle cell lymphoma (MCL) induces cell death by apoptosis and inhibits lymphoma growth in xenografted mice. (A) Quantification of the flow cytometry values of annexin V/PI incorporation (Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis Kit, Invitrogen) to show the proapoptotic effect of QTX125 on the MCL cell lines MINO, REC-1, IRM-2 and HBL-2. (B) Induction of programmed cell death upon QTX125 administration shown by the cleavage of caspase-9, caspase-8, caspase-3, and PARP in the western blot assay. Actin is used as a loading control. (C) Antitumoral activity of QTX125 in REC-1 and MINO xenografts in nude mice. For REC-1, tumor volume was monitored over time in vehicle and QTX125-treated (two regimens) and cyclophosphamide-treated mice (Mann–Whitney U-test). qd×5: daily dosing for 5 days; 5/2×4w: 5 days of dosing/2 days off for 4 weeks; qd×28: daily dosing for 28 days. For MINO, tumor volume was monitored over time in vehicle and and QTX125-treated mice (5/2×4w: 5 days of dosing/2 days off for 4 weeks). The vehicle group arm was stopped at 15 days for ethical reasons.
Figure 3.
QTX125 use in primary mantle cell lymphoma (MCL) samples and normal blood. (A) Effect of QTX125 in cell viability determined by flow cytometry of AnnexinV/7AAD negative cells in primary MCL samples from the patients AA3319 and AA9683. IC50 values are shown for each sample. (B) Effect of QTX125 in cell viability determined by flow cytometry of AnnexinV/7AAD negative cells in peripheral blood mononuclear cells (PBMCs), CD3+ and CD19+ cells obtained from healthy donors (n=4). IC50 values are shown for each sample.
Overall, our results show that the QTX125 compound obtained is a new HDAC6-specific inhibitor that inhibits cell-growth inhibition and causes programmed cell death in association with increased levels of acetylated α-tubulin, its most recognizable target. The antitumoral effect is particularly evident in MCL models, both in culture and in vivo, surpassing the efficacy of currently available HDAC6 inhibitors. We demonstrate, therefore, the efficacy of QTX125 in the pre-clinical setting and suggest it warrants further assessment as a novel candidate agent for use in epigenetic lymphoma therapy.
Supplementary Material
Footnotes
Funding: this work was supported by the Institute of Health Carlos III (ISCIII), co-financed by the European Development Regional Fund, ‘A way to achieve Europe’ ERDF, under I-PFIS contract no. IFI17/00006. M.P.S. was supported by a Formacion de Profesorado Universitario (FPU) fellowship from Spanish Ministery of Education.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Rodríguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med. 2011; 17(3):330–339. [DOI] [PubMed] [Google Scholar]
- 2.Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016; 17(10):630–641. [DOI] [PubMed] [Google Scholar]
- 3.Huertas D, Soler M, Moreto J, et al. Antitumor activity of a small-molecule inhibitor of the histone kinase Haspin. Oncogene. 2012; 318(11):1408–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filippakopoulos P, Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat Rev Drug Discov. 2014; 13(5):337–356. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Salvia M, Simó-Riudalbas L, Llinàs-Arias P, et al. Bromodomain inhibition shows antitumoral activity in mice and human luminal breast cancer. Oncotarget. 2017; 8(31):51621–51629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidel C, Schnekenburger M, Dicato M, Diederich M. Histone deacetylase 6 in health and disease. Epigenomics. 2015;7(1):103–118. [DOI] [PubMed] [Google Scholar]
- 7.Govindarajan N, Rao P, Burkhardt S, et al. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol Med. 2013; 5(1):52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lernoux M, Schnekenburger M, Dicato M, Diederich M. Anti-cancer effects of naturally derived compounds targeting histone deacetylase 6-related pathways. Pharmacol Res. 2018; 129:337–356 [DOI] [PubMed] [Google Scholar]
- 9.Zubia A, Ropero S, Otaegui D, et al. Identification of (1H)-pyrroles as histone deacetylase inhibitors with antitumoral activity. Oncogene 2009; 28(11):1477–1484. [DOI] [PubMed] [Google Scholar]
- 10.Aginagalde M, Bello T, Masdeu C, Vara Y, Arrieta A, Cossío FP. Formation of g−oxoacids and 1H-pyrrol-2(5H)-ones from α,β−unsaturated ketones and ethyl nitroacetate. J Org Chem. 2010; 75(21):7435–7438. [DOI] [PubMed] [Google Scholar]
- 11.Spurgeon SE, Till BG, Martin P, et al. Recommendations for clinical trial development in mantle cell lymphoma. J Natl Cancer Inst. 2016; 109(1):djw263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard OM, Gribben JG, Neuberg DS, et al. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002; 20(5):1288–1294. [DOI] [PubMed] [Google Scholar]
- 13.Lwin T, Zhao X, Cheng F, et al. A microenvironment-mediated c-Myc/miR-548m/HDAC6 amplification loop in non-Hodgkin B cell lymphomas. J Clin Invest. 2013; 123(11):4612–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Li N, Caron C, et al. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003; 22(5):1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bobrowicz M, Dwojak M, Pyrzynska B, et al. HDAC6 inhibition upregulates CD20 levels and increases the efficacy of anti-CD20 monoclonal antibodies. Blood. 2017; 130(14):1628–1638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.