We herein report the complete characterization of the monoclonal Ig fragments produced by the plasma cell clone in a 65-year-old patient with a typical heavy chain deposition disease (HCDD).1–3 The patient was referred for nephrotic syndrome and the main biological parameters are summarized in Online Supplementary Table S1. Kidney biopsy at diagnosis revealed a typical HCDD with nodular glomerulosclerosis and thickening of tubular and vascular basement membranes by a refractile ribbon-like material. HCDD was confirmed by immunofluorescence studies, showing linear γ1 heavy chain (HC) deposits along basement membranes and the absence of κ and λ light chain (LC) stainings, and later by electron microscopy with ultrastructural feature of monoclonal immunoglobulin deposition disease (MIDD) (Online Supplementary Figure S1). The patient was treated with vincristine, doxorubicin and oral dexamethasone. Overall, the treatment was well tolerated and laboratory tests conducted three months after chemotherapy showed a complete hematological and renal response (Online Supplementary Table S1). Ten years later, the patient relapsed with stage 3 multiple myeloma and died due to pulmonary infection.
We have recently published the most important series of HCDD cases,3 unraveling some pathophysiological mechanisms of the disease. However, the molecular events leading to HC deposits are still incompletely understood. Deletion of the CH1 domain is supposed to be a prerequisite for the secretion of HC in the absence of a LC since unassembled HC is normally trapped in the endoplasmic reticulum (ER) by a non-covalent association with BiP/GRP78 until its association with a LC.4 However, whether HC truncation in HCDD results from similar defect in LC production or other independent abnormalities remains to be determined. Our present study highlights the molecular events leading to the production of a monoclonal pathogenic HC in this HCDD patient.
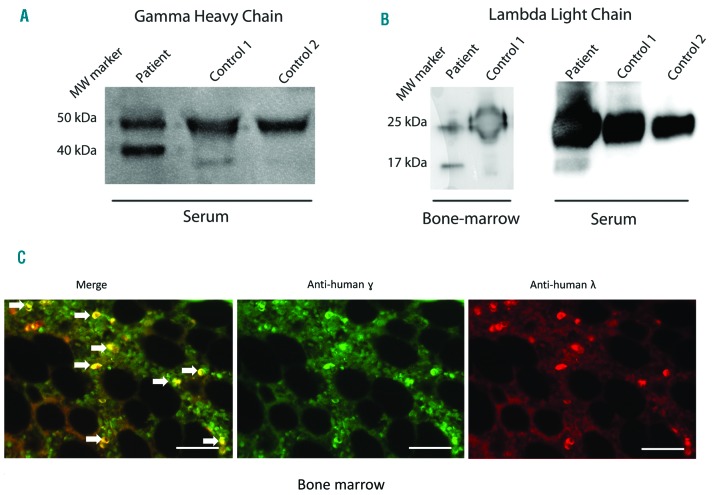
Western blot analysis carried out on serum collected at diagnosis confirmed the presence of a truncated γ1 HC corresponding to the deposited HC (Figure 1A). Despite not being detected by immunofixation in serum, a λ-type Bence-Jones protein was observed in urine. Therefore, we sought for a λLC in bone marrow protein extracts and serum by western blot. Interestingly, it revealed the presence of both a full-length λLC and a shortened λLC of approximatively 17 kDa (Figure 1B). Immunofluorescence studies carried out on medullary bone biopsy showed that both γHC and λLC are produced by the same plasma cell clone (Figure 1C).
Figure 1.
Detection of truncated γHC and λLC in bone marrow and serum. Western blot analysis of γHC (A) and λLC (B) in the serum and/or bone marrow (protein extracts) from the patient and controls. (A) The upper band (50 kDa) corresponds to the full-length γHC, the lower band (40 kDa) corresponds to the truncated γHC. (B) The upper band (25 kDa) corresponds to the full-length λLC and the lower band (around 17 kDa) corresponds to the truncated LC. The truncated LC chain is detectable in both bone marrow and serum. (C) Immunofluorescence studies of a dewaxed paraffin embedded sample of medullary bone biopsy co-stained with anti- γHC (middle) and anti-λLC (right), the merge (left) indicating that PCs are double positive for γ and λwhite arrows). Original magnification ×200, Bar = 100 μM).
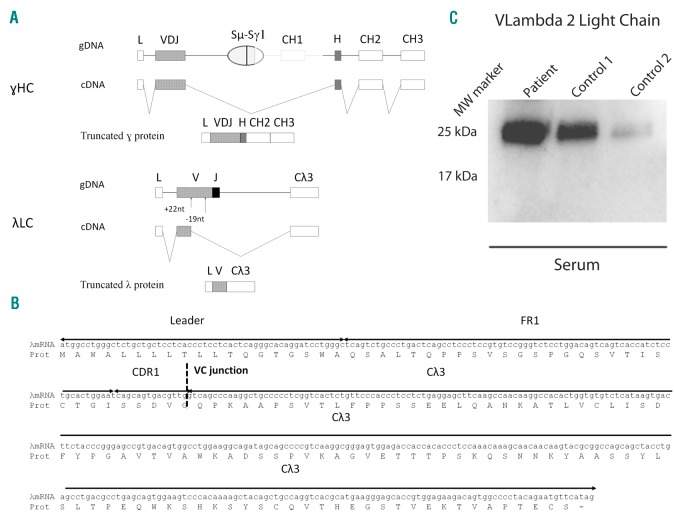
We determined the corresponding sequences from cDNAs derived from bone marrow extracts. We confirmed the presence of a monoclonal CH1-truncated HC sequence, composed of the rearranged VH3-30/DH2-15/JH4-derived germline genes (identity = 93.06 % for VH gene and 83.3 % for JH gene) directly spliced to the hinge/CH2/CH3 exons (Online Supplementary Figure 2). Further analysis of the monoclonal γ1 HC primary transcripts showed a large genomic deletion from the end of switch γ1 region to the middle of the CH1/hinge intron (Figure 2A). The PCR amplifications of λ LC cDNA confirmed the presence of a monoclonal truncated LC with a large deletion of the 3’ part of the variable (V) domain, from the end of the CDR1 to the junction (J) region (Figure 2A). The remaining 5’ part of the V domain, derived from the germline Vλ2-18 gene, was directly spliced onto the Cλ3 exon to form an in-frame truncated λ LC (Figure 2B and Online Supplementary Figure S2). We next analyzed the λ LC primary transcripts and found two abnormalities in the V domain: a 22 pb addition in FR2 and a 19 pb deletion in FR3. The 22 pb addition corresponded to a duplication of the CDR1/FR2 upstream region leading to a frameshift with the appearance of a stop codon at the beginning of the FR2 (Online Supplementary Figure 2). Frameshift in the Vλ exon may result from somatic hypermutations as it was previously shown for HC V domains.5 When occurring in such HC V regions, a frameshift may lead to a complete decay of the transcript,6 consequently resulting in free LC secretion (as seen in ~10% of plasma cell dyscrasias) since LCs are not retained in the ER in the absence of HC pairing. Alternatively, a frameshift may result in an alternative splicing leading to the production of a HC with a truncated V domain as observed in heavy chain diseases (HCD).5 In the present case, an alternative splicing in the Vλ exon using the cryptic site TG/GTAGT in CDR1 led to the skipping of the premature stop codon and the production of truncated in-frame mRNA λ LC (Online Supplementary Figure S2). Since most of the Vλ domain is missing in the truncated LC, we performed a western blot using an anti-Vλ2 domain antibody7 which proved positive for the full length polyclonal λ LC containing V λ2 domains but failed to detect the shortened monoclonal V domain (Figure 2C).
Figure 2.
Determination of LC and HC mutations. (A) Schematic representation of the transcripts and proteins alterations observed on the γHC and λLC. (B) λmRNA sequence and the deduced amino-acid sequence. (C) Western blot analysis of the circulating λLC with an anti-Vλ2 antibody compared to the western blot carried out with an anti-λantibody. Note the absence of the 17 kDa band, corresponding to the truncated λLC detected with the anti-λLC.
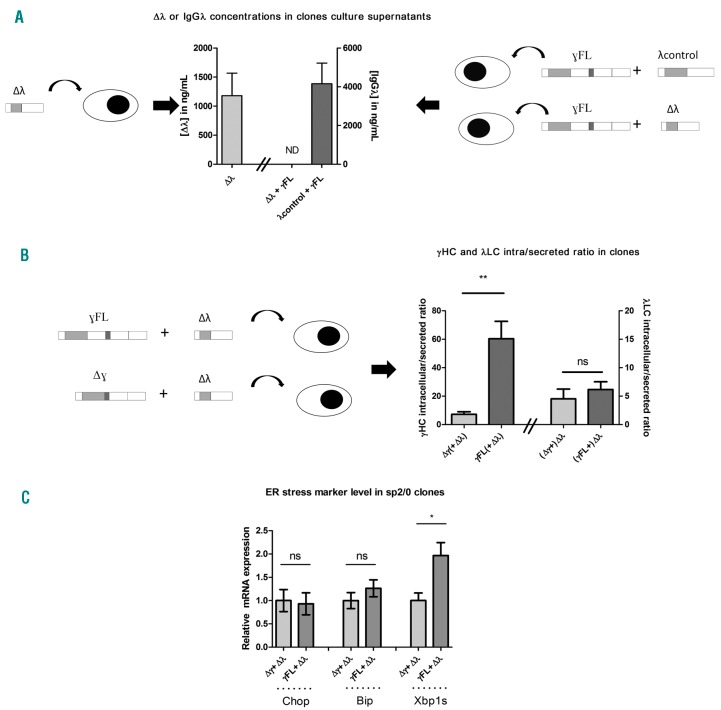
Given our findings, we decided to study the capacity of this truncated LC to associate with a HC in an in vitro model. For this purpose, we stably transfected the murine hybridoma/myeloma cell line SP2/0 with the truncated λ LC and the patient’s truncated HC or full-length HC obtained by the addition of the CH1 domain. A control full-length λ LC was also transfected with the full-length or the truncated HC. We confirmed that despite its abnormalities, the truncated λ LC can be secreted, as observed in the patient (Figure 3A). Then, to detect HC/LC association, we performed a hybrid ELISA using anti-γ HC antibodies for coating and anti-λ LC for revealing. Our results showed that the truncated LC do not associate with the full-length HC (Figure 3A). Finally, the intracellular/secreted ratio for the full-length HC in cell culture was nearly ten times higher compared to the truncated HC (Figure 3B). These results confirmed that in absence of a pairing LC, a complete HC is retained intracellulary and that CH1 domain deletion facilitates HC secretion. This result prompted us to determine if the deletion of the HC CH1 domain could benefit plasma cell fitness. We analyzed the transcriptional expression of endoplasmic reticulum stress markers Chop, Bip and Xbp1s on SP2/0 clones expressing complete or truncated HC. We found that clones expressing a solitary full-length HC present a two-fold increased rate of Xbp1s (P=0.01) compared to clones expressing the truncated one (Figure 3C). We also observed a slight but non-significant increase of Bip (P=0.3) while Chop was unchanged. As previously mentioned, in the absence of LC, the HC is retained in the ER by BiP binding to CH1 domain,4 a situation which was shown to lead to few CH1-truncated HC in a LC-deficient mouse model.8 Consequently, we suggest that in the present case, the defect of the LC could have been the first event promoting the deletion of the CH1 domain of the HC in order to avoid HC intracellular retention and alleviate reticulum stress. Recently, several studies have shown that excessive ER stress induced apoptosis of PCs,9 that inhibition of LC production by siRNA triggered apoptosis due to unpaired HC accumulation in ER and unfolded protein response10 and that truncated LC selectively inhibited plasma cell differentiation and survival.11 Consequently, we hypothesize that the CH1 deletion could act as a prosurvival event since the intracellular retention of the full-length HC would likely have caused the loss of the PC clone due to ER stress. Accordingly, the very rare occurrence of true nonsecretory plasma cell disorders likely accounts for the toxicity of solitary HCs, the loss of LC or H/L pairing being lethal for the plasma cell except in case of HC truncation.
Figure 3.
In vitro study of the truncated LC/HC mispairing and resulting higher ER stress in cells expressing a solitary complete γ HC in a SP2/0 cell line. model. (A, left) ELISA analysis of supernatants from SP2/0 cells transfected with the truncated λLC (Δλ) or (right) transfected with the full-length HC (γ FL) and the truncated λLC (Δλ) or a control λLC (λcontrol). Note the absence of association between the truncated λLC and the normal reconstituted HC (ND=non detectable). (B) Determination of the intracellular/secreted ratio of SP2/0 clones expressing the full-length (γ FL) or truncated HC (Δγ) co-transfected with the truncated λLC. The high ratio indicates that products were retained intracellularly. Note that the intracellular/secreted ratio of the truncated λLC was similar in both cases, demonstrating that the full-length HC retention is not due to a general secretory defect of the cells. (C) Quantitative expression of ER stress marker Chop, Bip and Xbp1s in a SP2/0 cell model containing a full-length HC (γ FL) or a truncated HC (Δγ) associated with the truncated λLC. Note the two-fold increased rate of Xbp1s in cells expressing the γ FL HC. Data are shown as mean ± SEM from 6 clones obtained per condition which express the highest levels of HC and/or LC. ** =P<0.01; *=P<0.05; ns: non-significant.
Truncated LC were not observed in other HCDD cases with LC characterization3 but shortened LC transcripts resulting from internal deletions of the VJ exon and/or of splicing aberrations were also found in cases of Burkitt’s lymphoma12 or in non-secreting myeloma13 and it cannot be excluded that point mutations without effects on the LC length could also impede H/L association. Our study gives an example of the relevance to monitor free LC in HCDD or other HC-related diseases since the same clone is producing both HC and LC. The intrinsic toxicity of isolated full-length HC in plasma cells also underpins the relevance of therapeutics aimed at inhibiting or altering LCs, for instance by siRNA10 or antisense oligonucleotide (AON)-induced exon skipping.11
To conclude, we hypothesize that in this patient the onset of HCDD started with the alteration of the LC, followed by the deletion of the CH1 domain of the HC. This eventually relieved ER stress and thus improved the fitness and survival of the abnormal plasma cells. Further investigations must be conducted on other cases, in order to appreciate on a larger series how often H/L mispairing occurs in HCDD and can thus be considered as the initial alterations affecting these malignant B-cell clones.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Aucouturier P, Khamlichi AA, Touchard G, et al. Heavy-chain deposition disease. N Engl J Med. 1993;329(19):1389–1393. [DOI] [PubMed] [Google Scholar]
- 2.Bridoux F, Leung N, Hutchison CA, et al. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87(4):698–711. [DOI] [PubMed] [Google Scholar]
- 3.Bridoux F, Javaugue V, Bender S, et al. Unravelling the immunopathological mechanisms of heavy chain deposition disease with implications for clinical management. Kidney Int. 2017;91(2):423–434. [DOI] [PubMed] [Google Scholar]
- 4.Feige MJ, Hendershot LM, Buchner J. How antibodies fold. Trends Biochem Sci. 2010;35(4):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goossens T, Klein U, Küppers R. Frequent occurrence of deletions and duplications during somatic hypermutation: implications for oncogene translocations and heavy chain disease. Proc Natl Acad Sci USA. 1998;95(5):2463–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tinguely A, Chemin G, Péron S, et al. Cross talk between immunoglobulin heavy-chain transcription and RNA surveillance during B cell development. Mol Cell Biol. 2012;32(1):107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davern S, Tang LX, Williams TK, et al. Immunodiagnostic capabilities of anti-free immunoglobulin light chain monoclonal antibodies. Am J Clin Pathol. 2008;130(5):702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou X, Osborn MJ, Bolland DJ, et al. Heavy chain-only antibodies are spontaneously produced in light chain-deficient mice. J Exp Med. 2007;204(13):3271–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bianchi G, Oliva L, Cascio P, et al. The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood. 2009;113(13):3040–3049. [DOI] [PubMed] [Google Scholar]
- 10.Zhou P, Ma X, Iyer L, Chaulagain C, Comenzo RL. One siRNA pool targeting the λconstant region stops λlight-chain production and causes terminal endoplasmic reticulum stress. Blood. 2014; 123(22):3440–3451. [DOI] [PubMed] [Google Scholar]
- 11.Srour N, Chemin G, Tinguely A, et al. A plasma cell differentiation quality control ablates B cell clones with biallelic Ig rearrangements and truncated Ig production. J Exp Med. 2016;213(1):109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cogné M, Mounir S, Aucouturier P, Preud’homme JL, Nau F, Guglielmi P. Immunoglobulin light chain transcripts with altered V regions in Burkitt’s lymphoma cell lines producing short mu chains. Eur J Immunol. 1990;20(9):1905–1910. [DOI] [PubMed] [Google Scholar]
- 13.Cogné M, Guglielmi P. Exon skipping without splice site mutation accounting for abnormal immunoglobulin chains in nonsecretory human myeloma. Eur J Immunol. 1993;23(6):1289–1293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.