Abstract
The ability to form endospores allows certain Gram-positive bacteria (e.g. Bacillus subtilis) to challenge the limits of microbial resistance and survival. Thus, B. subtilis is able to tolerate many environmental extremes by transitioning into a dormant state as spores, allowing survival under otherwise unfavorable conditions. Despite thorough study of spore resistance to external stresses, precisely how long B. subtilis spores can lie dormant while remaining viable, a period that potentially far exceeds the human lifespan; is not known although convincing examples of long term spore survival have been recorded. In this study, we report the first data from a 500-year microbial experiment, which started in 2014 and will finish in 2514. A set of vials containing a defined concentration of desiccated B. subtilis spores is opened and tested for viability every two years for the first 24 years and then every 25 years until experiment completion. Desiccated baseline spore samples were also exposed to environmental stresses, including X-rays, 254 nm UV-C, 10% H2O2, dry heat (120°C) and wet heat (100°C) to investigate how desiccated spores respond to harsh environmental conditions after long periods of storage. Data from the first 2 years of storage show no significant decrease in spore viability. Additionally, spores of B. subtilis were subjected to various short-term storage experiments, revealing that space-like vacuum and high NaCl concentration negatively affected spore viability.
Introduction
Microorganisms are virtually ubiquitous on Earth, capable of not only tolerating but adapting to nearly any environmental extreme. Discoveries on the vast distribution of microbial diversity have continuously challenged fundamental questions such as ‘what are the limitations of life?’ [1,2]. Our capacity to explore these questions and begin to understand the mechanisms of microbial survival are continuously improving [3,4]. One microbial survival strategy that has been the subject of thorough microbiological investigation is sporulation. It allows certain microbes including members of the Bacillus and Clostridium genera to form dormant, multi-layered endospores in response to nutrient depletion. In their inactive state, spores monitor their surrounding environment so that if conditions become favorable, they can break dormancy and resume vegetative cell growth [5]. Due to the applied importance of bacterial spores in the medical field [6–9], the food industry [10,11], and extraterrestrial environments [12–14], there has been extensive research on spore-forming bacteria.
Bacterial spores are able to endure a variety of prolonged external stresses such as desiccation, freezing, elevated temperatures in dry or wet conditions, a slew of toxic chemicals, high pressures, as well as UV and γ-radiation [10]. The extreme resistance of a spore is largely due to its multi-layer structure, consisting of (from out- to inside) the proteinaceous coat, the peptidoglycan cortex, the germ cell wall and the inner membrane, all of which surround the spore core that contains the DNA and other biomolecules. The spore coat constitutes the initial barrier to potentially harmful molecules, while the compressed inner membrane blocks the entry of many small DNA-damaging chemicals due to its low permeability [15]. The spore core’s properties also contribute to resistance to external stress agents, as it has a low water content, a high concentration of dipicolinic acid (DPA), and its DNA is saturated with α/β-type small acid-soluble spore proteins (SASPs) [16–18]. Additionally, spores contain efficient mechanisms for repair of DNA damage during revival, helping them to combat accumulated damage during the spores’ dormancy [19]. These characteristics allow spores to remain viable and outlast the majority of other organisms [20]. In fact, it has been posited that bacterial spores are among the most resistant life forms [10] and could be one of the longest-living cellular structures [21]. There are controversial reports of spores discovered in the guts of fossilized bees located in 25-million year-old amber [22], in ancient soils and aquatic sediments [23,24], and even in a 250-million year old primary salt crystal from the subterranean Salado Formation located near Carlsbad, NM [25]. Studies have also indicated that pathogenic spores (i.e. Bacillus anthracis) could potentially remain viable for extended periods of time [26–28].
In order to address current questions of spore resistance and longevity, spores of Bacillus subtilis represent a well-documented model system [20,29]. Thus, B. subtilis spores have been extensively explored as decontamination indicators in industrial settings [30–32], as well as for resistance to certain stress agents such as radiation [33], heat [34] and high salinity [35]. However, while we know that B. subtilis spores can remain in their dormant state for many years [10], the true longevity and limits of viable B. subtilis spores are not well understood. Further, to our knowledge, no systematic studies of spore desiccation resistance have been performed for an extended timescale. Studies of this nature also aid our understanding of what cellular components are most prone to degradation, presenting the opportunity to engineer microbes with greater capability to survive long-term stresses with applications to drug storage and storage during space exploration.
The 500-Year Microbial Experiment [36,37] is designed to address the following fundamental questions. (1) How long can a lifeform (e.g. B. subtilis spores) survive storage in extreme desiccation? (2) How are spores’ revival kinetics affected by this long-term storage? (3) What mathematical function describes the rate of spore death over long periods? The opportunity to carry out this experiment over a timescale more conducive with spore lifetimes might help us to begin answering these questions. Here, we report the first data from what will ultimately comprise a 500-year study, and as such, begin a record. By continuing to discover the limits of life, we may further understand how life has existed for over 3.5 billion years on Earth and the possibility of life existing elsewhere.
Materials and methods
Spore production and purification
All experiments were carried out with Bacillus subtilis trpC2 strain 168 (DSM 402) originally obtained from the German Collection of Microorganisms and Cell Cultures GmbH (DSMZ, Braunschweig, Germany). Spores were prepared by cultivation in double-strength liquid Schaeffer sporulation medium [38] with vigorous aeration at 37°C for 72 hours. Harvested spores were purified by washing with sterile water repeatedly followed by lysozyme and DNase I treatment for the removal of vegetative cells. Further, a heat inactivation step at 80°C for 10 min was performed to inactivate any remaining vegetative or germinating spores, and the spores were subsequently washed with sterile water and checked for purity by phase-contrast microscopy. Spore preparations were free (>99%) of vegetative cells, germinated spores and cell debris.
Storage experiment preparation
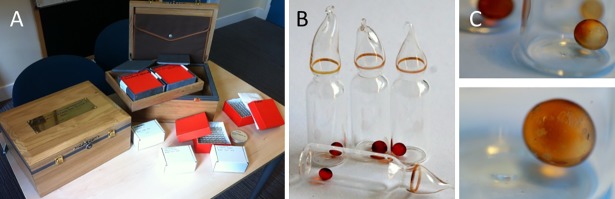
Spore samples were prepared in sealed glass vials harboring a defined amount of dried B. subtilis spores (106) with silica gel beads to maintain desiccation (Fig 1). To prepare the glass vials, 100 μL of B. subtilis spore stock solution with a concentration of 9.11 x 106 CFU/mL was pipetted into sterile glass vials with gel loading pipette tips to reduce splash onto the vial walls. The vials were then dried down in silicon bead desiccators for 1–2 weeks before being sealed. To prepare the packages for the experiment, the neck of the vial was heated, the vial lid was pulled to seal the glass vials containing the desiccated spores, and the vials were put in their respective sample containers. Each time point consisted of two sets of three separate glass vials where each set was contained in a cardboard box. The cardboard box is located in a large oak box at the University of Edinburgh with an experimental duplicate at the Natural History Museum in London, UK. To perform the 500-year experiment, a set of vials is removed from the box every two years for the first 24 years and then every 25 years for the next 475 years (S1 Fig). To achieve this, sufficient sample vials to last the study timescale (500 years) were prepared, totaling 400 vials of spore samples, and the University of Edinburgh and the Natural History Museum will arrange both sample collection and analysis for each sampling time point. Logistics for when the sampling regimen decreases to once every 25 years have not yet been determined. Each sample box contains information on sampling interval and instructions in both written and electronic form. At each 25-year time point, the researchers must copy the instructions to ensure longevity and keep instructions updated with regard to technological and linguistic development. Because preservation is of the utmost importance, paper and ink of archival quality must be utilized. The first two time points (2014 and 2016) were recorded in this study. At each time point, a set of glass vials (n = 3) were opened and dried spores were recovered and tested for viability.
Fig 1. Experimental design.
(A) The 500-Year Microbiology Experiment and its components. (B) Glass vials containing 100 μL of B. subtilis spore stock solution (106 CFU/mL) and then dried down on silicon bead desiccators before being sealed as described in Methods. (C) Silica beads to maintain desiccation.
In addition to initiating the 500-year storage experiment, smaller time scale storage experiments were conducted to investigate the impact of various environmental agents on spore longevity and viability. Spore samples were stored in different stresses including -80°C, space-like vacuum, ambient air, and anoxic conditions as well as in different media including halite, simulated Martian regolith powder, and various aqueous NaCl solutions (0, 1.2 M, and 3.6 M). Conditions for all storage experiments are described in Tables 1 and 2. All spore samples started with 107 spores, except for the aqueous NaCl incubations (pH of ~7), which started with 108 spores. For cold (-80°C), ambient air, and anoxic conditions, samples were stored as air-dried spores sealed in glass vials. Those in -80°C were monitored over the course of 360 days. Ambient air samples contained a controlled atmosphere of 80% N2 and 20% O2 and temperature of 4°C and anoxic samples maintained an atmosphere of 99.9% CO2 at 4°C. Spore viability of ambient and anoxic air samples was monitored every 2 years for ten years.
Table 1. Conditions for storage of air-dried B. subtilis spores*.
| Condition | Material additives | Amount of initial spores | Length of storage | Temperature (°C) | Atmospheric conditions |
|---|---|---|---|---|---|
| 500 year desiccation | n.a. | 106 | 2 years | 20 ± 3 | Ambient air |
| -80°C | n.a. | 107 | 360 days | -80 | Ambient air |
| Vacuum | n.a. | 107 | 450 days | 20 ± 3 | 10−7 Pa pressure |
| Controlled ambient air | n.a. | 107 | 10 years | 4 | 80% N2, 20% O2 |
| Anoxic | n.a. | 107 | 10 years | 4 | 99.9% CO2 |
| Simulated Mars regolith powder | 47.7% Na-montmorillonite, 9.9% kaolinite, 21.3% hematite, 13.0% anhydrite, 7.1% MgSO4, 1.0% NaCl, 2.5% Na2O, 3.4% MgO, 14.1% Al2O3, 34.6% SiO2, 5.1% SO3, 0.2% Cl-, 0.2% K2O, 6.1% CaO, 0.1% TiO, and 18.5% FeO | 107 | 10 years | 4 | Ambient air |
| Halite powder | 99.5% NaCl | 107 | 10 years | 4 | Ambient air |
*Unless otherwise noted, storage was performed in ambient air, 40 + /- 5% relative humidity, and normal laboratory conditions.
n.a. is defined as not-applicable
Table 2. Conditions for liquid storage of B. subtilis spores*.
| Concentration of NaCl [M] | Amount of initial spores | Length of storage (weeks) |
|---|---|---|
| 0 | 108 | 52 |
| 1.2 | 108 | 52 |
| 3.6 | 108 | 52 |
*Storage was carried out in 4°C, ambient air.
In additional experiments spanning ten years, spores were stored in two different powders. In these experiments 107 spores were to either (i) a powder composed of rock salt (halite containing 99.5% NaCl) or (ii) simulated Mars regolith powder containing a mineralogical composition according to the spectral data and information from the NASA Mars Exploration Rover Spirit and Opportunity [39] and OMEGA/Mars Express observations [40,41]. Spore-powder mixtures were air-dried, ground, and stored in reaction tubes at 4°C and monitored at two-year intervals for 10 years. For studying the effect of vacuum-induced extreme desiccation and space-like vacuum storage conditions, samples were exposed to ultrahigh vacuum produced by an ion-getter pumping system (400 liter/s; Varian SpA, Torino, Italy) that reached a final pressure of 10−7 Pa [42,43]. Samples remained at room temperature (20 ± 3°C) and were monitored over a period of 450 days. To assess spore viability over a gradient of NaCl concentrations, 108 spores were stored in 10 ml solutions of 0 M, 1.2 M, and 3.6 M NaCl in H2O over 52 weeks and checked at regular intervals (Table 2).
Live-cell imaging
To test whether individual spores recovered from the glass vials were capable of germination and outgrowth, spores were resuspended in the glass vial with 40 μL sterile water. A drop (7 μL) of this suspension was applied to a cell culture dish with a thin plastic bottom (μ-dish 35 mm, ibidi GmbH, Germany) and dried for 20 minutes at ambient room temperature. Then, spores were covered with a thin (~ 1 mm thickness) layer of 1.5% LB-agar to initiate germination and imaged at 37°C in a temperature-controlled incubation system by an automated inverted light microscope (TE2000-E Eclipse, Nikon) using phase-contrast and a NA of 1.3. Images were recorded with a digital color CCD camera (DS-2Mv, Nikon) at a resolution of 1600x1200 Pixel (12 bit) and with 5 seconds interval.
Spore resistance treatments
For baseline data, spore samples from time point zero (2014) of the 500-year study were exposed to various environmental factors that can negatively affect long-term spore survival. Measurements of spore resistance to X-rays, 254 nm UV-C, 10% H2O2, dry heat (120°C) and wet heat (100°C) were performed on 107 spores as previously described [16,32,44]. Ionizing radiation was administered to air dried spore samples in the form of X-rays (150 keV/19 mA) generated by an X-ray tube (Mueller type MG 150, MCN 165; Phillips, Hamburg, Germany) as described in Moeller et al. 2007 [44]. Dosimetry and dose calculations were performed as described previously [45]. Air-dried spore monolayers were exposed to monochromatic UV-C radiation from a low-pressure mercury lamp (NN 8/15; Heraeus, Berlin, Germany) with a major emission line at 254 nm. Heat (100°C) and oxidative (10% H2O2) stress were applied to spore samples in aqueous solution with 107 spores/mL. To expose samples to dry heat, the air-dried spore samples in monolayers were incubated at 120°C.
Recovery and evaluation of spore survival
Spores were recovered from glass vials by first sterilizing the vial exterior with ethanol and then placing a sterile Eppendorf microcentrifuge tube over the vial top to use as a lever to break the vial at the neck to open. 100 μL of buffered M9 medium was added to the vial and a small strip of ethanol sterilized parafilm was used to seal the vial. The vial was then was vortexed several times for short 10-second bursts for resuspension. With at least 106 spores per sample, we expect the spores to be uniformly deposited in a monolayer fashion thereby decreasing the possibility of spore aggregation during rehydration, which could affect measurements of spore viability. Each vial (3 replicates for each time point) was checked for colony forming ability. Colony forming units (CFU) were checked by spreading 50 μl aliquots of serial dilutions in sterile water on nutrient broth (NB) agar plates. CFU were counted after 1 day at 37°C.
Spore samples containing powdered materials were directly resuspended in 10 mL sterile distilled water, vortexed and sonicated to guarantee spore separation as described in detail by Brown et al. 2007 [46]. Spores were recovered from all air-dried storage samples by covering the samples with sterile 10% aqueous polyvinyl alcohol (PVA) solution and dried [45,47]. The PVA layer was then removed aseptically as described previously [44,48] and resuspended in 1 mL sterile distilled water, resulting in >95% recovery of the spores. This procedure has no geno- or cytotoxic effects on spore viability [47]. Spore survival was determined by standard colony formation assays; resuspended spores and spores stored in NaCl solutions were serially diluted in sterile distilled water, aliquots applied to nutrient broth agar plates (Difco, Detroit, MI) and colonies counted after incubation overnight at 37°C, all as described previously [44,48,49].
Numerical and statistical analysis
Spore survival was determined from the quotient N/N0, where N is the average CFU (colony forming units) of treated samples and N0 is the average CFU of untreated controls. The logarithm of N/N0 was plotted as a function of each treatment to obtain survival curves. Spore inactivation was expressed as the lethal dose at which 90% of the spore population is inactivated (LD90) [50]. Each experiment was performed in triplicate and all data are expressed as averages ± standard deviations. Significant differences in survival rates were calculated by single-factor analysis of variance (ANOVA), using SigmaPlot software Version 13.0 (Systat Software GmbH, Erkrath, Germany). Differences were considered statistically significant at P < 0.05.
Results
The dried and enclosed B. subtilis spores were able to germinate at the beginning of the storage experiment (baseline) as revealed by cultivation experiments and by live cell microscopy (Fig 2, S1 Movie). After two years of storage, B. subtilis spores in the 500-year experiment exhibited no significant decrease in viability– 2016 samples had an averaged surviving fraction of 86 ± 21%.
Fig 2. Live cell microscopy of germinating B. subtilis spores recovered from baseline samples in 500-year storage conditions.
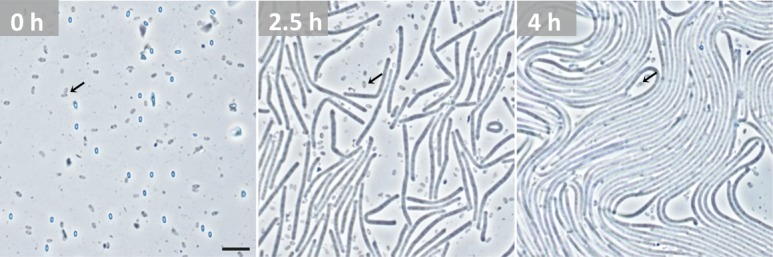
Images of three time points of germination are shown: 0, 2.5 and 4 h (see S1 Movie for an entire image sequence at 5 sec intervals). Note, that time point 0 h marks the beginning of the imaging. Activation of spores started 1–2 minutes before by adding a layer of LB-agar on top of the spores (see Materials & Methods section). A subpopulation of spores (~ 17%) was not capable of germination (arrows). Non-germinating spores appeared rather grey in phase-contrast without the bright core and darker ring-like boundary which is typical for dormant spores capable of germination (see S2 Fig). Scale bar = 5 μm.
When baseline (2014) B. subtilis spores were exposed to X-rays, UV-254 nm, 10% H2O2, wet heat and dry heat (S3, S4, S5, S6 and S7 Figs)), oxidative stress with 10% H2O2 was the most detrimental to spore populations with an LD90 value of just 9.54 ± 1.06 min (Table 3). Spores exposed to dry heat (120°C) showed greater resistance than those exposed to wet heat (100°C). In wet heat, spores reached 90% activation in one-fourth of the time (3.01 ± 0.39 min) compared to that of dry heat (14.75 ± 2.31 min), but dry heat resulted in more of a linear decrease in spore survival (S5 Fig). Radiation stress via X-ray and UV radiation was administered to air-dried spore monolayers and LD90 were 780.5 ± 62.4 Gy and 326.5 ± 29.5 J/m2, respectively. Resistance experiments were only conducted on 2014 baseline samples because 2016 desiccated spore samples did not yet show significant negative effects from storage. Table 3 displays all LD90 values from this study as well as comparisons to previously published values.
Table 3. Resistance of B. subtilis baseline spores of the 500-year experiment to various agents*.
| Treatment | Observed LD90 | Published LD90 | Reference |
|---|---|---|---|
| X-ray (Gy) | 780.5 ± 62.4 | 838.1 ± 98.0 | [44] |
| UV-254 nm (J/m2) | 326.5 ± 29.5 | 273.1 ± 52.2 | [44] |
| H2O2 (10%) (min) | 9.54 ± 1.06 | 43.7 ± 1.9 a | [50] |
| Wet heat, 100°C (min) | 3.01 ± 0.39 | 17.4 ± 3.5 b | [50] |
| Dry heat, 120°C (min) | 14.75 ± 2.31 | 19 or 4.6 ± 0.2 c | [16], [50] |
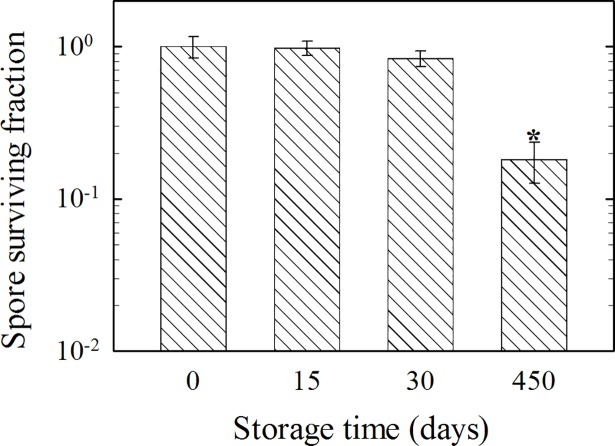
Ten-year storage experiments in dry conditions demonstrated that spore populations in ambient air (4°C), anoxic air (4°C), simulated Mars regolith powder and halite powder had no significant losses in spore viability (Table 4). Projections for LD90 were well over 300 years for each condition, ranging from 380.6 to 1789.7 years. Storage at -80°C for 360 days caused no significant decrease in B. subtilis spore viability–surviving fraction of 72.2 ± 11.8% after 360 days. However, when B. subtilis spores were stored for 450-days in ultrahigh vacuum (10−7 Pa), spore survival decreased by ~82% (p < 0.001) (Fig 3). The LD90 was estimated be to less than 2 years within these space-like conditions (Table 4).
Table 4. LD90 (90% spore inactivation) ranges for storage under different conditions*.
| Treatment | Storage time | LD90 (years)a |
|---|---|---|
| 500-year desiccation | 2 years | 29.5–45.3 |
| Halite | 10 years | 699–829.4 |
| Mars regolith | 380.6–524.2 | |
| Ambient air | 1616.9–1789.7 | |
| Anoxic air | 414.3–506.9 | |
| Vacuum (10−7 Pa) | 450 days | 1.54–1.78 |
| 0 M NaCl solution | 1 year | 38.2–45.6 |
| 1.2 M NaCl solution | n.a. | |
| 3.6 M NaCl solution | 3.1–3.7 |
*Spore storage experiments were carried out and spore survival measured all as described in Methods. The range was calculated using the standard deviation from the average of triplicate samples. However, the LD90 could not be calculated for 1.2 M NaCl solution storage because survival did not drop below 100%.
a Data obtained by extrapolation and assuming log-linearity. However, it is not known whether log linearity will be maintained over the long storage times.
n.a. denotes an uncalculated LD90
Fig 3. Spore survival during storage in space-like vacuum (10−7 Pa).
Dry spores were stored at 10−7 Pa and spore survival was determined as described in Methods. Error bars represent standard deviation. Significance by ANOVA between different incubation times is denoted by (*) with p<0.001.
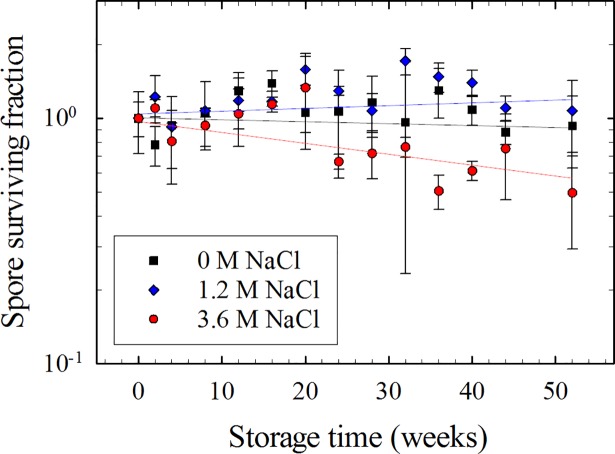
B. subtilis spores stored in 3.6 M NaCl also exhibited a loss in viability over 1 year. While spore viability in 0 M and 1.2 M NaCl did not decrease, significant differences were observed in 3.6 M NaCl (Fig 4 and S1 Table). Total spore survival decreased by about ~50% in 3.6M NaCl after one year, with a projected LD90 ranging from 3.1–3.7 years (Table 3).
Fig 4. Spore survival in various NaCl solutions.
Spores, 108 per sample, were stored in solutions with various NaCl concentrations, and spore survival was measured as described in Methods. Black squares denote spores in water (0 M NaCl); blue diamonds denote spores in 1.2 M NaCl; and red circles denote spores in 3.6 M NaCl. Error bars signify the standard deviation.
Discussion
The pursuit of understanding the limits of life, in terms of both longevity and extreme resistance, is still ongoing. B. subtilis spores, in particular, are documented to tolerate many environmental extremes and are thus useful for understanding the fundamental mechanisms of dormancy, resistance and revival. This study will address the longevity of desiccated B. subtilis spores as well as that of the photosynthetic Chroococcidiopsis sp.–performed by the Cockell laboratory. Project details have been outlined previously in [36,37].
Following two years of storage in the conditions for the 500-year experiment, there was no significant loss in spore viability. However, prolonged storage in desiccation can be damaging to cell components by causing (i) conformational changes in the lipid membranes, (ii) cross-linking within carbohydrates, proteins, and nucleic acids, or (iii) polymerization of biomolecules, all of which can negatively affect cellular functions [51]. Previous research reports that DNA protection by α/β-type SASPs and efficient mechanisms for repair of DNA damage contribute to B. subtilis spores’ ability to remain viable following exposure to extreme dryness [12]. With a reduced water content of 38 ± 7% total weight of spores within the stored glass vials, proteins are also immobilized and enzymes inactivated, which may allow for prolonged dormancy [52]. The extent to which these factors can protect and stabilize DNA to allow long periods of survival in desiccation cannot be stated this early in our study. While there are no significant differences in survivability, minor differences may lead to big differences as the 500-year storage study progresses. B. subtilis spores have survived ten-year storage in various environments with no significant decrease in spore survival (Table 4), as well as nearly six years in space vacuum (1–2% survival in monolayers) as described by Horneck et al., [51]. Upon exposure to other extreme conditions including X-ray and UV radiation, wet heat and dry heat, baseline B. subtilis spore samples demonstrated similar LD90 when compared to previously published values (Table 3). However, as storage time increases, spore resistance to these conditions may be dramatically affected.
B. subtilis spore viability appeared unaffected by storage in artificial settings such as Mars regolith and halite powders (Table 4). Conversely, storage in aqueous solutions with elevated salinity showed a significant decrease in spore survival as compared to storage in NaCl powder, suggesting that B. subtilis spores are particularly susceptible to very concentrated NaCl solutions. The detrimental effects of salinity on microbial communities [53] and specifically on B. subtilis vegetative cells [54] are well characterized. Further, recent studies indicate that high salinity can be inhibitory for B. subtilis spore germination by causing a delay in germination onset [55,56]. The LD90 for spores stored in 3.6 M NaCl was estimated at 3.4 ± 0.3 years, which is significantly less time when compared to those in halite powder (764.2 ± 65.2 years). Nonetheless, it reveals that B. subtilis spores in the short-term storage have a marginal osmoresistance, a quality that has been documented for various Bacillus spores [57].
Altogether, we hope that our study will shed light on whether spores of at least one species can realistically survive for centuries in desiccation and whether the potential compounding damage from prolonged desiccation can be combatted. Because this study is set for an extended timescale, it should be noted that spontaneous germination may occur, albeit at an extremely low frequency [58,59]. However, we expect the degree of desiccation within the glass vials to substantially reduce the likelihood of spontaneous germination of the stored spores. Importantly, with 498 more years left of this study, there is also room for project development as our capabilities for investigation advance. For example, it may become possible to sequence low amounts of nucleic acids from germinated (viable) recovered spores to ascertain what changes and mutations, if any, are occurring at the genome level as storage time increases e.g. every 25 years. In doing so, we can systematically quantify the way in which these spores die over extended timescales and understand the pathways and failures lying therein. This study may have associated implications of re-activating a microorganism that has potentially survived half a millennium of dormancy. Nevertheless, this experiment will provide valuable samples and project possibilities for the future. There is a great deal of time left for data collection, documentation and study development.
Supporting information
For the first 24 years (stage 1), spore viability tests will be performed every 2 years. For the remaining 475 years (stage 2), sampling will decrease to once every 25 years. Each sampling point is denoted by a vertical line. Dark blue signifies what data has been collected; light blue signifies data to be collected as the study continues.
(TIF)
Samples were fixed with 2.5% glutaraldehyde in HEPES buffer. A) Phase contrast light microscopy showed that spores with two morphologies are present in the baseline sample. Besides dormant spores, which showed the typical compressed ring-like morphology, grey or black spores could be detected (red arrows). Live-cell imaging demonstrated that these atypical spores did not germinate (see Fig 2). B) Scanning electron microscopy (SEM) showed that some of the spores (yellow arrow) possessed a collapsed shape and an unusual surface structure (i.e. the rucks of the coat are missing). Scale bar in A = 5 μm and in B = 200 nm.
(TIF)
The experiment was performed with baseline 500-yr storage samples in triplicate as described Methods with error bars representing the standard deviation from the average (n = 3). The loss in spore viability follows a linear (1st order) function with r2 = 0.9924.
(TIF)
The experiment was performed with baseline 500-yr storage samples in triplicate as described Methods with error bars representing the standard deviation from the average (n = 3). The loss in spore viability follows a 1st order function with r2 = 0.9817.
(TIF)
The experiment was performed with baseline 500-yr storage samples in triplicate as described Methods with error bars representing the standard deviation from the average (n = 3). The loss in spore viability follows a 1st order function with r2 = 0.9960.
(TIF)
The experiment was performed with baseline 500-yr storage samples in triplicate as described Methods with error bars representing the standard deviation from the average (n = 3). The loss in spore viability follows a 1st order function with r2 = 0.9957.
(TIF)
The experiment was performed with baseline 500-yr storage samples in triplicate as described Methods with error bars representing the standard deviation from the average (n = 3). The loss in spore viability follows a 2nd order function with r2 = 0.9441.
(TIF)
(DOCX)
Spores were deposited on ibidi-dishes from baseline 500-year storage glass vial samples, covered by LB-agar and imaged by phase-contrast microscopy at 5 sec intervals. Spores with the typical morphology (bright core with a dark ring-like boundary) germinated (switch from phase-bright to phase-dark core) and grew out. In contrast, grey spores did not change in their morphology (see also S2 Fig).
(AVI)
Acknowledgments
We thank Andrea Schröder, Marina Raguse, Toby S. Samuels, Marisa Mayer, Ellen Sirks, and Indiarose Friswell for their excellent technical assistance during the beginning of this study, and Kazimierz Madela for conducting the live cell imaging and the SEM. We express gratitude to Felix Fuchs for helpful discussion and critiques.
Data Availability
All data are included in the main manuscript and the supplemental information.
Funding Statement
N.J.U. and R.M. were supported by DLR grant DLR-FuE-Projekt ISS LIFE, Programm RF-FuW, Teilprogramm 475.
References
- 1.Rampelotto P. Extremophiles and extreme environments. Life. 2013;3: 482–485. 10.3390/life3030482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poli A, Finore I, Romano I, Gioiello A, Lama L, Nicolaus B. Microbial diversity in extreme marine habitats and their biomolecules. von Bergen M, editor. Microorganisms. MDPI; 2017;5: 1–30. 10.3390/microorganisms5020025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schloss PD, Handelsman J. Metagenomics for studying unculturable microorganisms: cutting the Gordian knot. Genome Biol. 2005;6 10.1186/gb-2005-6-8-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mojarro A, Ruvkun G, Zuber MT, Carr CE. Nucleic acid extraction from synthetic Mars analog soils for in situ life detection. Astrobiology. 2017;17: 747–760. 10.1089/ast.2016.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setlow P. Spore germination. Curr Opin Microbiol. 2003;6: 550–556. 10.1016/j.mib.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 6.Hong HA, Le HD, Cutting SM. The use of bacterial spore formers as probiotics. FEMS Microbiol Rev. 2005;29: 813–835. 10.1016/j.femsre.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Nerandzic MM, Donskey CJ. Triggering germination represents a novel strategy to enhance killing of Clostridium difficile spores. PLoS One. 2010;5: e12285 10.1371/journal.pone.0012285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards AN, Karim ST, Pascual RA, Jowhar LM, Anderson SE, McBride SM. Chemical and stress resistances of Clostridium difficile spores and vegetative cells. Front Microbiol. 2016;7: 1–13. 10.3389/fmicb.2016.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell AD. Bacterial resistance to disinfectants: present knowledge and future problems. J Hosp Infect. 1999;43: S57–S68. 10.1016/S0195-6701(99)90066-X [DOI] [PubMed] [Google Scholar]
- 10.Setlow P. The germination of spores of Bacillus species: what we know and don’t know. J Bacteriol. 2014;196: 1297–1305. 10.1128/JB.01455-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masiello SN, Kent D, Martin NH, Schukken YH, Wiedmann M, Boor KJ. Longitudinal assessment of dairy farm management practices associated with the presence of psychrotolerant Bacillales spores in bulk tank milk on 10 New York State dairy farms. J Dairy Sci. 2017; 10.3168/jds.2017-13139 [DOI] [PubMed] [Google Scholar]
- 12.Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiology Mol Biol Rev. 2000;64: 548–572. 10.1128/MMBR.64.3.548–572.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassmann M, Moeller R, Rabbow E, Panitz C, Horneck G, Reitz G, et al. Survival of spores of the UV-resistant Bacillus subtilis strain MW01 after exposure to low-Earth orbit and simulated Martian conditions: data from the space experiment ADAPT on EXPOSE-E. Astrobiology. 2012;12: 498–507. 10.1089/ast.2011.0772 [DOI] [PubMed] [Google Scholar]
- 14.Vaishampayan P a., Rabbow E, Horneck G, Venkateswaran KJ. Survival of Bacillus pumilus spores for a prolonged period of time in real space conditions. Astrobiology. 2012;12: 487–497. 10.1089/ast.2011.0738 [DOI] [PubMed] [Google Scholar]
- 15.Klobutcher L a, Ragkousi K, Setlow P. The Bacillus subtilis spore coat provides “eat resistance” during phagocytic predation by the protozoan Tetrahymena thermophila. Proc Natl Acad Sci U S A. 2006;103: 165–70. 10.1073/pnas.0507121102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popham DL, Sengupta S, Setlow P. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA-binding proteins. Appl Environ Microbiol. 1995;61: 3633–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moeller R, Schuerger AC, Reitz G, Nicholson WL. Protective role of spore structural components in determining Bacillus subtilis spore resistance to simulated Mars surface conditions. Appl Environ Microbiol. 2012;78: 8849–8853. 10.1128/AEM.02527-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setlow P. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu Rev Microbiol. 1995;49: 29–54. 10.1146/annurev.mi.49.100195.000333 [DOI] [PubMed] [Google Scholar]
- 19.Nicholson WL, Fajardo-Cavazos P, Rebeil R, Slieman TA, Riesenman PJ, Law JF, et al. Bacterial endospores and their significance in stress resistance. Antonie Van Leeuwenhoek. 2002;81: 27–32. 10.1023/A:1020561122764 [DOI] [PubMed] [Google Scholar]
- 20.Errington J. Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol. 2003;1: 117–126. 10.1038/nrmicro750 [DOI] [PubMed] [Google Scholar]
- 21.Kennedy MJ, Reader SL, Swierczynski LM. Preservation records of micro-organisms: evidence of the tenacity of life. Microbiology. 1994;140: 2513–2529. 10.1099/00221287-140-10-2513 [DOI] [PubMed] [Google Scholar]
- 22.Cano R, Borucki M. Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican amber. Science (80-). 1995;268: 1060–1064. 10.1126/science.7538699 [DOI] [PubMed] [Google Scholar]
- 23.Willerslev E, Hansen AJ, Poinar HN. Isolation of nucleic acids and cultures from fossil ice and permafrost. Trends Ecol Evol. 2004;19: 141–147. 10.1016/j.tree.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 24.Perron GG, Whyte L, Turnbaugh PJ, Goordial J, Hanage WP, Dantas G, et al. Functional characterization of bacteria isolated from ancient arctic soil exposes diverse resistance mechanisms to modern antibiotics. PLoS One. 2015;10: 1–19. 10.1371/journal.pone.0069533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vreeland RH, Rosenzweig WD, Powers DW. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature. 2000;407: 897–900. 10.1038/35038060 [DOI] [PubMed] [Google Scholar]
- 26.Wood JP, Meyer KM, Kelly TJ, Choi YW, Rogers J V., Riggs KB, et al. Environmental persistence of Bacillus anthracis and Bacillus subtilis spores. PLoS One. 2015;10: e0138083 10.1371/journal.pone.0138083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leishman ON, Johnson MJ, Labuza TP, Diez-Gonzalez F. Survival of Bacillus anthracis spores in fruit juices and wine. J Food Prot. 2010;73: 1694–1697. 10.4315/0362-028X-73.9.1694 [DOI] [PubMed] [Google Scholar]
- 28.Graham-Smith GS. The longevity of dry spores of B. anthracis. J Hyg (Lond). 1930;30: 213–215. 10.1017/S0022172400059787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro L, Losick R. Protein localization and cell fate in bacteria. Science (80-). 1997;276: 712–718. 10.1126/science.276.5313.712 [DOI] [PubMed] [Google Scholar]
- 30.Roth S, Feichtinger J, Hertel C. Characterization of Bacillus subtilis spore inactivation in low-pressure, low-temperature gas plasma sterilization processes. J Appl Microbiol. 2010;108: 521–531. 10.1111/j.1365-2672.2009.04453.x [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Jiang B, Liao X, Yi J, Hu X, Zhang Y. Inactivation of Bacillus subtilis spores by combining high-pressure thermal sterilization and ethanol. Int J Food Microbiol. 2012;160: 99–104. 10.1016/j.ijfoodmicro.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 32.Raguse M, Fiebrandt M, Stapelmann K, Madela K, Laue M, Lackmann J-W, et al. Improvement of biological indicators by using standardized Bacillus subtilis spore monolayers for the evaluation of study of enhanced spore decontamination technologies. Appl Environ Microbiol. 2016;49: AEM.03934-15. 10.1128/AEM.03934-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moeller R, Raguse M, Reitz G, Okayasu R, Li Z, Klein S, et al. Resistance of Bacillus subtilis spore DNA to lethal ionizing radiation damage relies primarily on spore core components and DNA repair, with minor effects of oxygen radical detoxification. Appl Environ Microbiol. 2014;80: 104–109. 10.1128/AEM.03136-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2006;101: 514–525. 10.1111/j.1365-2672.2005.02736.x [DOI] [PubMed] [Google Scholar]
- 35.Nagler K, Julius C, Moeller R. Germination of spores of astrobiologically relevant Bacillus species in high-salinity environments. Astrobiology. 2016;16: 500–512. 10.1089/ast.2015.1419 [DOI] [PubMed] [Google Scholar]
- 36.Cockell C. A 500-year experiment. Astron Geophys. 2015;56: 1.28–1.29. 10.1093/astrogeo/atv028 [Google Scholar]
- 37.The Cockell C. 500-year microbiology experiment. Microbiol Today. 2014; 95–96. [Google Scholar]
- 38.Schaeffer P, Millet J, Aubert JP. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci. 1965;54: 704–711. 10.1073/pnas.54.3.704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Squyres SW, Arvidson RE, Bell JF, Brückner J, Cabrol NA, Calvin W, et al. The Opportunity Rover’s Athena science investigation at Meridiani Planum, Mars. Science (80-). 2004;306: 1698–1703. 10.1126/science.1106171 [DOI] [PubMed] [Google Scholar]
- 40.Bibring J-P, Langevin Y, Gendrin A, Gondet B, Poulet F, Berthe M, et al. Mars surface diversity as revealed by the OMEGA/Mars Express observations. Science (80-). 2005;307: 1576–1581. 10.1126/science.1108806 [DOI] [PubMed] [Google Scholar]
- 41.Bibring J-P, Langevin Y, Mustard JF, Poulet F, Arvidson R, Gendrin A, et al. Global mineralogical and aqueous Mars history derived from OMEGA/Mars Express data. Science (80-). 2006;312: 400–404. 10.1126/science.1122659 [DOI] [PubMed] [Google Scholar]
- 42.Horneck G. Responses of Bacillus subtilis spores to space environment: results from experiments in space. Orig Life Evol Biosph. 1993;23: 37–52. 10.1007/BF01581989 [DOI] [PubMed] [Google Scholar]
- 43.Munakata N, Saitou M, Takahashi N, Hieda K, Morohoshi F. Induction of unique tandem-base change mutations in bacterial spores exposed to extreme dryness. Mutat Res—Genet Toxicol Environ Mutagen. 1997;390: 189–195. 10.1016/S0165-1218(97)00020-7 [DOI] [PubMed] [Google Scholar]
- 44.Moeller R, Stackebrandt E, Reitz G, Berger T, Rettberg P, Doherty AJ, et al. Role of DNA repair by nonhomologous-end joining in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV, and ionizing radiation. J Bacteriol. 2007;189: 3306–3311. 10.1128/JB.00018-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Micke U, Horneck G, Kozubek S. Double strand breaks in the DNA of Bacillus subtilis cells irradiated by heavy ions. Adv Sp Res. 1994;14: 207–211. 10.1016/0273-1177(94)90469-3 [DOI] [PubMed] [Google Scholar]
- 46.Brown GS, Betty RG, Brockmann JE, Lucero DA, Souza CA, Walsh KS, et al. Evaluation of a wipe surface sample method for collection of Bacillus spores from nonporous surfaces. Appl Environ Microbiol. 2007;73: 706–710. 10.1128/AEM.01082-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horneck G, Rettberg P, Reitz G, Wehner J, Eschweiler U, Strauch K, et al. Protection of bacterial spores in space, a contribution to the discussion on Panspermia. Orig Life Evol Biosph. 2001;31: 527–547. 10.1023/A:1012746130771 [DOI] [PubMed] [Google Scholar]
- 48.Moeller R, Horneck G, Rabbow E, Reitz G, Meyer C, Hornemann U, et al. Role of DNA protection and repair in resistance of Bacillus subtilis spores to ultrahigh shock pressures simulating hypervelocity impacts. Appl Environ Microbiol. 2008;74: 6682–6689. 10.1128/AEM.01091-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moeller R, Horneck G, Facius R, Stackebrandt E. Role of pigmentation in protecting Bacillus sp. endospores against environmental UV radiation. FEMS Microbiol Ecol. 2005;51: 231–236. 10.1016/j.femsec.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 50.Moeller R, Vlasic I, Reitz G, Nicholson WL. Role of altered rpoB alleles in Bacillus subtilis sporulation and spore resistance to heat, hydrogen peroxide, formaldehyde, and glutaraldehyde. Arch Microbiol. 2012;194: 759–767. 10.1007/s00203-012-0811-4 [DOI] [PubMed] [Google Scholar]
- 51.Horneck G, Moeller R, Cadet J, Douki T, Mancinelli RL, Nicholson WL, et al. Resistance of bacterial endospores to outer space for planetary protection purposes—experiment PROTECT of the EXPOSE-E mission. Astrobiology. 2012;12: 445–456. 10.1089/ast.2011.0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Setlow P. Mechanisms which contribute to the long‐term survival of spores of Bacillus species. J Appl Bacteriol. 1994;76: 49S–60S. 10.1111/j.1365-2672.1994.tb04357.x [DOI] [PubMed] [Google Scholar]
- 53.Yan N, Marschner P, Cao W, Zuo C, Qin W. Influence of salinity and water content on soil microorganisms. Int Soil Water Conserv Res. 2015;3: 316–323. 10.1016/j.iswcr.2015.11.003 [Google Scholar]
- 54.Bremer E. Adaptation to changing osmolanty In: Sonenshein A, Losick R, Hoch J, editors. Bacillus subtilis and Its Closest Relatives. Washington, DC: ASM Press; 2002. pp. 385–391. 10.1128/9781555817992.ch27 [Google Scholar]
- 55.Nagler K, Setlow P, Li YQ, Moeller R. High salinity alters the germination behavior of Bacillus subtilis spores with nutrient and nonnutrient germinants. Appl Environ Microbiol. 2014;80: 1314–1321. 10.1128/AEM.03293-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagler K, Moeller R. Systematic investigation of germination responses of Bacillus subtilis spores in different high-salinity environments. FEMS Microbiol Ecol. 2015;91: 1–10. 10.1093/femsec/fiv023 [DOI] [PubMed] [Google Scholar]
- 57.Tovar-Rojo F, Cabrera-Martinez RM, Setlow B, Setlow P. Studies on the mechanism of the osmoresistance of spores of Bacillus subtilis. J Appl Microbiol. 2003;95: 167–179. 10.1046/j.1365-2672.2003.01958.x [DOI] [PubMed] [Google Scholar]
- 58.Paidhungat M, Setlow P. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J Bacteriol. 2000;182: 2513–2519. 10.1128/JB.182.9.2513–2519.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ragkousi K, Eichenberger P, van Ooij C, Setlow P. Identification of a new gene essential for germination of Bacillus subtilis spores with Ca2+-dipicolinate. J Bacteriol. 2003;185: 2315–2329. 10.1128/JB.185.7.2315-2329.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For the first 24 years (stage 1), spore viability tests will be performed every 2 years. For the remaining 475 years (stage 2), sampling will decrease to once every 25 years. Each sampling point is denoted by a vertical line. Dark blue signifies what data has been collected; light blue signifies data to be collected as the study continues.
(TIF)
Samples were fixed with 2.5% glutaraldehyde in HEPES buffer. A) Phase contrast light microscopy showed that spores with two morphologies are present in the baseline sample. Besides dormant spores, which showed the typical compressed ring-like morphology, grey or black spores could be detected (red arrows). Live-cell imaging demonstrated that these atypical spores did not germinate (see Fig 2). B) Scanning electron microscopy (SEM) showed that some of the spores (yellow arrow) possessed a collapsed shape and an unusual surface structure (i.e. the rucks of the coat are missing). Scale bar in A = 5 μm and in B = 200 nm.
(TIF)
The experiment was performed with baseline 500-yr storage samples in triplicate as described Methods with error bars representing the standard deviation from the average (n = 3). The loss in spore viability follows a linear (1st order) function with r2 = 0.9924.
(TIF)
The experiment was performed with baseline 500-yr storage samples in triplicate as described Methods with error bars representing the standard deviation from the average (n = 3). The loss in spore viability follows a 1st order function with r2 = 0.9817.
(TIF)
The experiment was performed with baseline 500-yr storage samples in triplicate as described Methods with error bars representing the standard deviation from the average (n = 3). The loss in spore viability follows a 1st order function with r2 = 0.9960.
(TIF)
The experiment was performed with baseline 500-yr storage samples in triplicate as described Methods with error bars representing the standard deviation from the average (n = 3). The loss in spore viability follows a 1st order function with r2 = 0.9957.
(TIF)
The experiment was performed with baseline 500-yr storage samples in triplicate as described Methods with error bars representing the standard deviation from the average (n = 3). The loss in spore viability follows a 2nd order function with r2 = 0.9441.
(TIF)
(DOCX)
Spores were deposited on ibidi-dishes from baseline 500-year storage glass vial samples, covered by LB-agar and imaged by phase-contrast microscopy at 5 sec intervals. Spores with the typical morphology (bright core with a dark ring-like boundary) germinated (switch from phase-bright to phase-dark core) and grew out. In contrast, grey spores did not change in their morphology (see also S2 Fig).
(AVI)
Data Availability Statement
All data are included in the main manuscript and the supplemental information.