Abstract
Premature progesterone (P) elevation was commonly seen in IVF prior to the utilization of GnRH analogues for suppression of endogenous gonadotropin release. The cause and effect of premature P elevation has finally been better elucidated in the past decade. Although still occurring in 5–38% of all IVF cycles, the adverse effects of premature P elevation on pregnancy outcomes are now well known.
Keywords: progesterone, IVF, prematurely elevated progesterone, endometrium, implantation window, implantation
Introduction
Over the past several years, there has been an improved overall understanding of the adverse effect of prematurely elevated progesterone (P) on in vitro fertilization (IVF) cycles. Premature P elevation was commonly seen in IVF prior to the utilization of gonadotropin releasing hormone (GnRH) analogs for suppression of endogenous gonadotropin release [1]. The GnRH analogs were essential in pituitary suppression and prevention of a premature luteinizing hormone (LH) surge [1–4]. However, in contemporary IVF, premature P elevations still occur despite pituitary downregulation [1, 4, 5]. This occurs in 5–38% of all IVF cycles [1, 6–8]. When premature P elevation occurs, there is asynchrony between the endometrium and the embryo, resulting in decreased embryo implantation [9–15]. Although there are different cut-off values for an elevated P level described in the literature, two large retrospective cohort studies demonstrate that P levels >1.5–2 ng/mL on the day of human chorionic gonadotropin (hCG) trigger have a negative impact on pregnancy outcomes in women undergoing IVF [6, 16].
After two decades of disagreement in the literature regarding the adverse effects of premature P elevation on IVF outcomes, several recent studies have emerged providing more definitive evidence. This review summarizes the history and evolution of the literature concerning premature P elevation and what knowledge gaps still exist.
Biologic plausibility
The potential for premature luteinization was a common reality of IVF stimulation prior to the advent of GnRH analogs. As early as the 1980s, this was hypothesized to have the potential to negatively affect IVF outcomes [17, 18]. It was known that in natural reproduction, the rise of progesterone is inextricably linked to the LH surge and ovulation. This synchronizes the age of the embryo to the total time of endometrial progesterone exposure, optimizing the natural window of implantation. It was recognized that premature luteinization during IVF stimulation could lead to asynchrony. However, even after the advent of GnRH analogs effectively minimized the risk of premature luteinization, premature P elevation still occurred. Despite the occurrence of premature P elevation in clinical practice and the sound biologic plausibility for a negative effect, the systematic reviews and meta-analyses in the 1990s and 2000s failed to demonstrate a negative effect of premature P elevation. Additionally, although certain genetic defects such as 21-α-hydroxylase, 11-β-hydroxylase, and 17-α-hydroxylase deficiency cause an elevation of progesterone levels, this manuscript focuses on patients assumed to not have these genetic mutations [19].
Effect on the endometrium: scientific data
The putative negative effect of premature P elevation is embryo-endometrial asynchrony due to a prematurely advanced endometrium [5, 20]. There is scientific evidence to support this effect. Premature P elevation is associated with endometrial advancement on a histologic level [21]. Analyses of endometrial gene expression demonstrate that premature P elevation on the day of trigger leads to aberrant gene expression 7 days later, during the implantation window [13]. Furthermore, this phenomenon has been confirmed in other studies evaluating gene expression on the day of oocyte retrieval, day 2, and day 5 [13, 15, 22].
Additionally, Li et al. performed pathway analyses on microscopic RNA and microRNA genes that have been associated with poor endometrial receptivity [14, 23]. These microRNAs were evaluated in patients with premature P elevation. P is known to regulate the expression of endometrial epithelial cell osteopontin during the window of implantation to facilitate implantation [24]. Osteopontin and angiogenin expression in subjects with premature P elevation, leading to a decreased endometrial receptivity and increased risk for pregnancy loss [14].
1980s and 1990s
The earliest publications expressing concern of the effect of premature P elevation on IVF cycle outcomes date back to the late 1980s by Hamori et al. and Feldberg et al. [17, 18]. In 1991, Schoolcraft et al. demonstrated that P levels >0.5 ng/mL on the day of hCG trigger were significantly associated with lower pregnancy rates compared with <0.5 ng/mL (20% vs 54%, P < 0.005) [25]. This study proposed the idea of using serum P levels during IVF stimulation to assist with the timing of hCG triggers [25]. Similarly, Fanchin et al. found lower pregnancy rates with a P threshold over 0.9 ng/mL on the day of hCG trigger [11]. They further demonstrated that premature P elevation did not affect embryo quality, since patients above and below this threshold value had similar fertilization and cleavage rates [11, 26]. However, most of the other early studies failed to demonstrate a negative effect from premature P elevation [7–9, 27–29]. This is likely related to small sample sizes and utilization of lower thresholds to define premature P elevation (0.5–0.9 ng/mL) [7–9, 27–29]. Additionally, certain studies state an elevated P of 1.2 ng/mL and higher on the day of trigger in PCOS patients may be a predictor of IVF success [30].
2000s
During the 2000s, the majority of published studies continued to demonstrate no association of premature P elevation and IVF outcomes [29]. These studies continued to be small, with sample sizes averaging 125 subjects [9] and were at risk for type II error. They also continued to analyze premature P elevation as a dichotomous value, choosing predetermined low threshold values of 0.9–1.2 ng/mL on the day of trigger [9]. In 2007, Venetis et al. summarized the available literature in a systematic review and meta-analysis [9]. The authors concluded that there was no statistically significant association between premature P elevation on the day of hCG trigger and IVF outcomes based on the available literature, while highlighting the weaknesses in literature at that time [9].
Concurrently, the etiology of premature P elevation began to be elucidated more clearly. It was shown that premature P elevation occurred during IVF cycles despite GnRH-analog suppression. This suggested that premature luteinization was not the cause of premature P elevation. It was also established that several cycle characteristics were associated with premature P elevation, including higher stimulation doses of FSH, lower LH levels available to convert P to androgens, higher estradiol levels, and larger cohorts of follicles [1, 5, 20, 31]. Taken together, these findings suggested that premature P elevation resulted from FSH stimulation of a larger number of follicles, with progesterone being secreted as an intermediate substrate in the steroid pathway.
2010s
It was not until 2010 that large observational data sets demonstrated a negative effect of premature P elevation on the day of trigger. Bosch et al. analyzed over 4000 IVF cycles and demonstrated that a P > 1.5 ng/mL was negatively associated with clinical pregnancy [6]. It is notable that the sample size used in this study was almost six times larger than the combined world's literature summarized by Venetis et al. [9]. It was evident that even with 20 years of literature and several observational studies, the prior data were underpowered and at high risk for type II error.
Two years later, Xu et al. analyzed over 10,000 IVF cycles and also demonstrated that premature P elevation was negatively associated with IVF outcomes [32]. They proposed thresholds ranging from 1.5 to 2.25 ng/mL based on ovarian response. Two months later, Ochsenkuhn et al. published a study of 2555 subjects which again demonstrated a negative effect of premature P elevation >2.0 ng/mL on IVF outcomes [6, 16].
In a short 2-year period, this area of research was dramatically transformed. This is partially explained by more rigorous statistical analyses, which shifted the dichotomous threshold analyses from primarily 0.9 to 1.5–2.25 ng/mL. At these higher levels of premature P elevation, it became easier to demonstrate a negative effect. In addition, with more IVF programs measuring P levels on the day of trigger, the published data available for analysis substantially increased from 700 IVF cycles to over 17,000 IVF cycles in just 2 years. One year later, these data had increased to over 60,000 IVF cycles again summarized by Venetis et al. in a meta-analysis [33]. The risk of type II error was thus eliminated. Of note, these studies were almost entirely in GnRH agonist cycles using an hCG trigger [6, 16, 20, 34]. Additionally, some literature suggests that elevated P on the day of trigger in human menotrophin gonadotrophin and medroxyprogesterone acetate cycles have no impaired outcome on oocyte retrieval rates, fertilization rates, implantation rates, clinical pregnancy rates, and live birth rates [35]. Thus, while there was overwhelming evidence that premature P elevation was negatively associated with IVF outcomes, it was still unclear if this association translated to other pituitary downregulation and oocyte maturation protocols.
Association or cause?
By 2012, it was clear that premature P elevation was negatively associated with pregnancy rates with IVF [Figures 1–3] [5, 6, 16, 20, 34]. However, the question remained whether premature P elevation caused lower pregnancy rates or whether the larger body of observational data simply demonstrated a negative association, with the true cause of lower pregnancy rates yet to be elucidated. Despite the lack of randomized control trials, we argue a causal effect, supported by the current body of literature demonstrating clear biologic plausibility, substantial endometrial molecular changes, and overwhelming observational data showing a negative effect.
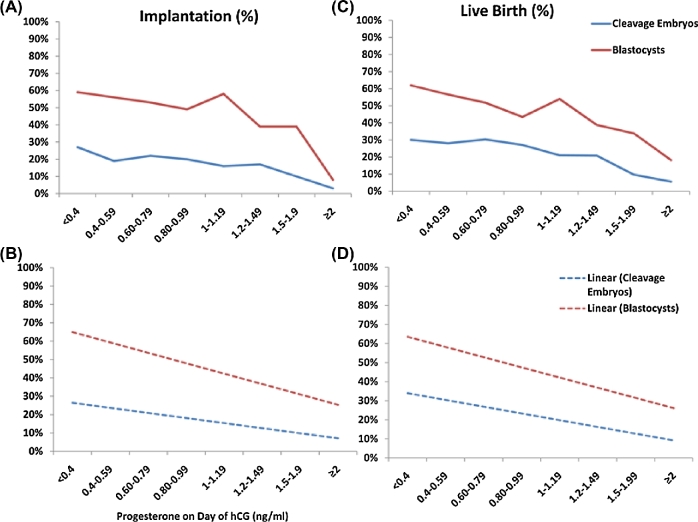
Figure 1.
Effect of serum P values on implantation and live birth in cleavage and blastocyst embryo transfers. P values (ng/mL) are plotted on the x-axis. Implantation and live birth are on the y-axis. Actual implantation (A) and live birth (C) are shown per serum P value. Linear trend lines for implantation (B) and live birth (D) are shown per serum P value. hCG = human chorionic gonadotropin; P = progesterone. Reprinted with permission from Elsevier on 02/08/2018. Hill, MJ, et al., Are good patient and embryo characteristics protective against the negative effect of elevated progesterone level on the day of oocyte maturation? Fertil Steril 2015;103(6):1477–84.e1–5 [5].
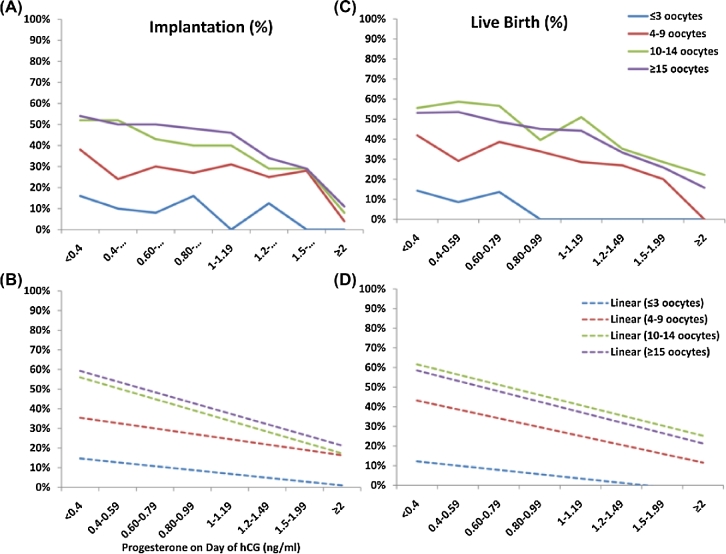
Figure 3.
Effect of serum P values on implantation and live birth based on ovarian response. P values (ng/mL) are plotted on the x-axis. Implantation and live birth are indicated on the y-axis. Actual implantation (A) and live birth (C) are shown per serum P value. Linear trend lines for implantation (B) and live birth (D) are shown per serum P value. hCG = human chorionic gonadotropin; P = progesterone. Reprinted with permission from Elsevier on 02/08/2018. Hill, MJ, et al., Are good patient and embryo characteristics protective against the negative effect of elevated progesterone level on the day of oocyte maturation? Fertil Steril 2015;103(6):1477–84.e1–5 [5].
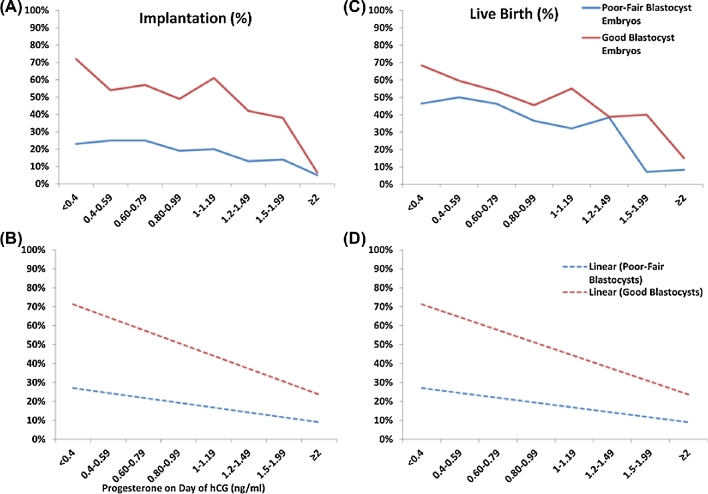
Figure 2.
Effect of serum P values on implantation and live birth in good blastocyst and fair or poor blastocyst embryo transfers. P values (ng/mL) are plotted on the x-axis. Implantation and live birth are indicated on the y-axis. Actual implantation (A) and live birth (C) are shown per serum P value. Linear trend lines for implantation (B) and live birth (D) are shown per serum P value. hCG = human chorionic gonadotropin; P = progesterone. Reprinted with permission from Elsevier on 02/08/2018. Hill, MJ, et al., Are good patient and embryo characteristics protective against the negative effect of elevated progesterone level on the day of oocyte maturation? Fertil Steril 2015;103(6):1477–84.e1–5 [5].
Premature P elevation and other in vitro fertilization protocols
The initial data demonstrating a negative effect of premature P were almost entirely in GnRH agonist cycles using hCG for oocyte maturation [6, 16, 20, 34]. Connell et al. studied 3222 IVF cycles comparing the effect of P values with hCG versus GnRH agonist oocyte maturation trigger [36]. This study concluded that premature P elevation negatively affected cycle outcomes with both trigger types, with premature P elevation resulting in live birth in only 28% in the hCG trigger group and 25% in the GnRH agonist group [36]. Furthermore, Kyrou et al. published data that demonstrated the premature P elevation had a significant detriment on ongoing pregnancy among GnRH antagonist cycles [37], later confirmed by Koo et al. [38].
Is premature P elevation detrimental to all patients?
Papanikolaou et al. published data in 482 subjects suggesting that premature P elevation had a negative impact on cleavage stage embryo transfers, but not in blastocyst transfers [20]. It was hypothesized that the endometrium could correct itself by day 5 or that a blastocyst was more robust and could tolerate the endometrial asynchrony. Researchers had also hypothesized that higher estradiol levels, younger age, advanced embryo stage, and more follicles were all protective from the negative effect of premature P elevation. This was made more intriguing by the thought that young age, estradiol, and follicle number were all positively associated with an increased risk of premature P elevation [5]. However, these good prognostic factors have proven not to be the protective. In 2015, Hill et al. demonstrated that premature P elevation had a similar negative interaction across embryo stage, embryo quality, patient age, and ovarian response [5].
Furthermore, other studies concluded that an increased ratio of progesterone to mature oocytes (P/O) was a better predictor of pregnancy outcome than the P level alone [39–41]. Contrary to this finding, Hill et al. demonstrated that the progesterone to oocyte ratio was not protective, indicating a negative effect regardless of ovarian response [42]. Finally, Hill et al. demonstrated that the negative effect of premature P elevation persisted among various ethnicities in a similar fashion, although ethnic minorities had a higher prevalence of premature P elevation [43]. Across all of these studies, interaction testing for live birth by P with each variable was statistically nonsignificant. In other words, young good responders with good embryos had a similar reduction in their odds of live birth compared to older, poor responders, with poor quality embryos. The increased embryo-endometrial asynchrony had a negative effect, independent of other predictors of IVF success.
This negative effect was primarily due to endometrial advancement from P exposure. Healy et al. investigated if this effect was further worsened if the embryo was slower to develop [44]. The authors concluded that day 5 and day 6 embryos had decreased live birth with premature P elevation, but the negative effect was twofold stronger in day 6 embryos. Interaction testing was significant, suggesting premature P elevation affected day 6 embryos more than day 5 embryos. Thus, it was demonstrated that the premature P elevation and the slow growing embryo were compounding risk factors for embryo-endometrial asynchrony [44]. Furthermore, embryo ploidy status was not evaluated in this study. While embryo-endometrial asynchrony appears to be largely attributed to prematurely elevated P, aneuploidy status of day 6 embryos likely plays a part of decreased live birth rates as well [44].
Cause of premature P elevation
Premature P elevation was initially called premature luteinization by Hamori et al. in 1987 and Feldberg et al. in 1989 [17, 18]. Prior to the advent of GnRH analogs, an endogenous LH surge leading to luteinization was a common cause of premature P elevation. Luteinization is caused by an LH surge leading to increased expression of steroidogenic activation factor, P450 side change cleavage, and 3B- hydroxysteroid dehydrogenase [45–47]. This alteration in gene expression, with concomitant increase in angiogenesis and cholesterol availability, leads to increased progesterone output from the luteinized follicle. However, even after the clinical introduction of GnRH analogs, premature P elevation still occurred despite the lack of an LH surge. Without an LH surge and luteinization, the cause of modern premature progesterone elevation is putatively not premature luteinization. However, studies as recently as 2015 still refer to premature P elevation as the historic misnomer of premature luteinization [38]. Since 2010, studies have demonstrated the cause of premature P elevation to be primarily due to an excess number of follicles present. Each of the follicles contribute a small amount of P production as an intermediary in the steroid pathway. With a few follicles, this is a minute contribution. However, with many follicles, the total level of progesterone increases to clinically relevant amounts.
The association of follicle number with P levels has been documented in numerous large studies [5, 6, 42]. Furthermore, FSH only protocols and total FSH dose increases the risk of premature P elevation [37, 38, 47]. Conversely, the addition of LH to protocols decreases the risk of premature P elevation [48]. This is due to the fact that additional LH upregulates 17-hydroxylase to convert P substrate to androgens, which are ultimately aromatized to estradiol [42]. Taken together, these data support that the common cause of premature P elevation in GnRH analog cycles is not the result of LH-induced luteinization, but rather a product of FSH induced P stimulation from a large number of follicles.
Clinical management of premature P elevation
Optimal timing of fresh embryo transfer cycles has been extensively studied [49]. The importance of transferring during the window of implantation has been linked to improved implantation rates and pregnancy rates [50, 51]. Current literature of premature P elevation supports the concern of an asynchronous relationship between the embryo age and the endometrial lining. More specifically, elevated P causes faster advancement of the endometrial lining, which can lead to an endometrium to no longer be in the window of implantation when it comes time to transfer either a cleavage stage embryo or blastocyst.
This detrimental phenomenon has been shown to be mitigated by a freeze all approach. Shapiro et al. concluded that in cycles affected by prematurely elevated P levels, vitrification of all embryos and performing a subsequent frozen embryo transfer yielded higher pregnancy rates [52]. Healy et al. [53] further investigated whether the adverse effects seen with elevated P on the day of trigger in autologous fresh IVF cycles carried over to subsequent FET cycles. Live birth rates in fresh and FET cycles with a P ≥ 2 ng/mL were compared to live birth rates in fresh and FET cycles where P was ≤2 ng/mL on day of trigger [53]. The authors demonstrated a negative association of P with live birth in fresh cycle transfers (OR 0.30; 95% CI, 0.23–0.39) but not in subsequent FET transfer cycles (OR 0.88; 95% CI 0.7–1.10) [53]. When P was ≥2 ng/mL, live birth rate was more likely in FET cycles compared with fresh cycles (47% vs. 10%; P = 0.02). These results support that the adverse effects of elevated P in the fresh cycle are ameliorated in a subsequent FET.
Another clinically relevant question is whether premature rise of P negatively affects oocyte quality and thus embryo quality or development. To evaluate this question, researchers have looked at oocyte donor cycles with premature P elevation. Current studies demonstrate that elevated P does not lead to a decline in pregnancy rates in oocyte recipients [10, 11, 20, 26, 27, 54]. This would indicate that elevated P does not affect oocyte quality intrinsically. In support of this conclusion, Venetis et al. published a systematic review and meta-analysis including over 60,000 IVF cycles concluding that a premature elevated P is not associated with oocyte quality. Premature P elevation does not seem to have an impact on donor–recipient cycle outcome or subsequent frozen-thawed embryo transfer [33].
Certain ovarian stimulation approaches should also be considered to prevent a premature rise in P. Werner et al. recommended that a gonadotropin dose with an LH-to-FSH ratio of 0.30:0.60 is associated with the lowest risk of late follicular phase P elevation. This allows adequate LH to convert progestins to androgens that are ultimately converted to estrogen, and minimize adverse IVF outcomes [48]. Additionally, cycle characteristics associated with premature P elevation that should be considered when making gonadotropin doses include higher stimulation doses of FSH, higher estradiol levels, and larger cohorts of follicles [1, 5, 20, 31].
In summary, though the literature is lacking large randomized controlled trials evaluating whether to freeze or transfer in cycles with premature elevated P, there exists strong observational evidence with large datasets that support the recommendation to offer vitrification of all embryos in a fresh cycle affected by elevated P with subsequent frozen embryo transfers.
Future directions
Great progress has been made over the past several years regarding the adverse effect of premature elevated P resulting in embryo-endometrial asynchrony. However, there remain several areas of research that require further development. First, there are no defined thresholds for premature P elevation. Venetis et al.’s meta-analysis of over 60,000 IVF cycles yielded 68 studies proposing over a dozen different threshold values [33]. To date there has been no dedicated publication exploring the appropriate P threshold value. The second issue is that commercially available steroid assays lack analytic sensitivity, especially at low hormone levels. Future developments should include investigating advanced methods of P measurement such as mass spectrometry [33]. The third future issue is that there is not good level I data demonstrating that vitrifying all embryos results in improved live birth outcomes. Hill et al. have argued that the overwhelming observational data and biologic plausibility for this course of action negate the need for RCTs, but others have disagreed and these RCT studies are underway [42]. The concern for premature P elevation was expressed 30 years ago. The cause and effect of premature P elevation has finally been better elucidated in the past decade. However, this story is not yet complete.
References
- 1. Al-Azemi M, Kyrou D, Kolibianakis EM, Humaidan P, Van Vaerenbergh I, Devroey P, Fatemi HM. Elevated progesterone during ovarian stimulation for IVF. Reprod Biomed Online 2012; 24(4):381–388. [DOI] [PubMed] [Google Scholar]
- 2. Olivennes F, Belaisch-Allart J, Emperaire JC, Dechaud H, Alvarez S, Moreau L, Nicollet B, Zorn JR, Bouchard P, Frydman R. Prospective, randomized, controlled study of in vitro fertilization-embryo transfer with a single dose of a luteinizing hormone-releasing hormone (LH-RH) antagonist (cetrorelix) or a depot formula of an LH-RH agonist (triptorelin). Fertil Steril 2000; 73(2):314–320. [DOI] [PubMed] [Google Scholar]
- 3. Westergaard LG, Erb K, Laursen SB, Rex S, Rasmussen PE. Human menopausal gonadotropin versus recombinant follicle-stimulating hormone in normogonadotropic women down-regulated with a gonadotropin-releasing hormone agonist who were undergoing in vitro fertilization and intracytoplasmic sperm injection: a prospective randomized study. Fertil Steril 2001; 76(3):543–549. [DOI] [PubMed] [Google Scholar]
- 4. Younis JS. Premature luteinization in the era of GnRH analogue protocols: time to reconsider. “Premature luteinization” in the era of GnRH analogue protocols: time to reconsider. J Assist Reprod Genet 2011; 28(8):689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hill MJ, Royster GDt, Healy MW, Richter KS, Levy G, DeCherney AH, Levens ED, Suthar G, Widra E, Levy MJ. Are good patient and embryo characteristics protective against the negative effect of elevated progesterone level on the day of oocyte maturation? Fertil Steril 2015; 103(6):1477–1484.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bosch E, Labarta E, Crespo J, Simon C, Remohi J, Jenkins J, Pellicer A. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod 2010; 25(8):2092–2100. [DOI] [PubMed] [Google Scholar]
- 7. Silverberg KM, Martin M, Olive DL, Burns WN, Schenken RS. Elevated serum progesterone levels on the day of human chorionic gonadotropin administration in in vitro fertilization cycles do not adversely affect embryo quality. Fertil Steril 1994; 61(3):508–513. [DOI] [PubMed] [Google Scholar]
- 8. Edelstein MC, Seltman HJ, Cox BJ, Robinson SM, Shaw RA, Muasher SJ. Progesterone levels on the day of human chorionic gonadotropin administration in cycles with gonadotropin-releasing hormone agonist suppression are not predictive of pregnancy outcome. Fertil Steril 1990; 54(5):853–857. [DOI] [PubMed] [Google Scholar]
- 9. Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update 2007; 13(4):343–355. [DOI] [PubMed] [Google Scholar]
- 10. Melo MA, Meseguer M, Garrido N, Bosch E, Pellicer A, Remohi J. The significance of premature luteinization in an oocyte-donation programme. Hum Reprod 2006; 21(6):1503–1507. [DOI] [PubMed] [Google Scholar]
- 11. Fanchin R, de Ziegler D, Taieb J, Hazout A, Frydman R. Premature elevation of plasma progesterone alters pregnancy rates of in vitro fertilization and embryo transfer. Fertil Steril 1993; 59(5):1090–1094. [DOI] [PubMed] [Google Scholar]
- 12. Shulman A, Ghetler Y, Beyth Y, Ben-Nun I. The significance of an early (premature) rise of plasma progesterone in in vitro fertilization cycles induced by a “long protocol” of gonadotropin releasing hormone analogue and human menopausal gonadotropins. J Assist Reprod Genet 1996; 13(3):207–211. [DOI] [PubMed] [Google Scholar]
- 13. Labarta E, Martinez-Conejero JA, Alama P, Horcajadas JA, Pellicer A, Simon C, Bosch E. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod 2011; 26(7):1813–1825. [DOI] [PubMed] [Google Scholar]
- 14. Li R, Qiao J, Wang L, Li L, Zhen X, Liu P, Zheng X. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod Biol Endocrinol 2011; 9(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Vaerenbergh I, Fatemi HM, Blockeel C, Van Lommel L, In’t Veld P, Schuit F, Kolibianakis EM, Devroey P, Bourgain C. Progesterone rise on HCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online 2011; 22(3):263–271. [DOI] [PubMed] [Google Scholar]
- 16. Ochsenkuhn R, Arzberger A, von Schonfeldt V, Gallwas J, Rogenhofer N, Crispin A, Thaler CJ, Noss U. Subtle progesterone rise on the day of human chorionic gonadotropin administration is associated with lower live birth rates in women undergoing assisted reproductive technology: a retrospective study with 2,555 fresh embryo transfers. Fertil Steril 2012; 98(2):347–354. [DOI] [PubMed] [Google Scholar]
- 17. Feldberg D, Goldman GA, Ashkenazi J, Dicker D, Shelef M, Goldman JA. The impact of high progesterone levels in the follicular phase of in vitro fertilization (IVF) cycles: a comparative study. J Assist Reprod Genet 1989; 6(1):11–14. [DOI] [PubMed] [Google Scholar]
- 18. Hamori M, Stuckensen JA, Rumpf D, Kniewald T, Kniewald A, Kurz CS. Premature luteinization of follicles during ovarian stimulation for in-vitro fertilization. Hum Reprod 1987; 2(8):639–643. [DOI] [PubMed] [Google Scholar]
- 19. Reichman DE, White PC, New MI, Rosenwaks Z. Fertility in patients with congenital adrenal hyperplasia. Fertil Steril 2014; 101(2):301–309. [DOI] [PubMed] [Google Scholar]
- 20. Papanikolaou EG, Kolibianakis EM, Pozzobon C, Tank P, Tournaye H, Bourgain C, Van Steirteghem A, Devroey P. Progesterone rise on the day of human chorionic gonadotropin administration impairs pregnancy outcome in day 3 single-embryo transfer, while has no effect on day 5 single blastocyst transfer. Fertil Steril 2009; 91(3):949–952. [DOI] [PubMed] [Google Scholar]
- 21. Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, Devroey P. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril 2002; 78(5):1025–1029. [DOI] [PubMed] [Google Scholar]
- 22. Haouzi D, Bissonnette L. Endometrial receptivity profile in patients with premature progesterone elevation on the day of HCG administration. Biomed Res Int 2014; 2014:951937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev 2004; 18(5):504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006; 147(3):1097–1121. [DOI] [PubMed] [Google Scholar]
- 25. Schoolcraft W, Sinton E, Schlenker T, Huynh D, Hamilton F, Meldrum DR. Lower pregnancy rate with premature luteinization during pituitary suppression with leuprolide acetate. Fertil Steril 1991; 55(3):563–566. [PubMed] [Google Scholar]
- 26. Fanchin R, Righini C, Olivennes F, de Ziegler D, Selva J, Frydman R. Premature progesterone elevation does not alter oocyte quality in in vitro fertilization. Fertil Steril 1996; 65(6):1178–1183. [DOI] [PubMed] [Google Scholar]
- 27. Bosch E, Valencia I, Escudero E, Crespo J, Simon C, Remohi J, Pellicer A. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril 2003; 80(6):1444–1449. [DOI] [PubMed] [Google Scholar]
- 28. Ubaldi F, Smitz J, Wisanto A, Joris H, Schiettecatte J, Derde MP, Borkham E, Van Steirteghem A, Devroey P. Oocyte and embryo quality as well as pregnancy rate in intracytoplasmic sperm injection are not affected by high follicular phase serum progesterone. Hum Reprod 1995; 10(12):3091–3096. [DOI] [PubMed] [Google Scholar]
- 29. Martinez F, Coroleu B, Clua E, Tur R, Buxaderas R, Parera N, Barri PN, Balasch J. Serum progesterone concentrations on the day of HCG administration cannot predict pregnancy in assisted reproduction cycles. Reprod Biomed Online 2004; 8(2):183–190. [DOI] [PubMed] [Google Scholar]
- 30. Doldi N, Marsiglio E, Destefani A, Gessi A, Merati G, Ferrari A. Elevated serum progesterone on the day of HCG administration in IVF is associated with a higher pregnancy rate in polycystic ovary syndrome. Hum Reprod 1999; 14(3):601–605. [DOI] [PubMed] [Google Scholar]
- 31. Fleming R, Jenkins J. The source and implications of progesterone rise during the follicular phase of assisted reproduction cycles. Reprod Biomed Online 2010; 21(4):446–449. [DOI] [PubMed] [Google Scholar]
- 32. Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, Zhu G. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril 2012; 97(6):1321–1327.e4. [DOI] [PubMed] [Google Scholar]
- 33. Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update 2013; 19(5):433–457. [DOI] [PubMed] [Google Scholar]
- 34. Lahoud R, Kwik M, Ryan J, Al-Jefout M, Foley J, Illingworth P. Elevated progesterone in GnRH agonist down regulated in vitro fertilisation (IVFICSI) cycles reduces live birth rates but not embryo quality. Arch Gynecol Obstet 2012; 285(2):535–540. [DOI] [PubMed] [Google Scholar]
- 35. Lu X, Chen Q, Fu Y, Ai A, Lyu Q, Kuang YP. Elevated progesterone on the trigger day does not impair the outcome of Human Menotrophins Gonadotrophin and Medroxyprogesterone acetate treatment cycles. Sci Rep 2016; 61:31112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Connell MT, Patounakis G, Healy MW, DeCherney AH, Devine K, Widra E, Levy MJ, Hill MJ. Is the effect of premature elevated progesterone augmented by human chorionic gonadotropin versus gonadotropin-releasing hormone agonist trigger? Fertil Steril 2016; 106(3):584–589.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kyrou D, Al-Azemi M, Papanikolaou EG, Donoso P, Tziomalos K, Devroey P, Fatemi HM. The relationship of premature progesterone rise with serum estradiol levels and number of follicles in GnRH antagonist/recombinant FSH-stimulated cycles. Eur J Obstet Gynecol Reprod Biol 2012; 162(2):165–168. [DOI] [PubMed] [Google Scholar]
- 38. Koo HS, Cha SH, Kim HO, Song IO, Min EG, Yang KM, Park CW. A high response to controlled ovarian stimulation induces premature luteinization with a negative impact on pregnancy outcomes in a gonadotropin-releasing hormone antagonist cycle. Clin Exp Reprod Med 2015; 42(4):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aflatoonian A, Davar R, Hojjat F. Elevated serum progesterone/ MII oocyte ratio on the day of human chorionic gonadotropin administration can predict impaired endometrial receptivity. Iran J Reprod Med 2014; 12(6):427–434. [PMC free article] [PubMed] [Google Scholar]
- 40. Roque M, Valle M, Sampaio M, Geber S, Checa MA. Ratio of progesterone-to-number of follicles as a prognostic tool for in vitro fertilization cycles. J Assist Reprod Genet 2015; 32(6):951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shufaro Y, Sapir O, Oron G, Ben Haroush A, Garor R, Pinkas H, Shochat T, Fisch B. Progesterone-to-follicle index is better correlated with in vitro fertilization cycle outcome than blood progesterone level. Fertil Steril 2015; 103(3):669–674.e3. [DOI] [PubMed] [Google Scholar]
- 42. Hill MJ, Healy MW, Richter KS, Widra E, Levens ED, DeCherney AH, Patounakis G, Whitcomb BW. Revisiting the progesterone to oocyte ratio. Fertil Steril 2017; 107(3):671–676.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hill MJ, Royster GDt, Taneja M, Healy MW, Zarek SM, Christy AY, DeCherney AH, Widra E, Devine K. Does elevated progesterone on day of oocyte maturation play a role in the racial disparities in IVF outcomes? Reprod Biomed Online 2017; 34(2):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Healy MW, Yamasaki M, Patounakis G, Richter KS, Devine K, DeCherney AH, Hill MJ. The slow growing embryo and premature progesterone elevation: compounding factors for embryo-endometrial asynchrony. Hum Reprod 2017; 32(2):362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ivell R, Bathgate R, Walther N. Luteal peptides and their genes as important markers of ovarian differentiation. J Reprod Fertil Suppl 1999; 54:207–216. [PubMed] [Google Scholar]
- 46. Boerboom D, Sirois J. Equine P450 cholesterol side-chain cleavage and 3 beta-hydroxysteroid dehydrogenase/delta(5)-delta(4)isomerase: molecular cloning and regulation of their messenger ribonucleic acids in equine follicles during the ovulatory process. Biol Reprod 2001; 64(1):206–215. [DOI] [PubMed] [Google Scholar]
- 47. Oktem O, Akin N, Bildik G, Yakin K, Alper E, Balaban B, Urman B. FSH Stimulation promotes progesterone synthesis and output from human granulosa cells without luteinization. Hum Reprod 2017; 32(3):643–652. [DOI] [PubMed] [Google Scholar]
- 48. Werner MD, Forman EJ, Hong KH, Franasiak JM, Molinaro TA, Scott RT Jr.. Defining the “sweet spot” for administered luteinizing hormone-to-follicle-stimulating hormone gonadotropin ratios during ovarian stimulation to protect against a clinically significant late follicular increase in progesterone: an analysis of 10,280 first in vitro fertilization cycles. Fertil Steril 2014; 102(5):1312–1317. [DOI] [PubMed] [Google Scholar]
- 49. Marek D, Langley M, Gardner DK, Confer N, Doody KM, Doody KJ. Introduction of blastocyst culture and transfer for all patients in an in vitro fertilization program. Fertil Steril 1999; 72(6):1035–1040. [DOI] [PubMed] [Google Scholar]
- 50. Navot D, Scott RT, Droesch K, Veeck LL, Liu HC, Rosenwaks Z. The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril 1991; 55(1):114–118. [DOI] [PubMed] [Google Scholar]
- 51. Teh WT, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet 2016; 33(11):1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Embryo cryopreservation rescues cycles with premature luteinization. Fertil Steril 2010; 93(2):636–641. [DOI] [PubMed] [Google Scholar]
- 53. Healy MW, Patounakis G, Connell MT, Devine K, DeCherney AH, Levy MJ, Hill MJ. Does a frozen embryo transfer ameliorate the effect of elevated progesterone seen in fresh transfer cycles? Fertil Steril 2016; 105(1):93–99.e1. [DOI] [PubMed] [Google Scholar]
- 54. Fanchin R, Righini C, Olivennes F, Ferreira AL, de Ziegler D, Frydman R. Consequences of premature progesterone elevation on the outcome of in vitro fertilization: insights into a controversy. Fertil Steril 1997; 68(5):799–805. [DOI] [PubMed] [Google Scholar]