We previously reported the safety of the novel, non-platinum combination of cetuximab, pemetrexed, and radiation therapy in head and neck cancer. In this randomized trial we evaluated this combination with or without bevacizumab. Both arms were superior to historical control but the bevacizumab arm had increased toxicities. The non-bevacizumab regimen is recommended for further development.
Keywords: bevacizumab, cetuximab, clinical trial, head and neck cancer, pemetrexed, radiation therapy
Abstract
Background
We previously reported the safety of concurrent cetuximab, an antibody against epidermal growth factor receptor (EGFR), pemetrexed, and radiation therapy (RT) in patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN). In this non-comparative phase II randomized trial, we evaluated this non-platinum combination with or without bevacizumab, an inhibitor of vascular endothelial growth factor (VEGF).
Patients and methods
Patients with previously untreated stage III–IVB SCCHN were randomized to receive: conventionally fractionated radiation (70 Gy), concurrent cetuximab, and concurrent pemetrexed (arm A); or the identical regimen plus concurrent bevacizumab followed by bevacizumab maintenance for 24 weeks (arm B). The primary end point was 2-year progression-free survival (PFS), with each arm compared with historical control. Exploratory analyses included the relationship of established prognostic factors to PFS and quality of life (QoL).
Results
Seventy-eight patients were randomized: 66 oropharynx (42 HPV-positive, 15 HPV-negative, 9 unknown) and 12 larynx; 38 (49%) had heavy tobacco exposure. Two-year PFS was 79% [90% confidence interval (CI) 0.69–0.92; P < 0.0001] for arm A and 75% (90% CI 0.64–0.88; P < 0.0001) for arm B, both higher than historical control. No differences in PFS were observed for stage, tobacco history, HPV status, or type of center (community versus academic). A significantly increased rate of hemorrhage occurred in arm B. SCCHN-specific QoL declined acutely, with marked improvement but residual symptom burden 1 year post-treatment.
Conclusions
RT with a concurrent non-platinum regimen of cetuximab and pemetrexed is feasible in academic and community settings, demonstrating expected toxicities and promising efficacy. Adding bevacizumab increased toxicity without apparent improvement in efficacy, countering the hypothesis that dual EGFR–VEGF targeting would overcome radiation resistance, and enhance clinical benefit. Further development of cetuximab, pemetrexed, and RT will require additional prospective study in defined, high-risk populations where treatment intensification is justified.
introduction
Patients with squamous cell carcinoma of the head and neck (SCCHN) usually present with locoregionally advanced disease necessitating combined modality treatment. Although optimal integration of surgery, radiation therapy (RT), and chemotherapy is debated, concomitant chemotherapy and RT (chemoRT) is a well-established approach supported by phase III randomized studies, especially with platinum-based regimens [1]. Although cisplatin–RT improves locoregional control (LRC) and overall survival (OS), it also causes considerable toxicity and is only curative for approximately half of patients. Patients with human papillomavirus (HPV)-positive versus HPV-negative tumors demonstrate better outcomes following chemoRT [2].
Over the last decade, incorporating novel targeted agents into combined modality regimens promised to improve efficacy without enhancing toxicity. Cetuximab, a monoclonal antibody against the epidermal growth factor receptor (EGFR), significantly improved LRC and OS when combined with RT compared with RT alone in locoregionally advanced SCCHN [3]. Similar to cisplatin trials, improved outcomes occurred in both HPV-positive and HPV-negative patients [4]. Notably, adding cetuximab to cisplatin–RT was not superior to cisplatin–RT [5]. The lack of additive activity may represent redundant mechanisms of radiation sensitization, as both inhibit repair of DNA double-strand breaks [6]. Thus, adding non-platinum cytotoxics to cetuximab–RT may be optimal.
Antifolates, like methotrexate, have long been used in SCCHN. Pemetrexed, a novel multi-targeted antifolate, results in fewer cumulative toxicities than cisplatin [7]. In a phase III trial in recurrent/metastatic SCCHN, cisplatin–pemetrexed was not superior to cisplatin alone; however, significant benefit was observed in a planned oropharyngeal subset analysis [8]. Pemetrexed is a potent, non-cell cycle-dependent radiation sensitizer in vitro [9, 10]. We reported a phase I trial investigating optimal dose and safety of full-dose pemetrexed when combined with standard cetuximab–RT in locoregionally advanced SCCHN [11].
Vascular endothelial growth factor (VEGF) is over-expressed in SCCHN, and associated with treatment resistance and poor prognosis [12]. Radiation-associated hypoxia induces VEGF expression, conferring radiation resistance [13]. Moreover, EGFR up-regulates VEGF signaling, an established mechanism of cetuximab resistance. Conversely, EGFR inhibition down-regulates VEGF [14]. Bevacizumab, an anti-VEGF monoclonal antibody, demonstrated preclinical activity against SCCHN angiogenesis and promising efficacy when added to pemetrexed or cetuximab in phase II, recurrent/metastatic trials [15–17]. We hypothesized that bevacizumab could further potentiate radiation sensitization in a cetuximab-based regimen. Thus, we designed this phase II randomized trial to evaluate the efficacy of cetuximab, pemetrexed, and RT, with or without bevacizumab, in locoregionally advanced SCCHN.
patients and methods
eligibility criteria
Key eligibility criteria included: age ≥18 years; previously untreated, HPV-positive or HPV-negative, stage III-IVb SCCHN; Eastern Cooperative Oncology Group performance status 0–1; adequate organ function; written informed consent. Institutional review board approval was obtained and the trial registered at clinicaltrials.gov (NCT00703976). Patients accrued at the University of Pittsburgh Medical Center (UPMC) Cancer Centers academic hub, Hillman Cancer Center, which served as coordinating center, as well as 10 UPMC community affiliates and St Joseph's Hospital (Orange, CA).
treatment plan
Patients were randomized to two experimental arms: (i) arm A, RT at 2 Gy/fraction/day for ∼35 treatments to a total dose of 70–74 Gy, concurrent cetuximab 250 mg/m2/week after a loading dose of 400 mg/m2, and pemetrexed 500 mg/m2 on days 1, 22, and 43; (2) arm B, identical regimen with concurrent bevacizumab 15 mg/kg on days 1, 22, and 43 followed by maintenance bevacizumab for 6 months. Based on phase I experience, patients took prophylactic ciprofloxacin on days 4–14 of each pemetrexed cycle. Patients received standard vitamin supplementation and dexamethasone prophylaxis.
Patients underwent CT-based treatment planning with three-dimensional conformal or intensity-modulated RT. Central RT quality control for the UPMC network included centralized contours evaluation and planning supported by D3 Oncology Solutions (Pittsburgh, PA).
Toxicities were assessed using National Cancer Institute Common Toxicity Criteria, version 4.0. Prophylactic gastrostomy tubes were routinely recommended, as was referral to speech and language pathology for swallowing therapy.
HPV testing
HPV was validated as a prognostic biomarker for oropharyngeal SCCHN during the conduct of this study, and became a clinical standard at participating sites. HPV status was collected retrospectively on oropharynx cases only, and classified by p16 immunohistochemistry or HPV DNA in situ hybridization (ISH) according to local results. A tumor was considered HPV-positive if demonstrating strong and diffuse nuclear and cytoplasmic p16 staining of ≥70% of tumor cells, or a punctate ISH nuclear signal. Oropharyngeal tumors were classified as HPV(unknown) if lacking HPV assessment; p16-negative oropharyngeal and non-oropharyngeal tumors were considered HPV-negative.
quality of life
Patients completed the Functional Assessment of Cancer Therapy-Head and Neck (FACT-H&N, version 4), a validated disease-specific health-related quality of life (QoL) instrument [18], at three time points: baseline, 3 months post-RT, and 1 year post-RT. Two QoL scores were computed. The FACT-G (general) is the summary of 27 items from four domains, including physical, social/family, emotional, and functional well-being [19]. Items are scored from 0 to 4, yielding a score range of 0–108, with higher scores suggesting better QoL. The 11-item H&N subscale includes mouth dryness, trouble swallowing, and voice quality. Items are scored from 0 to 4, yielding a score range of 0–44.
statistical analysis
This was a randomized, non-comparative, parallel cohort phase II trial. The primary objective was to evaluate the 2-year progression-free survival (PFS) against the historical control in each arm. Randomization was stratified by stage (III versus IV) and primary site (oropharynx versus other). PFS was defined as elapsed time between starting study treatment until disease progression or treatment-related death. Progression events were defined pathologically and/or in accordance with modified Response Evaluation Criteria in Solid Tumors, version 1.1 [20]. Patients who died without progression were censored. Assuming a historical control of 46% 2-year PFS for cetuximab–RT [3], we targeted a 2-year PFS of 64%. A one-tailed exponential test using the maximum likelihood estimator for the hazard rate of the exponential distribution at α = 0.10 provided 90% power to reject the null hypothesis. Secondary objectives were locoregional and distant PFS, OS, toxicities, and QoL. Estimates of PFS and OS used the Kaplan–Meier method. Exploratory group comparisons were conducted using the log-rank test with the Greenwood confidence intervals. The Cox proportional hazards regression was used to explore the associations of established prognostic factors (stage, HPV status, tobacco exposure), academic versus community treatment center, and bevacizumab with PFS. Changes in QoL were evaluated by paired t-tests using pre- and post-treatment FACT-G and H&N subscale scores.
results
baseline patient characteristics
Between January 2012 and September 2014, 80 patients were consented and randomized; 2 patients in arm A were found ineligible before treatment and withdrawn (Figure 1). Results are reported for the 78 eligible, treated patients: 37 on arm A and 41 on arm B. Baseline patient characteristics are presented in Table 1. Treatment arms were balanced for major prognostic factors, including stage, HPV status, and tobacco history.
Figure 1.
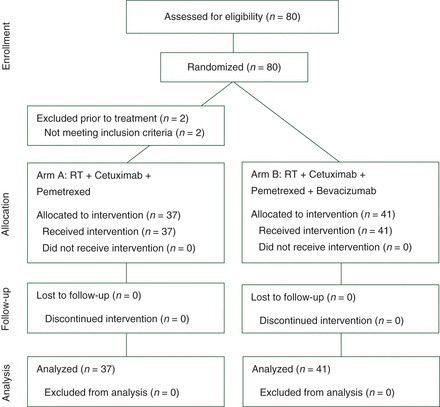
CONSORT diagram.
Table 1.
Patient characteristics
| Characteristic | All patients (n = 78) | Arm A CPem (n = 37) | Arm B CPem-B (n = 41) | Test of equality P valuea |
|---|---|---|---|---|
| Gender | ||||
| Male | 63 | 32 (86%) | 31 (76%) | 0.26 |
| Female | 15 | 5 (14%) | 10 (24%) | |
| Age | ||||
| Median | 56 | 57 | 56 | 0.94 |
| Range | 35–76 | 39–69 | 35–76 | |
| Disease site | ||||
| Oropharynx | 66 | 31 (84%) | 35 (85%) | 1.0 |
| Larynx | 12 | 6 (16%) | 6 (15%) | |
| ECOG performance status | 28 (76%) | 34 (83%) | ||
| 0 | 62 | 9 (24%) | 7 (17%) | 0.58 |
| 1 | 16 | |||
| AJCC stage | ||||
| III | 16 | 7 (19%) | 9 (22%) | 0.79 |
| T1N1 | 4 | 1 | 3 | |
| T2N1 | 2 | 1 | 1 | |
| T3N0 | 8 | 4 | 4 | |
| T3N1 | 2 | 1 | 1 | |
| IV | 62 | 30 (81%) | 32 (78%) | |
| T1N2 | 10 | 6 | 4 | |
| T2N2 | 32 | 15 | 17 | |
| T2N3 | 2 | 1 | 1 | |
| T3N2 | 10 | 2 | 8 | |
| T3N3 | 1 | 1 | 0 | |
| T4N0 | 1 | 1 | 0 | |
| T4N1 | 1 | 0 | 1 | |
| T4N2 | 4 | 3 | 1 | |
| T4N3 | 1 | 1 | 0 | |
| HPV status (66 oropharynx cases)b | ||||
| Positive | 42 | 16 (53%) | 26 (67%) | 0.54 |
| Negative | 27 | 14 (47%) | 13 (33%) | |
| Unknown | 9 | |||
| Smoking history | ||||
| <10 pack-years | 40 | 19 (51%) | 21 (51%) | 1.0 |
| ≥10 pack-years | 38 | 18 (49%) | 20 (49%) | |
| Site of accrual | ||||
| Academic center | 44 | 24 (65%) | 20 (49%) | 0.18 |
| Community center | 34 | 13 (35%) | 21 (51%) | |
aWilcoxon test for age, Fisher's exact test for all others.
bNine patients with an oropharyngeal primary were missing HPV status.
treatment delivery and toxicity
Treatment delivery of cetuximab and pemetrexed appeared similar between arms; the median number of cycles administered for cetuximab was 9 (range 2–11), pemetrexed 3 (1–3), and bevacizumab 3 (0–11). The cumulative RT dose (median 70 Gy in each arm) and number of elapsed days (median 51 days in each arm) were also similar.
Acute regimen-related adverse events are summarized in Table 2. Serious toxicities associated with cetuximab–RT, including mucositis and dysphagia, were comparable between arms. Hemorrhage was significantly more common on the bevacizumab arm. Other class toxicities associated with anti-angiogenic therapy, including gastrointestinal perforation and wound-healing complication, were rare but only occurred in the bevacizumab arm. The single treatment-related death was due to pulmonary hemorrhage complicating bronchoscopy. This event occurred within 30 days of completing bevacizumab maintenance and resulted in elimination of maintenance bevacizumab for the remainder of the study. Seventy-three patients (94%) received prophylactic gastrostomy tubes. Among 68 patients alive and without locoregional recurrence at 12 months, 10 (15%) remained gastrostomy tube-dependent; 5 (7.5%) remained dependent at 24 months.
Table 2.
Acute treatment-related adverse events
| Non-hematologic toxicities | Arm A CPem (n =
37) |
Arm B CPem-B (n =
41) |
||
|---|---|---|---|---|
| Grade 1–2 [n (%)] | Grade 3–4a [n (%)] | Grade 1–2 [n (%)] | Grade 3–4a [n (%)] | |
| Non-vascular | ||||
| Mucositis | 28 (68) | 19 (51) | 21 (51) | 17 (41) |
| Radiation dermatitis | 22 (59) | 5 (14) | 22 (54) | 11 (27) |
| Dysphagia | 18 (49) | 10 (27) | 20 (49) | 12 (29) |
| Pain | 27 (73) | 8 (22) | 28 (68) | 11 (27) |
| Nausea | 19 (51) | 4 (11) | 22 (54) | 4 (10) |
| Weight loss | 22 (59) | 2 (5) | 21 (51) | 5 (12) |
| Osteonecrosis | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Infusion reaction | 1 (3) | 0 (0) | 2 (4) | 0 (0) |
| Rash | 31 (68) | 3 (8) | 35 (85) | 3 (7) |
| Infection | 19 (51) | 5 (14) | 11 (28) | 4 (10) |
| Neutropenic fever | 0 (0) | 2 (5) | 0 (0) | 0 (0) |
| Gastrointestinal perforation | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Wound complication | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Erectile dysfunction | 0 (0) | 0 (0) | 1 (2) | 1 (2) |
| Vascular toxicities | ||||
| Thrombosis | 0 (0) | 0 (0) | 0 (0) | 2 (5) |
| Hemorrhageb | 1 (3) | 0 (0) | 14 (34) | 3 (7) |
| Epistaxis | 0 (0) | 0 (0) | 7 (17) | 0 (0) |
| Hematuria | 0 (0) | 0 (0) | 4 (10) | 0 (0) |
| Other | 1 (3) | 0 (0) | 3 (8) | 3 (7) |
aAll comparisons of grade 3/4 toxicities between arms were non-significant (Fisher's exact test).
bThe rate of hemorrhage (all grades) was significantly increased in arm B (Fisher's exact test P = 0.0005 with the Bonferroni correction).
efficacy
With a median follow-up of 32 months among 58 censored patients, 19 progression events were observed: 8 in arm A (3 locoregional, 4 distant, and 1 both locoregional and distant) and 11 in arm B (3 locoregional, 4 distant, 4 both, 1 treatment-related death). The Kaplan–Meier curves for PFS and OS are presented in Figure 2. Each treatment arm met the primary efficacy end point. The 2-year PFS in arm A was 79% (90% CI 0.69–0.92); a one-tailed exponential test rejected the null hypothesis (P < 0.0001). The 2-year PFS in arm B was 75% (90% CI 0.64–0.88), also statistically significant (P < 0.0001). Eight deaths occurred due to progression. Two patients died without progression, one from unknown cause, and one from treatment-related pulmonary hemorrhage. In the combined cohorts, the probability of 2-year OS was 0.88 (95% CI = 0.81–0.96). In exploratory analysis, no univariate prognostic factor for PFS (stage, HPV status, tobacco exposure, academic versus community center, or bevacizumab) was identified (supplementary Table S1, available at Annals of Oncology online).
Figure 2.
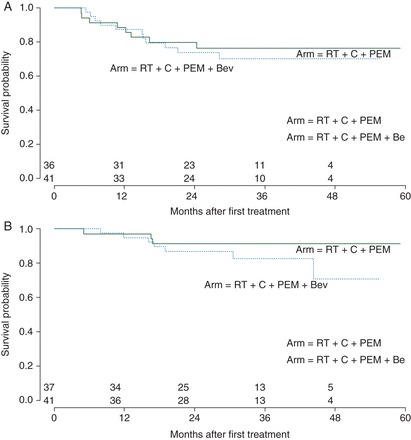
(A) The Kaplan–Meier estimates of progression-free survival by treatment arm. Tick marks indicate censored times. (B) The Kaplan–Meier estimates of overall survival by treatment arm. Tick marks indicate censored times.
quality of life
QoL data were available at baseline for 59 patients (76%), at 3 months post-RT for 50 patients, and at 1-year post-RT for 35 patients. Patients reported essentially stable general QoL over time. There was no difference between baseline (M = 85.06, SD = 13.41) and 3-month post-RT FACT-G scores [M = 83.72, SD = 15.36; t(42) = 1.30; P = 0.20]. However, QoL improved at 1 year post-RT relative to baseline [M = 90.53, SD = 12.65; t(27) = −2.78, P = 0.010].
Patients reported significantly decreased head and neck-specific QoL at 3 months post-RT (M = 26.75, SD = 7.2) compared with baseline [M = 20.27, SD = 6.49; t(43) = 6.54, P < 0.0001]. This difference was less pronounced but still evident by 1 year post-RT [M = 23.28, SD = 5.93; t(28) = 2.13, P = 0.042] (supplementary Figure S1, available at Annals of Oncology online).
discussion
Strong preclinical evidence supports dual EGFR and VEGF pathway inhibition in SCCHN. The up-regulation of VEGF is associated with resistance to EGFR inhibitors, and cetuximab-resistant tumors exhibit extensive neo-vascularization [14, 21]. The combination of bevacizumab with cetuximab or pemetrexed has demonstrated promising efficacy in phase II, recurrent/metastatic trials [16, 17]. Moreover, VEGF blockade may abrogate radiation-induced VEGF production and normalize tumor vasculature, improving blood flow and drug delivery [22]. These data prompted us to incorporate bevacizumab into a curative RT regimen containing cetuximab.
Because concurrent cisplatin–RT is toxic, identifying alternative agents that can maintain efficacy with reduced toxicity is clinically important. Based on our previous phase I study defining the safety of RT, cetuximab, and pemetrexed [11], we conducted a multicenter phase II randomized trial evaluating this regimen, with or without bevacizumab, against historical control. Both non-platinum-containing regimens were facile to administer in community centers, and demonstrated promising 2-year PFS. However, adding bevacizumab did not appear to enhance efficacy, countering the hypothesis that dual EGFR–VEGF targeting would overcome radiation resistance and increase clinical benefit. Our study predominantly enrolled patients with oropharyngeal primaries. Notably, no significant difference in 2-year PFS was observed between patients classified as HPV-positive or negative in secondary analysis, although the event rate was low and such an analysis underpowered.
Bevacizumab has been safely added to several chemoRT regimens in previously untreated, locoregionally advanced SCCHN, where systemic therapy has included 5-fluorouracil and hydroxyurea [23], cisplatin [24], docetaxel [25], or cetuximab–cisplatin [26]. However, a phase II randomized trial investigating hyperfractionated RT, 5-fluorouracil, and hydroxyurea with or without bevacizumab was terminated early, as LRC appeared reduced with bevacizumab [27]. A similar detrimental signal was not observed in our study, as relapse rates and patterns appeared numerically similar between arms. However, bevacizumab was associated with a significant, expected increase in the rate of hemorrhage, largely grade 1–2 events. Although concurrent bevacizumab was safe, maintenance bevacizumab was dropped after a treatment-related death. Otherwise, toxicities in both arms were consistent with historical patterns, albeit not reduced as hypothesized by this non-platinum approach [3, 5]. Consistent with the class of anti-angiogenic therapeutics, bevacizumab is associated with a 3.5% incidence of grade 3–5 hemorrhage [28]. Although the observed hemorrhage rate is in line with prior safety results, the additional toxicity is not justified and further development of arm B is not recommended.
General QoL and head and neck-specific QoL were compatible with patterns observed in trials of conventional cisplatin–RT or cetuximab–RT [29]. While general QoL was stable, FACT-H&N revealed increased head and neck-specific symptom burden 3 months post-RT and significant residual symptom burden 12 months post-RT. Given the 50% drop off in questionnaire adherence, these data may not be representative.
In summary, the combination of concurrent cetuximab, pemetrexed, and RT is safe and superior to the historical control of cetuximab–RT in an HPV-unselected group. Although this represents a positive study as designed, an important limitation must be recognized. Modern chemoRT trials in locoregionally advanced SCCHN are subject to time trends bias, where control groups are consistently outperforming historical controls [30, 31]. This is likely due to three factors: (i) the increased proportion of HPV-associated SCCHN associated with favorable prognosis; (ii) decreased pack-years of smoking among SCCHN patients, also an independent, positive prognostic factor; and (iii) major improvements in precision RT techniques [2]. Although these factors may have influenced the observed PFS, our results are sufficiently robust to merit further investigation. Although cetuximab did not add to cisplatin–RT in RTOG 0522, concluding that biologic–cytotoxic combinations are non-synergistic would be premature [5]. The best cytotoxic chemotherapy to combine with cetuximab–RT may be a non-platinum agent, since cisplatin and cetuximab have overlapping mechanisms of radiation sensitization [6]. Currently, the NRG Oncology cooperative group is conducting a randomized phase II/III trial in postoperative, high-risk SCCHN comparing adjuvant RT with docetaxel and cetuximab, a non-platinum combination showing promise in phase II testing [32], with cisplatin–RT or docetaxel–RT (NCT01810913). Similarly, the cytotoxic-biologic combination of pemetrexed–cetuximab warrants randomized investigation in higher risk settings, such as intermediate-risk HPV-positive or high-risk HPV-negative SCCHN, where treatment intensification can be justified.
funding
This project was supported by the Investigator-Sponsored Trial programs of Genentech, Inc. and Eli Lilly, Inc., the University of Pittsburgh Cancer Institute Biostatistics Facility (supported in part by award P30CA047904), and the PA Department of Health. UD was supported in part by funds from the Department of Veterans Affairs, BLR&D.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
This work does not reflect the views of the U.S. Government or the Department of Veterans Affairs.
references
- 1. Adelstein DJ, Li Y, Adams GL et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21: 92–98. [DOI] [PubMed] [Google Scholar]
- 2. Ang KK, Harris J, Wheeler R et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonner JA, Harari PM, Giralt J et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354: 567–578. [DOI] [PubMed] [Google Scholar]
- 4. Rosenthal DI, Harari PM, Giralt J et al. Association of human papillomavirus and p16 status with outcomes in the IMCL-9815 Phase III registration trial for patients with locoregionally advanced oropharyngeal squamous cell carcinoma of the head and neck treated with radiotherapy with or without cetuximab. J Clin Oncol 2016; 34: 1300–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ang KK, Zhang Q, Rosenthal DI et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014; 32: 2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dittmann K, Mayer C, Rodemann HP. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother Oncol 2005; 76: 157–161. [DOI] [PubMed] [Google Scholar]
- 7. Argiris A, Pennella E, Koustenis A et al. Pemetrexed in head and neck cancer: a systematic review. Oral Oncol 2013; 49: 492–501. [DOI] [PubMed] [Google Scholar]
- 8. Urba S, van Herpen CM, Sahoo TP et al. Pemetrexed in combination with cisplatin versus cisplatin monotherapy in patients with recurrent or metastatic head and neck cancer: final results of a randomized, double-blind, placebo-controlled, phase 3 study. Cancer 2012; 118: 4694–4705. [DOI] [PubMed] [Google Scholar]
- 9. Bischof M, Weber KJ, Blatter J et al. Interaction of pemetrexed disodium (ALIMTA, multitargeted antifolate) and irradiation in vitro. Int J Radiat Oncol Biol Phys 2002; 52: 1381–1388. [DOI] [PubMed] [Google Scholar]
- 10. Mauceri HJ, Seetharam S, Salloum RM et al. Treatment of head and neck and esophageal xenografts employing Alimta and concurrent ionizing radiation. Int J Oncol 2001; 19: 833–835. [DOI] [PubMed] [Google Scholar]
- 11. Argiris A, Karamouzis MV, Smith R et al. Phase I trial of pemetrexed in combination with cetuximab and concurrent radiotherapy in patients with head and neck cancer. Ann Oncol 2011; 22: 2482–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith BD, Smith GL, Carter D et al. Prognostic significance of vascular endothelial growth factor protein levels in oral and oropharyngeal squamous cell carcinoma. J Clin Oncol 2000; 18: 2046–2052. [DOI] [PubMed] [Google Scholar]
- 13. Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res 2003; 9: 1957–1971. [PubMed] [Google Scholar]
- 14. Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res 2007; 5: 203–220. [DOI] [PubMed] [Google Scholar]
- 15. Jenab-Wolcott J, Giantonio BJ. Bevacizumab: current indications and future development for management of solid tumors. Expert Opin Biol Ther 2009; 9: 507–517. [DOI] [PubMed] [Google Scholar]
- 16. Argiris A, Karamouzis MV, Gooding WE et al. Phase II trial of pemetrexed and bevacizumab in patients with recurrent or metastatic head and neck cancer. J Clin Oncol 2011; 29: 1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Argiris A, Kotsakis AP, Hoang T et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol 2013; 24: 220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. List MA, D'Antonio LL, Cella DF et al. The performance status scale for head and neck cancer patients and the functional assessment of cancer therapy-head and neck scale. A study of utility and validity. Cancer 1996; 77: 2294–2301. [DOI] [PubMed] [Google Scholar]
- 19. Cella DF, Tulsky DS, Gray G et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993; 11: 570–579. [DOI] [PubMed] [Google Scholar]
- 20. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 21. Benavente S, Huang S, Armstrong EA et al. Establishment and characterization of a model of acquired resistance to epidermal growth factor receptor targeting agents in human cancer cells. Clin Cancer Res 2009; 15: 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao S, Durrani FA, Toth K et al. Bevacizumab enhances the therapeutic efficacy of irinotecan against human head and neck squamous cell carcinoma xenografts. Oral Oncol 2011; 47: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seiwert TY, Haraf DJ, Cohen EE et al. Phase I study of bevacizumab added to fluorouracil- and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. J Clin Oncol 2008; 26: 1732–1741. [DOI] [PubMed] [Google Scholar]
- 24. Fury MG, Lee NY, Sherman E et al. A phase 2 study of bevacizumab with cisplatin plus intensity-modulated radiation therapy for stage III/IVB head and neck squamous cell cancer. Cancer 2012; 118: 5008–5014. [DOI] [PubMed] [Google Scholar]
- 25. Yao M, Galanopoulos N, Lavertu P et al. Phase II study of bevacizumab in combination with docetaxel and radiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck 2015; 37: 1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fury MG, Xiao H, Sherman EJ et al. Phase II trial of bevacizumab +cetuximab + cisplatin with concurrent intensity-modulated radiation therapy for patients with stage III/IVB head and neck squamous cell carcinoma. Head Neck 2016; 38(Suppl 1): E566–E570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salama JK, Haraf DJ, Stenson KM et al. A randomized phase II study of 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy compared with bevacizumab plus 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy for intermediate-stage and T4N0-1 head and neck cancers. Ann Oncol 2011; 22: 2304–2309. [DOI] [PubMed] [Google Scholar]
- 28. Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology 2010; 79: 27–38. [DOI] [PubMed] [Google Scholar]
- 29. Ringash J. Survivorship and quality of life in head and neck cancer. J Clin Oncol 2015; 33: 3322–3327. [DOI] [PubMed] [Google Scholar]
- 30. Cohen EE, Karrison TG, Kocherginsky M et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol 2014; 32: 2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haddad R, O'Neill A, Rabinowits G et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 2013; 14: 257–264. [DOI] [PubMed] [Google Scholar]
- 32. Harari PM, Harris J, Kies MS et al. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: Radiation Therapy Oncology Group RTOG-0234. J Clin Oncol 2014; 32: 2486–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


