Abstract
BACKGROUND
Hypertension in African Americans is characterized by greater systemic vascular resistance (SVR) compared with Caucasian Americans, but the responsible mechanisms are not known. The present study sought to determine if peripheral vascular hypertrophy is a potential mechanism contributing to elevated SVR in African Americans with high blood pressure (BP).
METHODS
In a biracial sample of 80 men and women between the ages of 25 and 45 years, with clinic BP in the range 130/85–160/99mm Hg, we assessed cardiac output and SVR, in addition to BP. Minimum forearm vascular resistance (MFVR), a marker of vascular hypertrophy, also was assessed.
RESULTS
SVR was elevated in African Americans compared with Caucasians (P < 0.001). Regression models indicated that age, body mass index, 24-hour diastolic BP, and ethnicity were significant predictors of SVR. There was also a significant interaction between ethnicity and MFVR in explaining SVR in the study sample. In particular, there was a significant positive association between MFVR and SVR among African Americans (P = 0.002), whereas the association was inverse and not statistically significant among Caucasians (P = 0.601).
CONCLUSION
Hypertrophy of the systemic microvasculature may contribute to the elevated SVR that is characteristic of the early stages of hypertension in African American compared with Caucasians.
Keywords: blood pressure, ethnicity, hypertension, hypertrophy, vascular resistance.
Research on the natural history of hypertension has emphasized that its early stages typically involve a hyperkinetic circulatory state, characterized hemodynamically by elevated cardiac output; with the progression of hypertensive disease, this hemodynamic profile ultimately transitions into elevated systemic vascular resistance (SVR). 1 However, among African Americans, for whom hypertension is more prevalent than Caucasians and other ethnic groups and is associated with greater target organ damage, the hemodynamic profile may be characterized by elevated SVR from the onset. 2–4 Several studies have shown that even at similar levels of blood pressure (BP), SVR is greater among African Americans compared to Caucasians. 5,6 African Americans also exhibit a propensity to vasoconstrictive responses to a wide range of environmental, physical, and psychosocial stressors, which may further contribute to the progression of hypertension and its adverse manifestations. 5–9
The physiological basis for elevated SVR in the early stages of hypertension in African Americans is not well understood. Impaired vascular function is implicated as the predominant mechanism underlying the high rates of cardiovascular disease in African Americans. 10 There is some evidence that blunted beta-adrenergic receptor vasodilator activity and heightened vascular alpha-adrenergic receptor sensitivity may be contributing factors. 8,11–13 Impaired endothelial vasodilator function also may contribute to increased SVR in African Americans. 14–17 Vascular hypertrophy, characterized by alterations (i.e., thickening) of the medial layer of the vascular wall, is another potential etiology of an elevated SVR that typically emerges with the progression of hypertension but may also occur in its early stages. 18,19 Evidence from studies in normotensive samples indicates that in addition to elevated SVR, African Americans also tend to exhibit structural microvascular differences, including greater wall thickness and a narrower lumen diameter, compared to Whites. 6 Minimal forearm vascular resistance (MFVR), obtained using venous occlusion plethysmography, is a measure of the structural component of vascular resistance. 20 Previous research has shown MFVR to be highly correlated (i.e., r ≈ 0.70) with small artery media:lumen ratio, 21 and other research has conceptualized MFVR as a surrogate index of vascular hypertrophy. 22–25 Given that even small differences in vascular structure may have significant functional consequences, 19,26 the purpose of the present study was to examine the association between SVR and a noninvasive marker of vascular hypertrophy and to determine whether this relationship differed among African Americans and Caucasians.
METHODS
Study population
Subjects were 80 men and women between the ages of 25 and 45 years who participated in the Duke Biobehavioral Investigation of Hypertension (BIOH) study. Details of the study and primary results were published previously. 27 The present study included individuals with untreated clinic BP in the range 130–160/85–99mm Hg. The study protocol and all procedures were approved by Duke University Medical Center Institutional Review Board. Written informed consent was obtained from all subjects prior to participation.
BP and hemodynamics
Clinic BPs were taken on 3 separate visits, each approximately 1 week apart. On each visit, 3 seated BP readings were taken, each 2 minutes apart, using an appropriate-sized occlusion cuff, mercury column sphygmomanometer, and stethoscope. The systolic BP was recorded coincident with the first occurrence of Korotkoff sounds (phase I), and diastolic BP (DBP) with their disappearance (phase V). Hemodynamic measurements were made between 9:00 am and noon.
Cardiac output (CO) was estimated using impedance cardiography in accordance with published guidelines. 28 Impedance cardiography signals were recorded via a Minnesota Impedance Cardiograph (Model HIC-1, Bio-Impedance Technology, Chapel Hill, NC) using a tetrapolar band-electrode configuration. The recording electrode bands were positioned around the base of the neck and around the thorax over the xiphisternal junction. The current electrode bands were positioned to encompass the neck and thorax, at least 3cm away from each of the recording electrodes. The electrocardiogram was recorded from the Minnesota Impedance Cardiograph using disposable electrocardiogram electrodes. The basal thoracic impedance (Zo), the first derivative of the pulsatile impedance (dZ/dt) and the electrocardiogram waveforms were processed using specialized ensemble-averaging software (COP, BIT), which was used to derive stroke volume using the Kubicek equation. 28 To account for potential sex and ethnic differences in body size, CO and SVR were indexed by body surface area.
Ambulatory BP was also assessed during a typical workday for all participants. The AccuTracker II ABP Monitor (Suntech AccuTracker II), an auscultatory, noninvasive device, was worn for approximately 24 hours, usually starting between 8:00 am and 10:00 am until the same time the following morning. The device was programmed to take 4 BP measurements per hour at random intervals during the day and to take 2 BP readings per hour during sleeping hours. All BP readings were reviewed and artifactual readings were deleted following criteria previously described. 27 Mean 24-hour systolic BP and DBP values were computed based on all valid readings obtained during waking hours and during nighttime sleep.
Minimum forearm vascular resistance
Evidence of vascular hypertrophy was assessed according to MFVR, calculated from measurements of BP and forearm blood flow during reactive hyperemia, as described previously. 29 Forearm blood flow was measured in the left arm by mercury-in-silastic strain gauge using a Hokanson (Issaquah, WA) plethysmograph and rapid cuff inflator. With the subject reclining, the left forearm was suspended at the wrist and elbow slightly above heart level. An occlusion cuff was applied to the upper arm and a pediatric cuff was applied at the wrist. A mercury-in-silastic strain gauge encircled the forearm approximately 5cm distal to the antecubital crease and was coupled to an electronically calibrated plethysmograph. To produce forearm ischemia, the upper occlusion cuff was inflated to a suprasystolic pressure (200mm Hg) for 10 minutes, during which time the subject was instructed to contract the hand for 5 seconds of every 30-second interval. The pediatric wrist cuff was inflated to a suprasystolic pressure 1 minute before and during blood flow measurements in order to arrest circulation to the hand. Beginning 10 seconds after the release of arterial occlusion, the upper arm occlusion cuff was rapidly inflated to 40mm Hg and deflated in l0-second cycles. BP was measured simultaneously in the right arm. Forearm blood flow in ml/100ml forearm volume/min was determined by averaging the results from 3 plethysmographic curves during the 40–50 seconds immediately after ischemia. Mean arterial pressure was calculated as DBP + 1/3 pulse pressure. MFVR was calculated as mean arterial pressure/forearm blood flow.
Data analysis
Analysis of variance and chi-square tests were implemented to document any differences in demographic and hemodynamic characteristics between African American and Caucasian subjects. Correlational analyses were used to assess the strength of associations among MFVR, demographics, clinic BP, and hemodynamic variables. Hierarchical regressions models were implemented to assess the association between SVR and MFVR, after controlling for potential confounds. All analyses were performed using the SAS (Cary, NC) software system; significance was set at P < 0.05 for all tests.
RESULTS
Demographic, anthropometric, and hemodynamic characteristics
The 80 subjects included 41 African Americans (22 females; 19 males) and 39 Caucasians (10 females; 29 males), with a mean age of 35±6 years and an average screening BP of 138±11/89±9mm Hg. Characteristics of the African American and Caucasian subjects are summarized in Table 1. There were no ethnic differences in body surface area or ambulatory BP. Caucasian subjects were younger, on average, and had greater stoke volume and cardiac index compared to African Americans, and body mass index (BMI) and clinic BP were marginally higher in African Americans. Despite similar BPs, African Americans exhibited higher SVR and greater MFVR.
Table 1.
Demographic characteristics and hemodynamics in African American and Caucasian subjects
| African American (n = 41) | Caucasian (n = 39) | P value | |
|---|---|---|---|
| Age (years) | 36±6 | 33±6 | 0.033 |
| Sex (% female) | 56 | 26 | 0.009 |
| Body mass index (kg/m 2 ) | 27.9±4 | 26.2±3.2 | 0.022 |
| Body surface area (m 2 ) | 1.97±0.20 | 1.97±0.19 | 0.934 |
| Clinic systolic blood pressure (mm Hg) | 140±13 | 136±9 | 0.093 |
| Clinic diastolic blood pressure (mm Hg) | 91±10 | 87±7 | 0.018 |
| 24-hour systolic blood pressure (mm Hg) | 133±11 | 131±8 | 0.324 |
| 24-hour diastolic blood pressure (mm Hg) | 83±7 | 82±5 | 0.239 |
| Heart rate (bpm) | 67±11 | 71±12 | 0.129 |
| Stroke volume index (ml/m 2 ) | 42±11 | 51±16 | 0.007 |
| Cardiac index (l/min/m 2 ) | 2.7±0.6 | 3.5±0.8 | <0.001 |
| Systemic vascular resistance index (dyne-s-cm -5 /m 2 ) | 2,853±662 | 2,263±566 | <0.001 |
| Minimum forearm vascular resistance | 3.72±1.4 | 2.88±1.05 | 0.004 |
Correlations with SVR
SVR was positively correlated with age (r = 0.32, P ≤ 0.01), BMI (r = 0.35, P ≤ 0.01), clinic DBP (r = 0.34, P ≤ 0.01), and ambulatory DBP (r = 0.31, P ≤ 0.01) and inversely associated with stroke volume index (r = −0.70, P ≤ 0.001) and cardiac index (r = −0.89, P ≤ 0.001). MFVR was inversely correlated with sex (r = −0.50, P ≤ 0.001). MFVR was positively associated with older age (r = 0.22, P ≤ 0.05), clinic systolic BP (r = 0.27, P ≤ 0.05), and SVR (r = 0.23, P ≤ 0.05). Both SVR (r = 0.43, P ≤ 0.001) and MFVR (r = 0.33, P ≤ 0.01) were significantly correlated with ethnicity indicating higher values in African Americans.
Multivariate associations with SVR
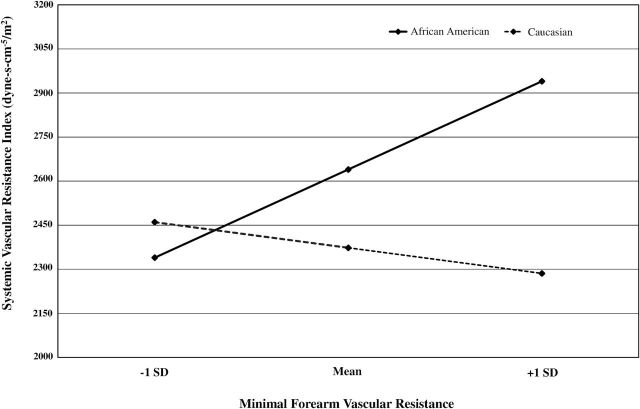
To further examine the association between SVR and MFVR, a series of hierarchical regression models were conducted (see Table 2). In model 1, which included age, sex, and ethnicity, age (b = 0.25, P = 0.017) and ethnicity (b = 0.42, P ≤ 0.001) were significant predictors of SVR. BMI and ambulatory BP were added in model 2, with both BMI (b = 0.30, P = 0.002) and 24-hour DBP (b = 0.37, P = 0.003) emerging as the only additional significant predictors. Model 3 added MFVR and the MFVR × ethnicity interaction. Additionally, we tested for potential interactions of sex with ethnicity and/or MFVR by including sex × ethnicity and MFVR × sex cross-product terms in model 3. As depicted in Table 2, the effects of BMI and 24-hour DBP remained significant; however, the effect for ethnicity was fully attenuated with the inclusion of MFVR and the interaction terms. Neither the sex × ethnicity (b = 0.29, P = 0.13) nor the sex × MFVR interaction term (b = −0.51, P = 0.08) were significant; however, the MFVR × ethnicity interaction was significant (b = 0.93, P = 0.023). As depicted in Figure 1, the association between SVR and MFVR differed as a function of ethnicity. Whereas increasing MFVR was inversely associated with SVR among Caucasians, for African Americans, greater SVR was associated with higher MFVR.
Table 2.
Hierarchical regression model predicting systemic vascular resistance (N = 80)
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Estimate (β) | P value | Estimate (β) | P value | Estimate (β) | P value | |
| Age | 0.25 | 0.017 | 0.22 | 0.03 | 0.13 | 0.15 |
| Sex | 0.09 | 0.40 | 0.06 | 0.51 | 0.40 | 0.27 |
| Ethnicity | 0.42 | <0.001 | 0.34 | 0.001 | −0.53 | 0.20 |
| BMI | 0.30 | 0.002 | 0.24 | 0.009 | ||
| SBP24 | −0.21 | 0.09 | −0.22 | 0.06 | ||
| DBP24 | 0.37 | 0.003 | 0.36 | 0.003 | ||
| MFVR | −0.13 | 0.60 | ||||
| Sex × ethnicity | 0.29 | 0.13 | ||||
| MFVR × sex | −0.51 | 0.08 | ||||
| MFVR × ethnicity | 0.93 | 0.023 | ||||
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; MFVR, minimum forearm vascular resistance; SBP, systolic blood pressure.
Figure 1.
Model-estimated change in systemic vascular resistance index (SVRI) for 1 SD change in minimal forearm vascular resistance (MFVR) in African Americans compared with Caucasians. The simple slope for MFVR predicting SVRI was significant for African Americans (P = 0.002) but not Caucasians (P = 0.601).
In order to ascertain whether the findings described above held for participants with hypertension that was defined rigorously, we repeated the multivariate analyses in a subgroup restricted to those participants with average daytime ambulatory BP ≥135/85mm Hg. As depicted in Table 3, the ethnicity × MFVR interaction remains significant (b = 1.33, P = 0.042) in this hypertensive subsample.
Table 3.
Hierarchical regression model predicting systemic vascular resistance in hypertensive participants (N = 59)
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Estimate (β) | P value | Estimate (β) | P value | Estimate (β) | P value | |
| Age | 0.37 | 0.006 | 0.38 | <0.001 | 0.27 | 0.03 |
| Sex | 0.11 | 0.43 | 0.03 | 0.79 | −0.68 | 0.27 |
| Ethnicity | 0.34 | 0.01 | 0.18 | 0.13 | −1.35 | 0.05 |
| BMI | 0.39 | 0.001 | 0.33 | 0.007 | ||
| SBP24 | −0.12 | 0.32 | −0.15 | 0.19 | ||
| DBP24 | 0.32 | 0.006 | 0.35 | 0.004 | ||
| MFVR | −0.44 | 0.22 | ||||
| Sex × ethnicity | 1.45 | 0.054 | ||||
| MFVR × sex | 0.33 | 0.50 | ||||
| MFVR × ethnicity | 1.33 | 0.042 | ||||
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; MFVR, minimum forearm vascular resistance; SBP, systolic blood pressure.
DISCUSSION
We observed that MFVR was differentially associated with resting SVR in African American and Caucasian adults with elevated BPs, independent of the influence of age, sex, and BMI. Specifically, we found that MFVR was positively associated with SVR among African Americans; however, this pattern was inverse and not significant among Caucasians. Importantly, the present results support prior hypothesis that greater SVR among African Americans may be due to early structural changes in the peripheral vasculature. 11,12,29
Although elevated SVR is typically associated with a more advanced hypertensive state, 1 greater vascular resistance among African Americans develops early in life and irrespective of hypertensive status. 6,30 Studies of cardiovascular reactivity to behavioral challenges have demonstrated not only greater resting SVR among young, normotensive African Americans compared to Caucasians, but also a greater SVR response to the cold pressor and other laboratory-based stressors. 3,30 In accord with previous work, 30 our findings also raise the possibility that high SVR among African Americans may not be preceded by the typical “hyperkinetic” pattern of elevated CO. Alternatively, the transition from high CO to a state of high vascular resistance may occur at a much younger age among African Americans, which may further enhance the risk for early vascular remodeling.
Although BPs were comparable among African Americans and Caucasians in our study, both SVR and MFVR were significantly higher in African Americans. Increased MFVR is considered an early marker of hypertension-related remodeling of the small arteries and vascular damage. 14,31,32 Notably, Eftekhari et al. examined hemodynamic differences between patients with either stage 1 or stage 2 hypertension and a normotensive control group. Compared to controls, both hypertensive groups exhibited greater SVR and MFVR. Importantly, while elevations in 24-hour BP were up to 28% greater among the hypertensives relative to controls, the degree of elevation in MFVR was 58% and 87% greater for stage 1 and stage 2 groups, respectively. 31 As these authors suggest, for those in the early stages of hypertension, the degree of BP elevation does not necessarily indicate the level of underlying microvascular impairment.
While it is apparent that vascular resistance more strongly influences BP among African Americans, the origins of this pattern remain unclear. There is evidence that genetic factors may play a role in elevated vascular resistance. 33 For example, a positive family history of hypertension has been related to both greater SVR and MFVR. 14,34 In addition, twin research has shown that the relative contribution of genetic factors to resting SVR may be greater among African Americans compared to Caucasians. 33 There is also some indication that genetic factors may have a larger influence on SVR during stress among African American males, compared to females. 35 Other work has shown that stress-related changes in MFVR may be similar in African American males, irrespective of family history of hypertension. 36
Despite African Americans in our sample being slightly older and having a modestly greater BMI than Caucasians, analyses indicated that these factors did not account for our observed racial differences in MFVR. Nonetheless, future research on ethnic differences in vascular resistance and hypertrophy should more closely examine the influence of these and other nongenetic factors. For instance, it has been suggested that the cumulative impact of several factors including more frequent exposure to environmental and psychosocial stressors, contributes to accelerated biological aging in African Americans. 37 Compatible with this view, MFVR values among African Americans in our sample were consistent with previously reported levels among an older European cohort with established hypertension. 31 As other work has shown, there are a number of complex pathways through which greater BMI may influence microvascular function, 10 and there is some evidence that obesity is associated with vascular hypertrophy in young African Americans. Notably, previous research found that obese African American adolescents exhibited greater MFVR compared to nonobese African American and obese Caucasian adolescents. 38 Although age and ethnicity are nonmodifiable factors, there is prospective evidence that vascular hypertrophy can be effectively reversed using antihypertensive medications. 39 These findings suggest the possibility that monitoring changes in small artery structure may prove useful in determining treatment efficacy in the management of hypertension in African Americans.
Limitations
Because this was a cross-sectional study in which assessments of SVR and MFVR were obtained concurrently, we are unable to address the cause–effect relationship between them. This temporal distinction is important in determining whether structural microvascular changes should be regarded as a risk factor or simply as a disease marker. We also did not examine the relationship between MFVR and SVR during stress. Stress-induced changes in SVR have previously been linked to myocardial ischemia during stress. 40 Although MFVR was found to be unrelated to SVR during stress in a previous study of Caucasians, 41 our findings suggest that the MFVR relationship to stress SVR merits further investigation in African Americans. In the present study, SVR was derived from estimates of CO obtained via impedance cardiography. Impedance cardiography is more widely accepted as a measure of change in CO than of absolute levels; however, in lieu of more advanced and/or invasive methods, and with adequate sample size, resting estimates have been considered acceptable. 28 Our sample contained a larger proportion of males compared to females, so it is possible that sex differences may have at least partially accounted for our findings. However, we did not find sex to be a significant predictor of SVR index independently or interactively with ethnicity or MFVR. Nonetheless, future work should more closely examine interactions between ethnicity and sex in relation to SVR and vascular hypertrophy. Finally, based upon previous literature 22–25 we interpreted increased MFVR to indicate greater vascular hypertrophy among African Americans compared to Whites; however, other potential mechanisms including differences in vascular contractility, adrenergic receptor sensitivity, microvascular rarefaction, and nitric oxide bioavailability may all contribute to elevated SVR. 6,10,15,19,20
In conclusion, our observations suggest that the relationship between SVR and MFVR is moderated by ethnicity among young to middle-aged adults with moderately elevated BP. Moreover, for African Americans with high BP, among whom elevated SVR is more common, the degree of SVR elevation appears closely related to microvascular impairment. Contemporary perspectives conceptualize hypertension as a vascular disease and vascular dysfunction has been characterized as the primary determinant of the excessive cardiovascular disease burden faced by African Americans. 10,20 Consistent with these views, it is plausible that the greater prevalence of hypertension seen in African Americans may reflect a larger underlying pattern of vascular remodeling.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENT
This work was supported by funding from the National Heart, Blood and Lung Institute (HL49427, HL50774, and HL121708).
REFERENCES
- 1. Julius S. Transition from high cardiac output to elevated vascular resistance in hypertension. Am Heart J 1988; 116:600–606. [DOI] [PubMed] [Google Scholar]
- 2. Ergul A. Hypertension in Black patients: an emerging role of the endothelin system in salt-sensitive hypertension. Hypertension 2000; 36:62–67. [DOI] [PubMed] [Google Scholar]
- 3. Treiber FA, Musante L, Braden D, Arensman F, Strong WB, Levy M, Leverett S. Racial differences in hemodynamic responses to the cold face stimulus in children and adults. Psychosom Med 1990; 52:286–296. [DOI] [PubMed] [Google Scholar]
- 4. Musante L, Turner JR, Treiber FA, Davis H, Strong WB. Moderators of ethnic differences in vasoconstrictive reactivity in youth. Ethn Dis 1996; 6:224–234. [PubMed] [Google Scholar]
- 5. Sherwood A, Hughes JW, McFetridge J. Ethnic differences in the hemodynamic mechanisms of ambulatory blood pressure regulation. Am J Hypertens 2003; 16:270–273. [DOI] [PubMed] [Google Scholar]
- 6. Taherzadeh Z, Brewster LM, van Montfrans GA, VanBavel E. Function and structure of resistance vessels in Black and White people. J Clin Hypertens (Greenwich) 2010; 12:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calhoun DA, Mutinga ML, Collins AS, Wyss JM, Oparil S. Normotensive Blacks have heightened sympathetic response to cold pressor test. Hypertension 1993; 22:801–805. [DOI] [PubMed] [Google Scholar]
- 8. Sherwood A, May CW, Siegel WC, Blumenthal JA. Ethnic differences in hemodynamic responses to stress in hypertensive men and women. Am J Hypertens 1995; 8:552–557. [DOI] [PubMed] [Google Scholar]
- 9. Kahn DF, Duffy SJ, Tomasian D, Holbrook M, Rescorl L, Russell J, Gokce N, Loscalzo J, Vita JA. Effects of Black race on forearm resistance vessel function. Hypertension 2002; 40:195–201. [DOI] [PubMed] [Google Scholar]
- 10. Patel PD, Velazquez JL, Arora RR. Endothelial dysfunction in African-Americans. Int J Cardiol 2009; 132:157–172. [DOI] [PubMed] [Google Scholar]
- 11. Adefurin A, Ghimire LV, Kohli U, Muszkat M, Sofowora GG, Paranjape SY, Stein CM, Kurnik D. Blacks have a greater sensitivity to α1-adrenoceptor-mediated venoconstriction compared with Whites. Hypertension 2013; 61:915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stein CM, Lang CC, Singh I, He HB, Wood AJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in Blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension 2000; 36:945–951. [DOI] [PubMed] [Google Scholar]
- 13. Thomas KS, Nelesen RA, Ziegler MG, Bardwell WA, Dimsdale JE. Job strain, ethnicity, and sympathetic nervous system activity. Hypertension 2004; 44:891–896. [DOI] [PubMed] [Google Scholar]
- 14. Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GC. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest 1997; 99:1873–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med 2003; 139:761–776. [DOI] [PubMed] [Google Scholar]
- 16. Andrawis N, Jones DS, Abernethy DR. Aging is associated with endothelial dysfunction in the human forearm vasculature. J Am Geriatr Soc 2000; 48:193–198. [PubMed] [Google Scholar]
- 17. Lang CC, Stein CM, Brown RM, Deegan R, Nelson R, He HB, Wood M, Wood AJ. Attenuation of isoproterenol-mediated vasodilatation in Blacks. N Engl J Med 1995; 333:155–160. [DOI] [PubMed] [Google Scholar]
- 18. Folkow B. “Structural factor” in primary and secondary hypertension. Hypertension 1990; 16:89–101. [DOI] [PubMed] [Google Scholar]
- 19. Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res 2015; 116:1007–1021. [DOI] [PubMed] [Google Scholar]
- 20. Fernandez C, Sander GE, Giles TD. Prehypertension: defining the transitional phenotype. Curr Hypertens Rep 2016; 18:2. [DOI] [PubMed] [Google Scholar]
- 21. Rosei EA, Rizzoni D, Castellano M, Porteri E, Zulli R, Muiesan ML, Bettoni G, Salvetti M, Muiesan P, Giulini SM. Media: lumen ratio in human small resistance arteries is related to forearm minimal vascular resistance. J Hypertens 1995; 13:341–347. [PubMed] [Google Scholar]
- 22. Egan B, Julius S. Vascular hypertrophy in borderline hypertension: relationship to blood pressure and sympathetic drive. Clin Exp Hypertens A 1985; 7:243–255. [DOI] [PubMed] [Google Scholar]
- 23. Rizzoni D, Muiesan ML, Montani G, Zulli R, Calebich S, Agabiti-Rosei E. Relationship between initial cardiovascular structural changes and daytime and nighttime blood pressure monitoring. Am J Hypertens 1992; 5:180–186. [DOI] [PubMed] [Google Scholar]
- 24. Olsen MH, Fossum E, Hjerkinn E, Wachtell K, Høieggen A, Nesbitt SD, Andersen UB, Phillips RA, Gaboury CL, Ibsen H, Kjeldsen SE, Julius S. Relative influence of insulin resistance versus blood pressure on vascular changes in longstanding hypertension. ICARUS, a LIFE sub study. Insulin Carotids US Scandinavia. J Hypertens 2000; 18:75–81. [DOI] [PubMed] [Google Scholar]
- 25. Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol 2004; 15:1983–1992. [DOI] [PubMed] [Google Scholar]
- 26. Bund SJ, Lee RM. Arterial structural changes in hypertension: a consideration of methodology, terminology and functional consequence. J Vasc Res 2003; 40:547–557. [DOI] [PubMed] [Google Scholar]
- 27. Sherwood A, Gullette EC, Hinderliter AL, Georgiades A, Babyak M, Waugh RA, Blumenthal JA. Relationship of clinic, ambulatory, and laboratory stress blood pressure to left ventricular mass in overweight men and women with high blood pressure. Psychosom Med 2002; 64:247–257. [DOI] [PubMed] [Google Scholar]
- 28. Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology 1990; 27:1–23. [DOI] [PubMed] [Google Scholar]
- 29. Hinderliter AL, Sager AR, Sherwood A, Light KC, Girdler SS, Willis PW., 4th Ethnic differences in forearm vasodilator capacity. Am J Cardiol 1996; 78:208–211. [DOI] [PubMed] [Google Scholar]
- 30. Alpert BS, Barnard M. Prevention of essential hypertension in minority populations. Prog Pediatr Cardiol 2001; 12:189–193. [DOI] [PubMed] [Google Scholar]
- 31. Eftekhari A, Mathiassen ON, Buus NH, Gotzsche O, Mulvany MJ, Christensen KL. Disproportionally impaired microvascular structure in essential hypertension. J Hypertens 2011; 29:896–905. [DOI] [PubMed] [Google Scholar]
- 32. Nazzaro P, Seccia T, Vulpis V, Schirosi G, Serio G, Battista L, Pirrelli A. Measures of total stress-induced blood pressure responses are associated with vascular damage. Am J Hypertens 2005; 18:1226–1232. [DOI] [PubMed] [Google Scholar]
- 33. Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension 2003; 41:1196–1201. [DOI] [PubMed] [Google Scholar]
- 34. Boutcher YN, Park YJ, Boutcher SH. Vascular and baroreceptor abnormalities in young males with a family history of hypertension. Eur J Appl Physiol 2009; 107:653–658. [DOI] [PubMed] [Google Scholar]
- 35. Hill LK, Sollers Iii JJ, Edwards CL, Thayer JF, Whitfield KE. A validation of estimated total peripheral resistance using twin data. Biomed Sci Instrum 2014; 50:210–218. [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson EH, Nazzaro P, Gilbert DC. Cardiovascular reactivity to stress in Black male offspring of hypertensive parents. Psychosom Med 1991; 53:420–432. [DOI] [PubMed] [Google Scholar]
- 37. Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. Am J Public Health 2006; 96:826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoffman RP. Effect of adolescent obesity on cardiometabolic risk in African-Americans and Caucasians. ISRN Obes 2012; 2012:603205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eftekhari A, Mathiassen ON, Buus NH, Gotzsche O, Mulvany MJ, Christensen KL. Changes in blood pressure and systemic vascular resistance do not predict microvascular structure during treatment of mild essential hypertension. J Hypertens 2012; 30:794–801. [DOI] [PubMed] [Google Scholar]
- 40. Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski J, Stone PH, Strother D, Taylor H, Sheps DS. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation 1996; 94:2402–2409. [DOI] [PubMed] [Google Scholar]
- 41. Paine NJ, Ring C, Bosch JA, McIntyre D, Veldhuijzen van Zanten JJ. The effect of acute mental stress on limb vasodilation is unrelated to total peripheral resistance. Psychophysiology 2013; 50:680–690. [DOI] [PubMed] [Google Scholar]