Abstract
BACKGROUND
Radiofrequency ablation of the renal arteries (RF-ABL) has been shown to decrease blood pressure (BP) in drug-resistant hypertensive patients who receive antihypertensive drug therapy. However, there remain questions regarding how RF-ABL influences BP independent of drug therapy and whether complete renal denervation is necessary to maximally lower BP. To study these questions, we examined the cardiovascular, sympathetic, and renal effects produced by RF-ABL of the proximal renal arteries in spontaneously hypertensive rats (SHR) with established hypertension.
METHODS
SHR were instrumented (telemetry) for measurement of systolic/diastolic BP (SBP/DBP). Rats then underwent Sham-ABL or RF-ABL adjacent to the renal ostium and BP was recorded for 8 weeks. Changes in sympathetic activity, 24-hour water/sodium excretion, and levels of urinary angiotensinogen (AGT), plasma renin activity, and kidney renin content (KRC) were measured in SHR.
RESULTS
Compared with Sham-ABL, RF-ABL produced a sustained decrease in BP. At 8 weeks, SBP/DBP was 171±6/115±3 and 183±4/129±3mm Hg for RF-ABL and Sham-ABL SHR, respectively. Correlating with the reduction in BP, RF-ABL significantly decreased the low frequency/total and low frequency/high frequency of BP variability and attenuated the hypotensive response to chlorisondamine. Kidney norepinephrine levels were markedly decreased at 8 weeks in RF-ABL vs. Sham-ABL SHR. There were no group differences in 24-hour sodium/water excretion or urinary AGT excretion rate (6 weeks) or plasma renin activity or KRC (8 weeks). In other studies, concurrent RF-ABL plus surgical denervation initially decreased BP to a greater level than RF-ABL alone, but thereafter the reduction in BP between groups was not different.
CONCLUSIONS
In hypertensive SHR, bilateral RF-ABL of the proximal renal arteries produced a sustained decease in sympathetic activity and BP without changes in sodium/water excretion or activity of the systemic/renal renin–angiotensin system.
Keywords: blood pressure, hypertension, kidney norepinephrine, radiofrequency renal nerve ablation, renal denervation, renal nerves, spontaneously hypertensive rats, sympathetic nerve activity, urinary sodium excretion.
Percutaneous catheter-based radiofrequency renal nerve ablation (RF-ABL) is being developed as an approach to denervate the renal sympathetic nerves in humans. Using this approach, early clinical studies have shown that RF-ABL is a safe and long-lasting treatment for decreasing blood pressure (BP) in patients with drug-resistant hypertension.1–4 Despite these findings, over the last couple of years, the use of RF-ABL for treatment of resistant hypertension has come under considerable debate since this approach has been shown to lower BP in some, but not all drug-resistant hypertensive patients.5–9
To clarify reasons for the discrepancies, it is important to understand the underlying mechanisms by which RF-ABL decreases BP in hypertension independent of the confounding influence of antihypertensive drugs, which themselves influence and/or block regulatory pathways that control cardiovascular and renal function. Further, there is ongoing debate as to whether ablation of the renal nerves in the proximal (renal ostium) or distal (branches) segments of the renal arteries of resistant hypertensive patients will achieve the maximal decrease in kidney norepinephrine (NE) content and therapeutic reduction in BP.8–10
To study these questions, we examined the effects of RF-ABL on cardiovascular and renal excretory function, sympathetic nerve activity, and the systemic and intrarenal renin–angiotensin system (RAS) in spontaneously hypertensive rats (SHR) with established hypertension. SHR were selected since this rat strain is an established model of essential hypertension, which in part is due to a defect in autonomic control similar to that observed in resistant hypertensive patients.11,12 To avoid the influence of antihypertensive medicine on the effects of RF-ABL, no drugs were administered to SHR in this study. RF-ABL was performed in SHR via an external approach to selectively target and ablate the renal nerves located within the wall of the renal arteries (circumferential) at a location close to the renal artery ostium, thus mimicking the ablation procedures which have been used in humans. For comparison, studies were also performed in hypertensive SHR to determine the maximal changes in BP that could be achieved by total renal denervation, i.e., a combination of RF-ABL plus surgical renal denervation. In these studies, we hypothesized that RF-ABL will produce a sustained decrease in BP via inhibition of sympathetic activity and modulation of the water/sodium excretion and the systemic/tissue renin–angiotensin system (RAS).
METHODS
Details for Material and Methods are available in Supplementary Data.
Animals
Male SHR and Wistar-Kyoto (WKY) rats (Charles Rivers) and Sprague–Dawley rats (Envigo) were used in the present studies. All procedures were conducted in accordance with the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health and the LSU Health Sciences Center Institutional Animal Care and Use Committee.
Radiotelemetry transmitter implantation
Radiotelemetry transmitters were implanted into the abdominal aorta of SHR or WKY at 19 weeks of age (Data Sciences International, DSI, St. Paul, MN) for chronic measurement of BP and heart rate using established procedures (see Supplementary Data). Baseline BP was then recorded in 20-week-old SHR or WKY for 4 consecutive days.
RF-ABL, Sham-ABL, and surgical renal denervation procedures
SHR or WKY were randomly divided into either a RF-ABL or Sham-ABL group. For these procedures, rats were anesthetized with either (i) sodium methohexital (SHR; 75mg/kg, i.p., and supplemented with 10mg/kg, i.v., as needed; King Pharmaceuticals, Bristol, TN) or (ii) isoflurane (SHR, WKY, Sprague–Dawley; 2%) and a flank incision was made to expose the left or right (random) renal artery. As described in detail in the Supplementary Data, a segment (~3mm) of the proximal renal artery close to the renal artery ostium then underwent RF-ABL (4 quadrants, 20 seconds each, 10W) or Sham-ABL (same procedure, 0W) using a Stockert 70 radiofrequency generator and probes graciously provided by Biosense Webster. In certain studies, SHR also received RF-ABL and surgical renal denervation (i.e., total denervation). The flank incision was then closed and the same procedure(s) was repeated on the contralateral renal artery. After the Sham-ABL or RF-ABL procedure, BP recording was performed daily for the first 2 weeks and then 3 times each week for the remaining study.
Note that in pilot studies we observed that in hypertensive SHR, bilateral RF-ABL produced an immediate decrease in BP, a response not reported to occur in drug-resistant patients who have RF-ABL under conscious sedation. Thus, in these studies, we used 2 different types of anesthetic agents (barbiturate vs. gaseous) to determine whether the immediate effects of RF-ABL on BP were anesthetic dependent.
Assessment of RF-ABL induced changes in sympathetic activity
Spectral density analysis of BP variability.
Arterial BP waveforms from the radiotelemetry recordings (500 Hz) were used to assess the low vs. high frequency components of BP variability by spectral analysis before (control) and after (1 week, 1 month, 2 months) RF-ABL or Sham-ABL as an index of changes in sympathetic nerve activity at these time periods.13–15 (Supplementary Data).
BP responses to ganglionic blockade.
At the end of the 8-week telemetry study, the contribution of sympathetic control of vascular tone and resting BP (telemetry) was determined in SHR by examining the change in BP 2 hours after administration of the ganglionic blocker, chlorisondamine (5mg/kg. i.p.).
Kidney NE content.
High-performance liquid chromatography was used to measure kidney NE content and thereby assess the extent of renal sympathetic denervation 2 weeks (n = 5/group) and 6 weeks (n = 7/group) following RF-ABL or Sham-ABL as detailed in Supplementary Data.
Daily water and sodium excretion
Studies were performed to examine Sham/RF-ABL induced changes in daily sodium and water excretion in hypertensive SHR placed in individual metabolic cages using established procedures (Supplementary Data).
Plasma renin activity, kidney renin content, and urinary angiotensinogen and creatinine assays
Plasma renin activity and kidney renin content (KRC) and urinary angiotensinogen (AGT) and creatinine levels were measured using established methods.16 (Supplementary Data).
Statistical analysis
Data are expressed as mean ± SEM. The magnitude of the changes in cardiovascular parameters at different time points after RF-ABL or Sham-ABL were compared between treatment groups (main effect) by a 2-way analysis of variance, followed by a Bonferroni’s post hoc test to compare variations among the treatment (interaction effects). Pearson product correlation was used to analyze the relationship between change in BP and baseline BP. Where appropriate, an unpaired Student’s t test was used to compare means between 2 groups. Statistical analysis was carried out using a software program (GraphPad Prism version 5; GraphPad Software, CA). Statistical significance was defined as P < 0.05.
RESULTS
RF-ABL produces a sustained reduction in BP in hypertensive SHR
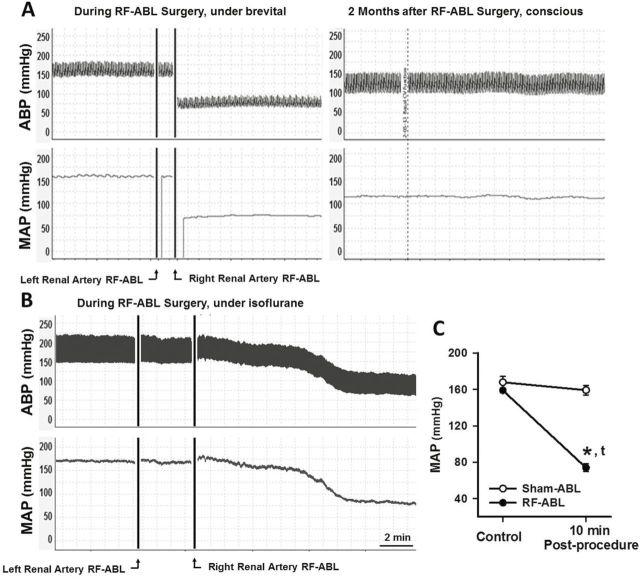
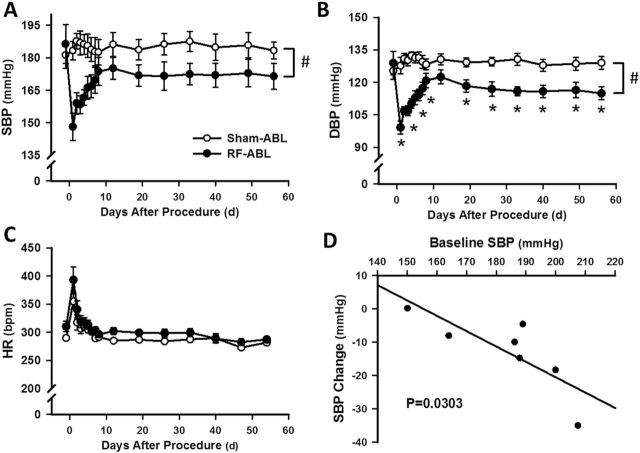
Studies were performed to examine the acute and chronic (8 weeks) changes in systemic cardiovascular function produced by bilateral RF-ABL in SHR with established hypertension. Figure 1a depicts an original tracing showing the changes in pulsatile and mean arterial pressure (MAP; telemetry) evoked by RF-ABL of the left, and then right, renal artery in a hypertensive SHR anesthetized with sodium methohexital. As shown, MAP was not altered following RF-ABL of the first (left) renal artery. However, immediately following RF-ABL of the contralateral (right) renal artery, MAP was markedly diminished from 155mm Hg (pre-ABL) to ~70mm Hg. In comparison to these findings, bilateral RF-ABL also produced a significant decrease in MAP in hypertensive SHR anesthetized with isoflurane, although the response was delayed by 6–10 minutes (Figure 1b,c). Group data demonstrating the time course of the decrease in systolic BP (SBP) and diastolic BP (DBP) produced by bilateral RF-ABL in hypertensive SHR is shown in Figure 2a,b. Over the first 10–14 days post RF-ABL, SBP and DBP progressively increased toward preablation control levels. Thereafter, over the remaining duration of the 8-week study, SBP and DBP stabilized at levels that were significantly below those of Sham-ABL-treated rats and below the respective group baseline levels of SBP/DBP prior to RF-ABL. Sham-ABL did not alter SBP/DBP in SHR over the 2-month study (Figure 2a,b). As compared to respective group control values, RF-ABL and Sham-ABL both produced a significant but transient (3–4 days) increase in heart rate in hypertensive SHR, after which levels were not different from respective control levels over the remaining 8-week study. The magnitude decrease in SBP was shown to be correlated with the baseline SBP (Figure 2d). RF-ABL or Sham-ABL did not alter SBP or DBP in normotensive WKY rats over 1 month (Supplementary Data).
Figure 1.
(a) Original tracing for a single hypertensive SHR showing pulsatile ABP and MAP responses (telemetry) produced during bilateral RF-ABL of the renal arteries under sodium methohexital anesthesia and 2-months later while conscious. (b) Original BP tracing for a single SHR during the RF-ABL procedure under isoflurane anesthesia. (c) Group data illustrating the change in MAP measured 10 minutes following completion of RF-ABL or Sham-ABL of the second renal artery conducted under isoflurane anesthesia. Sham, n = 6; RF-ABL, n = 9. Values are means ± SE. *P < 0.05, compared with Sham-ABL; tP < 0.05, compared with baseline MAP. Abbreviations: ABP, arterial blood pressure; MAP, mean arterial pressure; RF-ABL, radiofrequency ablation of the renal arteries; SHR, spontaneously hypertensive rats.
Figure 2.
Chronic effects of RF-ABL on systemic cardiovascular function in hypertensive SHR. Values are means ± SE, illustrating the chronic (2-months) changes in SBP (a), DBP (b), and HR (c) produced by RF-ABL (n = 7) or Sham-ABL (n = 7). #P < 0.05, compared with Sham-ABL (2-way ANOVA, main effect); *P < 0.05, compared with Sham-ABL value at same time point (significant interaction, Bonferroni’s post hoc test). (d) Pearson correlation between baseline and change in SBP in the same group of SHR 8 weeks after RF-ABL. Abbreviations: ANOVA, analysis of variance; DBP, diastolic blood pressure; HR, heart rate; RF-ABL, radiofrequency ablation of the renal arteries; SBP, systolic blood pressure; SHR, spontaneously hypertensive rats.
RF-ABL decreases indices of sympathetic activity in hypertensive SHR
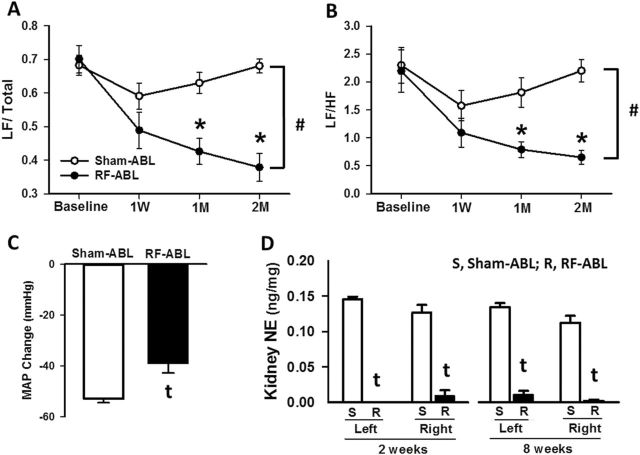
To investigate changes in autonomic cardiovascular control, changes in the frequency components of BP variability were measured by spectral analysis at baseline, 1-week, 1 month, and 2 months following Sham/RF-ABL in the same groups of hypertensive SHR for which BP data is shown in Figure 2. As compared to values for Sham-ABL rats, RF-ABL produced a significant and sustained decrease in the sympathetic component of BP variability shown as decreases in the low frequency/total and low frequency/high frequency ratios at 1- and 2-month post-RF-ABL (Figure 3a,b). Eight weeks after Sham/RF-ABL, these same groups of SHR were administered the ganglionic blocker, chlorisondamine, and changes in BP (telemetry) were measured. BP gradually decreased after drug administration and reached nadir 20–30 minutes postinjection. At the dose of 5mg/kg, i.p., chlorisondamine produces a sympathetic ganglionic blockade effect, which is demonstrated as a significant fall on BP due to inhibition of sympathetic control on vasculature. As illustrated in Figure 3c, the peak decrease in MAP produced by chlorisondamine in the RF-ABL group (39±4mm Hg) was significantly (P < 0.01) less than the maximal reduction in MAP produced by chlorisondamine in Sham-ABL-treated SHR (53±2mm Hg).
Figure 3.
Chronic effects of RF-ABL on low and high frequency components of BPV as assessed by spectral analysis in hypertensive SHR. Values are means ± SE demonstrating changes in the frequency components of BPV expressed as (a) LF/total and (b) LF/HF at baseline, 1W, 1M, and 2M after RF-ABL or Sham-ABL. (c) MAP response to chlorisondamine (5mg/kg, i.p.) administration in SHR 8 weeks following RF-ABL or Sham-ABL (n=7/group). (D) Norepinephrine content measured in left and right kidney cortex from groups of SHR at 2 weeks (n = 5/group) and 8 weeks (n = 7/group) following Sham-ABL (S) or RF-ABL (R). Values are means ± SE. #P < 0.05, compared with Sham-ABL (2-way ANOVA, main effect); *P < 0.05, compared with Sham-ABL value at same time point (significant interaction, Bonferroni’s post hoc test). tP < 0.05, compared with Sham-ABL (Student’s t test). Abbreviations: ANOVA, analysis of variance; BPV, BP variability; DBP, diastolic blood pressure; HF, high frequency; HR, heart rate; LF, low frequency; M, month; MAP, mean arterial pressure; RF-ABL, radiofrequency ablation of the renal arteries; SBP, systolic blood pressure; SHR, spontaneously hypertensive rats; W, week.
The effects of RF-ABL and Sham-ABL on kidney (cortex) NE levels from SHR at the completion of the 8-week telemetry study are shown in Figure 3d. For comparison, values for renal cortical NE levels are also shown for separate groups of SHR that underwent RF-ABL or Sham-ABL 2 weeks prior to catecholamine measurement. As compared to levels in Sham-ABL rats, NE content was dramatically (P < 0.001) decreased in both the left and right kidneys of hypertensive SHR 2 weeks and 8 weeks following RF-ABL.
BP response produced by combination of RF-ABL and surgical renal denervation (i.e., total denervation) in hypertensive SHR
Changes in mean SBP and DBP produced by Sham-ABL, RF-ABL, and the combination of RF-ABL and surgical renal denervation (i.e., total denervation) in groups of hypertensive SHR are shown in Figure 4. Compared to RF-ABL, the total denervation group had a greater decrease in DBP, but not SBP, for the first 5 days after the procedure. However, at subsequent time points DBP levels were not different between these groups. Interestingly, the variation in BP observed in individual rats over the course of the first 2-week postprocedure was less in rats from the total denervation group as compared to animals that only received RF-ABL (Supplementary Data).
Figure 4.
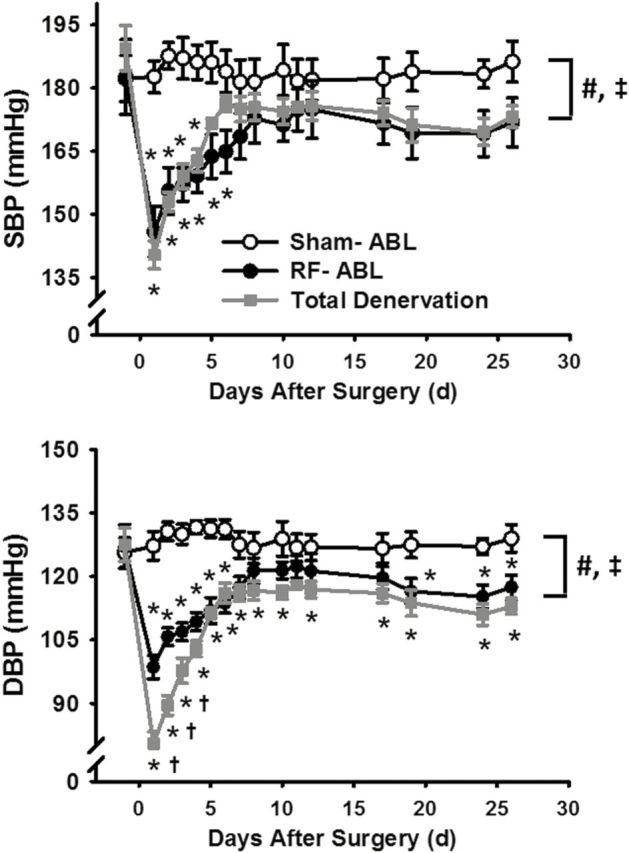
Changes in SBP and DBP produced by Sham-ABL (n = 7), RF-ABL (n = 8), and total renal denervation (RF-ABL plus surgical renal denervation; n = 5) in hypertensive SHR. Values are means ± SE for each group; #P < 0.05, RF-ABL compared with Sham-ABL (2-way ANOVA, main effect); ‡P < 0.05, total denervation compared with Sham-ABL; *P < 0.05, compared with Sham-ABL value at same time point; †P < 0.05, compared with RF-ABL value at same time point (significant interaction, Bonferroni’s post hoc test). Abbreviations: ANOVA, analysis of variance; DBP, diastolic blood pressure; SBP, systolic blood pressure; RF-ABL, radiofrequency ablation of the renal arteries; SHR, spontaneously hypertensive rats.
Effects of RF-ABL on daily sodium and water excretion and activity of the RAS in SHR under basal conditions
Studies were performed in hypertensive SHR to examine changes in the 24-hour renal excretion of water/sodium and food intake at time points 2 weeks and 6 weeks following RF-ABL. As depicted in Table 1, 2 weeks following RF-ABL there was a slight but significant increase in 24-hour urinary sodium excretion and increase in food (and thus salt) intake as compared to respective levels observed in hypertensive Sham-ABL-treated rats. However, RF-ABL did not change urinary protein, AGT or creatinine levels. Six-weeks following RF-ABL the levels for urinary sodium excretion, food intake, urinary protein, AGT, and creatinine levels were not different than those observed in Sham-ABL-treated SHR (Table 2). Further, in other studies, there were no differences in plasma renin activity or KRC between groups of RF-ABL and Sham-ABL SHR measured 8 weeks following treatment (Supplementary Data).
Table 1.
Effects of Sham/RF-ABL on BP and renal excretory parameters measured 2 weeks postprocedure in SHR
| 24 hours | Sham-ABL | RF-ABL |
|---|---|---|
| Systolic BP (mm Hg) | 182±3 | 173±6 |
| Diastolic BP (mm Hg) | 125±6 | 117±3 |
| Food intake (g) | 16.9±0.78 | 21.0±0.8* |
| Water intake (ml) | 24.6±2.7 | 26.5±1.8 |
| Urine volume (ml) | 5.56±0.58 | 5.73±0.66 |
| Urinary Na+ excretion (mEq) | 0.25±0.03 | 0.33±0.02* |
| Urinary protein (mg) | 10.5±0.4 | 10.6±0.6 |
| Urinary AGT (ng) | 135.3±12.0 | 131.7±16.0 |
| Urinary creatinine (mg) | 15.0±0.7 | 15.7±0.6 |
Abbreviations: AGT, angiotensinogen; BP, blood pressure; RF-ABL, radiofrequency ablation; SHR, spontaneously hypertensive rats; Sham-ABL, Sham ablation.
*P < 0.05, compared with Sham-ABL; N = 8/group.
Table 2.
Effects of Sham/RF-ABL on BP and renal excretory parameters measured 6 weeks postprocedure in SHR
| 24 hours | Sham-ABL | RF-ABL |
|---|---|---|
| Systolic BP (mm Hg) | 192±5 | 173±5* |
| Diastolic BP (mm Hg) | 131±4 | 118±2* |
| Food intake (g) | 17.1±0.7 | 16.9±0.6 |
| Water intake (ml) | 25.8±1.8 | 26.0±1.6 |
| Urine volume (ml) | 9.13±1.6 | 8.82±1.3 |
| Urinary Na+ excretion (mEq) | 0.43±0.03 | 0.45±0.02 |
| Urinary protein (mg) | 12.0±1.01 | 10.9±0.31 |
| Urinary AGT (ng) | 143±21.9 | 144±11.7 |
| Urinary creatinine (mg) | 21.0±1.53 | 20.1±0.97 |
Abbreviations: AGT, angiotensinogen; BP, blood pressure; RF-ABL, radiofrequency ablation; SHR, spontaneously hypertensive rats; Sham-ABL, Sham ablation.
*P < 0.05, compared with Sham-ABL; N = 8/group.
DISCUSSION
In the present study, we examined the cardiovascular and renal responses produced by bilateral RF-ABL of the renal arteries in SHR with established hypertension. Using this approach, RF-ABL, but not Sham-ABL, decreased BP in hypertensive SHR for the course of the 2-month study. This is in contrast to findings which demonstrate that surgical renal denervation delays the development of high BP in young (7 weeks of age) SHR,12,17 transiently (2 weeks) lowers BP in 12-week-old SHR,18 but did not alter BP in older SHR (18 weeks old) with established hypertension.12
RF-ABL produced a characteristic pattern of acute and chronic changes in BP in hypertensive SHR. Upon RF-ABL of the first renal artery, BP remained unchanged. However, after ablating the contralateral renal artery, BP markedly decreased with the time course of the response dependent on the anesthesia used during the procedure. In SHR anesthetized with sodium methohexital, RF-ABL of the second renal artery produced an immediate (seconds) decrease in BP. With isoflurane, the decrease in BP to RF-ABL occurred over a 5- to 10-minute period, yet the decrease was of similar magnitude. With either anesthetic, Sham treatment did not alter BP in SHR. The observation that BP rapidly and dramatically decreased in SHR only after ablation of the second renal artery and was independent of barbiturate or gaseous anesthesia, served in our hands as a functional biomarker to indicate that the RF-ABL procedure was successful in denervating both kidneys. This was later confirmed by the observation that after completion of the 2-month study, postmortem renal cortex NE levels remained markedly decreased in RF-ABL vs. Sham-ABL SHR. These findings are of importance since previous studies investigating the effects of RF-ABL in humans or other animal models have not previously documented an immediate decrease in BP during the RF-ABL procedure. One important exception is the finding by Heuser et al., who demonstrated that in resistant hypertensive patients undergoing gaseous general anesthesia, nonvascular (intraureteral) delivery of RF energy to the renal pelvis (Verve Medical NephroBlate) decreased BP within 30 seconds from ablating the first kidney.19 Thus, the immediate depressor effects of RF-ABL may be dependent upon the type of anesthesia (e.g., conscious sedation or general anesthesia) used during the procedure.
In the present studies, all visible renal nerve bundles were carefully dissected away from the segment of the renal artery that was to be ablated and were left intact. Therefore, we speculated that activity of these intact renal nerves, which innervate the renal artery and kidney distal to our ablation site, would remain functionally active and therefore could prevent the maximal decrease in BP produced by RF-ABL from occurring. Accordingly, in SHR that underwent “total” renal denervation (RF-ABL plus surgical denervation) the initial peak decrease in BP was significantly greater in magnitude than that produced by RF-ABL alone. Despite these observations, by the end of the 4-week study, the level of BP in the total denervation group was not significantly lower than that attained by RF-ABL alone. These observations are of merit since in separate investigations we histologically analyzed the innervation of the kidney following Sham-ABL or RF-ABL in hypertensive SHR using the same treatment procedure (circumferential RF-ABL of the proximal renal artery).20 In these studies, 5-weeks following RF-RDN it was observed that tyrosine hydroxylase staining of the renal artery, which is used as an indicator of renal nerve viability, was significantly reduced (≃50%), but variable, as compared to arteries from the Sham-RDN group.20 Thus, in hypertensive SHR, bilateral RF-ABL of the proximal renal arteries was sufficient to markedly and chronically decrease kidney NE content and produce the maximal decrease in BP that can be achieved via renal denervation (i.e., as compared to total renal denervation). These findings are of clinical interest since there is ongoing debate as to whether ablation of the renal nerves in the proximal (renal ostium) or distal (branches) segments of the renal arteries of resistant hypertensive patients will achieve the maximal renal denervation, decrease in kidney NE content, and therapeutic reduction in BP.8–10
It has been speculated that by preventing heightened renal afferent signals to the brain, RF-ABL lowers BP in resistant hypertensive patients by decreasing central sympathetic outflow.19,21–23 Support for this premise stems from studies demonstrating that RF-ABL produced a decrease in NE overflow from the kidneys5 and a decrease in mesenteric sympathetic nerve activity in some19,24 but not all25,26 resistant hypertensive patients, who also were continued on their antihypertensive therapy. In our studies, 3 lines of evidence support the premise that RF-ABL decreases BP in hypertensive SHR via inhibiting central sympathetic outflow. First, at 1- and 2-month postprocedure, the low-frequency (sympathetic) component of BP fluctuations was significantly decreased in SHR treated with RF-ABL. Similarly, RF-ABL in patients with resistant hypertension has been shown to decrease the low-frequency component of BP signals.27 Second, the decrease in BP produced by the ganglionic blocker, chlorisondamine, was attenuated in SHR that had previously (8 weeks) undergone RF-ABL. Third, we observed a dramatic and sustained (8 weeks) decrease in kidney cortex NE content in RF-ABL as compared to Sham-ABL-treated SHR. In regards to the latter findings, Winternitz et al.12 reported that surgical renal denervation decreased kidney NE content in SHR by 94% at week 1 but the levels increased to ~57% of that in Sham SHR at week 6. In line with these findings, pivotal studies by Mulder et al.28 showed that in normotensive rats, reinnervation of the renal sensory and renal sympathetic nerves occurs over the same time course, both being complete by 9–12 weeks following surgical renal denervation.
Activation of the renal sympathetic nerves produces a frequency-dependent increase in renin secretion, renal tubular reabsorption of sodium, and decrease in renal blood flow.29,30 Thus, we speculated that in the SHR, an animal model of essential and neurogenic hypertension, the sympathoinhibitory effects produced by RF-ABL may potentially contribute to a sustained decrease in BP via chronic modulation of the renal excretion of sodium/water and/or alteration in the systemic/tissue RAS. In accord with this premise, we observed a slight but significant increase in urinary sodium excretion measured 2 weeks after RF-ABL. However, when measured at 6-week postablation, there were no differences in any renal excretory parameters between Sham- and RF-ABL groups. Further, RF-ABL did not alter plasma renin concentration or KRC. Consistent with these findings in SHR, plasma renin and aldosterone levels were unchanged from baseline levels following RF-ABL in resistant hypertensive patients.29 Despite a further increase in BP with age, urinary AGT levels did not change in SHR measured 2 weeks vs. 6 weeks after Sham-ABL (rats 22 vs. 26 weeks of age). It has been suggested that increased urinary AGT levels serve as a marker of hypertension evoked by a heightened intrarenal angiotensin II system.30,31 Although these findings suggest that SHR have a heightened RAS,30 our findings showed that the RF-ABL procedure did not alter the systemic or renal RAS. Thus, in contrast to our original hypothesis, these findings indicate that the sustained decrease in BP produced by RF-ABL in SHR is not mediated by persistent alterations in the renal excretion of sodium/water or alteration in the systemic/kidney RAS. However, it is important to note that a major source of increased sympathetic outflow, which can influence BP via constriction of mesenteric resistance vessels, occurs via activation of the central RAS.32,33 Whether RF-ABL decreases sympathetic activity and BP in SHR via blocking an afferent nerve pathway involved in stimulation of the central RAS remains to be investigated.
In conclusion, these findings indicate that in hypertensive SHR, RF-ABL of the renal arteries at a site adjacent to the renal artery ostium is sufficient to markedly reduce renal tissue NE concentration and produce the maximal sustained decrease in BP achievable by renal denervation. Our findings also provide strong evidence that in this hypertensive animal model, the decrease in BP produced by RF-ABL occurred, at least in part, due to inhibition of central sympathetic nerve activity. Interestingly, despite the known roll of the renal nerves in fluid-electrolyte homeostasis, the effects of RF-ABL on BP occurred independent of changes in the renal excretion of water/sodium or the circulating-kidney RAS.
SUPPLEMENTARY MATERIAL
Supplementary Data are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of General Medical Sciences IDeA Program (COBRE, P30GM106392 to D.R.K. and P30GM103337 to L.G.N.), Biosense Webster (IIS-175 to D.R.K. and F.S.), and the American Heart Association (12GRNT12060613 to D.R.K. and 14POST20450173 to J.G.).
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC).Vital signs: prevalence, treatment, and control of hypertension—United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep 2011; 60:103–108. [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC).Vital signs: awareness and treatment of uncontrolled hypertension among adults--United States, 2003–2010. MMWR Morb Mortal Wkly Rep 2012; 61:703–709. [PubMed] [Google Scholar]
- 3. Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T; American Heart Association/American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation 2006; 113:e409–e449. [PubMed] [Google Scholar]
- 4. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 5. Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 6. Heuser RR, Mhatre AU, Buelna TJ, Berci WL, Hubbard BS. A novel non-vascular system to treat resistant hypertension. EuroIntervention 2013; 9:135–139. [DOI] [PubMed] [Google Scholar]
- 7. Esler M. Renal denervation for hypertension: observations and predictions of a founder. Eur Heart J 2014; 35:1178–1185. [DOI] [PubMed] [Google Scholar]
- 8. Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015; 28:909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahfoud F, Tunev S, Ewen S, Cremers B, Ruwart J, Schulz-Jander D, Linz D, Davies J, Kandzari DE, Whitbourn R, Böhm M, Melder RJ. Impact of lesion placement on efficacy and safety of catheter-based radiofrequency renal denervation. J Am Coll Cardiol 2015; 66:1766–1775. [DOI] [PubMed] [Google Scholar]
- 10. Cohen-Mazor M, Mathur P, Stanley JR, Mendelsohn FO, Lee H, Baird R, Zani BG, Markham PM, Rocha-Singh K. Evaluation of renal nerve morphological changes and norepinephrine levels following treatment with novel bipolar radiofrequency delivery systems in a porcine model. J Hypertens 2014; 32:1678–1691; discussion 1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamori Y. Pathogenesis of spontaneous hypertension as a model for essential hypertension. Jpn Circ J 1977; 41:259–266. [DOI] [PubMed] [Google Scholar]
- 12. Winternitz SR, Katholi RE, Oparil S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J Clin Invest 1980; 66:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol 2007; 34:362–368. [DOI] [PubMed] [Google Scholar]
- 14. Baltatu O, Janssen BJ, Bricca G, Plehm R, Monti J, Ganten D, Bader M. Alterations in blood pressure and heart rate variability in transgenic rats with low brain angiotensinogen. Hypertension 2001; 37:408–413. [DOI] [PubMed] [Google Scholar]
- 15. Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991; 84:482–492. [DOI] [PubMed] [Google Scholar]
- 16. Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol 2009; 296:F1067–F1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenberg S, Osborn JL. Relationship between sodium balance and renal innervation during hypertension development in the spontaneously hypertensive rat. J Hypertens 1994; 12:1359–1364. [PubMed] [Google Scholar]
- 18. Pires NM, Igreja B, Moura E, Wright LC, Serrão MP, Soares-da-Silva P. Blood pressure decrease in spontaneously hypertensive rats following renal denervation or dopamine β-hydroxylase inhibition with etamicastat. Hypertens Res 2015; 38:605–612. [DOI] [PubMed] [Google Scholar]
- 19. Hering D, Marusic P, Walton AS, Lambert EA, Krum H, Narkiewicz K, Lambert GW, Esler MD, Schlaich MP. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension 2014; 64:118–124. [DOI] [PubMed] [Google Scholar]
- 20. Polhemus DJ, Gao J, Scarborough A, Trivedi RK, McDonough KH, Goodchild TT, Smart F, Kapusta DR, Lefer DJ. Radiofrequency renal denervation protects the ischemic heart via inhibition of GRK2 and increased nitric oxide signaling. Cir Res 2016; 119:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ye S, Gamburd M, Mozayeni P, Koss M, Campese VM. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens 1998; 11:723–728. [DOI] [PubMed] [Google Scholar]
- 22. Campese VM, Ku E, Park J. Sympathetic renal innervation and resistant hypertension. Int J Hypertens 2011; 2011:814354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol 2015; 308:R79–R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 2013; 61:457–464. [DOI] [PubMed] [Google Scholar]
- 25. Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J, Tank J. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension 2012; 60:1485–1490. [DOI] [PubMed] [Google Scholar]
- 26. Grassi G, Seravalle G, Brambilla G, Trabattoni D, Cuspidi C, Corso R, Pieruzzi F, Genovesi S, Stella A, Facchetti R, Spaziani D, Bartorelli A, Mancia G. Blood pressure responses to renal denervation precede and are independent of the sympathetic and baroreflex effects. Hypertension 2015; 65:1209–1216. [DOI] [PubMed] [Google Scholar]
- 27. Ewen S, Dörr O, Ukena C, Linz D, Cremers B, Laufs U, Hamm C, Nef H, Bauer A, Mancia G, Böhm M, Mahfoud F. Blood pressure variability after catheter-based renal sympathetic denervation in patients with resistant hypertension. J Hypertens 2015; 33:2512–2518. [DOI] [PubMed] [Google Scholar]
- 28. Mulder J, Hökfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 2013; 304:R675–R682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ezzahti M, Moelker A, Friesema EC, van der Linde NA, Krestin GP, van den Meiracker AH. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens 2014; 32:135–141. [DOI] [PubMed] [Google Scholar]
- 30. Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol 2005; 16:2073–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59:251–287. [DOI] [PubMed] [Google Scholar]
- 32. DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens 2002; 11:197–200. [DOI] [PubMed] [Google Scholar]
- 33. Raizada MK, Lu D, Sumners C. AT1 receptors and angiotensin actions in the brain and neuronal cultures of normotensive and hypertensive rats. Adv Exp Med Biol 1995; 377:331–348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.