Abstract
Aims
To investigate the possibility that vorticity assessed by four-dimensional flow cardiac magnetic resonance (4D-Flow CMR) in the left ventricle of patients with mild-to-moderate chronic obstructive pulmonary disease (COPD) is a potential marker of early LV diastolic dysfunction (LVDD) and more sensitive than standard echocardiography, and whether changes in vorticity are associated with quantitative computed tomography (CT) and clinical markers of COPD, and right ventricular (RV) echocardiographic markers indicative of ventricular interdependency.
Methods and results
Sixteen COPD patients with presumptive LVDD and 10 controls underwent same-day 4D-Flow CMR and Doppler echocardiography to quantify early and late diastolic vorticity as well as standard evaluation for LVDD. Furthermore, all patients underwent detailed CT analysis for COPD markers including percent emphysema and air trapping. The 4D-Flow CMR derived diastolic vorticity measures were correlated with CT measures, standard clinical and CMR markers, and echocardiographic diastolic RV metrics. Early diastolic vorticity was significantly reduced in COPD patients (P < 0.0001) with normal left ventricular (LV) mass, geometry, systolic function, and no or mild signs of Doppler LVDD when compared with controls. Vorticity significantly differentiated COPD patients without echocardiographic signs of LVDD (n = 11) from controls (P < 0.0001), and from COPD patients with stage I LVDD (n = 5) (P < 0.0180). Vorticity markers significantly correlated with CT computed measures, CMR-derived RV ejection fraction, echocardiographic RV diastolic metrics, and 6-minute walk test.
Conclusion
4D-Flow CMR derived diastolic vorticity is reduced in patients with mild-to-moderate COPD and no or mild signs of LVDD, implying early perturbations in the LV flow domain preceding more obvious mechanical changes (i.e. stiffening and dilation). Furthermore, reduced LV vorticity appears to be driven by COPD induced changes in lung tissue and parallel RV dysfunction.
Keywords: COPD, 4D-flow CMR, left ventricle diastolic dysfunction
Introduction
Chronic obstructive pulmonary disease (COPD) is a known risk factor for left ventricular (LV) remodelling and dysfunction.1,2 The pathophysiology of LV diastolic dysfunction (LVDD) in the setting of COPD is currently thought to be mediated primarily by means of lung hyperinflation and ventricular interdependency due to increased right ventricular (RV) afterload.3–5 Importantly, LVDD has been shown to significantly worsen survival in COPD patients, emphasizing the need for an accurate and early screening diagnostic technique.2 However, non-invasive early characterization and diagnosis of LVDD in the presence of COPD has proved to be a difficult task due to associated comorbidities and the fact that LVDD may occur prior to development of LV hypertrophy and stiffness, i.e. noticeable tissue mechanical changes.6–9
While the Doppler echocardiography is the most widely used and clinically accessible technique, it is limited by its inability to measure velocity in all spatial directions, variable velocity acquisition assessment due to probe alignment, and interpretation of velocity metrics due to simplified flow theorems.10–14 In contrast, four-dimensional flow cardiac magnetic resonance (4D-Flow CMR) can provide complete spatiotemporal information about velocity and allows for quantitative and qualitative analysis in any given anatomical region of interest.13,15,16 Furthermore, early detection of LVDD has recently been suggested to be investigated by means of fluid-tissue interactions and large scale vortex formations with the premise that changes in the ventricular flow domain would be detectable sooner than early stage tissue stiffening.17,18 Accordingly, we sought to investigate vorticity and vortex formations in patients with mild-to-severe COPD and presumptive LVDD via 4D-Flow CMR. Specifically, we aimed to evaluate LV diastolic vorticity previously shown to be a sensitive measure of ventricular interdependency and diastolic dysfunction in pulmonary hypertension.19 We further sought to correlate LV vorticity with: (i) metrics of lung hyperinflation using chest computed tomography (CT) markers and (ii) ventricular interdependency via standard volumetric CMR and echocardiographic indices.
Methods
Patients with diagnosed COPD and presumptive LVDD (n = 16) underwent a prospective Institutional Review Board approved same-day echocardiography study followed by 4D-Flow CMR. Additionally, control subjects with the same protocol (n = 10) were retrospectively selected in order to establish physiologic range of 4D-Flow CMR derived diastolic markers. COPD was defined by means of ratio between forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) < 0.70.20 Severity of COPD was determined using spirometry GOLD criteria.21 Included patients had no prior history of cardiovascular surgery, arrhythmia, and myocardial ischaemia. All control subjects were without prior history of cardiovascular disease. Informed consent was obtained in all subjects.
Echocardiography
All subjects underwent standard tissue Doppler ECHO using a Vivid 7 ultrasound system (General Electric Medical Systems, Milwaukee, WI). All ventricular diastolic markers were acquired according to consensus based recommendations of the American Society of Echocardiography (ASE) and the European Association of Echocardiography (EAE).10,22,23 The following standard parameters were collected from the mitral valve (MV) and tricuspid valve (TV) inflow: (i) peak early diastolic filling velocity (E), (ii) peak late atrial filling velocity (A), and (iii) corresponding E/A ratio. Furthermore, mitral annular Doppler tissue imaging parameters e’ and a’ were recorded in each subject. The stage of diastolic dysfunction for each ventricle was categorized using consensus recommendations.23
Quantitative CT markers of COPD
All COPD patients underwent thoracic CT on Siemens 64-slice helical scanner (120 kVp, 100 mA at exposure time 0.5 s) with 0.75-mm slice thickness and standard (b31f) reconstruction kernel. Quantitative analysis of CT-included semiautomatic lung segmentation and lung voxel histogram calculation on inspiratory and expiratory series.24 Whole-lung emphysema score was computed as the percentage of lung voxels with intensity less than −950 Hounsfield units (HU) on inspiratory scans. In addition, the 15th percentile of the lung voxel histogram, i.e. the threshold at which 15% of voxels have a lower density, was computed for each inspiratory series.25 Air trapping was quantified on expiratory CT by calculation of the percentage of lung voxels with intensity value less than the established threshold of −856 HU.
CMR and 4D-flow CMR protocol
CMR with 4D-Flow technique was performed using a 1.5 T MRI Siemens system (MAGNETOM, Avanto, Erlangen, Germany) with 8-channel phased array coil. The field of view was oriented to cover the entire mediastinum and great vessels. 4D-Flow CMR was performed with interleaved 3D velocity encoding (spatial resolution = 2.4–2.6 × 2.4–2.6 x 2.4–3.0 mm, α = 14–15°, TE/TR = 2.85/48.56 ms). Velocity encoding values ranged between 100 and 150 cm/s. 4D Flow CMR images were acquired using a RF-spoiled gradient echo pulse sequence, prospective ECG gating, and respiratory navigators using bellows.
A cine steady-state free precession (SSFP) technique with retrospective gating was used to image the heart from the base to apex during brief end-expiratory breath-holds using contiguous short-axis slices in 8 mm increments. Ventricular volumetric and functional analyses were performed off-line by a blinded reader using commercially available software (Argus, MR B17, Siemens AG Healthcare Sector, Erlangen, Germany). Ventricular end-diastolic and end-systolic contours were manually traced in the axial view for each slice. LV and RV ejection fraction (LVEF and RVEF) were determined using the modified Simpson’s rule.
Vorticity computation
The raw 4D-Flow CMR datasets were preprocessed and corrected for phase offset errors, noise, and anti-aliasing as described previously using standard recommendations.13 The preprocessed 4D-Flow CMR datasets were then converted for quantitative and qualitative analysis (Paraview, Kitware, Clifton, NY, USA) using a custom-made MATLAB program (Mathworks, Natick, MA, USA).26
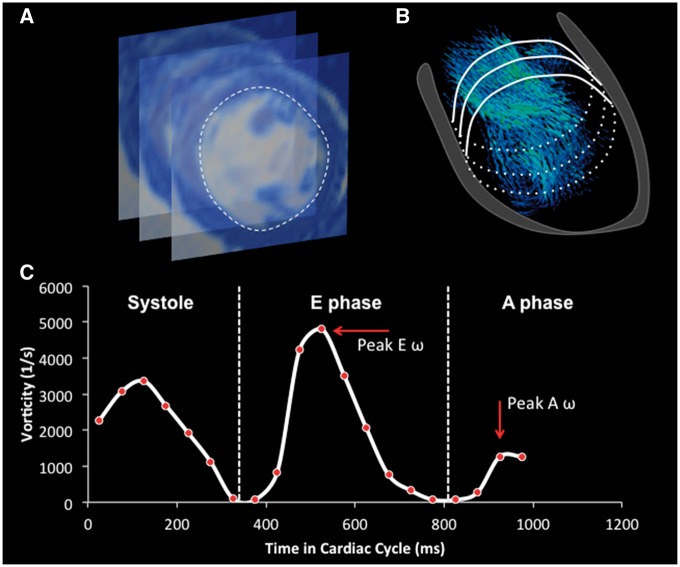
Vorticity within the specific ventricular compartment was calculated as shown previously.19 Briefly, time sampled vorticity was spatially integrated along the entire ventricular volume defined on the basal side by annular rings and by the endocardial segmentation contours created from SSFP short axis images (see Figure 1). Peak E and A phase vorticity were then defined as the maximum vorticity values within the corresponding diastolic phase.
Figure 1.
The work-flow process towards vorticity computation. The SSFP images served for delineation of the LV endocardial border in diastole (A). Segmented contours were then superimposed on the velocity vector field (B). Vorticity was computed within the isolated LV flow domain from the corresponding velocity vector field throughout the entire cardiac cycle, and peak E and A wave vorticity were sampled from each subject (C).
In order to investigate large flow formations within the ventricles, we have also evaluated every subject for the presence of vortex formation. Prominent diastolic vortices in healthy individuals are formed during diastolic phase along the mitral valve leaflets. Every subject was evaluated then for the presence of anterior and posterior mitral valve leaflets (AML and PML). A fully developed vortex was defined as the complete concentric set of rings detected by either streamline or vector projection at any point in diastolic cardiac phase.
Statistical analysis
Analyses were performed using JMP 10.0 (SAS Institute, Cary, NC, USA). Variables were checked for the distributional assumption of normality using normal plots, in addition to Kolmogorov–Smirnov and Shapiro–Wilks tests. Variables that were positively skewed (e.g. vorticity and chest CT metrics) were naturally log-transformed for the analyses. All normally distributed group specific data sets are reported as mean with corresponding standard deviations or as median values with interquartile range if non-uniformly distributed. Demographic and clinical characteristics among COPD patients and controls were compared using a student’s t-test for normally distributed continuous variables, Wilcoxon ranked sum test for non-uniform distributed variables, and χ2 for categorical variables. One-way analysis of variance was applied to investigate variability in subgroup analysis. Generalized linear regression models were employed to evaluate the associations between natural log of vorticity metrics and known COPD risk predictors [chest CT metrics, RVEF, and 6-minute walk test (6-MWT)]. Significance was based on an α-level of 0.05.
Results
All subjects successfully underwent same-day 4D-Flow CMR and Doppler Echo evaluation. For each subject, the LV velocity and vorticity vector fields were successfully generated to perform both qualitative and quantitative diastolic inflow evaluation (see Figure 2). The standard haemodynamic, demographic, and pulmonary function data are summarized in Table 1. Based on FEV1 values, 3 COPD patients had stage I and 13 had stage II disease per GOLD spirometry criteria. The mean 6MWT distance (m) was 1329 ± 302. The CMR-derived LV systolic and volumetric measures were not different between evaluated groups, except significantly increased CO in the COPD group (4.6 ± 0.8 vs. 3.8 ± 0.6, P < 0.05). Correspondingly, heart rate was elevated in the COPD subjects when compared with healthy controls (78 ± 11 vs. 57 ± 7, P < 0.001). The RV volumetric measures did not reveal any significant variabilities between considered cohorts. However, RVEF (%) was significantly reduced in the COPD group (51 ± 7 vs. 59 ± 9, P < 0.01).
Figure 2.
Visualization of velocity and vorticity vector fields during the early diastolic phase in the left ventricle in a control subject. Short-axis (SA) visualization of the vorticity vector field shows strong vortex ring generated at the early filling phase (top right).
Table 1.
Demographics, standard haemodynamics, spirometry
| COPD (n = 16) |
|||
|---|---|---|---|
| Variable | Control (n = 10) | No LVDD (n = 11) | LVDD (n = 5) |
| Age (year) | 57 ± 9 | 64 ± 7 | 65 ± 2* |
| Sex (female) | 30% | 27% | 40% |
| BSA (m2) | 1.87 ± 0.24 | 1.82 ± 0.20 | 1.78 ± 0.21 |
| LV CO (L/min) | 3.8 ± 0.6 | 4.9 ± 0.8* | 4.2 ± 0.5 |
| LVEF (%) | 70 ± 7 | 70 ± 8 | 67 ± 13 |
| LV SV (mL) | 68 ± 11 | 65 ± 18 | 54 ± 13 |
| LV EDV (mL) | 98 ± 24 | 94 ± 21 | 81 ± 17 |
| LV ESV (mL) | 30 ± 13 | 29 ± 15 | 26 ± 16 |
| LV mass (g) | 62.2 ± 15.6 | 69 ± 19 | 70 ± 18 |
| LAVI (mL/m2) | 32 ± 5 | 31 ± 8 | 33 ± 7 |
| RV EF (%) | 59 ± 9 | 49 ± 6* | 52 ± 11 |
| RV SV (mL) | 66 ± 16 | 61 ± 8 | 64 ± 19 |
| RV EDV (mL) | 112 ± 28 | 123 ± 22 | 121 ± 14 |
| RV ESV (mL) | 46 ± 15 | 62 ± 17 | 56 ± 10 |
| HR (bpm) | 57 ± 7 | 78 ± 11** | 79 ± 13* |
| FEV1 predicted (%) | N/A | 61 ± 12 | 59 ± 10 |
| FVC (%) | N/A | 83 ± 10 | 84 ± 9 |
| FEV1/FVC | N/A | 55 ± 9 | 53 ± 9 |
| 6-MWT (m) | N/A | 426 ± 82 | 353 ± 90 |
All values are reported as means with corresponding ± SD.
BSA, body surface area; LV CO, left ventricular cardiac output; LVEF, left ventricular ejection fraction; LV SV, left ventricular stroke volume; LV EVD, left ventricular end diastolic volume; LV ESV, left ventricular end systolic volume; LAVI, left atrial volume index; RVEF, right ventricular ejection fraction; RV SV, right ventricular stroke volume; RV EDV, right ventricular end diastolic volume; RV ESV, right ventricular end systolic volume; HR, heart rate; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; 6-MWT, six minute walk test; COPD, chronic obstructive pulmonary disease; LVDD, left ventricular diastolic dysfunction.
P < 0.05.
P < 0.001 with respect to controls.
Doppler echocardiography
Table 2 contains a comprehensive summary of all transvalvular and tissue Doppler velocities. Five COPD patients presented Grade I LV diastolic dysfunction according to ASE/EAE criteria.23 The mean values of LV transmitral velocity and tissue Doppler indices (all in cm/s) for both groups were considered within the normal range.10 However, MV A velocity was increased in the COPD group (72 ± 18 vs. 49 ± 15, P < 0.01) and correspondingly MV E/A ratio was lower in this group (0.97 ± 0.29 vs. 1.46 ± 0.30, P < 0.01). Furthermore, COPD patients showed significantly higher MV lateral a’ velocity (13.6 ± 4.0 vs. 9.0 ± 1.6, P < 0.001) and MV septal a’ velocity (11.2 ± 2.0 vs. 8.7 ± 1.6, P < 0.01). Three COPD patients presented a mild mitral regurgitation.
Table 2.
Ventricular diastolic parameters
| Variable | Control (n = 10) | COPD (n = 16) | P-value |
|---|---|---|---|
| MV E (cm/s) | 70 ± 17 | 65 ± 16 | NS |
| MV A (cm/s) | 49 ± 15 | 72 ± 18 | <0.01 |
| MV E/A ratio | 1.46 ± 0.30 | 0.97 ± 0.29 | <0.01 |
| MV lat e’ (cm/s) | 11.7 ± 3.2 | 10.5 ± 1.6 | NS |
| MV lat a’ (cm/s) | 9.0 ± 1.6 | 13.6 ± 4.0 | <0.001 |
| MV sept e’ (cm/s) | 9.9 ± 2.7 | 9.5 ± 1.8 | NS |
| MV sept a’ (cm/s) | 8.7 ± 1.6 | 11.2 ± 2.0 | <0.01 |
| MV sep E/e’ ratio | 7.2 ± 2.1 | 6.9 ± 1.8 | NS |
| MV lat E/e’ ratio | 6.1 ± 2.6 | 6.2 ± 1.8 | NS |
| TV E (cm/s) | 43 ± 7 | 44 ± 13 | NS |
| TV A (cm/s) | 25 ± 7 | 41 ± 34 | <0.05 |
| TV E/A ratio | 1.7 ± 0.5 | 1.0 ± 0.3 | <0.01 |
| TV e’ (cm/s) | 13 ± 4 | 11 ± 4 | NS |
| TV E/e’ ratio | 3.3 ± 0.9 | 4.1 ± 1.6 | NS |
All values are reported as means with corresponding ± SD.
SD, standard deviation; NS, not significant; COPD, chronic obstructive pulmonary disease; MV, mitral valve; TV, tricuspid valve; E, peak early diastolic filling velocity; A, peak late atrial filling velocity.
Two and three COPD patients showed Grade I and Grade II RV diastolic dysfunction, respectively according to ASE guidelines.23 The TV A velocity was significantly increased in the COPD group (52 ± 34 vs. 25 ± 7, P < 0.05) and correspondingly TV E/A ratio was lower in the same group (1.0 ± 0.3 vs. 1.7 ± 0.5, P < 0.01). Additionally, TV E/e’ ratio was higher in the COPD group (5 ± 2 vs. 3 ± 1, P < 0.05).
4D-flow CMR and chest CT
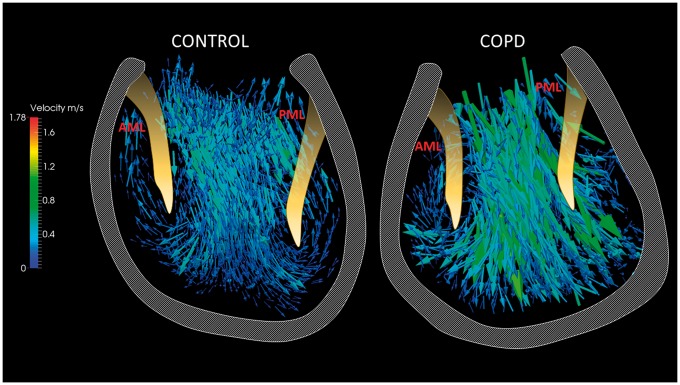
4D-Flow CMR and quantitative CT measures are summarized in Table 3. Figure 2 illustrates the visualization of segmented velocity and vorticity vector fields in one of the control subjects inside the LV. The LV peak E vorticity (1/s) in COPD patients was significantly depressed when compared with healthy subjects (3248 vs. 7947, P < 0.0001), while the mean COPD LV A peak vorticity (1/s) failed to show any difference (2285 vs. 3686, P < 0.440). The macroscopic quantitative evaluation of the flow formations in the LV in control subjects revealed prominent vortex formations along both mitral valve leaflets during early filing phase. Contrarily, the LV in COPD patients presented vortex formation only in 8 patients along the AML (50%, P < 0.05) and in 5 patients along the PML (31%, P < 0.05) (see Figure 3).
Table 3.
4D-flow CMR and chest CT parameters
| Variable | Control (n = 10) | COPD (n = 16) | P-value |
|---|---|---|---|
| LV peak E vorticity (1/s) | 7947 (7148–8548) | 3248 (2876–4578) | <0.0001 |
| LV peak A vorticity (1/s) | 3686 (2719–5389) | 2285 (1885–4519) | NS |
| AML vortex presence | 10 (100%) | 8 (50%) | <0.05 |
| PML vortex presence | 10 (100%) | 5 (31%) | <0.05 |
| Emphysema % < −950 HU | 6.7 (1.4–15.0) | ||
| Emphysema 15th percentile | 940 (921–951) | ||
| Air trapping % < −856 HU | 27.3 (11.2–42.3) | ||
| Air trapping 15th percentile | 894 (916–843) | ||
All 4D-Flow CMR and chest CT values are reported as medians with corresponding IQR.
HU, Hounsfield unit; PML, posterior mitral leaflet; AML, anterior mitral leaflet; CMR, cardiac magnetic resonance; CT, computed tomography; LV, left ventricular; NS, not significant; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; E, peak early diastolic filling velocity; A, peak late atrial filling velocity.
Figure 3.
Visualization of the velocity vector field in the LV at the peak E wave, focused on the presence of the mitral leaflet associated vortices. An exemplary control subject presents distinct formation of the mitral valve vortices which were present in each healthy control. On the right side is shown a typical COPD patient with reduced vortex formation along the mitral leaflets. In total, only 47% of COPD patients showed distinct vortex formation along the AML and 33% showed vortex present along the PML. AML, anterior mitral leaflet, PML, posterior mitral leaflet.
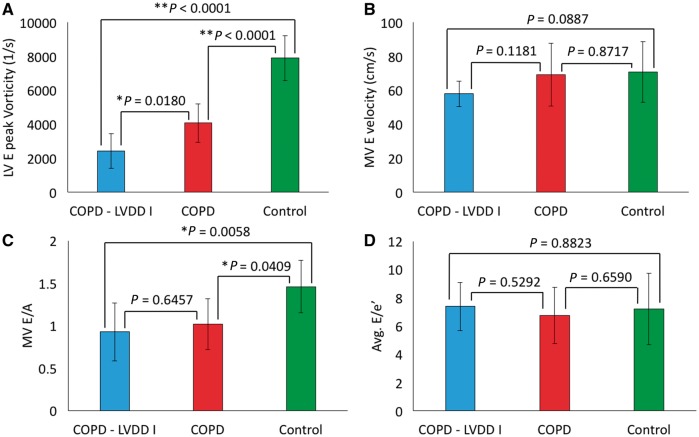
To further test the sensitivity of vorticity in COPD patients with presumptive LVDD, we compared vorticity between (i) COPD patients with detected LVDD (n = 5), (ii) COPD patients with no evidence of LVDD (n = 11), all assessed by echocardiography, and (iii) healthy control subjects (n = 10). We found significant differences between all considered groups (see Figure 4A). Specifically, patients with COPD and detected LVDD presented significantly decreased vorticity when compared with controls (2419 vs. 7891, P < 0.0001) and COPD patients without signs of LVDD (2419 vs. 4075, P = 0.0180). Most importantly, significant differences were observed between patients with COPD and no echocardiographic signs of LVDD and the control group (4075 vs. 7891, P < 0.0001). Similar comparison of Doppler echocardiography markers failed to show inter-group variability (see Figure 4C–D). Both quantitative and qualitative perspectives of this inter-group variability can be viewed in Figure 5 comparing representative subjects in each group. All intergroup variabilities remained significant when adjusted to heart rate.
Figure 4.
Subgroup analysis indicating sensitivity of vorticity toward detecting LVDD in patients with COPD. (A) Significant variability existed between patients with evidenced LVDD by means of echocardiography (n = 5) and patients with COPD and no signs of LVDD (n = 11). Furthermore, both group presented significantly decreased LV peak early diastolic vorticity when compared with control group. (B) Parallel Doppler echocardiography metric MV E velocity failed to reveal any inter-group variability. (C) MV E/A revealed only significance between COPD groups and controls, but failed to present any difference between COPD—LVDD and COPD groups. (D) Average E/e’ was not different between among considered groups.
Figure 5.
Comparative analysis of flow patterns and the vorticity field generated during the early LV diastolic phase between representative control, COPD without LVDD, and COPD with stage I LVDD subjects. Streamline representation of the flow through the LV mitral valve shows two visible vortices along both mitral leaflets in control subject. The vorticity vector field covers the entire chamber in the control case, opposed to incomplete coverage seen in COPD and COPD + LVDD subjects.
Regression analysis
The LV vorticity metrics correlated significantly with quantitative CT measures (see Table 4). The LV E wave vorticity correlated in negative fashion with whole lung percent emphysema (<−950 HU) (β ± SE: −55 ± 28, P = 0.041) and emphysema 15th percentile (β ± SE: 29 ± 14, P = 0.043). Additionally, the LV E vorticity correlated in positive trend with the 15th percentile of air trapping measure (β ± SE: 16 ± 7, P = 0.035). No significant correlations existed between chest CT indices reflective of air trapping and 4D-flow CMR derived vorticity metrics.
Table 4.
Determinants of LVDD in general linear regression model analysis
| 4D Flow CMR LVDD measure |
||
|---|---|---|
| LV E vorticity | ln (LV E/A vorticity) | |
| RVEF | 140 ± 49 (0.009) | 0.030 ± 0.014 (0.029) |
| TV E/A | 2917 ± 1086 (0.013) | 1.05 ± 0.45 (0.031) |
| TV E | −7.93 ± 20.55 (0.703) | 0.001 ± 0.022 (0.973) |
| TV A | −133 ± 32 (0.022) | −0.068 ± 0.048 (0.171) |
| TV E/e’ | −34 ± 273 (0.901) | −0.400 ± 0.294 (0.188) |
| TV e’ | 3176 ± 1525 (0.022) | 4.86 ± 1.59 (0.006) |
| 6-MWT | 2.201 ± 0.85 (0.024) | 0.0011 ± 0.0003 (0.012) |
| Emphysema % < −950 HU | −55 ± 28 (0.041) | −0.018 ± 0.015 (0.256) |
| Emphysema 15th percentile | 29 ± 14 (0.043) | 0.010 ± 0.008 (0.267) |
| Air trapping % < −856 | −29 ± 20 (0.077) | −0.009 ± 0.007 (0.210) |
| Air trapping 15th percentile | 16 ± 7 (0.035) | 0.005 ± 0.003 (0.094) |
Data are beta coefficients ± SE (P-values). Significant associations are in bold. All correlations are adjusted for age and sex.
RVEF, right ventricular ejection fraction; TV, tricuspid valve; 6-MWT, six minute walk test; HU Hounsfield unit; CMR, cardiac magnetic resonance; LVDD, left ventricular diastolic dysfunction; LV, left ventricular; E, peak early diastolic filling velocity; A, peak late atrial filling velocity.
To test the involvement of ventricular interdependency, we correlated the RV functional/volumetric CMR derived indices as well as standard RV echocardiographic diastolic indices with the LV vorticity measures. The RVEF significantly and positively correlated with the LV E vorticity (β ± SE: 140 ± 49, P = 0.009), and similarly with the log LV E/A ratio (β ± SE: 0.030 ± 0.014, P = 0.029) (see Figure 6). Multiple indices of RV diastolic function correlated with markers of LV diastolic function. TV E/A correlated positively with the LV E peak vorticity (β ± SE: 2917 ± 1086, P = 0.013) and log LV E/A (β ± SE: 1.05 ± 0.45, P = 0.031). In addition, TV A correlated negatively with the LV E peak vorticity (β ± SE: −133 ± 32, P < 0.001). TV e’ also correlated in in positive fashion with the LV E peak vorticity (β ± SE: 3170 ± 1590, P = 0.022) and log LV E/A (β ± SE: 4.86 ± 1.59, P < 0.002). No statistically significant correlations existed between the CMR derived ventricular volumetric and the LV vorticity indices. Finally, the LV E wave vorticity correlated with the 6MWT in positive trend (β ± SE: 2.201 ± 0.85, P = 0.024) and with log LV E/A (β ± SE: 0.0011 ± 0.0003, P = 0.012) (see Figure 7). All correlations remained significant when adjusted to heart rate.
Figure 6.
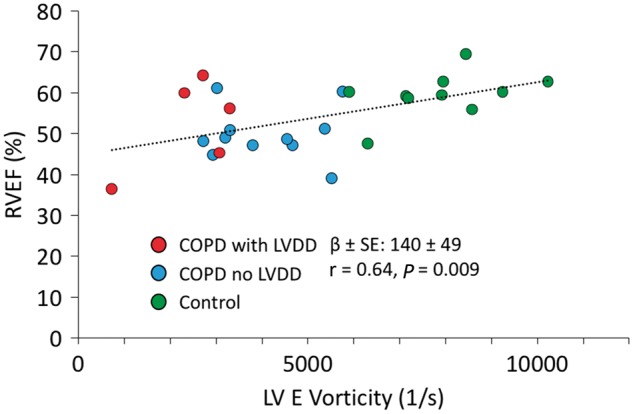
Positive correlation between RV ejection fraction and LV E phase vorticity. The association indicative of RV-LV interdependency segregated controls from specific COPD groups.
Figure 7.
Correlations between LV vorticity metrics and standard clinical marker—6-MWT. 6-MWT positively correlated with the LV E phase vorticity (left) and with the Ln LV E/A ratio (right).
Discussion
The present study demonstrates that patients with mild-to-moderate COPD present different LV vortex dynamics than controls despite having comparable cardiac function and geometry. Diastolic vortex formation has been previously described, predominantly using contrast echocardiography, as a physiological sign of a healthy compliant LV and an important component of the fluid energetic transfer between cardiovascular compartments.18,27–29 Importantly, impaired LV vortex formation and reduced vorticity have been described in patients with non-ischaemic dilated and hypertrophic cardiomyopathies.17 Furthermore, the LV vortex strength has been investigated in terms of kinetic energy dissipation between late diastole and iso-volumetric contraction in patients with heart failure.30,31 Other complex flow dynamic measures derived by echocardiographic techniques and single plane phase contrast MRI, i.e. vortex formation time metrics, were previously shown to be non-reflective of the LVDD.28 Just recently, a study on the feasibility of detecting ventricular diastolic vortex formation by means of 4D-Flow CMR has been completed.27 We significantly extend the impact of this study with our current work, in that we have shown that, in addition to differences in qualitatively described vortex formations, patients with mild-to-moderate COPD present decreased 4D-Flow CMR derived vorticity. 4D-Flow CMR can then provide graphical representation of both qualitative and quantitative flow haemodynamic condition inside the LV (Figure 4). Given the spatial and temporal localization of its measurement, this vorticity provides a quantitative marker of the LV inflow condition19; thus we speculate that it may reflect on very early stage ventricular remodelling associated with the restrictive nature of hyperinflation on mediastinal structures. Given the associations between LV vorticity and RV function, we further speculate that LV diastolic vorticity reflects LV–RV interdependency effects in COPD which occur due to structural changes earlier in the disease process.20,32
Effect of hyperinflation on vorticity
Severity of lung hyperinflation in patients with COPD has been previously described in association with cardiovascular comorbidities and specifically with LVDD.4,33 Importantly, the severity of hyperinflation is a well-recognized independent predictor of mortality in patients with mild COPD.34,35 The elevated ventricular mass and stiffness in patients with progressed COPD has been recently positively correlated with the severity of hyperinflation determined by standard chest CT metrics.1,20 However, investigation of subclinical LVDD in association with COPD has yet received only limited attention. Previous study indicated the presence of LVDD in selected patients with severe COPD and showed associations with 6MWT and echocardiographic E/A ratio.36 In our present study, we observed association between the diastolic vorticity and chest CT metrics descriptive of percent emphysema and air trapping in patients with normal LV mass and volumes. In parallel with previous studies, it appears that in early stage COPD reduced systolic and diastolic ventricular function occurs in association with restrictive/overextended lung parenchyma.32,33 However, further studies considering the involvement of systemic inflammation and vascular remodelling are required to fully understand the pathophysiology behind the early ventricular dysfunction in COPD.
Effect of ventricular interdependency on vorticity
Ventricular interdependency is a recurrent pathophysiologic theme in numerous cardiovascular diseases and was also shown to play a role in COPD patients.37–39 Therefore, we investigated the relationship between the RV volumetric and functional metrics and LV diastolic vorticity. We found no significant correlations between the RV volumetric metrics and LV vorticity implying that RV preload might not be playing crucial role in the mild stages of COPD. However, associations between RV dimensional parameters and LV filling parameters have been previously described in patients with severe COPD.37 Additionally, a strong relationship between the pulmonary arterial pressure and LV diastolic function was described in advanced stages of COPD.5 In this study we found correlations between RV diastolic indices (TV E/A, TV A, and TV e’) and LV vorticity. Most interestingly, we observed an association between RV systolic function (RVEF) and the LV vorticity. This non-invasively described early involvement of ventricular interdependency in COPD has not yet been described. However, the investigation of this phenomenon in early stage COPD would require a larger homogenous group of patients and chronological observation. Additionally, the effect of the COPD induced tachycardia on ventricular relaxation properties has been shown to be rate-dependent.40 We speculate that with progressive worsening of COPD and LVDD we would observe more profound decrease in diastolic vorticity, but additional factors such as decreased LV filling due to reduced RV stroke volume and septal flattening would play important role in this process. Contrary, isolated LVDD would then reduce diastolic vorticity primarily by elevated ventricular stiffness compromising diastolic recoil. Future studies combining 4D-Flow CMR with tissue deformation and myocardial perfusion imaging would provide more thorough insights into the LVDD pathophysiology and subsequent effects on impaired diastolic filling.
We acknowledge several limitations to this study. The number of COPD patients who could undergo same-day echocardiographic and 4D-Flow CMR evaluation mainly due to physical intolerance limited the size of this study. Furthermore, our control group did not receive spirometry evaluation due to their retrospective recruitment. Finally, the major limitation of 4D-Flow CMR haemodynamic evaluation is its large temporal resolution and use of prospective ECG gating, which does not permit more discrete investigation of diastolic function in tachycardic patients. However, our main findings focus on early diastolic phase, when the LV relaxation properties impaired by LVDD express most dramatic changes in flow and mechanical haemodynamics. Our future study will include both cardio-pulmonary exercise protocol, investigation of specific patients with mild COPD and normal RV dysfunction, along with collection of the blood biomarkers to fully investigate the effect of COPD on diastolic dysfunction. Furthermore, we will investigate the feasibility of 4D-Flow CMR assessment of early and presumptive LVDD in routine clinical setting in larger patient population.
Conclusion
In this study, we demonstrate that 4D-flow CMR derived vorticity is significantly altered in the LV in diastole in mild-to-moderate COPD patients. Given the important prognostic aspect of LVDD, this technique may provide a novel technique for identifying and investigating the pathophysiologic mechanisms for diastolic dysfunction. Specifically, the pathophysiologic origins of reduced vorticity requires further investigation particularly given that our COPD cohort did not present reduced LV EF and LV volumetric metrics. The effect of hyperinflation and ventricular interdependency on LVDD and may ultimately inform clinical approaches that improve diastolic function through the treatment of COPD.
Conflict of interest: none declared.
Funding
Funded by John C. Carson Foundation and partially by NIH K25-HL094749.
References
- 1. Smith BM, Kawut SM, Bluemke DA, Basner RC, Gomes AS, Hoffman E. et al. Heart failure the multi-ethnic study of atherosclerosis COPD study. Circulation 2013;127:1503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macchia A, Rodriguez Moncalvo JJ, Kleinert M, Comignani PD, Gimeno G. et al. Unrecognised ventricular dysfunction in COPD. Eur Respir J 2012;39:51–8. [DOI] [PubMed] [Google Scholar]
- 3. Rossi A, Aisanov ZR, Avdeev S, Di Maria G, Donner CF, Izquierdo JL. et al. Mechanisms, assessment and therapeutic implications of lung hyperinflation in COPD. Respir Med 2015;109:785–802. [DOI] [PubMed] [Google Scholar]
- 4. Watz H, Waschki B, Meyer T, Kretschmar G, Kirsten A, Claussen M. et al. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest 2010;138:32–8. [DOI] [PubMed] [Google Scholar]
- 5. Funk GC, Lang I, Schenk P, Valipour A, Hartl S, Burghuber OC.. Left ventricular diastolic dysfunction in patients with COPD in the presence and absence of elevated pulmonary arterial pressure. Chest 2008;133:1354. [DOI] [PubMed] [Google Scholar]
- 6. Wan SH, Vogel MW, Chen HH.. Pre-clinical diastolic dysfunction. J Am Coll Cardiol 2014;63:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Jemtel TH, Padeletti M, Jelic S.. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2007;49:171–80. [DOI] [PubMed] [Google Scholar]
- 8. Scharf SM, Iqbal M, Keller C, Criner G, Lee S, Fessler HE.. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med 2002;166:314–22. [DOI] [PubMed] [Google Scholar]
- 9. Chaouat A, Naeije R, Naeije R, Weitzenblum E, Weitzenblum E.. Pulmonary hypertension in COPD. Eur Respir J 2008;32:1371–85. [DOI] [PubMed] [Google Scholar]
- 10. Flachskampf FA, Biering-Sorensen T, Solomon SD, Duvernoy O, Bjerner T, Smiseth OA.. Cardiac imaging to evaluate left ventricular diastolic function. JACC Cardiovasc Imaging 2015;8:1071–93. [DOI] [PubMed] [Google Scholar]
- 11. Chen S, Yuan J, Qiao S, Duan F, Zhang J, Wang H.. Evaluation of left ventricular diastolic function by global strain rate imaging in patients with obstructive hypertrophic cardiomyopathy: a simultaneous speckle tracking echocardiography and cardiac catheterization study. Echocardiography 2014;31:615–22. [DOI] [PubMed] [Google Scholar]
- 12. Puwanant S, Park M, Popović ZB, Tang WHW, Farha S, George D. et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation 2010;121:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhäll C-J, Ebbers T. et al. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 2015;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O.. 4D flow MRI. J Magn Reson Imaging 2012;36:1015–36. [DOI] [PubMed] [Google Scholar]
- 15. Rodriguez Muñoz D, Markl M, Moya Mur JL, Barker A, Fernández-Golfín C, Lancellotti P. et al. Intracardiac flow visualization: current status and future directions. Eur Heart J Cardiovasc Imaging 2013;14:1029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sengupta PP, Pedrizzetti G, Kilner PJ, Kheradvar A, Ebbers T, Tonti G. et al. Emerging trends in CV flow visualization. JACC Cardiovasc Imaging 2012;5:305–16. [DOI] [PubMed] [Google Scholar]
- 17. Martinez-Legazpi P, Bermejo J, Benito Y, Yotti R, Perez Del Villar C, Gonzalez-Mansilla A. et al. Contribution of the diastolic vortex ring to left ventricular filling. J Am Coll Cardiol 2014;64:1711–21. [DOI] [PubMed] [Google Scholar]
- 18. Pedrizzetti G, La Canna G, Alfieri O, Tonti G.. The vortex–an early predictor of cardiovascular outcome? Nat Rev Cardiol 2014;11:545–53. [DOI] [PubMed] [Google Scholar]
- 19. Fenster BE, Browning J, Schroeder JD, Schafer M, Podgorski CA, Smyser J. et al. Vorticity is a marker of right ventricular diastolic dysfunction. Am J Physiol Heart Circ Physiol 2015;309:H1087–93. [DOI] [PubMed] [Google Scholar]
- 20. Kawut SM, Poor HD, Parikh MA, Hueper K, Smith BM, Bluemke DA. et al. Cor pulmonale parvus in chronic obstructive pulmonary disease and emphysema. J Am Coll Cardiol 2014;64:2000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–55. [DOI] [PubMed] [Google Scholar]
- 22. Focardi M, Cameli M, Carbone SF, Massoni A, De Vito R, Lisi M. et al. Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur Hear J Cardiovasc Imaging 2014;16:47–52. [DOI] [PubMed] [Google Scholar]
- 23. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T. et al . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 24. Estepar RSJ, Ross JC, Harmouche R, Onieva J, Diaz AA, Washko GR.. Chest imaging platform: an open-source library and workstation for quantitative chest imaging. C66. Lung imaging ii: new probes and emerging technologies. Am Thorac Soc 2015;191:A4975. [Google Scholar]
- 25. Lynch DA, Al-qaisi ML.. Quantitative CT in COPD. J Thorac Imaging 2013;28:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bock J, Markl M, Kreher B, Henning J.. Optimized pre-processing of time-resolved 2D and 3D phase contrast MRI data. Proc 15th Annu Meet ISMRM, 2007. Abstract, p.3138. International Society for Magnetic Resonance in Medicine, Berlin, Germany. [Google Scholar]
- 27. Elbaz MSM, Calkoen EE, Westenberg JJM, Lelieveldt BPF, Roest AAW, van der Geest RJ. Vortex flow during early and late left ventricular filling in normal subjects: quantitative characterization using retrospectively-gated 4D flow cardiovascular magnetic resonance and three-dimensional vortex core analysis. J Cardiovasc Magn Reson 2014;16:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart KC, Charonko JC, Niebel CL, Little WC, Vlachos PP.. Left ventricle filling vortex formation is unaffected by diastolic impairment. AJP Hear Circ Physiol 2012;303:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hong GR, Pedrizzetti G, Tonti G, Li P, Wei Z, Kim JK. et al. Characterization and quantification of vortex flow in the human left ventricle by contrast echocardiography using vector particle image velocimetry. JACC Cardiovasc Imaging 2008;1:705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abe H, Caracciolo G, Kheradvar A, Pedrizzetti G, Khandheria BK, Narula J. et al. Contrast echocardiography for assessing left ventricular vortex strength in heart failure: a prospective cohort study. Eur Heart J Cardiovasc Imaging 2013;14:1049–60. [DOI] [PubMed] [Google Scholar]
- 31. Kheradvar A, Assadi R, Falahatpisheh A, Sengupta PP.. Assessment of transmitral vortex formation in patients with diastolic dysfunction. J Am Soc Echocardiogr 2012;25:220–7. [DOI] [PubMed] [Google Scholar]
- 32. Hilde JM, Skjørten I, Grøtta OJ, Hansteen V, Melsom MN, Hisdal J. et al. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol 2013;62:1103–11. [DOI] [PubMed] [Google Scholar]
- 33. Thomas M, Decramer M, O’Donnell DE.. No room to breathe: the importance of lung hyperinflation in COPD. Prim Care Respir J 2013;22:101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D. et al. COPD as a lung disease with systemic consequences–clinical impact, mechanisms, and potential for early intervention. COPD 2008;5:235–56. [DOI] [PubMed] [Google Scholar]
- 35. Decramer M, Cooper CB.. Treatment of COPD: the sooner the better? Thorax 2010;65:837–41. [DOI] [PubMed] [Google Scholar]
- 36. Lopez-Sanchez M, Munoz-Esquerre M, Huertas D, Gonzalez-Costello J, Ribas J, Manresa F. et al. High prevalence of left ventricle diastolic dysfunction in severe COPD associated with a low exercise capacity: a cross-sectional study. PLoS One 2013;8:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boussuges A, Pinet C, Molenat F, Burnet H, Ambrosi P, Badier M. et al. Left atrial and ventricular filling in chronic obstructive pulmonary disease. An echocardiographic and Doppler study. Am J Respir Crit Care Med 2000;162:670–5. [DOI] [PubMed] [Google Scholar]
- 38. Hsia HH, Haddad F.. Pulmonary hypertension: a stage for ventricular interdependence? J Am Coll Cardiol 2012;59:2203–5. [DOI] [PubMed] [Google Scholar]
- 39. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ.. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008;117:1436–48. [DOI] [PubMed] [Google Scholar]
- 40. Selby DE, Palmer BM, Lewinter MM, Meyer M.. Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J Am Coll Cardiol 2011;58:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]