Abstract
The phosphoinositide 3-kinase/AKT (protein kinase B) signaling pathway negatively regulates follicle activation via the forkhead box O (FOXO) transcription factor in rodents. FOXO3 knockout mice exhibit global activation of primordial follicles leading to early depletion of ovarian follicles and subsequent infertility. Whether a similar mechanism for follicle activation exists in the primate ovary is unclear. In the current study, protein localization of FOXO1, 3, and 4 as well as their upstream regulator, AKT/p-AKT, was examined in rhesus macaque ovaries of three developmental stages: fetal, prepubertal, and adult. FOXO1 protein is expressed in granulosa cells of fetal, prepubertal, and adult ovaries. FOXO3 is distributed sparsely in the mitotically active germ cells, but its expression decreases following follicle formation in the macaque fetal ovary. In addition, FOXO3 is seldom with interanimal variation in the prepubertal ovary and is absent in the adult ovary. FOXO4 is nondetectable in fetal ovaries, although it is expressed in some theca cells of antral follicles and some stromal cells in prepubertal and adult ovaries. Our results suggest that the regulation and/or function of FOXO3 in the primate primordial follicle may differ than that of the rodent. Nevertheless, AKT/p-AKT is expressed in macaque primordial oocytes, suggesting that similar upstream events but different downstream effects may regulate primordial follicle activation in nonhuman primates compared to rodents. Elucidation of the mechanism responsible for follicle activation in primates will be crucial for understanding primary ovarian insufficiency, improving female fertility, and applying techniques for in vitro maturation of follicles for fertility preservation in cancer survivors.
Keywords: FOXO1, FOXO3, FOXO4, AKT, primordial follicle, ovary, nonhuman primate
Summary Sentence
FOXO3 protein localizes in the oocyte of fetal and prepubertal but not adult ovaries while FOXO1 and 4 are expressed in granulosa and theca cells, receptively in the rhesus macaque.
Introduction
It is generally believed that women are born with a finite number of oocytes that declines with age. The primordial follicle represents the most abundant follicle population in the ovary and comprises a dormant oocyte arrested in prophase of meiosis surrounded by a layer of flattened (pre-) granulosa cells. During each menstrual cycle in primates, a small population (∼1000) of the primordial follicles is activated by an unknown process and begins to grow and develop, ultimately producing a mature and fertilizable oocyte [1]. Follicle activation is an irreversible process; when the primordial follicle pool is depleted, ovarian function ceases followed by onset of menopause at an average age of 51. This natural decline of ovarian reserve is accelerated in women with primary ovarian insufficiency (POI; 1% prevalence rate), defined as women with primary (absence of menarche) or secondary amenorrhea (premature depletion of ovarian follicles before the age of 40 [2]). In addition, POI is a common side effect in cancer patients facing cytotoxic chemotherapy and radiation [3, 4]. Regulation of the transition of a primordial follicle from quiescence to activation is crucial for maintaining ovarian function and fertility, and regulates the length of the female reproductive lifespan. However, the mechanisms controlling this process are poorly understood, especially in primates.
Using mouse models, the phosphatase and tensin homolog deleted on chromosome 10 (PTEN)-phosphatidylinositol 3-kinase (PI3K)-Akt-forkhead box O3 (FOXO3) signaling pathway has been proposed as a key regulator for follicle activation/quiescence [5–7]. PI3K is a lipid kinase and converts phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3) which activates AKT. PTEN inhibits PI3K/Akt (protein kinase B) signaling by dephosphorylating PIP3. The PI3K/Akt signaling cascade plays a pivotal role in cell survival, proliferation, and differentiation in a wide range of cellular processes [8], and dysregulation of this pathway is involved in various malignancies [9]. FOXO3, a forkhead transcription factor, is a downstream effector of PI3K/Akt. In rodents, FOXO3 exerts its transcriptional activity in the nucleus of quiescent primordial follicles and suppresses follicular growth; upon phosphorylation, FOXO3 is translocated to the cytoplasm, resulting in follicle activation. In a pioneering study by Castrillon et al., knockout mice lacking Foxo3 exhibited global activation of primordial follicles and subsequent POI by 12 weeks of age [7]. Later studies generated conditional knockout mice with oocyte-specific Foxo3 deletion and confirmed the role of intra-oocyte Foxo3 in primordial follicle activation [6]. A similar phenotype was observed in oocyte-specific PTEN knockout mice which exhibited premature primordial follicle activation and POI in young adult mice [10]. The role of FOXO3 in the primate ovary is controversial as FOXO3 mutations are rarely observed in women with POI [11–13]. In addition, while FOXO3 is globally expressed in the mouse primordial oocyte, conflicting data exist with regard to the presence (women [5]) or absence (monkey and women [14]) of FOXO3 in the primate primordial oocyte. The latter study suggests that other closely related FOXOs such as FOXO1 or FOXO4 may serve as a major regulator within primordial follicles in the primate ovary, instead of FOXO3.
While the forkhead family of transcription factors is highly conserved in evolution, it should not be surprising that different mechanisms govern follicle activation in rodents and primates due to their differences in reproductive physiology. A fundamental difference between rodents and primates is the timing of primordial follicle activation. In rodents, primordial follicles are not assembled until 3 days after birth and follicle activation occurs shortly after the assembly [15]. In contrast, follicle activation in primates starts in utero during the second trimester with ongoing germ cell division and primordial follicle assembly [16, 17]. Due to the scarcity of human ovarian specimens from young females as well as the inability to fertilize mature human oocytes due to ethical concerns, research in nonhuman primates becomes extremely important due to their similar reproductive functions compared to women (i.e., mono-ovulatory, similar control, and regulation of the hypothalamic–pituitary–ovarian axis during monthly reproductive cycle). In mammals, there are four FOXO isoforms, FOXO1, 3, 4, and 6. Only FOXO1, 3, and 4 are widely expressed in a variety of organs including the ovary, while FOXO6 is only expressed in the developing brain [18]. To further understand the signaling pathways that potentially regulate primordial follicle activation in primates, the current study evaluates protein expression of FOXO1, 3, and 4 as well as their upstream regulator, AKT/p-AKT, in the ovary of rhesus macaques of three different developmental stages: fetal, prepubertal, and adult.
Material and methods
Animals and ovary collection
The general care and housing of rhesus macaques (Macaca mulatta) provided by the Division of Comparative Medicine at the Oregon National Primate Research Center (ONPRC) was previously described [19]. Animals were fed food twice a day and water ad libitum and housed in a temperature-controlled (22°C) light-regulated room (12L:12D). Ovaries were collected from three age groups at necropsy for reasons unrelated to reproductive health or during the early follicular phase of a spontaneous menstrual cycle (adult females): fetuses (n = 3; gestational day 130, third trimester), prepubertal females (n = 4; 1–3 years old), and adult females (n = 3 at necropsy and n = 3 during early follicular phase; 9–12 years old). Ovaries from three B6;129 cross female mice (normal cycling 21-day-old B6) were also collected. All protocols used in this study were approved the ONPRC Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All chemicals used in the study were purchased from Sigma-Aldrich unless otherwise noted.
Ovarian tissue processing and immunohistochemistry
Ovaries were fixed in 4% paraformaldehyde at 4°C overnight, embedded in paraffin, and serial sectioned at 5 μm thickness. Immunostaining was performed as previously described [20]. Briefly, sections were treated with boiling citrate buffer (10 mM pressure cooker, 10 min) for antigen retrieval and incubated with 0.3% H2O2 (30 min) and serum (60 min) to prevent nonspecific binding of the primary and secondary antibodies. Nonimmune serum or primary antibodies including FOXO1 (1:200, rabbit monoclonal, targets C-terminus of human FOXO1 with proven reactivity to monkey tissues, Cell Signaling, Boston, MA, USA), FOXO3 (first antibody: 1:200, rabbit polyclonal, targets human FOXO3 with proven reactivity to monkey tissues, Cell Signaling; second antibody: 1:100, rabbit polyclonal, targets human FOXO3 with proven reactivity to primate tissues, Novus Biologicals, Littleton, CO, USA), FOXO4 (AFX1, 1:100, goat polyclonal, targets C-terminus of human FOXO4 with proven reactivity to human tissues, Santa Cruz, Dallas, TX, USA), AKT (1:100; rabbit monoclonal, Cell Signaling), and p-AKT (1:100; rabbit monoclonal, Cell Signaling) were applied to sections on adjacent slides and incubations were carried out at 4°C overnight. Additional sections from fetal ovaries were incubated with primary antibody against VASA (1:50, goat polyclonal, Santa Cruz) for identification of germ cells. Primary antibody binding was visualized with biotinylated secondary antibodies and 3,3΄-diaminobenzidine (DAB) (Vector, Burlingame, CA, USA). Images were taken with a DP72 camera attached to an Olympus BX40 microscope and CellSens imaging software (Olympus, Center Valley, PA, USA). Dark brown DAB staining was considered positive staining for each antibody examined. Mouse (n = 3; normal cycling 21-day-old B6;129 cross female) ovarian tissue sections were used for positive controls for FOXO3 immunoreactivity. Primary antibody titration was performed in all primary antibodies to select the optimal primary antibody concentration for macaque ovarian tissue immunohistochemistry.
Results
FOXO1 localization in the ovary
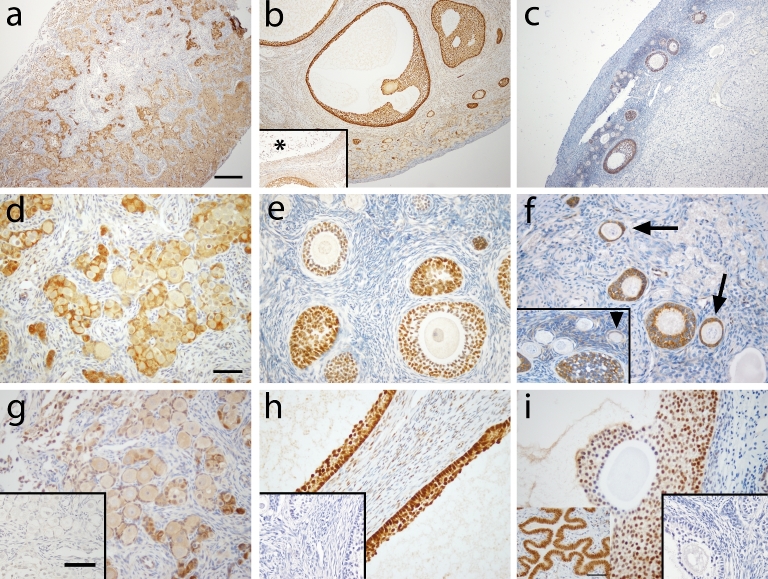
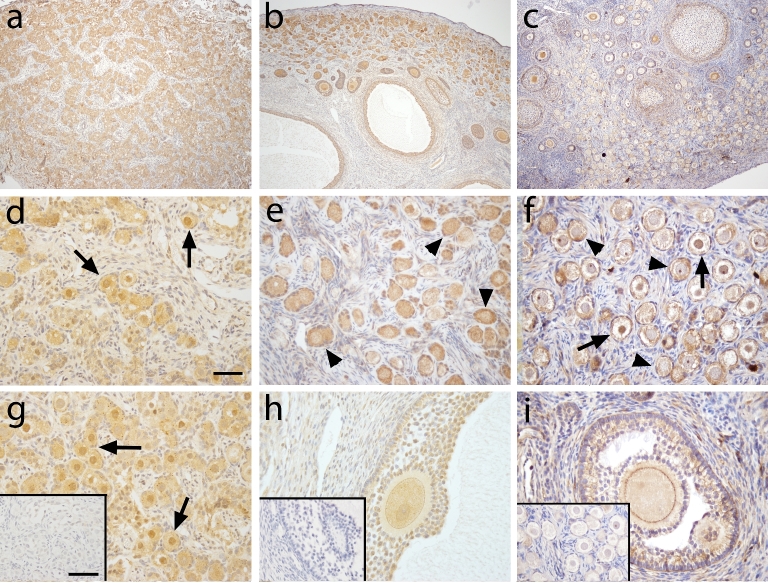
Figure 1 shows FOXO1 immunoreactivity in macaque ovarian tissue during fetal (a, d, g) prepubertal (b, e, h), and adult (c, f, i) life. At gestation day 130, FOXO1 immunoreactivity (ir; brown staining) was found in the nuclei of pregranulosa cells in germ cell nests (Figure 1d) as well as in some newly assembled primordial follicles (Figure 1g). Adjacent sections of the fetal ovary were labeled for VASA for identification of germ cells (Figure 2). Similar FOXO1 expression was found in ovaries from prepubertal and adult macaque monkeys. FOXO1 was located in all granulosa cells of healthy growing follicles including primary, secondary, multilayer, and antral follicles (Figure 1b, e, h [prepubertal]; Figure 1c, f, I [adult]). In large antral follicles, staining intensity seemed to decrease in cumulus granulosa cells compared to mural granulosa cells (Figure 1i). FOXO1 staining was not observed in granulosa cells of atretic antral follicles (Figure 1b, asterisk). In preantral follicles, FOXO1 tends to locate in the cytoplasm of granulosa cells (Figure 1e, f), whereas nuclear FOXO1 localization was found in granulosa cells in the majority of large antral follicles (Figure 1h, i). Consistently among animals, FOXO1 expression is absent in most primordial follicles (Figure 1f, insert), but can be seen in a few primordial follicles (Figure 1f, insert, arrowhead) as well as transitional primary follicles (Figure 1f, arrow). Weak and sporadic FOXO1 staining was also observed in thecal and stromal cells of prepubertal and adult monkey ovaries. It is worth nothing that ovarian tissues sections used in the current study were from ovaries collected, fixed, and processed at different time points. This might contribute to different degree of background staining in different ovaries.
Figure 1.
FOXO1 immunoreactivity (brown) in macaque ovarian tissue during fetal (a, d, and g), prepubertal (b, e, and h), and adult (c, f, and i) life. Pictures are from one representative animal within age, but results were consistent among all animals within age. Low magnification (×100) photomicrographs (a–c) show overall expression in the ovary, while high magnification (×400) photomicrographs (d–i) show staining details within follicles. Asterisk in panel b shows an atretic antral follicle. Arrowhead (insert in f) shows pregranulosa cells in primordial follicle positive for FOXO1 staining. Arrow in panel f denotes transitional follicle with FOXO1 localization in granulosa cells. Panel h captures the follicular wall of two adjacent healthy antral follicles positive for FOXO1 staining. Primary antibody omission negative control (inserts in g–i) showed a lack of nonspecific binding. Scale bar = 500 μm (a), 50 μm (d), and 65 μm (inserts in g).
Figure 2.
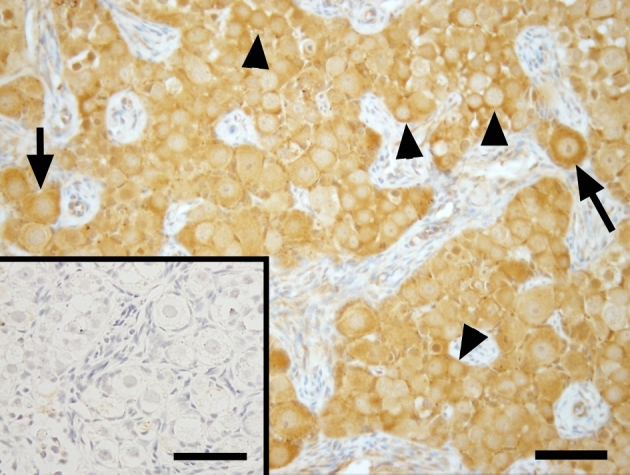
Photomicrographs of VASA expression in ovarian tissue of a representative fetal macaque under ×400 magnification. Scale bar = 50 μm and 65 μm (inserts). Arrow = newly formed primordial follicles. Arrowheads = germ cells still in germ cell nest. Primary antibody omission negative control (inserts) showed a lack of nonspecific binding.
VASA localization in the ovary
Localization of germ cells in the monkey fetal ovary (Figure 2) was confirmed by VASA immunostaining. VASA was expressed in the cytoplasm of all germ cells, both prior to follicle assembly within germ cells clusters (Figure 2, arrowheads) and in primordial follicles (Figure 2, arrows).
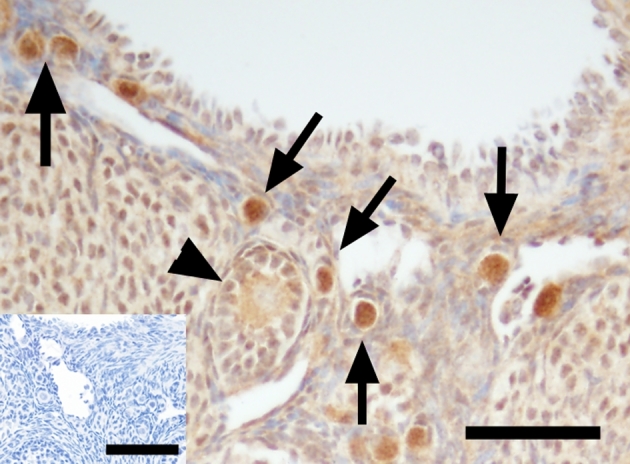
FOXO3 localization in the ovary
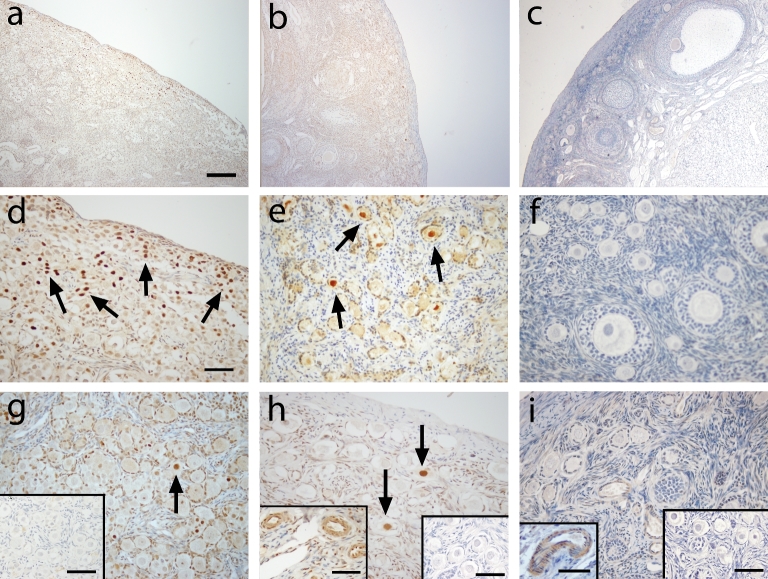
At gestation day 130 (Figure 3a), mitotically active germ cells located within syncitial clusters in the cortical region of the ovary showed sporadic nuclear expression of FOXO3 (Figure 3d). However, very few of the newly assembled primordial follicles in the medullary region of the fetal ovary expressed FOXO3 (Figure 3g). As expected, mouse ovarian tissue exhibited nuclear expression of FOXO3 in oocytes of all primordial follicles (Figure 4). FOXO3 expression in the prepubertal ovary (Figure 3b) was found in the oocyte nucleus of few primordial follicles and this varies among different animals (from few follicles [Figure 3e] to very rare [Figure 3h]). FOXO3 was consistently not expressed in adult ovaries (Figure 3 c, f, and i). FOXO3-positive staining was found in vascular smooth muscle in ovaries of prepubertal and adult ovaries and serves as an internal positive control (Figure 3h and i, inserts) as previously shown in the ovary [14]. Weak FOXO3 staining was also found in few stromal cells in prepubertal and adult monkey ovaries.
Figure 3.
FOXO3 immunoreactivity (brown, arrows) in macaque ovarian tissue from a representative animal during fetal (a, d, and g), prepubertal (b, e, and h), and adult (c, f, and i) life under low (×100; a–c) and high (×400; d–i) magnification. Similar localization patterns were observed in all animals within age. Arrow in d, e, g, and h denotes positive FOXO3 staining in the nucleus of primordial oocytes. Positive controls: macaque ovarian blood vessels (inserts in h and i). Primary antibody omission negative control (inserts in g, right inserts in h and i) showed a lack of nonspecific binding. Scale bar = 500 μm (a), 50 μm (d; left inserts in h, i), and 65 μm (right inserts in g–i).
Figure 4.
FOXO3 immunoreactivity (brown, arrows) in B6;129 mouse ovarian tissue in a ×400 magnification image. Arrows indicate positive FOXO3 staining in the nucleus of primordial oocytes. Arrowheads show cytoplasmic staining of FOXO3 in the mouse primordial oocyte. Primary antibody omission negative control (insert) showed a lack of nonspecific binding. Scale bar = 50 μm and 65 μm (insert).
FOXO4 localization in the ovary
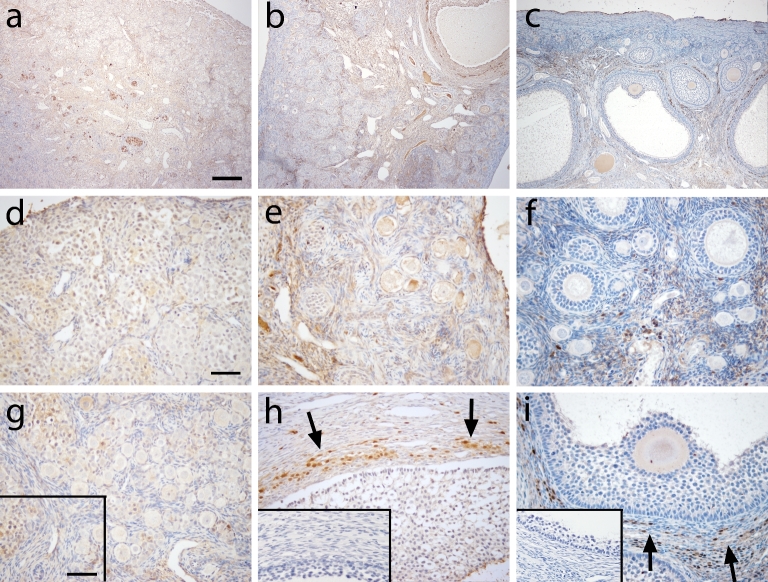
At gestation day 130, the ovary was devoid of FOXO4-positive cells in germ cells, newly assembled primordial follicles, and surrounding stroma (Figure 5a, d, g). In prepubertal ovaries (Figure 5b), FOXO4 was found in few stromal cells in the cortex (Figure 5e) and in some theca cells in the theca interna layer (Figure 5h). Similar FOXO4 expression was observed in the adult ovary (Figure 5c) compared to the prepubertal ovary. FOXO4 protein was localized in some stromal (Figure 5f) and theca interna cells (Figure 5i) in all adults.
Figure 5.
FOXO4 immunoreactivity in macaque ovarian tissue from a representative animal during fetal (a, d, and g), prepubertal (b, e, and h), and adult (c, f, and i) life under low (×100; a–c) and high (×400; d–i) magnification. Similar immunoreactivity was observed in all animals within age. Arrows indicate positive FOXO4 staining in the theca layer of an antral follicle. Primary antibody omission negative control (insert in g–i) showed a lack of nonspecific binding. Scale bar = 500 μm (a) and 50 μm (d), and 65 μm (insert).
AKT and p-AKT localization in the ovary
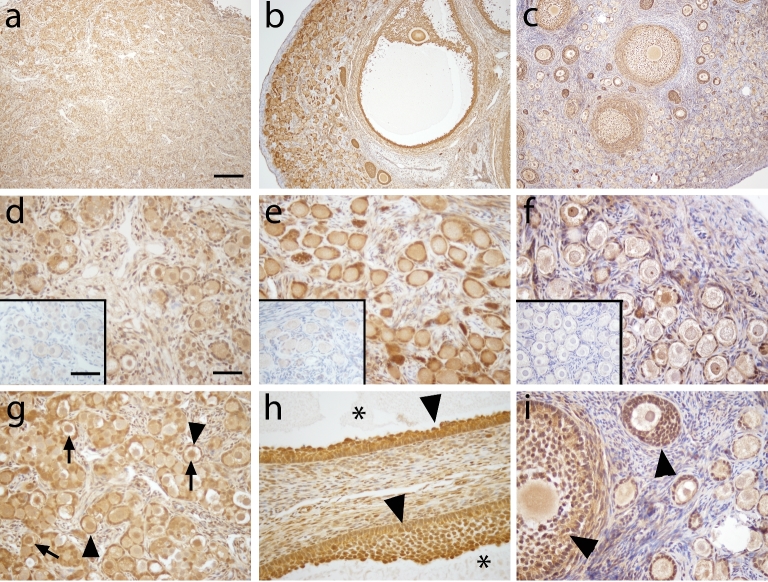
Since FOXO3 was not found in any of the macaque adult ovaries, we examined the expression of its upstream regulator, AKT, and p-AKT. Similar expression patterns of AKT were found in fetal (Figure 6a, d, g), prepubertal (Figure 6b, e, h), and adult (Figure 6 c, f, i) macaque ovaries. Strong AKT immunoreactivity was observed in granulosa cells of follicles of all stages as well as oocytes of secondary and antral follicles. Weak AKT immunoreactivity was observed in the cytoplasm and sometimes nucleus of oocytes in primordial and primary follicles. AKT was also present in some stromal cells. Similar to AKT, p-AKT was observed in fetal (Figure 7a, d, g), prepubertal (Figure 7b, e, h), and adult (Figure 7c, f, i) macaque ovaries. Cytoplasmic localization of p-Akt was evident in the oocytes of all primordial follicles (Figure 7d, e, f, g). Some nuclear localization of p-AKT was also apparent in primordial follicles of the fetal and prepubertal macaque ovary (Figure 7d, e, g; arrow).
Figure 6.
AKT expression (brown) in macaque ovarian tissue during fetal (a, d, and g), prepubertal (b, e, and h), and adult (c, f, and i) life under low (×100; a–c) and high (×400; d–i) magnification. AKT expression patterns were similar among animals of all age groups studied. Arrows show oocytes with positive AKT staining in the oocyte (g and i), arrowheads show granulosa cells with positive AKT staining in antral follicles (h), and asterisk shows an atrum (h). Primary antibody omission negative control (insert in d–f) showed a lack of nonspecific binding. Scale bar = 500 μm (a) and 50 μm (d) and 65 μm (insert).
Figure 7.
p-AKT expression (brown) in macaque ovarian tissue during fetal (a, d, and g), prepubertal (b, e, and h), and adult (c, f, and i) life under low (×100; a–c) and high (×400; d–i) magnification. Arrows indicate p-AKT staining in the nucleus of primordial oocytes (d, e, and g). Arrowheads depict cytoplasmic p-AKT staining in the primordial oocyte (e, f, and i). Note the positive staining on the surface of the oocyte of antral follicles (h, i). Primary antibody omission negative control (insert in g–i) showed a lack of nonspecific binding. Scale bar = 500 μm (a) and 50 μm (d), and 65 μm (insert).
Discussion
In the current study, we found that FOXO1 and FOXO4 are expressed in granulosa and theca cells, respectively, in the monkey ovary, similar to that of rodents [14, 21]. However, FOXO3 is not consistently expressed in the oocyte of primordial follicles as reported in the rodent model. In addition, while FOXO1 and FOXO4 seem to maintain their localization throughout different stages of development, FOXO3 expression varied with ovarian age. FOXO3 was found in the germ cells during fetal life, decreased dramatically once primordial follicles are assembled in the fetal and prepubertal ovaries, and are lost in the adult ovaries. This is the first report of FOXO3 expression during different developmental stages including fetal, prepubertal, and adult. The discrepancy of the FOXO3 protein expression between the mouse and macaque ovary suggests that there may be different downstream factors responsible for regulating primordial follicle activation. However, it is also possible that FOXO3 protein is only expressed in activating primordial follicles in primates, unlike its universal expression in all primordial follicles in mice.
FOXO1 expression in the granulosa cells of growing follicles has been reported in several species including rodents, ruminants, cats, dogs, pigs, monkeys, and human [14]. Foxo1-null mouse is embryonic lethal resulting from deficiency in vascular development [22]. Studies have suggested the role of FOXO1 as a key regulator of granulosa cell survival, proliferation, and function in FSH, insulin growth factor-1, and estrogen signaling pathways in rats and pigs [21–25]. On the contrary, there is also evidence that FOXO1 is elevated in mouse granulosa cells during follicle atresia [26, 27]. Our results showed that FOXO1 is expressed in all granulosa cells of growing macaque follicles and absent in atretic follicles, suggesting a survival and proliferative role of FOXO1 in the macaque ovary. However, it is possible that the initial mechanism in follicle atresia involves FOXO1 and its expression has disappeared following morphological degradation of the follicle. In addition, FOXO1 is located in the cytoplasm of granulosa cells in macaque preantral follicles and translocates to the nucleus in antral follicles with differential expression in the mural and cumulus granulosa cells, suggesting a functional role of FOXO1 in granulosa cell differentiation (i.e. hormonal production) in primates.
In rodents, FOXO3 is globally expressed in primordial oocytes and its translocation from the nucleus to cytoplasm coincides with follicle activation and growth [5]. Foxo3 deletion leads to global follicle activation and premature ovarian failure in the mouse [7]. There appears to be a species-specific difference in the role of FOXO3 during follicle development in rodent and primate ovaries. Our finding is consistent with studies done by Tarnawa et al. [14] in the ovaries of diverse mammalian species (rodents, ruminants, pigs, monkeys, and human) wherein FOXO1 expression in granulosa cells was conserved in all species examined, but FOXO3 expression in the oocyte was not. Specifically, FOXO3 was only expressed in rodent oocytes and not in any other species they examined, including nonhuman primates and human [14]. FOXO3 expression was observed in two separate studies in human ovaries from patients with begin ovarian tumor [5] as well as adult women undergoing elective Caesarean section and with nonmalignant gynecological conditions [28]. However, only one follicle with FOXO3 staining was shown in both studies and neither mentioned whether FOXO3 was globally expressed in primordial oocytes as observed in rodents. In the current study, we obtained ovaries from reproductively healthy rhesus macaques of different developmental stages and found that FOXO3 expression decreases dramatically following follicle formation in the macaque fetal ovary, is consistently localized to oocyte nuclei of only a few primordial follicles, and is absent in the adult ovary. It is interesting that there is a differential expression of FOXO3 in germ cells prior to follicle assembly (highest prevalence) and primordial follicles in fetal (low prevalence), prepubertal (very low prevalence), and adult (absent) monkey ovaries. We cannot rule out that FOXO3 is only present when primordial follicles are activated in primates and this remains an interesting idea for future studies. Alternatively, it is possible that instead of promoting primordial follicle activation and growth, FOXO3 may be associated with apoptosis in primates, since the rate of apoptosis also drops significantly from germ cells prior to follicle assembly compared to primordial oocytes after birth and to the lowest in adult primordial oocytes [29, 30]. Foxo3-induced apoptosis is a known cellular event in several systems [31], including the granulosa cell of mice [32] and pigs [33], as well as human endothelial [34] and cancer cells [35]. The correlation between FOXO3 expression and apoptosis in primate primordial oocytes will be examined in future studies.
Li et al. [5] treated human ovarian tissue with a PTEN inhibitor, bpV, for 1 h in vitro prior to transplantation into immunodeficient SCID mice for 6 months. They showed that bpV increased the number of growing follicles compared to the control treatment. Similarly, McLaughlin et al. [28] subjected human ovarian biopsies to bpV exposure for 24 h followed by five additional days of culture without bpV, and isolated secondary follicles from the cultured tissues for further growth. They also demonstrated an increased number of growing follicles following bpV exposure compared to the control treatment; however, secondary follicles isolated from tissues exposed to bpV grew poorly and had compromised survival [28], indicating abnormal follicle development following bpV-induced follicle growth. While these studies suggest a role of the PTEN/PI3K/AKT pathway in primordial follicle activation in the human ovary, whether FOXO3 acts as a downstream effecter of the AKT-mediated pathway is still unclear. Our data demonstrated that while lacking FOXO3, macaque primordial follicles express its upstream regulator, AKT, and its phosphorylated form p-AKT. Thus, it is possible that similar upstream events but different downstream effects may regulate primordial follicles activation in rodents and nonhuman primates.
FOXO4, similar to the other FOXO proteins, is a downstream target of the PI3 kinase pathway. However, while Foxo4 gene expression has been found in the rodent and human ovary [21, 36], the role of Foxo4 in the ovary is unclear and rarely studied, in part due to a lack of abnormal phenotype in Foxo4 knockout mice. Foxo4 null mice are born viable, fertile with morphologically normal ovaries [22]. To date, there is only one study that examined the localization of Foxo4 in the ovary. Richards et al. [21] detected Foxo4 transcripts in granulosa, theca, and luteal cells of the mouse ovary, and showed that Foxo4 mRNA was elevated in ovaries after luteinization and during pregnancy. In our study, FOXO4 protein was localized in a subpopulation of the theca interna layer and some stromal cells in prepubertal and adult ovaries, but was absent in fetal ovaries in the rhesus macaque. FOXO4 expression in the theca cell is novel and suggests a functional role, but the precise identity of cells in the theca interna (i.e. hormone producing cells, immune cells, etc.) expressing FOXO4 remains to be determined.
Species divergence is evident in many aspects of mammalian reproductive physiology including ovarian function. Unlike fetal mouse ovaries, it is difficult to study follicle activation in ovarian cortical pieces from adult primates. Heterogeneity of preantral follicles at different development stages makes it impossible to discern whether bona fide primordial follicle activation occurs unless the number of primordial follicles in the cortical piece is known prior to in vitro treatments. Furthermore, current culture conditions are suboptimal for cortical fragments with dense stroma. Prolonged culture of pieces of ovarian cortex (over 7 days) is associated with increased number of atretic follicles wherein oxygen and nutrient uptake in vitro are limited to the edges of the cortical pieces (our observation, data not shown; [37]). Human ovarian tissue used in studies often originates from women with a large range of age and reproductive history and contains both nongrowing and growing follicles. Nonhuman primates are a clinically relevant model to study human reproduction compared to rodents since women and female monkeys share similar reproductive functions. For example, primordial follicle activation begins after birth in rodents [15], but starts in utero before the completion of germ cell division in primates [17]. Furthermore, primordial follicle activation must occur over decades of the reproductive lifespan in women, but only over a year in mice. Thus, it is not surprising that different mechanisms regulate primordial follicle activation in rodents and primates. Future emphasis on identifying (1) a role for FOXO3 in primate primordial oocytes, (2) the function of FOXO4 in the primate ovary, and (3) in particular, the downstream effects leading to primordial follicle activation in primates is needed. Understanding the mechanism for primordial follicle activation will give insights to the cause for physiological and pathological decline of female fertility and provide possible intervention for POI, preserving fertility in cancer patients facing gonadotoxic treatment and novel contraceptive targets that may help conserve the follicle reserve.
Acknowledgments
We thank Ms. Maralee Lawson for technical assistance and Dr. Carol Hanna for providing mouse ovarian tissue samples. We are also grateful to the ONPRC Division of Comparative Medicine and Pathology Services Unit for assisting animal surgeries and providing tissue samples.
References
- 1. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996; 17:121–155. [DOI] [PubMed] [Google Scholar]
- 2. Cox L, Liu JH. Primary ovarian insufficiency: an update. Int J Womens Health 2014; 6:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol 1996; 14:1718–1729. [DOI] [PubMed] [Google Scholar]
- 4. Schmidt KT, Larsen EC, Andersen CY, Andersen AN. Risk of ovarian failure and fertility preserving methods in girls and adolescents with a malignant disease. BJOG 2010; 117:163–174. [DOI] [PubMed] [Google Scholar]
- 5. Li J, Kawamura K, Cheng Y, Liu S, Klein C, Duan EK, Hsueh AJ. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA 2010; 107:10280–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol 2008; 321:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003; 301:215–218. [DOI] [PubMed] [Google Scholar]
- 8. Hopkins BD, Hodakoski C, Barrows D, Mense SM, Parsons RE. PTEN function: the long and the short of it. Trends Biochem Sci 2014; 39:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim HJ, Crowe P, Yang JL. Current clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment of human cancer. J Cancer Res Clin Oncol 2014; 141(4):671–689. [DOI] [PubMed] [Google Scholar]
- 10. Jagarlamudi K, Liu L, Adhikari D, Reddy P, Idahl A, Ottander U, Lundin E, Liu K. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS One 2009; 4:e6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang B, Mu Y, Ni F, Zhou S, Wang J, Cao Y, Ma X. Analysis of FOXO3 mutation in 114 Chinese women with premature ovarian failure. Reprod Biomed Online 2010; 20:499–503. [DOI] [PubMed] [Google Scholar]
- 12. Gallardo TD, John GB, Bradshaw K, Welt C, Reijo-Pera R, Vogt PH, Touraine P, Bione S, Toniolo D, Nelson LM, Zinn AR, Castrillon DH. Sequence variation at the human FOXO3 locus: a study of premature ovarian failure and primary amenorrhea. Hum Reprod 2008; 23:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watkins WJ, Umbers AJ, Woad KJ, Harris SE, Winship IM, Gersak K, Shelling AN. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertil Steril 2006; 86:1518–1521. [DOI] [PubMed] [Google Scholar]
- 14. Tarnawa ED, Baker MD, Aloisio GM, Carr BR, Castrillon DH. Gonadal expression of foxo1, but not foxo3, is conserved in diverse Mammalian species. Biol Reprod 2013; 88:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirshfield AN. Heterogeneity of cell populations that contribute to the formation of primordial follicles in rats. Biol Reprod 1992; 47:466–472. [DOI] [PubMed] [Google Scholar]
- 16. Sforza C, Forabosco A. A morphometric approach to the study of human ovarian organogenesis. Ital J Anat Embryol 1998; 103:51–62. [PubMed] [Google Scholar]
- 17. McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000; 21:200–214. [DOI] [PubMed] [Google Scholar]
- 18. Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem 2003; 278:35959–35967. [DOI] [PubMed] [Google Scholar]
- 19. Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL. In vitro fertilization-embryo transfer in nonhuman primates: the technique and its applications. Mol Reprod Dev 1990; 27:261–280. [DOI] [PubMed] [Google Scholar]
- 20. Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod 2011; 26:2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol 2002; 16:580–599. [DOI] [PubMed] [Google Scholar]
- 22. Hosaka T, Biggs WH 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA 2004; 101:2975–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cunningham MA, Zhu Q, Hammond JM. FoxO1a can alter cell cycle progression by regulating the nuclear localization of p27kip in granulosa cells. Mol Endocrinol 2004; 18:1756–1767. [DOI] [PubMed] [Google Scholar]
- 24. Cunningham MA, Zhu Q, Unterman TG, Hammond JM. Follicle-stimulating hormone promotes nuclear exclusion of the forkhead transcription factor FoxO1a via phosphatidylinositol 3-kinase in porcine granulosa cells. Endocrinology 2003; 144:5585–5594. [DOI] [PubMed] [Google Scholar]
- 25. Liu Z, Rudd MD, Hernandez-Gonzalez I, Gonzalez-Robayna I, Fan HY, Zeleznik AJ, Richards JS. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol Endocrinol 2009; 23:649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Z, Castrillon DH, Zhou W, Richards JS. FOXO1/3 depletion in granulosa cells alters follicle growth, death and regulation of pituitary FSH. Mol Endocrinol 2013; 27:238–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shen M, Lin F, Zhang J, Tang Y, Chen WK, Liu H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J Biol Chem 2012; 287:25727–25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McLaughlin M, Kinnell HL, Anderson RA, Telfer EE. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol Hum Reprod 2014; 20:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rabinovici J, Jaffe RB. Development and regulation of growth and differentiated function in human and subhuman primate fetal gonads. Endocr Rev 1990; 11:532–557. [DOI] [PubMed] [Google Scholar]
- 30. Baker TGA. Quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci 1963; 158:417–433. [DOI] [PubMed] [Google Scholar]
- 31. Brosens JJ, Wilson MS, Lam EW. FOXO transcription factors: from cell fate decisions to regulation of human female reproduction. Adv Exp Med Biol 2009; 665:227–241. [DOI] [PubMed] [Google Scholar]
- 32. Li L, Ji SY, Yang JL, Li XX, Zhang J, Zhang Y, Hu ZY, Liu YX. Wnt/beta-catenin signaling regulates follicular development by modulating the expression of Foxo3a signaling components. Mol Cell Endocrinol 2014; 382:915–925. [DOI] [PubMed] [Google Scholar]
- 33. Matsuda F, Inoue N, Maeda A, Cheng Y, Sai T, Gonda H, Goto Y, Sakamaki K, Manabe N. Expression and function of apoptosis initiator FOXO3 in granulosa cells during follicular atresia in pig ovaries. J Reprod Dev 2011; 57:151–158. [DOI] [PubMed] [Google Scholar]
- 34. Wang F, Wang YQ, Cao Q, Zhang JJ, Huang LY, Sang TT, Liu F, Chen SY. Hydrogen peroxide induced impairment of endothelial progenitor cell viability is mediated through a FoxO3a dependant mechanism. Microvasc Res 2013; 90:48–54. [DOI] [PubMed] [Google Scholar]
- 35. Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem 2003; 278:49795–49805. [DOI] [PubMed] [Google Scholar]
- 36. Pisarska MD, Kuo FT, Tang D, Zarrini P, Khan S, Ketefian A. Expression of forkhead transcription factors in human granulosa cells. Fertil Steril 2009; 91:1392–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hovatta O, Wright C, Krausz T, Hardy K, Winston RM. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum Reprod 1999; 14:2519–2524. [DOI] [PubMed] [Google Scholar]