First-line nab-paclitaxel versus dacarbazine significantly improved progression-free survival (PFS) (primary) and disease control rate in a phase III trial of patients with metastatic melanoma (NCT00864253). No statistical difference in OS was observed between arms. There was no correlation between SPARC expression and PFS in either arm. Toxicities associated with nab-paclitaxel were manageable and as expected.
Keywords: BRAF, chemotherapy-naïve, dacarbazine, metastatic melanoma, nab-Paclitaxel
Abstract
Background
The efficacy and safety of nab-paclitaxel versus dacarbazine in patients with metastatic melanoma was evaluated in a phase III randomized, controlled trial.
Patients and methods
Chemotherapy-naïve patients with stage IV melanoma received nab-paclitaxel 150 mg/m2 on days 1, 8, and 15 every 4 weeks or dacarbazine 1000 mg/m2 every 3 weeks. The primary end point was progression-free survival (PFS) by independent radiologic review; the secondary end point was overall survival (OS).
Results
A total of 529 patients were randomized to nab-paclitaxel (n = 264) or dacarbazine (n = 265). Baseline characteristics were well balanced. The majority of patients were men (66%), had an Eastern Cooperative Oncology Group status of 0 (71%), and had M1c stage disease (65%). The median PFS (primary end point) was 4.8 months with nab-paclitaxel and 2.5 months with dacarbazine [hazard ratio (HR), 0.792; 95.1% confidence interval (CI) 0.631–0.992; P = 0.044]. The median OS was 12.6 months with nab-paclitaxel and 10.5 months with dacarbazine (HR, 0.897; 95.1% CI 0.738–1.089; P = 0.271). Independently assessed overall response rate was 15% versus 11% (P = 0.239), and disease control rate (DCR) was 39% versus 27% (P = 0.004) for nab-paclitaxel versus dacarbazine, respectively. The most common grade ≥3 treatment-related adverse events were neuropathy (nab-paclitaxel, 25% versus dacarbazine, 0%; P < 0.001), and neutropenia (nab-paclitaxel, 20% versus dacarbazine, 10%; P = 0.004). There was no correlation between secreted protein acidic and rich in cysteine (SPARC) status and PFS in either treatment arm.
Conclusions
nab-Paclitaxel significantly improved PFS and DCR compared with dacarbazine, with a manageable safety profile.
introduction
Historically, dacarbazine and high-dose interleukin-2 were the only US Food and Drug Administration (FDA)–approved options for patients with metastatic melanoma [1]. However, several effective new treatment options, including immunotherapy (anti-CTLA-4 and anti PD-1) and targeted therapy (BRAF and MEK inhibitors) for patients with BRAF-mutated melanoma have recently been approved by the FDA [2–8]. Despite these therapeutic advances, chemotherapy retains a role in the treatment of patients with metastatic melanoma, including those without a targetable mutation [9]. However, neither single agents [10, 11] nor combination chemotherapy regimens [12] have demonstrated a clear advantage over dacarbazine alone, and improved therapeutic options are needed.
Although taxanes have shown limited efficacy in metastatic melanoma [11, 13–17], paclitaxel formulated as albumin-bound nanoparticles [nab-paclitaxel (Abraxane); Celgene, Summit, New Jersey] demonstrated a promising response rate (21.6%), median progression-free survival (PFS) of 4.5 months, and median overall survival (OS) of 9.6 months in a phase II study of chemotherapy-naïve patients [18]. Based on the promising utility of nab-paclitaxel in metastatic melanoma, this phase III study compared the efficacy and safety of single-agent nab-paclitaxel versus dacarbazine in chemotherapy-naïve patients.
patients and methods
This study was approved by the independent ethics committees of the participating medical institutions and was conducted in compliance with the protocol, the World Medical Association Declaration of Helsinki, Good Clinical Practice, and the Guidelines of the International Conference on Harmonization [19]. Written informed consent was obtained from all patients before study initiation.
patients
Adults with histologically/cytologically confirmed stage IV malignant melanoma were eligible if they had received no prior cytotoxic therapy and had ≥1 radiographically measurable lesion, based on Response Evaluation Criteria in Solid Tumor (RECIST) v1.0 [20]. Previous treatments with kinase inhibitors or cytokines were permitted if they were completed 4 weeks before enrollment. Patients with history of in situ, basal, or squamous cell skin cancer were eligible. Patients with other malignancies were also eligible if they were cured by surgery and/or radiation and had been continuously disease free for ≥5 years. Patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, a life expectancy of >12 weeks, and lactate dehydrogenase (LDH) levels ≤2× the upper limit of normal (ULN) were eligible. Patients were excluded from the study if they had prior/current brain metastases.
study design
In this open-label, multicenter phase III study, eligible patients were randomized 1 : 1 via a centralized system to nab-paclitaxel 150 mg/m2 administered i.v. on days 1, 8, and 15 every 28 days or dacarbazine 1000 mg/m2 administered on day 1 every 21 days. Randomization was stratified by disease stage (M1a, M1b, M1c), geographic region (Australia, North America, Western Europe), and baseline LDH levels (<0.8× ULN, 0.8–1.1× ULN, >1.1–2× ULN). Blood counts and chemistries were obtained before each drug administration, and dose modifications were carried out per protocol. Weekly review of patients on treatment was scheduled, irrespective of treatment allocation, to evaluate safety and efficacy. Patients were treated until disease progression, unacceptable toxicity, or patient/physician decision.
assessment of efficacy and safety end points
Patients were evaluated for response and progression using RECIST criteria v1.0. Radiographic evaluation by computed tomography scan was carried out at baseline (within 7 days of starting treatment) and then every 8 weeks in both arms.
Safety and tolerability were monitored through reporting of adverse events (AEs), serious AEs (SAEs), laboratory abnormalities, and incidence of patients experiencing dose modifications and/or premature discontinuation of study drug.
end points and statistical methods
The primary efficacy end point was PFS based on an independent radiological review and the secondary efficacy end point was OS; both were summarized by median time [including 95% confidence interval (CI)] for each treatment arm along with the hazard ratio (HR, including 95.1% CI for PFS and 99.9% CI for OS). The differences in Kaplan–Meier curves were tested using stratified log-rank test. The summary of censoring is described in the CONSORT diagram (supplementary Figure S1, available at Annals of Oncology online). All randomized patients were evaluated for efficacy [intent-to-treat (ITT) population]. For PFS, 514 planned patients with 379 events provided ≥80% power to detect a HR of 0.750 (two-sided α, 0.049). The final OS analysis was planned with at least 417 events, which provided ≥80% power to detect an HR of 0.76 (two-sided α, 0.049). Other end points, including overall response rate [ORR, confirmed complete response (CR) or partial response (PR)] and disease control rate [DCR; CR + PR + stable disease (SD) ≥16 weeks], were tested using χ2 test. The protocol was modified in 2011 for the collection of BRAF mutational status, after results showing that BRAF mutational status could be related to prognosis and response to other therapies [21]. The statistical plan was amended before database lock to include PFS and OS by BRAF status as a prespecified analysis.
All treated patients were evaluated for safety. All AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v3.0, coded using Medical Dictionary for Regulatory Activities v12.1 and summarized by System Organ Class and Preferred Term. Statistical testing of AE differences between nab-paclitaxel and dacarbazine were compared using the Fisher's exact test (overall) and the Cochran–Mantel–Haenszel test (by grade). The NCI CTCAE grades for hematology and chemistry laboratory results were summarized by the most severe grade.
exploratory biomarker analyses
SPARC immunohistochemistry (IHC) was carried out and scored as previously described (see supplementary Materials, available at Annals of Oncology online) [22]. H&E stained slides were scored for tumor-infiltrating lymphocytes and the score was correlated with survival outcomes (see supplementary Materials, available at Annals of Oncology online).
results
patients
A total of 529 patients were randomized between April 2009 and June 2011, 264 to nab-paclitaxel and 265 to dacarbazine (ITT population; see CONSORT diagram in supplementary Figure S1, available at Annals of Oncology online). The two treatment arms were generally well balanced for relevant baseline characteristics (Table 1). Only 8% of patients received prior therapy for metastatic disease, such as immunostimulants (6%) and antineoplastic agents (2%), including kinase inhibitors.
Table 1.
Baseline patient demographics and characteristics
| Variable | nab-Paclitaxel (N = 264) | Dacarbazine (N = 265) | All patients (N = 529) |
|---|---|---|---|
| Age | |||
| Median years (min, max) | 62 (21, 85) | 64 (28, 87) | 63 (21, 87) |
| <65, n (%) | 154 (58) | 135 (51) | 289 (55) |
| Sex | |||
| Male, n (%) | 173 (66) | 174 (66) | 347 (66) |
| Region | |||
| North America, n (%) | 115 (44) | 116 (44) | 231 (44) |
| Western Europe, n (%) | 114 (43) | 114 (43) | 228 (43) |
| Australia, n (%) | 35 (13) | 35 (13) | 70 (13) |
| Ethnicity | |||
| White, n (%) | 251 (95) | 252 (95) | 503 (95) |
| Latino, n (%) | 12 (5) | 12 (5) | 24 (95) |
| Asian, n (%) | 1 (<1) | 1 (<1) | 2 (<1) |
| ECOG PS | |||
| 0, n (%) | 195 (74) | 181 (68) | 367 (71) |
| 1, n (%) | 68 (26) | 82 (31) | 150 (28) |
| 2, n (%) | 1 (<1) | 2 (<1) | 3 (<1) |
| Metastatic stage | |||
| M1a, n (%) | 27 (10) | 21 (8) | 48 (9) |
| M1b, n (%) | 66 (25) | 69 (26) | 135 (26) |
| M1c, n (%) | 171 (65) | 175 (66) | 346 (65) |
| LDH category | |||
| <0.8× ULN, n (%) | 138 (52) | 139 (52) | 277 (52) |
| 0.8–1.1× ULN, n (%) | 72 (27) | 69 (26) | 141 (27) |
| >1.1–2× ULN, n (%) | 51 (19) | 56 (21) | 107 (20) |
| >2× ULN, n (%) | 3 (1) | 1 (<1) | 4 (<1) |
| BRAF status | |||
| Known, n (%) | 181 (69) | 175 (66) | 356 (67) |
| Mutant | 65 (36) | 67 (38) | 132 (37) |
| Wild type | 116 (64) | 108 (62) | 224 (63) |
| Unknown, n (%) | 83 (31) | 90 (34) | 173 (33) |
| Prior therapy | |||
| Metastatic, n (%) | 18 (7) | 24 (9) | 42 (8) |
ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PS, performance status; ULN, upper limit of normal.
efficacy results
progression-free survival
In the final PFS analysis, 152 patients (58%) in the nab-paclitaxel and 170 patients (64%) in the dacarbazine arm had progressed or died. Median PFS was 4.8 and 2.5 months, respectively (HR, 0.792; 95.1% CI 0.631–0.992; P = 0.044; Figure 1A; Table 2). The PFS estimate at 6 months was 37% with nab-paclitaxel versus 30% with dacarbazine. The robustness of the PFS analysis was supported with various sensitivity analyses related to off-schedule response assessments or missed study visits (supplementary Table S1, available at Annals of Oncology online). Investigator-assessed median PFS was 3.7 months with nab-paclitaxel and 2.1 months with dacarbazine (HR, 0.845; 95.1% CI 0.696–1.025; P = 0.086; supplementary Figure S2, available at Annals of Oncology online).
Figure 1.
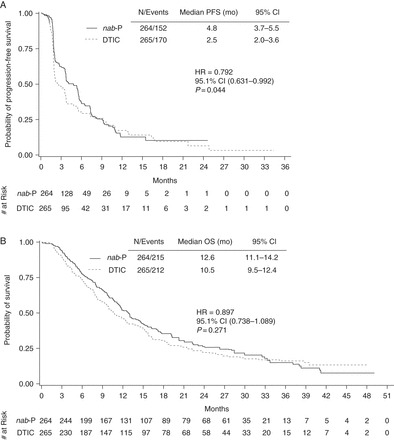
Independent radiologist-assessed progression-free survival (A) and final overall survival (B) Kaplan–Meier curves for the intent-to-treat population.
Table 2.
Response rates, progression-free survival, and overall survival for the intent-to-treat population based on independent radiological assessment
| Blinded radiology assessment | nab-Paclitaxel (N = 264) | Dacarbazine (N = 265) | Response rate ratioa (Pnab-P/PDTIC) | P b |
|---|---|---|---|---|
| ORR, n (%) | 39 (15) | 30 (11) | 1.305 (0.837–2.035) | 0.239 |
| 95% CI | 10.5–19.1 | 7.5–15.1 | ||
| DCR,cn (%) | 102 (39) | 71 (27) | 1.442 (1.123–1.852) | 0.004 |
| 95% CI | 32.8–44.5 | 21.5–32.1 | ||
| PR, n (%) | 39 (15) | 30 (11) | ||
| SD ≥16 weeks, n (%) | 63 (24) | 41 (15) | ||
| Best response, n (%) | 0.0017d | |||
| PR | 39 (15) | 30 (11) | ||
| SD | 67 (25) | 41 (16) | ||
| PD | 93 (35) | 128 (48) | ||
| Not evaluablee | 65 (25) | 65 (25) | 0.005f | |
| HR (HRnab-P/DTIC) | ||||
| PFS, median (95% CI) based on independent radiology review (months) | 4.8 (3.7–5.5) | 2.5 (2.0–3.6) | 0.792 (0.631–0.992)g | 0.044 |
| BRAF mutant | 5.3 (3.5–7.5) | 3.5 (1.9–5.5) | 0.883 (0.515–1.513) | 0.656 |
| BRAF wild type | 5.4 (3.5–5.7) | 2.5 (1.9–3.7) | 0.715 (0.492–1.040) | 0.088 |
| BRAF unknown | 3.7 (2.8–5.6) | 2.2 (1.9–3.6) | 0.684 (0.457–1.024) | 0.066 |
| PFS, median (95% CI) based on investigator review (months) | 3.7 (3.1–3.9) | 2.1 (1.9–2.5) | 0.845 (0.696–1.025) | 0.086 |
| OS, median (95% CI) (months) | 12.6 (11.1–14.2) | 10.5 (9.5–12.4) | 0.897 (0.738–1.089)h | 0.271 |
aThe 95% CI for response rate ratios is calculated according to the asymptotic 95% CI of the relative risk of nab-paclitaxel to dacarbazine.
bThe P values are based on the χ2 test.
cDCR includes CR + PR + stable disease (SD) ≥16 weeks.
dIncludes confirmed PR, SD, and PD.
eNonassessable patients had (i) scans that were not done, (ii) scans that were done but not fully evaluable, or (iii) scans that were done and evaluable but a single response of CR, PR, or SD was not confirmed at a later assessment.
fComparison of PD rate between arms.
g95.1% CI is provided given that two-sided type I error of 0.049 was allocated for final PFS analysis.
h95.1% CI is provided given that two-sided type I error of 0.049 was allocated for final OS analysis.
DCR, disease control rate; DTIC, dacarbazine; HR, hazard ratio; nab-P, nab-paclitaxel; ORR, objective response rate; OS, overall survival; P, proportion of improved patients; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
overall survival
At the time of the final OS analysis (data cutoff 20 September 2013), 427 patients (81%) died [215 (81%) in the nab-paclitaxel and 212 (80%) in the dacarbazine arms]. Median OS was 12.6 months with nab-paclitaxel and 10.5 months with dacarbazine (HR, 0.897; 95.1% CI 0.738–1.089; P = 0.271; Figure 1B; Table 2).
Most (75%) patients received subsequent therapies (77% nab-paclitaxel; 73% dacarbazine): 13% and 10% of patients received a BRAF inhibitor, and 31% and 32% received ipilimumab, in the nab-paclitaxel and dacarbazine arms, respectively. Additionally, 15% and 11% of patients received other immunotherapy or targeted therapy, 18% and 23% of patients received subsequent chemotherapy (other than nab-paclitaxel-based therapy), and 25% and 22% of patients received radiotherapy in the nab-paclitaxel and dacarbazine arms, respectively. The median time to the start of poststudy therapy was 26 and 21 days, respectively.
overall response and disease control rates
Independently assessed ORR was 15% versus 11% (response rate ratio, 1.305; 95% CI 0.837–2.035; P = 0.239) with nab-paclitaxel versus dacarbazine (Table 2). For patients who had a confirmed CR or PR, the median time to response was 2.2 versus 3.6 months, respectively (P = 0.44). Treatment with nab-paclitaxel versus dacarbazine resulted in a significant improvement in DCR (P = 0.004) and best ORR (P = 0.002; Table 2). Significantly less progressive disease was observed with nab-paclitaxel (35%) versus dacarbazine (48%), P = 0.005.
analyses by subgroups
In general, most subgroup analyses indicated an improvement in favor of the nab-paclitaxel arm (Figure 2). Improvement in PFS with nab-paclitaxel occurred in all patients regardless of age, region, baseline LDH, BRAF mutation status, and patients with M1c/poor prognosis. Of note, nab-paclitaxel produced longer PFS (HR, 0.734; 95% CI 0.558–0.965; P = 0.028) compared with dacarbazine for patients with the most advanced melanoma (M1c). Trends toward longer PFS favoring nab-paclitaxel were observed in all BRAF subgroups (Table 2).
Figure 2.
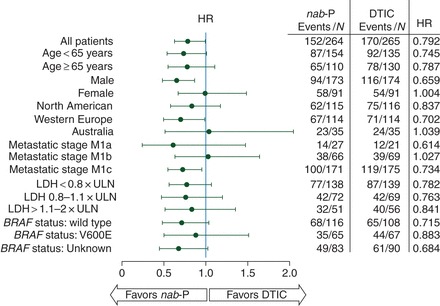
Subgroup analyses of progression-free survival by independent review.
treatment exposure and dose reductions
The median treatment duration was 11.1 weeks for nab-paclitaxel and 6.4 weeks for dacarbazine. The median number of cycles was 3 in each arm. Median percentage of protocol dose was 98% (min, max: 50%, 105%) and 100% (min, max: 48%, 105%) in the nab-paclitaxel and dacarbazine arms, respectively. Median dose intensity was 146.5 and 333.3 mg/m2/week, respectively, noting that dacarbazine was given every 3 weeks. More dose reductions occurred with nab-paclitaxel (32%) versus dacarbazine (20%), all of which were due to AEs, mainly neuropathy.
safety results
Both agents produced expected AE profiles (Table 3). Specifically, 50% versus 27% of patients had ≥1 treatment-related AE (TRAE) and 9% versus 7% patients had ≥1 treatment-related SAE in the nab-paclitaxel arm versus dacarbazine arm, respectively. The most common grade ≥3 TRAEs were neuropathy (25% versus 0%), neutropenia (20% versus 10%), and leukopenia (12% versus 7%) in the nab-paclitaxel versus dacarbazine arm, respectively (Table 3). No patients experienced grade ≥3 thrombocytopenia during treatment with nab-paclitaxel compared with 6% of patients receiving dacarbazine. Of the grade ≥3 treatment-related peripheral neuropathy events, all occurred in the nab-paclitaxel arm; 2 events were grade 4. The median onset of grade ≥3 peripheral neuropathy was 101 days (95% CI 85–113) after start of treatment. After treatment modification, median times for grade ≥3 peripheral neuropathy to improve by ≥1 grade and to reduce to grade ≤1 were 28 and 67 days, respectively. Thirty-two percent of patients never developed treatment-related neuropathy in the nab-paclitaxel arm.
Table 3.
Most common treatment-related grade ≥2 adverse events reported in ≥5% patients
| Preferred terms |
nab-Paclitaxel (N = 257) |
Dacarbazine (N =
258) |
||||
|---|---|---|---|---|---|---|
| Treatment-related death | 2 (<1) |
1 (<1) |
||||
| Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 | |
| Hematologic AEs, n (%)a | ||||||
| Neutropenia | 67 (26) | 42 (17) | 8 (3) | 34 (14) | 14 (6) | 11 (4) |
| Leukopenia | 93 (37) | 30 (12) | 1 (<1) | 48 (20) | 14 (6) | 3 (1) |
| Lymphocytopenia | 63 (25) | 18 (7) | 1 (<1) | 71 (29) | 23 (9) | 4 (2) |
| Thrombocytopenia | 0 ( | 0 ( | 0 ( | 14 (6) | 9 (4) | 6 (2) |
| Anemia | 52 (21) | 4 (2) | 0 ( | 31 (13) | 12 (5) | 0 ( |
| Nonhematologic AEs, n (%)a | ||||||
| Alopecia | 101 (39) | 12 (5) | 0 ( | 0 ( | 0 ( | 0 ( |
| Peripheral neuropathyb | 42 (16) | 62 (24) | 2 (<1) | 1 (<1) | 0 ( | 0 ( |
| Fatigue | 47 (18) | 21 (8) | 0 ( | 33 (13) | 4 (2) | 0 ( |
| Diarrhea | 24 (9) | 3 (1) | 0 ( | 11 (4) | 1 (<1) | 0 ( |
| Nausea | 22 (9) | 1 (<1) | 0 ( | 20 (8) | 3 (1) | 0 ( |
| Rash | 23 (9) | 1 (<1) | 0 ( | 1 (<1) | 0 ( | 0 ( |
| Nail disorder | 21 (8) | 3 (1) | 0 ( | 0 ( | 0 ( | 0 ( |
aBased on central laboratory values. Except for lymphocytopenia, all events across all grades (Cochran–Mantel–Haenszel test) and for grade 3 and 4 (Fisher's exact test) P < 0 (.05.
bPeripheral neuropathy was classified based on Standardized MedDRA Queries (SMQ) (broad scope).
AE, adverse event; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
exploratory biomarker analyses
SPARC IHC data were evaluable in 194 patient tumor samples (100 for nab-paclitaxel and 94 for dacarbazine). Baseline characteristics for this patient subset were similar to the ITT population. Patients were classified into high SPARC (n = 53 for nab-paclitaxel; n = 50 for dacarbazine) or low SPARC (n = 47 for nab-paclitaxel; n = 44 for dacarbazine). Independently assessed PFS was similar between patients with high SPARC and low SPARC scores who were in the nab-paclitaxel (median PFS 3.71 versus 3.94 months; P = 0.783) or dacarbazine (median PFS 3.71 versus 1.91 months; P = 0.182) arms.
Results from a post hoc analysis of TILs are reported in the supplementary Materials, available at Annals of Oncology online.
discussion
nab-Paclitaxel demonstrated clinically meaningful superiority compared with dacarbazine, with a near doubling of median PFS and a 44% improvement in DCR (includes patients with SD for ≥16 weeks) in chemotherapy-naïve patients with metastatic melanoma. Compared with dacarbazine, nab-paclitaxel reduced the risk of disease progression or death by >20%. The results observed for dacarbazine in this study were consistent with recent phase III trials [3–5, 8, 10, 11]. Early separation of the survival curves at 3 months provided evidence of early treatment effect, which was maintained for more than 30 months. Although a significant difference in PFS was observed with nab-paclitaxel versus dacarbazine, a significant treatment effect of nab-paclitaxel on OS may have been limited by the equivalent and high rate (75%) of use of poststudy therapy, including newer agents, such as BRAF inhibitors and ipilimumab, by patients in both treatment arms.
SPARC, an albumin-binding protein, has both protumorigenic and antitumorigenic properties [23]. SPARC expression may be associated with positive clinical outcomes in patients receiving nab-paclitaxel, as it may help to enrich nab-paclitaxel in the tumor and/or tumor microenvironment (reviewed in Yardley) [24]. However, no correlation was found between tumor SPARC expression and PFS with nab-paclitaxel treatment in this trial. A recent analysis of a large phase III trial of metastatic pancreatic cancer similarly found no correlation between SPARC expression, nab-paclitaxel treatment, and clinical outcome [25].
All AEs were manageable, and no new or unexpected AEs were noted for nab-paclitaxel in patients with metastatic melanoma [26–28]. Grade ≥3 treatment-related peripheral neuropathy was seen only in patients receiving nab-paclitaxel and was consistent with the incidence observed in patients receiving the agent for the approved indications [26–28]. Peripheral neuropathy was the primary reason for the higher rate of treatment discontinuation in the nab-paclitaxel arm than in the dacarbazine arm. Despite the high rate of grade ≥3 peripheral neuropathy, a number of patients were able to resume treatment with nab-paclitaxel following dose modification procedures that improved peripheral neuropathy by at least 1 grade in half the patients within 1 month. Thus, neuropathy management with treatment modifications remains important for patients to be able to receive the maximum benefit from nab-paclitaxel. Other strategies may include using drugs such as pregabalin or duloxetine, which may ameliorate chemotherapy-induced peripheral neuropathy [29].
One limitation of this study was that patients with >2× ULN of LDH were excluded; however, attempts were made to mirror the general population within the LDH categories. It has been established that exceedingly high levels of LDH may also make melanoma cells resistant to certain treatments [30]. Collection of quality-of-life data may have helped to more fully assess the clinical benefit of nab-paclitaxel in this patient population.
The higher efficacy observed for nab-paclitaxel versus historical trials of sb-paclitaxel in metastatic melanoma [15–17] may be explained by the intrinsic benefit of albumin-based nab technology and its distinct pharmacokinetic profile versus sb-paclitaxel [31]. The lack of solvent, which alone contributes to neuropathy [32] and hypersensitivity reactions [33], may contribute to an improved tolerability profile and allow for higher dose delivery and intensity of nab-paclitaxel compared with sb-paclitaxel [26, 27]. Efficacy results with single-agent nab-paclitaxel in our study compared favorably with the commonly used regimen of sb-paclitaxel plus carboplatin reported in a phase III study of patients with metastatic melanoma, producing similar efficacy outcomes (18% ORR; median PFS and OS of 4.2 and 11.3 months, respectively) [34]. Neutropenia, leukopenia, and sensory neuropathy were the most common grade ≥3 TRAEs observed with sb-paclitaxel plus carboplatin in that study. In a recent phase II study, nab-paclitaxel plus bevacizumab as first-line therapy in patients with metastatic melanoma produced a 36% ORR and a median PFS and OS of 7.6 and 16.8 months, respectively [35], suggesting that nab-paclitaxel may synergize with other therapeutics, including immunotherapy, and should be further explored in clinical trials. A phase II trial is underway to study nab-paclitaxel in combination with ipilimumab (NCT01827111) in patients with advanced or metastatic melanoma [36].
Chemotherapy remains an important treatment option for patients with BRAF wild-type melanoma who are not candidates for ipilimumab and patients with BRAF mutant disease resistant to BRAF inhibitors [9]. In the present trial, nab-paclitaxel benefited patients regardless of BRAF mutation status. Additionally, in a post hoc analysis of this trial, nab-paclitaxel was shown to benefit a subgroup of patients with low or absent TILs (see supplementary Materials, available at Annals of Oncology online), a poor prognostic factor in melanoma [37].
In conclusion, nab-paclitaxel demonstrated a clinical benefit versus dacarbazine and produced a manageable safety profile. Thus, nab-paclitaxel can be considered in the treatment armamentarium for chemotherapy-naïve patients with metastatic melanoma. The National Comprehensive Cancer Network guidelines recommend nab-paclitaxel as a single agent for the treatment of advanced or metastatic melanoma (category 2A) [38]. Results of ongoing trials of nab-paclitaxel in combination with targeted therapies or novel immunotherapies may help expand this recommendation in the future, as nab-paclitaxel may provide a good backbone regimen to build upon given its safety profile.
funding
This study was sponsored by Celgene Corporation, Summit, NJ (no grant number).
disclosure
EMH: research funding, Celgene Corp.; MDV: research funding, Celgene Corp.; MPB: consultant or advisory role and research funding, Celgene Corp.; CL: consultant or advisory role and research funding, Celgene Corp.; AT: consultant or advisory role, BMS, Roche and honoraria BMS; RG: consultant or advisory role Roche, MSD, BMS, Novartis, and GSK, honoraria, Roche, BMS, MSD, Janssen, Amgen, GSK, Almirall, Novartis, MerckSenono, and Pfizer, research funding, Roche Novartis and Pfizer; CR: consultant or advisory role, Celgene Corp.; ML: employment or leadership position and stock ownership, Celgene Corp.; AC: employment or leadership position and stock ownership, Celgene Corp.; CB: employment or leadership position and stock ownership, Celgene Corp.; MK: employment or leadership position and stock ownership, Celgene Corp.; IE: employment or leadership position and stock ownership, Celgene Corp.; MFR: employment or leadership position and stock ownership, Celgene Corp.; AH: consultant or advisory role, honoraria, and research funding, Celgene Corp. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank all the investigators worldwide who enrolled patients in this phase III study, the patients who participated in the study, as well as the patient families that supported them, Karthi Natarajan and Ruta Slepetis, Celgene Corporation for their coordination of the clinical trial sites. Medical writing assistance was provided by Anita N. Schmid, Celgene Corporation, and Kerry R. Garza, MediTech Media, Ltd, funded by Celgene Corporation. The authors are fully responsible for all content and editorial decisions for this manuscript.
references
- 1. Boyle GM. Therapy for metastatic melanoma: an overview and update. Expert Rev Anticancer Ther 2011; 11: 725–737. [DOI] [PubMed] [Google Scholar]
- 2. Hodi FS, O'Day SJ, McDermott DF et al. . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert C, Thomas L, Bondarenko I et al. . Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–2526. [DOI] [PubMed] [Google Scholar]
- 4. Chapman PB, Hauschild A, Robert C et al. . Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hauschild A, Grob JJ, Demidov LV et al. . Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380: 358–365. [DOI] [PubMed] [Google Scholar]
- 6. Flaherty KT, Robert C, Hersey P et al. . Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012; 367: 107–114. [DOI] [PubMed] [Google Scholar]
- 7. Robert C, Ribas A, Wolchok JD et al. . Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomized dose-comparison cohort of a phase 1 trial. Lancet 2014; 384: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 8. Robert C, Long GV, Brady B et al. . Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–330. [DOI] [PubMed] [Google Scholar]
- 9. Kaufman HL, Kirkwood JM, Hodi FS et al. . The Society for Immunotherapy of Cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nat Rev Clin Oncol 2013; 10: 588–598. [DOI] [PubMed] [Google Scholar]
- 10. Patel PM, Suciu S, Mortier L et al. . Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur J Cancer 2011; 47: 1476–1483. [DOI] [PubMed] [Google Scholar]
- 11. Bedikian AY, DeConti RC, Conry R et al. . Phase 3 study of docosahexaenoic acid-paclitaxel versus dacarbazine in patients with metastatic malignant melanoma. Ann Oncol 2011; 22: 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atkins MB, Hsu J, Lee S et al. . Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 2008; 26: 5748–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aamdal S, Wolff I, Kaplan S et al. . Docetaxel (Taxotere) in advanced malignant melanoma: a phase II study of the EORTC Early Clinical Trials Group. Eur J Cancer 1994; 30A: 1061–1064. [DOI] [PubMed] [Google Scholar]
- 14. Bedikian AY, Weiss GR, Legha SS et al. . Phase II trial of docetaxel in patients with advanced cutaneous malignant melanoma previously untreated with chemotherapy. J Clin Oncol 1995; 13: 2895–2899. [DOI] [PubMed] [Google Scholar]
- 15. Walker L, Schalch H, King DM et al. . Phase II trial of weekly paclitaxel in patients with advanced melanoma. Melanoma Res 2005; 15: 453–459. [DOI] [PubMed] [Google Scholar]
- 16. Legha SS, Ring S, Papadopoulos N et al. . A phase II trial of Taxol in metastatic melanoma. Cancer 1990; 65: 2478–2481. [DOI] [PubMed] [Google Scholar]
- 17. Einzig AI, Hochster H, Wiernik PH et al. . A phase II study of Taxol in patients with malignant melanoma. Invest New Drugs 1991; 9: 59–64. [DOI] [PubMed] [Google Scholar]
- 18. Hersh EM, O'Day SJ, Ribas A et al. . A phase 2 clinical trial of nab-paclitaxel in previously treated and chemotherapy-naïve patients with metastatic melanoma. Cancer 2010; 116: 155–163. [DOI] [PubMed] [Google Scholar]
- 19. American Society of Clinical Oncology. Good clinical practice research guidelines reviewed, emphasis given to responsibilities of investigators: second article in a series. J Oncol Pract 2008; 4: 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Therasse P, Arbuck SG, Eisenhauer EA et al. . New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216. [DOI] [PubMed] [Google Scholar]
- 21. Arkenau HT, Kefford R, Long GV. Targeting BRAF for patients with melanoma. Br J Cancer 2011; 104: 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Von Hoff DD, Ramanathan R, Borad MJ et al. . Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011; 29: 4548–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Podhajcer OL, Benedetti LG, Girotti MR et al. . The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev 2008; 27: 691–705. [DOI] [PubMed] [Google Scholar]
- 24. Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release 2013; 170: 365–372. [DOI] [PubMed] [Google Scholar]
- 25. Hidalgo M, Plaza C, Illei PB et al. . SPARC analysis in the phase III MPACT trial of nab-paclitaxel plus gemcitabine vs gemcitabine alone for patients with metastatic pancreatic cancer. In Oral presentation at: 16th World Congress on Gastrointestinal Cancer, 25–28 June 2014 Barcelona, Spain(abstract O-0004). [Google Scholar]
- 26. Gradishar WJ, Tjulandin S, Davidson N et al. . Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005; 23: 7794–7803. [DOI] [PubMed] [Google Scholar]
- 27. Socinski MA, Bondarenko I, Karaseva NA et al. . Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012; 30: 2055–2062. [DOI] [PubMed] [Google Scholar]
- 28. von Hoff DD, Ervin T, Arena F et al. . Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hershman DL, Lacchetti C, Dworkin RH et al. . Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2014; 32: 1941–1967. [DOI] [PubMed] [Google Scholar]
- 30. Keilholz U, Suciu S, Bedikian A et al. . LDH is a prognostic factor in stage IV melanoma patients (pts) but is a predictive factor only for bcl2 antisense treatment efficacy: re-analysis of GM301 and EORTC18951 randomized trials. J Clin Oncol 2007; 25(18s): abstr 4552. [Google Scholar]
- 31. Chen N, Li Y, Ye Y et al. . Pharmacokinetics and pharmacodynamics of nab-paclitaxel in patients with solid tumors: disposition kinetics and pharmacology distinct from solvent-based paclitaxel. J Clin Pharmacol 2014; 54: 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mielke S, Sparreboom A, Mross K. Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. Eur J Cancer 2006; 42: 24–30. [DOI] [PubMed] [Google Scholar]
- 33. Irizarry LD, Luu TH, McKoy JM et al. . Cremophor EL-containing paclitaxel-induced anaphylaxis: a call to action. Commun Oncol 2009; 6: 132–134. [PMC free article] [PubMed] [Google Scholar]
- 34. Flaherty KT, Lee SJ, Zhao F et al. . Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 2013; 31: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spitler L, Boasberg P, O'Day S et al. . Phase II study of nab-paclitaxel and bevacizumab as first-line therapy for patients with unresectable stage III and IV melanoma. Am J Clin Oncol 2015; 38: 61–67. [DOI] [PubMed] [Google Scholar]
- 36. ClinicalTrials.gov. Phase II study of abraxane plus ipilimumab in patients with metastatic melanoma. https://clinicaltrials.gov/ct2/show/NCT01827111 (11 March 2015, date last accessed).
- 37. Oble DA, Loewe R, Yu P, Mihm MC Jr. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun 2009; 9: 3. [PMC free article] [PubMed] [Google Scholar]
- 38. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Melanoma. Version 2.2015 www.nccn.org (10 March 2015, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


