The randomized phase II study ARCHER 1042 explored the impact of prophylactic treatment on select dermatologic and gastrointestinal adverse events (AE) of interest and patient-reported outcomes. Doxycycline reduced the incidence of grade ≥2 select dermatologic AEs of interest by 50% (P = 0.016). Both doxycycline and alclometasone reduced the negative impact in patient-reported dermatologic AEs.
Keywords: dacomitinib, non-small-cell lung cancer, epidermal growth factor receptor, dermatologic adverse events, gastrointestinal adverse events
Abstract
Background
ARCHER 1042, a randomized phase II trial, explored the impact of prophylactic treatment on select dermatologic adverse events of interest (SDAEI), diarrhea, and mucositis associated with dacomitinib, an oral irreversible pan-human epidermal growth factor receptor (HER) inhibitor, in development for advanced non-small-cell lung cancer (NSCLC).
Patients and methods
Patients with advanced NSCLC treated with dacomitinib were enrolled in two cohorts. Cohort I patients were randomized 1:1 to receive oral doxycycline or placebo (4 weeks). Cohort II patients received oral VSL#3 probiotic plus topical alclometasone. Primary end points for Cohorts I and II were incidence of all grade and grade ≥2 SDAEI in the first 8 weeks of treatment and quality of life (QoL) assessed by the Skindex-16 survey. Additional primary end points for Cohort II were incidence of all grade and grade ≥2 diarrhea and mucositis in the first 8 weeks of treatment; QoL regarding diarrhea and mucositis incidence was assessed by the modified-Oral Mucositis Daily Questionnaire.
Results
Cohort I randomized 114 evaluable patients: 56 in the doxycycline arm, 58 in the placebo arm. Cohort II enrolled 59 evaluable patients. Doxycycline significantly reduced the incidence of grade ≥2 SDAEI by 50% (P = 0.016) compared with placebo. The incidence of all grade SDAEI was lower with doxycycline than with placebo but did not reach statistical significance. Doxycycline was associated with less deterioration in QoL compared with placebo. Alclometasone was associated with less deterioration in QoL compared with placebo but did not statistically significantly reduce the incidence of all grade or grade ≥2 SDAEI. VSL#3 did not reduce the incidence of all grade or grade ≥2 diarrhea and did not impact mucositis scores.
Conclusions
Doxycycline was effective as a prophylactic treatment for dacomitinib-induced grade ≥2 SDAEI. Both doxycycline and alclometasone reduced the negative impact in patient-reported dermatologic AEs. The probiotic was not effective for preventing diarrhea or mucositis.
introduction
Dacomitinib (PF-00299804) is an orally administered, irreversible, small-molecule inhibitor of the human epidermal growth factor receptor (HER) family tyrosine kinase receptors [HER-1/epidermal growth factor receptor (EGFR), HER-2, and HER-4] [1]. It is currently in development for the treatment of patients with locally advanced or metastatic non-small-cell lung cancer (NSCLC) with EGFR activating mutations [1].
Dacomitinib has a toxicity profile comparable with that of other EGFR inhibitors, the most frequent adverse events (AEs) being skin toxicity, diarrhea, stomatitis, and mucosal inflammation [1]. Dermatologic toxicities associated with EGFR-targeted therapy include acneiform rash, desquamation, dry skin, skin fissures, and paronychia, with papulopustular (acneiform) rash being reported in nearly 90% of patients [2]. Diarrhea, the most common gastrointestinal toxicity seen with EGFR inhibitors, occurs in up to 76% of patients receiving first-generation EGFR tyrosine kinase inhibitors (TKIs) (gefitinib, erlotinib) and up to 96% of patients receiving second-generation EGFR TKIs (dacomitinib, afatinib) [3].
Although rarely life-threatening, AEs reported with EGFR TKIs can negatively impact a patient's quality of life (QoL) and lead to reduced treatment adherence which, in the long term, can jeopardize treatment efficacy. In this context, we designed the randomized phase II study ARCHER 1042 to explore the impact of prophylactic treatment on (i) select dermatologic adverse events of interest (SDAEI), diarrhea, and mucositis; and (ii) patient-reported outcomes (PRO) in patients treated with dacomitinib for advanced NSCLC.
methods
patients
To be eligible for the study, patients had to meet the following criteria: (i) age ≥18 years; (ii) evidence of measurable or non-measurable advanced (stage IIIB or IV) NSCLC for which there was no curative therapy; (iii) at least one prior systemic therapy including at least one standard chemotherapy for advanced NSCLC and failed standard therapy for advanced or metastatic disease; (iv) no prior EGFR-targeted or HER-targeted therapy; (v) Eastern Cooperative Oncology Group performance status of 0–2; and (vi) adequate renal and hepatic function.
Patients with any of the following were excluded from the study: (i) surgery, chemotherapy, radiotherapy, or biological/investigational agents within 2 weeks of study registration; (ii) leptomeningeal or symptomatic brain metastases; (iii) clinically significant gastrointestinal abnormalities; (iv) known diffuse interstitial lung disease; and (iv) uncontrolled or significant cardiovascular disease.
The study was done in accordance with the International Conference on Harmonisation and Good Clinical Practice standards. Approval from institutional review board/ethics committee at each participating institution was obtained. Patients provided written informed consent before the start of study-specific procedures. The study is registered with ClinicalTrials.gov, number NCT01465802.
study design and treatment schedule
This was a multicenter, triple cohort, phase II clinical study conducted in the USA and the Republic of Korea. Results from Cohorts I and II are presented in this manuscript. All patients in Cohorts I and II were from the USA with the exception of one patient in Cohort II who was from Korea. Cohort III enrolled patients so that the pharmacokinetics of dacomitinib could be characterized following a planned dose interruption; the results on Cohort III will be presented in a separate manuscript. All patients in the study received dacomitinib 45 mg orally on a continuous basis.
In Cohort I, patients were randomized 1:1 to receive prophylactic treatment of either oral doxycycline 100 mg twice daily for 4 weeks or matching placebo at the same frequency and duration. Patients were blinded to the prophylactic treatment.
In Cohort II, patients received prophylactic treatment of oral VSL#3 probiotic [4 capsules once daily (for patients in the USA) or one sachet daily (for patients in Korea)] for up to 5 weeks plus open-label topical alclometasone diproprionate cream 0.05% for 4 weeks. Patients were instructed to apply the cream to their face, hands, feet, neck, back, and chest at bedtime daily.
Any patient could receive treatment reactively for AEs. Treatment guidelines recommended the use of loperamide for diarrhea, and moisturizers (before or at the start of dosing) for preventing dry skin. Recommendations for reactive treatment of acneiform rash in the placebo arm included the use of topical steroids, topical antibiotics, and oral antibiotics.
Patients were allowed to continue treatment with dacomitinib on this study as long as there was evidence of clinical benefit in the investigator's judgment. Dacomitinib dose modification for toxicity was allowed for up to two dose reductions (30 or 15 mg/day). Dacomitinib treatment was discontinued for intolerance to the drug (defined as Grade 4, Grade 3, or intolerable Grade 2 AEs that do not return to Grade 1 or baseline after a 2 week interruption of dacomitinib treatment) or patient withdrawal.
study assessments
AE and treatment assessments are described in the supplementary material, available at Annals of Oncology online. PRO of health-related quality of life (HRQoL) and disease/treatment-related symptoms were assessed using the Skindex-16 (Dermatologic Survey) [4] and the modified-Oral Mucositis Daily Questionnaire (OMDQ) [5]. Further details are in the supplementary material, available at Annals of Oncology online.
study objectives
The primary objective was to estimate the AEs and PRO for dermatologic and gastrointestinal end points. There were no formal statistical hypotheses.
Secondary objectives were to (i) evaluate the safety and tolerability in each cohort as measured using Common Terminology Criteria for Adverse Events (CTCAE) version 4; (ii) evaluate the relative dose intensity in each cohort for the first 8 weeks of treatment; (iii) evaluate the use of concomitant treatment for select AEs of interest, and (iv) characterize the pharmacokinetics of dacomitinib in Cohorts I and II.
outcomes
Primary end points for Cohorts I and II were (i) incidence of all-causality, all grade and grade ≥2 SDAEI in the first 8 weeks of treatment; and (ii) Skindex-16 Scale scores. SDAEI included dermatitis acneiform, dry skin, exfoliative rash, nail discoloration, nail disorder, paronychia, pruritus, rash, skin exfoliation, skin fissures, skin infection, skin laceration, and skin ulcer. Additional primary end points for Cohort II were (i) incidence of all-causality, all grade and grade ≥2 diarrhea AEs in the first 8 weeks of treatment; and (ii) modified-OMDQ scores.
Secondary end points for Cohorts I and II were (i) overall safety profile as characterized by type, frequency, severity of AEs as graded by National Cancer Institute CTCAE v4; (ii) concomitant medication (both prescription and non-prescription) used for SDAEI, diarrhea, and mucositis; and (iii) plasma trough concentrations of dacomitinib and its metabolite, PF-05199265, as determined from pre-dose plasma samples at steady state. In addition, for Cohort I, plasma exposure parameters [area under the curve from 0 time to τ (end of dosing interval) (AUCτ) and maximum plasma concentration (Cmax)] of dacomitinib and its metabolite were measured at steady state when dacomitinib was administered alone or in combination with doxycycline to confirm that pharmacokinetics of dacomitinib are not altered by co-administration with doxycycline.
statistical analysis
The primary objective of this study was to estimate AEs and PRO. Point estimates and confidence intervals were calculated for the specified primary end points, and descriptive statistics were used to summarize secondary end points. Further details are in the supplementary material, available at Annals of Oncology online.
results
patients
All patients in the study received dacomitinib. A total of 132 patients were enrolled in Cohort I and randomized 1:1 to receive either a prophylactic treatment of doxycycline (n = 66) or matching placebo (n = 66). The evaluable population (those who did not discontinue treatment for reasons of progression or death <6 weeks after starting dacomitinib dosing) in Cohort I consisted of 56 patients in the doxycycline arm and 58 in the placebo arm. A total of 72 patients were enrolled in Cohort II to receive a prophylactic treatment of alclometasone diproprionate cream and VSL#3 probiotic; the evaluable population in this cohort consisted of 59 patients.
Treatment durations were protocol-driven. The median treatment duration was 8.0 weeks (range 0.6–8.0 weeks for Cohort I, 0.7–8.0 weeks for Cohort II) with dacomitinib, 4.0 weeks (0.6–5.3 weeks) with doxycycline, 4.0 weeks (0.7–4.9 weeks) with alclometasone, and 4.7 weeks (1.9–11.6 weeks) with the probiotic (treatment durations with doxycycline, alclometasone, and probiotic for some patients were longer than the protocol-specified durations because of delayed visits or site/patient error).
Demographics and baseline characteristics of Cohorts I and II were, in general, well balanced (supplementary Table S1, available at Annals of Oncology online).
primary end points
SDAEI in the evaluable population
Doxycycline demonstrated statistically significant reduction in the incidence of all-causality, grade ≥2 SDAEI by 50% (P = 0.016) when compared with placebo (Table 1). The incidence of all-causality, all grade SDAEI was also lower with doxycycline than with placebo, but this did not reach statistical significance (Table 1). The incidence of all-causality, grade ≥2 dermatitis acneiform, dry skin, paronychia, and rash was lower with doxycycline than with placebo (Figure 1).
Table 1.
Incidence and risk difference of select dermatologic adverse events of interest and diarrhea in the first 8 weeks of treatment for Cohorts I and II (evaluable population)
| Incidence | SDAEIa, all grade, n (%) (95%?CI) | SDAEI, grade ≥2, n (%) (95%?CI) | Diarrhea, all grade, n (%) (95%?CI) | Diarrhea, grade ≥2, n (%) (95%?CI) |
| Placebo (Cohort I; n = 58) | 46 (79.3) (66.6, 88.8) | 27 (46.6) (33.3, 60.1) | 49 (84.5) (72.6, 92.7) | 24 (41.4) (28.6, 55.1) |
| Doxycycline (Cohort I; n = 56) | 42 (75.0) (61.6, 85.6) | 13 (23.2) (13.0–36.4) | 46 (82.1) (69.6, 91.1) | 19 (33.9) (21.8, 47.8) |
| Alclometasone + probiotic (Cohort II; n = 59) | 47 (79.7) (67.2, 89.0) | 21 (35.6) (23.6, 49.1) | 49 (83.1) (71.0, 91.6) | 23 (39.0) (26.5, 52.6) |
| Risk difference | SDAEI, all grade (90% CI) | SDAEI, grade ≥2 (90% CI) | Diarrhea, all grade (90% CI) | Diarrhea, grade ≥ 2 (90% CI) |
| Doxycycline versus placebo (Cohort I) | −4.31 (−18.99, 10.37); P = 0.745 | −23.34 (−39.31, −7.36); P = 0.016 | −2.34 (−15.58, 10.90); P = 0.933 | −7.45 (−24.09, 9.19); P = 0.530 |
| Alclometasone + probiotic (Cohort II) versus placebo (Cohort I) | 0.35 (−13.64, 14.34); P = 1.000 | −10.96 (−27.54, 5.62); P = 0.309 | −1.43 (−14.35, 11.49); P = 1.000 | −2.40 (−19.01, 14.22); P = 0.940 |
| Alclometasone + probiotic (Cohort II) versus doxycycline (Cohort I) | 4.66 (−9.92, 19.24); P = 0.708 | 12.38 (−3.19, 27.95); P = 0.211 | 0.91 (−12.47, 14.29); P = 1.000 | 5.05 (−11.43, 21.54); P = 0.712 |
aSDAEI defined as dermatitis acneiform, dry skin, exfoliative rash, nail discoloration, nail disorder, paronychia, pruritus, rash, skin exfoliation, skin fissures, skin laceration, skin infection, and skin ulcer.
Confidence intervals for incidences are exact. Confidence intervals for risk differences and P values are based on the Wald asymptotic test with continuity correction.
SDAEI, select dermatologic adverse events of interest; CI, confidence interval.
Figure 1.
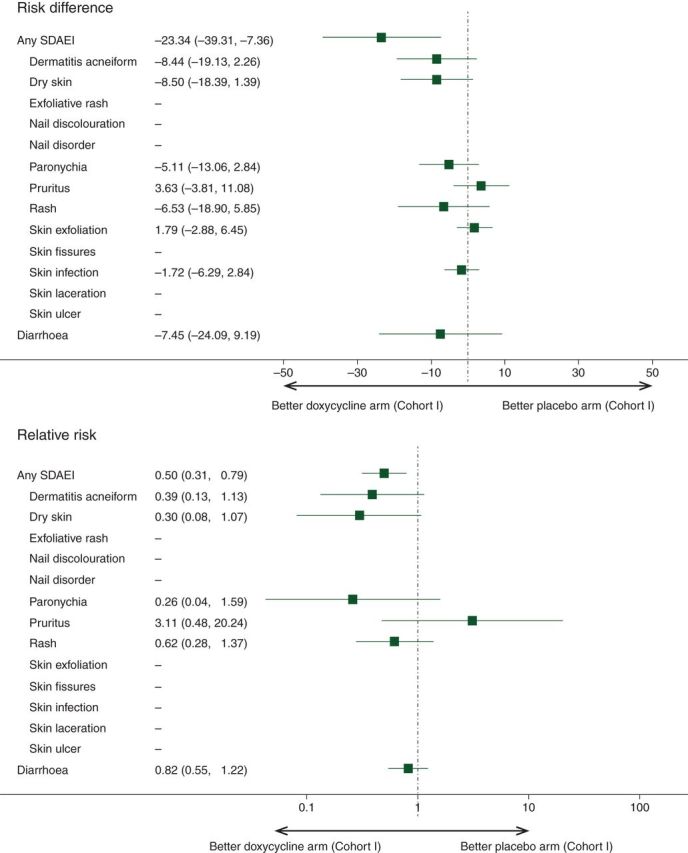
Forest plots of risk difference and relative risk of select dermatologic adverse events of interest and diarrhea (all-causality, grade ≥2) in the first 8 weeks of treatment for the evaluable population of Cohort I placebo arm versus Cohort I doxycycline arm. The confidence interval is based on the Wald asymptomatic test with continuity correction. The reference arm is Cohort I placebo arm.
The incidence of all-causality, all grade SDAEI with alclometasone was similar to that with placebo and with doxycycline (Table 1). Alclometasone reduced the incidence of all-causality, grade ≥2 SDAEI when compared with placebo, although this did not reach statistical significance. The incidence of all-causality, grade ≥2 SDAEI was lower, albeit not statistically significant, with doxycycline than with alclometasone (Table 1).
diarrhea in the evaluable population
The VSL#3 probiotic did not reduce the incidence of either all-causality, all grade diarrhea or all-causality, grade ≥2 diarrhea when compared with either placebo or doxycycline (Table 1). The incidence of grade ≥2 diarrhea was lower with doxycycline than with placebo, although the decrease did not reach statistical significance.
PRO on skindex-16 survey and modified-OMDQ
PROs of HRQoL and disease/treatment-related symptoms were assessed using the Skindex-16 dermatologic survey and the modified-OMDQ.
For the Skindex-16, exceeding the minimum clinically important difference of 10 points indicates a clinically relevant sign of deteriorating symptoms [4]. The change in Skindex-16 Total scores from baseline over the first 8 weeks of treatment (supplementary Figure S1, available at Annals of Oncology online) showed that there was clinically meaningful deterioration in the scores for (i) six of seven visits in the placebo arm, (ii) one of seven visits in the doxycycline arm, and (iii) three of seven visits in the alclometasone arm. Hence, doxycycline and alclometasone were associated with less deterioration in the Skindex-16 Total scores than placebo. Further details on the Skindex-16 subdomain scores are provided in the supplementary material and in supplementary Figure S2, available at Annals of Oncology online.
The change in the modified-OMDQ scores for mouth and throat soreness (question 2 in the modified-OMDQ) from baseline over the first 8 weeks of treatment showed that the scores for all three treatment arms had overlapping 95% confidence intervals (supplementary Figure S3A, available at Annals of Oncology online). This trend was also observed for the OMDQ scores for diarrhea (supplementary Figure S3B, available at Annals of Oncology online). Hence, neither doxycycline nor probiotic improved OMDQ scores.
safety
In the first 8 weeks, the median relative dose intensity of dacomitinib in the evaluable population was ≥75% in the three treatment arms (Table 2). A summary of all-causality treatment-emergent adverse events (TEAEs) reported for the evaluable population in the three treatment arms is shown in Table 2. All patients in Cohort I and all except one in Cohort II experienced at least one TEAE. The incidence of all-causality grade 3 or 4 AEs was lowest in the doxycycline arm (32.1% versus 43.1% in the placebo arm and 49.2% in the alclometasone/probiotic arm). A similar trend was observed for the incidence of permanent discontinuations due to AEs (7.1% in the doxycycline arm versus 13.8% in the placebo arm and 8.5% in the alclometasone/probiotic arm). Neither doxycycline nor alclometasone/probiotic treatment had an impact in reducing either temporary discontinuations or dose reductions due to AEs. A summary of all-causality TEAEs reported for the as-treated population is shown in supplementary Table S2, available at Annals of Oncology online. The trends observed in the as-treated population were not as pronounced as that in the evaluable population. A summary of dacomitinib-related/doxycycline-related/alclometasone-related/probiotic-related TEAEs is shown in supplementary Table S2, available at Annals of Oncology online.
Table 2.
Summary of safety in Cohorts I and II (evaluable population)
| Cohort I |
Cohort II | ||
|---|---|---|---|
| Placebo (n = 58) | Doxycycline (n = 56) | Alclometasone + probiotic (n = 59) | |
| Median relative dose intensitya (RDI) of dacomitinib in the first 8 weeks, % (range) | 78.87 (12.5–100) | 82.74 (12.5–100) | 75.00 (8.9–100) |
| Treatment-emergent adverse events (all causalities) in the first 8 weeks of treatment | |||
| Patients with adverse events, n (%) | 58 (100) | 56 (100) | 58 (98.3) |
| Patients with serious adverse events, n (%) | 12 (20.7) | 13 (23.2) | 11 (18.6) |
| Patients with grade 3 or 4 adverse events, n (%) | 25 (43.1) | 18 (32.1) | 29 (49.2) |
| Patients with grade 5 adverse events, n (%) | 3 (5.2) | 3 (5.4) | 6 (10.2) |
| Permanent discontinuations due to adverse events, n (%) | 8 (13.8) | 4 (7.1) | 5 (8.5) |
| Temporary discontinuations due to adverse events, n (%) | 18 (31.0) | 19 (33.9) | 26 (44.1) |
| Dose reductions due to adverse events, n (%) | 15 (25.9) | 16 (28.6) | 23 (39.0) |
| Concomitant drug treatment for adverse events of interest in the first 8 weeks of treatment | |||
| SDAEI, diarrhea, and mucositis, n (%) | 43 (74.1) | 37 (66.1) | 49 (83.1) |
| SDAEI, n (%) | 38 (65.5) | 28 (50.0) | 31 (52.5) |
| Diarrhea, n (%) | 36 (62.1) | 26 (46.4) | 41 (69.5) |
| Mucositis, n (%) | 14 (24.1) | 18 (32.1) | 17 (28.8) |
| Diarrhea burden index in the first 8 weeks of treatment | |||
| Diarrhea burden index, mean (standard deviation) | 2.3 (2.27) | 1.9 (1.55) | 2.4 (2.55) |
| Mean difference in diarrhea burden index (95% CI), P value | |||
| Doxycycline versus placebo | −0.5 (−1.2, 0.3), P = 0.198 | ||
| Alclometasone + probiotic versus placebo | 0.1 (−0.8, 1.0), P = 0.860 | ||
| Alclometasone + probiotic versus doxycycline | 0.5 (−0.2, 1.3), P = 0.164 | ||
aRelative dose intensity is defined as the total actual received dose during the first 8 weeks from the date of first dacomitinib dose divided by (45 mg dacomitinib × 56 days).
Diarrhea burden index is defined as the sum of daily maximum CTCAE grade of diarrhea AE patient experienced over day 1 to day 56, regardless of dosing interruption and/or missed dose. The maximum CTCAE grade of diarrhea is 0 for a day if no diarrhea AE was experienced on the day.
SDAEI, select dermatologic adverse events of interest; CI, confidence interval; CTCAE, common terminology criteria for adverse events; AE, adverse event.
When patients discontinued treatment, they were followed for 28 days after the last dose of dacomitinib, at which time, if they were alive, they were deemed to have completed the study. There were 25 deaths from all causes ≤28 days after the last dacomitinib dose in the evaluable population: 10 in the placebo arm, 7 in the doxycycline arm, and 8 in the alclometasone/probiotic arm. Seventeen deaths were due to disease progression, two were study treatment-related (one respiratory failure in the doxycycline arm, one pneumonia in the alclometasone/probiotic arm), and six were due to other reasons.
The most frequently reported all-causality TEAEs in the three treatment arms included diarrhea, rash, dry skin, nausea, decreased appetite, and fatigue (supplementary Table S3, available at Annals of Oncology online). There was a lower incidence of grade ≥2 diarrhea, rash, dry skin, dermatitis acneiform, and paronychia in the doxycycline arm than in the placebo arm. There was also a lower incidence of grade ≥2 rash and dry skin in alclometasone/probiotic arm than in the placebo arm. There was no grade 4 or grade 5 diarrhea in any of the arms; nor were there grade 4 or grade 5 SDAEI in any of the arms. The incidence of grade 3 diarrhea and grade 3 SDAEI are shown in supplementary Table S4, available at Annals of Oncology online.
Both doxycycline and alclometasone therapy were associated with a decrease in the incidence of concomitant drug treatment for SDAEI in the first 8 weeks of treatment when compared with placebo (Table 2). Furthermore, doxycycline use was associated with a decrease in the incidence of concomitant drug treatment for diarrhea when compared with placebo. In contrast, the probiotic had no impact on reducing the incidence of concomitant drug treatment for diarrhea.
The diarrhea burden index (DBI) was an exploratory end point that took into account both the duration and CTCAE grade for all episodes of diarrhea. Doxycycline reduced the mean DBI when compared with either placebo or probiotic, although the decrease did not reach statistical significance (Table 2). In contrast, the probiotic had no impact on reducing the DBI when compared with placebo.
pharmacokinetics of dacomitinib in Cohorts I and II
The observed exposure of dacomitinib and its metabolite, PF-05199265, on cycle 2 day 1 for the doxycycline arm was similar to that for the placebo arm (supplementary Figure S4, available at Annals of Oncology online). The observed median AUCτ values for dacomitinib in both arms were similar (1820.00 ng.h/ml for the doxycycline arm versus 1860 ng.h/ml for the placebo arm) as were the observed median Cmax values (88.10 ng/ml for the doxycycline arm versus 87.70 ng/ml for the placebo arm) (supplementary Table S5, available at Annals of Oncology online). Hence, co-administration of doxycycline did not appear to affect the pharmacokinetics of dacomitinib.
For patients in Cohorts I and II, observed median plasma Ctrough concentrations of dacomitinib and its metabolite on day 1 for cycles 3–10 appeared to be similar across cycles (data not shown).
discussion
The ARCHER 1042 study was designed to explore the impact of prophylactic treatment on SDAEI, diarrhea, and QoL in patients treated with dacomitinib—a pan-HER inhibitor—for advanced NSCLC. The study showed that doxycycline, compared with placebo, (i) reduced the incidence of all-causality, grade ≥2 SDAEI by 50% (23.2% with doxycycline versus 46.6% with placebo; P = 0.016); (ii) was associated with less deterioration in Skindex-16 Total scores; (iii) decreased the incidence of permanent discontinuation of dacomitinib due to TEAEs (7.1% versus 13.8% with placebo); and (iv) decreased the incidence of concomitant drug treatment for SDAEI (50.0% versus 65.5% with placebo). EGFR TKIs impair keratinocyte growth, migration, and chemokine expression, which leads to inflammatory cell recruitment and cutaneous injury [2]. Because of their anti-inflammatory properties, the antibiotics doxycycline [6], tetracycline [7], and minocycline [8] have been evaluated in the prophylactic management of skin toxicities associated with EGFR inhibitors. In the Skin Toxicity Evaluation Protocol with Panitumumab (STEPP) trial where colorectal cancer patients were treated with panitumumab, the incidence of grade ≥2 skin toxicities was 29% in the group given a prophylactic treatment of topical steroids and doxycycline (100 mg twice daily for 6 weeks) and 62% in the group given reactive treatment [6]. Furthermore, prophylactically treated patients showed less QoL impairment than reactively treated patients. The ARCHER 1042 results with doxycycline are in agreement with the results from the STEPP trial and the Pan Canadian Rash trial [8]. The doxycycline dose used in the ARCHER 1042 study was the same as that used in the STEPP trial but the duration of use was shorter in the ARCHER 1042. Although the optimal duration of use with doxycycline cannot be determined from the ARCHER 1042 data, a beneficial effect was seen in 4 weeks.
The ARCHER 1042 study is, to our knowledge, the first to evaluate a topical steroid as a single prophylactic treatment option for EGFR inhibitor-induced skin toxicities. We evaluated alclometasone dipropionate since it reduces cutaneous inflammation and pruritus. Our results show that alclometasone did not statistically significantly reduce the incidence of all-causality, grade ≥2 SDAEI when compared with placebo. However, alclometasone had a positive impact when compared with placebo in the following aspects: alclometasone (i) was associated with less deterioration in Skindex-16 Total scores; (ii) decreased the incidence of permanent discontinuation of dacomitinib due to TEAEs (8.5% versus 13.8% with placebo); and (iii) decreased the incidence of concomitant drug treatment for SDAEI (52.5% versus 65.5% with placebo).
As mentioned previously, both doxycycline and alclometasone reduced the negative impact in patient-reported dermatologic AEs. This reduction occurred during the first 4 weeks of treatment, a period when peak AEs of dacomitinib typically occur. The Skindex-16 scores for the three treatment arms appeared to converge at week 8; whether this was due to a plateau of symptoms or to less impact of prophylaxis cannot be concluded.
We evaluated the probiotic VSL#3 as a prophylactic treatment of diarrhea since this probiotic had been shown to reduce the overall incidence and grade 3–4 incidence of radiation-induced diarrhea [9]. Our results showed that the probiotic, when compared with placebo, had no impact on (i) the incidence of all-causality, all grade or grade ≥2 diarrhea; (ii) the incidence of concomitant drug treatment use for diarrhea; (iii) the QoL as assessed by the modified-OMDQ; and (iv) the mean DBI. In contrast, doxycycline, when compared with placebo, (i) reduced the incidence of all-causality, grade ≥2 diarrhea, although this did not reach statistical significance; (ii) decreased the incidence of concomitant drug treatment use for diarrhea (46.4% versus 62.1% with placebo); and (iii) reduced the mean DBI, although this did not reach statistical significance. It remains to be seen if radiation-induced diarrhea and dacomitinib-induced diarrhea occur through different mechanisms and if the latter is possibly inflammation-mediated. Our results with doxycycline are consistent with results from the STEPP trial which showed that there was a decrease in the overall incidence of diarrhea and grade ≥2 diarrhea with doxycycline.
In conclusion, this study showed that doxycycline is a promising prophylactic treatment in managing dacomitinib-induced grade ≥2 SDAEI and reducing deterioration in QoL with respect to dermatologic AEs. The absence of a positive impact with VSL#3 probiotic in managing dacomitinib-induced diarrhea AEs underscores the need for other prophylactic strategies.
funding
The study described in the manuscript was not funded by a grant. Therefore, there is no grant number applicable.
disclosure
MEL is funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and has served as a consultant to Pfizer, Roche, and Genentech and has received research funding from Genentech, Roche, Bristol-Myers Squibb, and Berg. DMK has received research funding from Helsinn and Entera Health, has been on a speaker bureau for Teva and Merck, and is a co-investigator on a research project funded by Pfizer. AJ has received research funding from Entera Health, Amgen, Boston Biologics, and Aveo Pharmaceuticals. BP is an employee of and owns stock of Merck. EBG has received clinical trial funding to his institution from Pfizer, AstraZeneca, Eli Lilly, Genentech, Merck, Novartis, and Bristol-Myers Squibb. DG, TW, JPD, NG, SN, JOC, and ES are employees of and own stock of Pfizer. SS has no conflict of interest.
Supplementary Material
acknowledgements
This study was sponsored by Pfizer Inc. We would like to thank all of the participating patients and their families, as well as the investigators, research nurses, study coordinators, and operations staff.
references
- 1. Mok T, Lee K, Tang M, Leung L. Dacomitinib for the treatment of advanced or metastatic non-small-cell lung cancer. Future Oncol 2014; 10: 813–822. [DOI] [PubMed] [Google Scholar]
- 2. Melosky B, Leighl NB, Rothenstein J et al. . Management of EGFR TKI-induced dermatologic adverse events. Curr Oncol 2015; 22: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirsh V, Blais N, Burkes R et al. . Management of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitors. Curr Oncol 2014; 21: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chren MM, Lasek RJ, Quinn LM et al. . Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol 1996; 107: 707–713. [DOI] [PubMed] [Google Scholar]
- 5. Stiff PJ, Erder H, Bensinger WI et al. . Reliability and validity of a patient self-administered daily questionnaire to assess impact of oral mucositis (OM) on pain and daily functioning in patients undergoing autologous hematopoietic stem cell transplantation (HSCT). Bone Marrow Transplant 2006; 37: 393–401. [DOI] [PubMed] [Google Scholar]
- 6. Lacouture ME, Mitchell EP, Piperdi B et al. . Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010; 28: 1351–1357. [DOI] [PubMed] [Google Scholar]
- 7. Jatoi A, Rowland K, Sloan JA et al. . Tetracycline to prevent epidermal growth factor receptor inhibitor-induced skin rashes: results of a placebo-controlled trial from the North Central Cancer Treatment Group (N03CB). Cancer 2008; 113: 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Melosky B, Anderson H, Burkes RL et al. . Pan Canadian Rash trial: a randomized phase III trial evaluating the impact of a prophylactic skin treatment regimen on epidermal growth factor receptor-tyrosine kinase inhibitor-induced skin toxicities in patients with metastatic lung cancer. J Clin Oncol 2016; 34: 810–815. [DOI] [PubMed] [Google Scholar]
- 9. Delia P, Sansotta G, Donato V et al. . Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol 2007; 13: 912–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


