Abstract
Aims
The impact of coronary computed tomographic angiography (CTA) on management of anomalous origin of the coronary artery arising from the opposite sinus (ACAOS) remains uncertain. We examined the prevalence, anatomical characterization, and outcomes of ACAOS patients undergoing CTA.
Methods and results
Among 5991 patients referred for CTA at two tertiary hospitals between January 2004 and June 2014, we identified 103 patients (1.7% prevalence) with 110 ACAOS vessels. Mean age was 52 years (range 5–83, 63% male), with 55% previously known ACAOS and 45% discovered on CTA. ACAOS subtypes included: 39% interarterial (n = 40 anomalous right coronary artery, n = 3 anomalous left coronary artery), 38% retroaortic, 15% subpulmonic, 5% prepulmonic, and 2% other. ACAOS patients were assessed for symptoms, ischaemic test results, revascularization, all-cause or cardiovascular (CV) death, and myocardial infarction. CTAs were reviewed for ACAOS course, take-off height and angle, length and severity of proximal narrowing, intramural course, and obstructive coronary artery disease (CAD). In follow-up (median 5.8 years), there were 20 surgical revascularizations and 3 CV deaths. After adjusting for obstructive CAD (n = 21/103, 20%), variables associated with ACAOS revascularization included the following: CV symptoms, proximal vessel narrowing ≥50%, length of narrowing >5.4 mm, and an interarterial course.
Conclusion
The prevalence of ACAOS on CTA was 1.7%, including 45% of cases discovered incidentally. CTA provided excellent characterization of ACAOS features associated with coronary revascularization, including the length and severity of proximal vessel narrowing.
Keywords: anomalous coronary artery, coronary computed tomographic angiography, prognosis, revascularization, sudden cardiac death
Introduction
Anomalous origin of the coronary artery arising from the opposite sinus (ACAOS) has variable presentations ranging from a benign, incidental finding to sudden cardiac death (SCD).1 Coronary computed tomographic angiography (CTA) provides an accurate, non-invasive technique to assess anomalous coronary artery origin, course, destination, luminal narrowing, relationship to surrounding structures and coronary artery disease (CAD).2 To date, limited data exist regarding the extent to which CTA features may impact management. Additionally, prior evidence has suggested that subclinical ischaemia may persist following revascularization for interarterial anomalous right coronary artery (ARCA) and anomalous left coronary artery (ALCA).3 With uncertainty regarding the ideal management of these ACAOS subtypes, there exists a growing need to examine the potential impact of CTA-identified ACAOS features on patient management and outcomes.
We therefore aimed to evaluate (i) the prevalence of ACAOS among patients referred for clinical CTA at two tertiary referral centres, (ii) CTA-identified ACAOS features and their association with coronary revascularization, and (iii) the incidence of symptoms and ischaemia in the subset of patients without obstructive coronary artery disease undergoing ARCA revascularization.
Methods
Study population
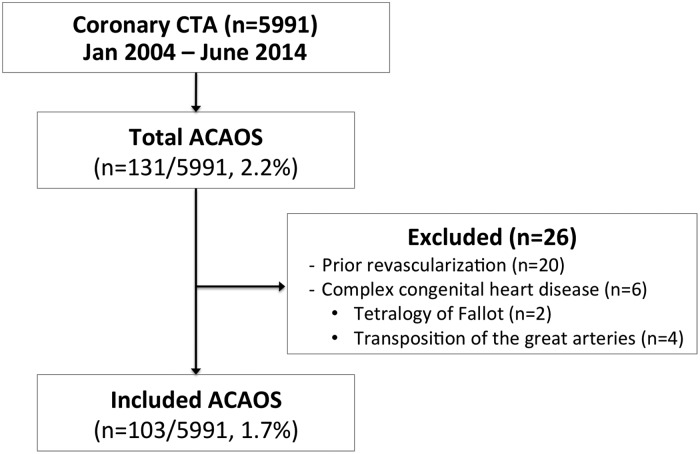
The initial population consisted of 5991 consecutive patients who underwent contrast-enhanced coronary CTA between January 2004 and June 2014 at Brigham and Women's Hospital or Massachusetts General Hospital. From this population, we identified 129 patients with ≥1 ACAOS vessel with the following course subtypes: prepulmonic, subpulmonic, interarterial, retroaortic, and retrocardiac. Attention was given in distinguishing the subpulmonic course subtype with a course below the pulmonic valve, and an interarterial subtype with a course at or above the pulmonic valve (Figure 1). To examine the potential impact of CTA findings on management with correlation to native ACAOS features, we excluded patients with prior ACAOS revascularization (n = 20) or complex congenital heart disease including tetralogy of Fallot (n = 2) and transposition of the great arteries (n = 4). The final cohort consisted of 103 patients with ≥1 ACAOS vessel (Figure 2). The study was approved by the Partners Healthcare Institutional Review Board and was conducted in accordance with institutional guidelines.
Figure 1.
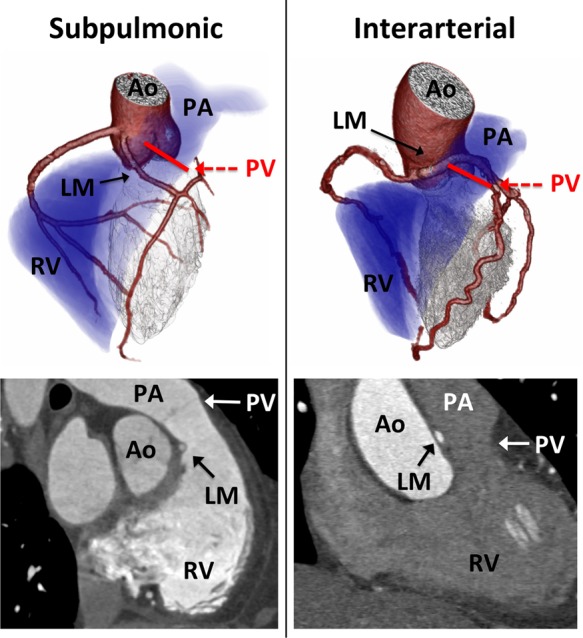
Distinguishing a subpulmonic vs. interarterial course. (Left panel) Three-dimensional volume rendering (top) and multiplanar image reconstruction (bottom) demonstrating an anomalous left main (LM) coronary artery arising from the right coronary cusp and following a subpulmonic course below the pulmonic valve (PV). (Right panel) Three-dimensional volume rendering (top) and multiplanar image reconstruction (bottom) demonstrating an anomalous left main coronary artery (ALCA) with an interarterial course above the pulmonic valve. ALCA, interarterial anomalous left main coronary artery; Ao, aorta; LM, left main coronary artery; PA, pulmonary artery; PV, pulmonic valve; RV, right ventricle.
Figure 2.
Study design. ACAOS, anomalous origin of the coronary artery arising from the opposite sinus; CTA, computed tomographic angiography.
Clinical information
Baseline demographics, clinical history, results of prior cardiac testing, symptoms, and indications for CTA were collected by a review of electronic medical records, including physician notes and procedures. A history of known CAD was defined as prior percutaneous coronary intervention, coronary artery bypass grafting (CABG), or myocardial infarction (MI).4 Prior aborted SCD was defined as resuscitated nontraumatic and unexpected sudden death that may occur from cardiac arrest within 6 h of a previously normal state of health,5,6 and without another known cardiovascular (CV) abnormality, excluding respiratory, cerebrovascular, and drug-related causes.7
Follow-up symptom status and results of cardiac testing were collected by a review of electronic medical records. Consistent with prior studies,3 symptoms were considered CV in origin if they included the following: chest pain, presyncope or syncope provoked by exertion, or if the patient experienced aborted SCD. To ensure that cardiac testing and events outside of our healthcare network were captured, a standardized questionnaire was mailed to each patient. In addition, patients had the option to complete a web-based version of the questionnaire via the Research Electronic Data Capture system,8 which is encrypted, secure, and Health Insurance Portability and Accountability Act compliant. For patients who did not reply to the questionnaire on repeated mailings, scripted phone interviews were performed based on the questionnaire. All self-reported events were verified via outside medical record review by two cardiologists blinded to coronary CTA results, with discordant events adjudicated by consensus. Clinical follow-up information was available for 93% of patients (n = 96/103) included in the present study.
Cardiovascular outcomes
All patient charts and vital status were reviewed by two cardiologists blinded to test findings for the adjudication of CV events by previously described methods.4 Non-fatal MI was defined using universal criteria.9 Incident coronary revascularization of ACAOS vessels was recorded as percutaneous coronary intervention, CABG, coronary unroofing (including modified unroofing and neo-ostia formation), or reimplantation. Surgical outcomes for each patient undergoing ACAOS revascularization were reviewed to ascertain death from any cause or surgical complications. Surgical complications consisted of any significant event requiring a therapeutic intervention during the index hospitalization. Deaths were considered to be of CV origin if the primary cause was acute MI, atherosclerotic coronary disease, congestive heart failure, valvular heart disease, arrhythmic origin, stroke, or sudden death of unknown cause.10
Ischaemic testing
When performed, results of ischaemic testing were interpreted by experienced cardiologists as part of the patient's clinical care. Exercise treadmill testing (ETT) utilized a symptom-limited Bruce protocol,11 and results were categorized as positive, negative, or inconclusive by previously described methods.12 Single photon emission computed tomography, positron emission tomography, and stress magnetic resonance imaging (MRI) were performed and interpreted according to standard guidelines using a semi-quantitative scale and a 17-segment model for the presence and severity of reversible perfusion defects.13–15 Exercise stress echocardiography reporting was performed by 17-segment analysis of regional wall motion abnormalities by standard criteria.16
Coronary CTA
All scans were performed using contrast-enhanced ≥64-slice multidetector CT according to established guidelines.17 Images were reconstructed in multiphase data sets and interpreted by Level III trained cardiologists or radiologists as described previously.4 Using an 18-segment model, each coronary segment with a >1.5 mm diameter was visualized by axial and multiplanar reformations for the presence of coronary atherosclerotic plaque and stenosis by visual grading defined as: normal (no plaque and no stenosis), non-obstructive (1–49% stenosis), or obstructive (≥50% stenosis). For the purposes of the current study, in order to avoid bias and ensure consistency of measurements, all CTA studies were re-read blinded to clinical data and patient outcomes for the following ACAOS features (Figures 3–5):
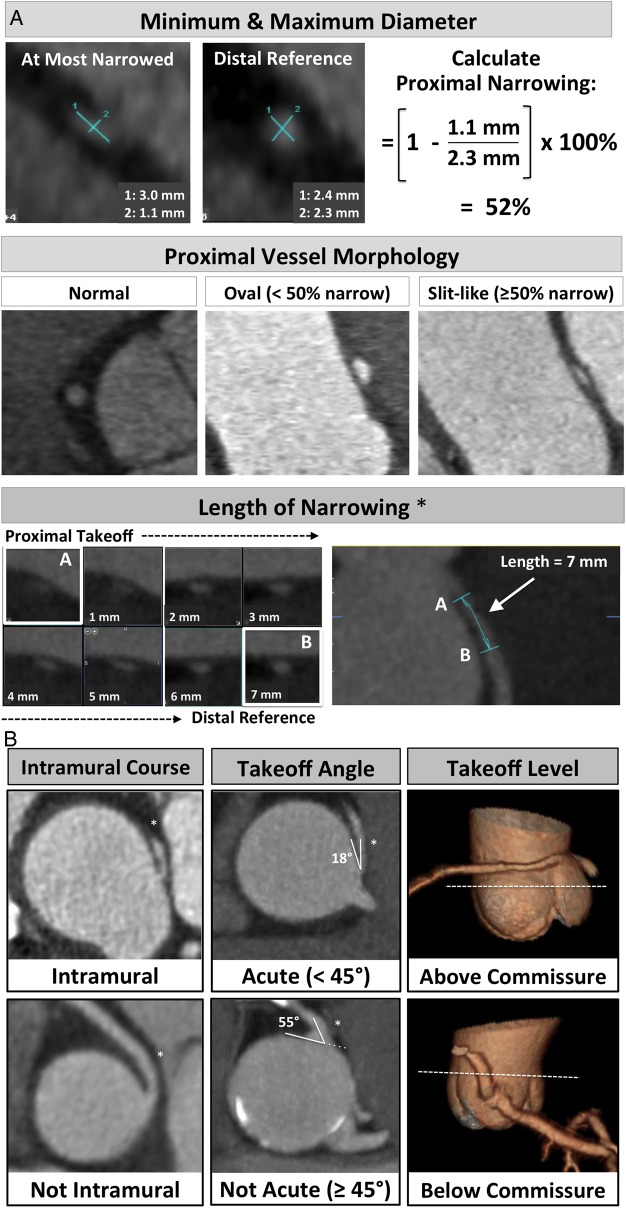
Minimum and maximum diameters (Figure 3A): at the most narrowed location and the normal distal reference segment, used to categorize proximal vessel morphology as: (i) normal, (ii) ‘oval’ (<50%), and (iii) ‘slit-like’ narrowing (≥50% reduction in minimum diameter in the absence of coronary artery disease).18,19
Length of narrowing: centreline length of vessel narrowing extending from the most proximal segment to the normal calibre distal reference (Figure 3A).
Acute angle: defined as the presence or absence of acute angle take-off <45° between (a) the plane formed by the ostium centre to a point 5 mm along the vessel centreline, and (b) a plane tangent to the aorta in multiplanar axial reconstruction at the level of the ACAOS ostium19,20 (Figure 3B).
Intramural course: defined as (i) present, (ii) absent, or (iii) indeterminate. Consistent with prior research, an intramural course (i.e. within the aortic wall) was suspected in cases with (a) proximal vessel narrowing,21 (b) acute take-off (<45°),21 and (c) separate ostium of the vessel from the aorta.22 We also incorporated direct visualization of the vessel within the aortic wall (optimized by window width/level ≈ 1000/300), and the absence of adjacent epicardial fat (tissue region of interest mean signal <−30 Hounsfield Units) (Figure 3B).
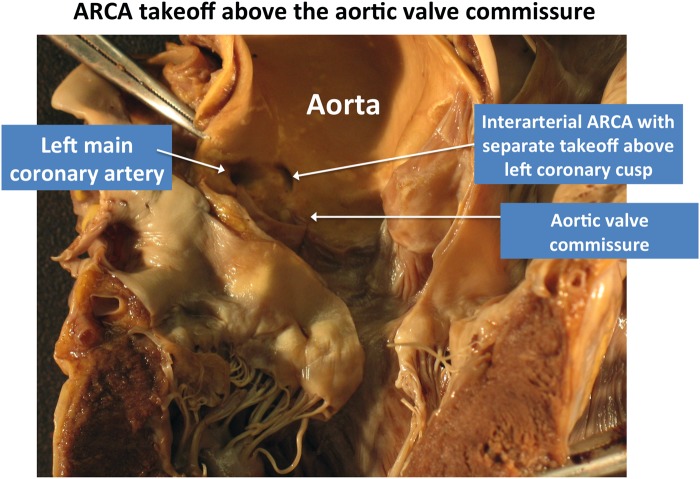
Vessel take-off level: categorized as at/above or below the aortic valve commissure. This feature has importance for surgical planning as noted by the Congenital Heart Surgeons' Society registry,23 which described the frequent occurrence of ALCA/ARCA take-off at or above the aortic commissure in 88% of cases undergoing revascularization (Figure 3B and Figure 4).
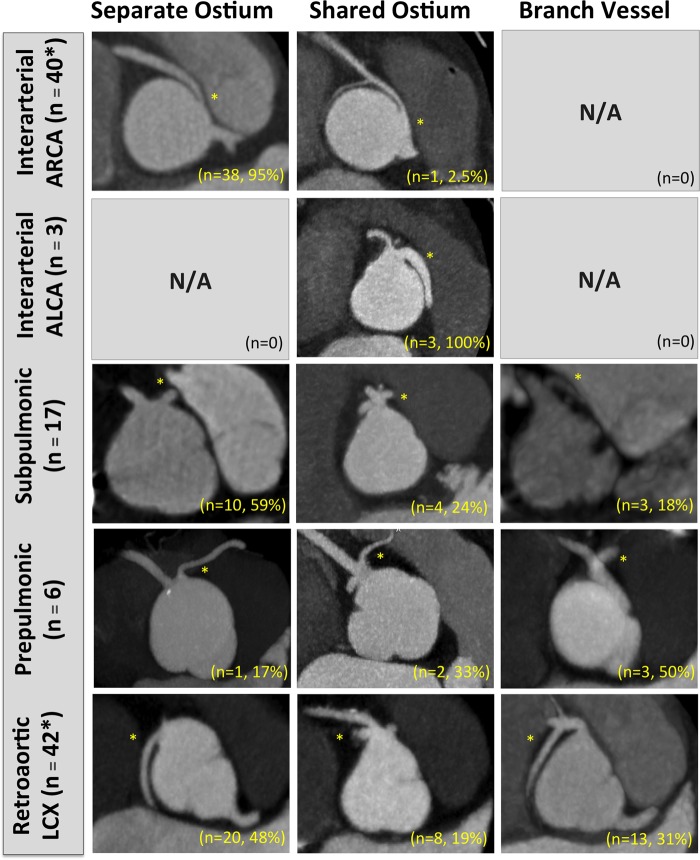
Ostia type: defined as (i) separate, (ii) shared, or (iii) branch vessel (Figure 5). This feature has importance in surgical planning, as patients with ARCA and a separate ostium from the aorta are more likely to have an intramural course.22
Figure 3.
(A) CTA-identified ACAOS features. Lumen diameters obtained in double oblique view, taking the maximum and minimum diameters of the vessel at the most narrowed proximal location and the distal reference using the smallest available slice thickness (0.5–0.625 mm isotropic resolution). *Centreline length of vessel narrowing shown in double oblique and curved multiplanar views extending from (a) ACAOS vessel take-off to (b) normal calibre distal reference. ACAOS, anomalous origin of the coronary artery arising from the opposite cusp. (B) Intramural location and take-off angles obtained in multiplanar axial reconstructions at the level of the ACAOS ostium using the smallest available slice thickness (0.5–0.625 mm). Vessel take-off level (above/below commissure) shown in 3D reformatted image.
Figure 5.
ACAOS course subtype stratified by the prevalence of CTA-identified ostia type. Note the most common take-off of an interarterial ARCA is a separate ostia. *Note here that numbers do not add to 100% given indeterminate take-offs resulting from a bioprosthetic valve limiting take-off visualization in one patient with interarterial ARCA, and misalignment artefact in one patient with a retroaortic left circumflex. Subtype images obtained by multiplanar axial reconstructions at the level of the ACAOS ostium using the smallest available slice thickness (0.5–0.625 mm).
Figure 4.
Example of ACAOS take-off above the aortic valve commissure. Post-mortem autopsy study of patient who died from idiopathic pulmonary fibrosis while awaiting lung transplant. Autopsy demonstrates an interarterial ARCA with separate take-off from the left coronary cusp above the aortic valve commissure. Image courtesy of Dr Robert Padera (Department of Pathology, Brigham and Women's Hospital, Boston, MA).
Statistical analysis
Continuous variables with normal distributions are expressed as mean ± 1 SD and compared with Student's t-test for independent groups and one-way analysis of variance for between-group comparisons. Continuous variables with non-normal distributions are expressed as median ± interquartile range (IQR) and compared with the Wilcoxon rank-sum. Categorical variables are expressed as frequencies (%) and compared by the Pearson χ2 test. Receiver operating characteristic (ROC) analysis was performed to determine the optimal cut-off for ACAOS length of narrowing to discriminate between patients who were treated with subsequent revascularization from those without revascularization. Logistic regression analysis was performed to determine ACAOS features associated with revascularization after CTA, adjusted for CTA-identified obstructive CAD. Statistical analysis was performed using Stata (Version 12.1, Statacorp, TX), and a two-tailed P-value of <0.05 was considered significant.
Results
Baseline characteristics
The study population consisted of 103 patients with 110 ACAOS vessels (per-patient prevalence 1.7% on CTA), including 7 patients with multiple ACAOS. Fifty-seven patients (55%) had previously known ACAOS, including 45 (44%) identified on prior invasive angiography and 12 (11%) recognized on prior transthoracic echocardiography (n = 4) or cardiac MRI (n = 8). In these cases, ACAOS were known prior to CTA for a median of 5 days (IQR: 2–27.5 days). When excluding the 57 patients (55%) with previously known ACAOS, the per-patient prevalence of ACAOS on CTA was 0.8%. Baseline patient characteristics are shown in Table 1, stratified by ACAOS revascularization (n = 20, 19%) vs. no revascularization (n = 83, 81%). Mean age of the study population was 52 ± 17 years (5–83, 63% male). Patients referred for ACAOS revascularization were more likely to have known CAD, hyperlipidaemia, known ACAOS prior to CTA, prior invasive angiography, and prior aborted SCD or CV symptoms (all P < 0.05) (Table 1). The majority of patients had symptoms (83% of cohort), including 95% (n = 19/20) of those referred for ACAOS revascularization.
Table 1.
Baseline patient characteristics stratified by ACAOS revascularization
| All patients (n = 103) | No Revasc (n = 83) | Revasc (n = 20) | P-value | |
|---|---|---|---|---|
| Age, years, mean ± SD | 52 ± 17 | 52 ± 17 | 51 ± 15 | 0.93 |
| Follow-up, years, median (IQR) | 5.8 (3.8–7.8) | 5.6 (3.4–7.8) | 6.4 (5.3–7.5) | 0.48 |
| Male, n (%) | 65 (63%) | 53 (64%) | 12 (60%) | 0.75 |
| Diabetes mellitus, n (%) | 19 (19%) | 17 (20%) | 2 (10%) | 0.28 |
| Hyperlipidaemia, n (%) | 51 (50%) | 37 (45%) | 14 (70%) | 0.04 |
| Hypertension, n (%) | 47 (46%) | 35 (42%) | 12 (60%) | 0.15 |
| Current or prior smoker, n (%) | 31 (30%) | 23 (28%) | 8 (26%) | 0.71 |
| Family history early CAD, n (%) | 22 (21%) | 15 (18%) | 7 (35%) | 0.10 |
| Known CAD, n (%) | 7 (7%) | 3 (4%) | 4 (20%) | 0.009 |
| ACAOS known before CTA, n (%) | 57 (55%) | 40 (48%) | 17 (85%) | 0.003 |
| Prior cardiac testing, n (%) | ||||
| Invasive angiography | 45 (44%) | 29 (35%) | 16 (80%) | 0.001 |
| Myocardial perfusion imaging | 42 (41%) | 31 (37%) | 11 (55%) | 0.15 |
| Exercise treadmill test | 19 (18%) | 15 (18%) | 4 (20%) | 0.60 |
| Cardiac MRI (rest only) | 10 (10%) | 9 (11%) | 1 (5%) | 0.30 |
| Stress echocardiogram | 4 (4%) | 2 (2%) | 2 (10%) | 0.19 |
| Stress MRI | 1 (1%) | 1 (1%) | 0 (0%) | 0.53 |
| Any ischaemia on prior testinga | 28/53 (53%) | 19/40 (48%) | 9/13 (69%) | 0.17 |
| Reason for initial evaluation + CTAb | ||||
| Chest pain | 62 (60%) | 48 (58%) | 14 (70%) | 0.32 |
| Dyspnoea | 18 (17%) | 15 (18%) | 3 (15%) | 0.75 |
| Asymptomatic | 18 (17%) | 17 (20%) | 1 (5%) | 0.10 |
| Preoperative evaluation | 13 (13%) | 11 (13%) | 2 (10%) | 0.69 |
| Light-headedness/syncope | 6 (6%) | 5 (6%) | 1 (5%) | 0.86 |
| Research | 5 (5%) | 5 (6%) | 0 (0%) | 0.26 |
| Palpitations/arrhythmia | 4 (4%) | 4 (5%) | 0 (0%) | 0.31 |
| Aborted SCD | 4 (4%) | 1 (1%) | 3 (15%) | 0.004 |
| CV symptomc | 12 (12%) | 14 (15%) | 6 (50%) | 0.004 |
ACAOS, anomalous coronary artery arising from the opposite sinus; CAD, coronary artery disease; CTA, computed tomographic angiography; MRI, magnetic resonance imaging. Revasc, revascularization of ACAOS.
aAmong patients who underwent prior ischaemic testing.
bNote: % do not sum to 100% as some patients had multiple indications for initial evaluation + CTA.
cAborted SCD, or any typical chest pain, presyncope, or syncope provoked by exertion.
CTA findings
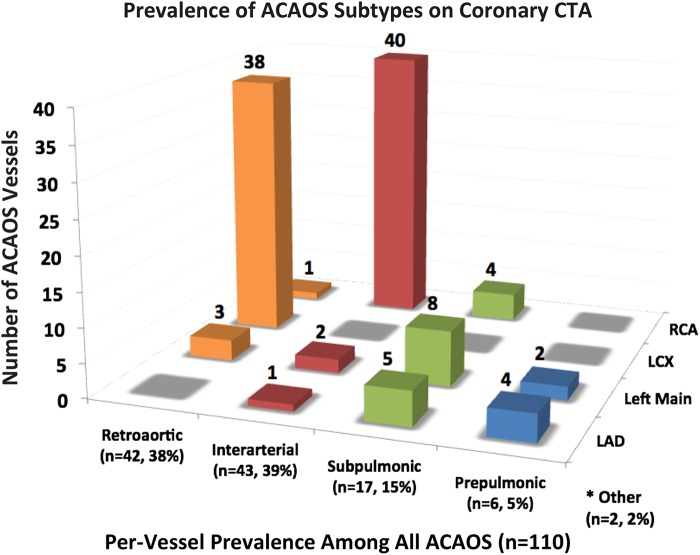
CTA-identified ACAOS subtypes and their prevalence are demonstrated in Figure 6. Out of 110 ACAOS vessels, there were 43 interarterial (39%; 40 ARCA, 3 ALCA), 42 (38%) retroaortic, 17 (15%) subpulmonic, 6 (5%) prepulmonic, 1 retrocardiac, and 1 other course subtype in a patient with anomalous RCA arising from the non-coronary cusp. The age distribution of ACAOS patients at the time of CTA is shown in Supplementary data online, Figure S1, stratified by interarterial vs. other course subtypes.
Figure 6.
Prevalence of ACAOS subtypes on coronary CTA. The number of ACAOS vessels by subtype, and per-vessel prevalence. Most common subtypes were interarterial RCA (n = 40) and retroaortic LCX (n = 38). *Other ACAOS include retrocardiac LCX (n = 1) and RCA arising from the non-coronary cusp with an otherwise normal course (n = 1). ACAOS, anomalous origin of the coronary artery arising from the opposite sinus; CTA, computed tomographic angiography; LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery.
CTA results are shown in Table 2 stratified by ACAOS revascularization. Patients referred for revascularization were more likely to have an interarterial course, proximal vessel narrowing, and more severe CAD (all P < 0.05). Excluding patients with obstructive CAD, ROC analysis identified a length of narrowing ≥5.4 mm (sensitivity 83%, specificity 74%) as the optimal cut-off to discriminate patients who were treated with ACAOS revascularization. After adjusting for obstructive CAD, baseline variables associated with ACAOS revascularization included the following: CV symptoms, ‘slit-like’ proximal narrowing, length of narrowing >5.4 mm, and an interarterial course (Table 3).
Table 2.
CTA results stratified by ACAOS revascularization
| All patients (n = 103) | No Revasc (n = 83) | Revasc (n = 20) | P-value | |
|---|---|---|---|---|
| ACAOS features* | ||||
| Proximal vessel morphology | ||||
| Normal | 47 (46%) | 43 (52%) | 4 (20%) | 0.06 |
| Oval (<50% narrowing) | 28 (27%) | 20 (24%) | 8 (40%) | |
| Slit-like (≥50% narrowing) | 22 (21%) | 15 (18%) | 7 (35%) | |
| Uninterpretable | 6 (6%) | 5 (6%) | 1 (5%) | |
| % Proximal narrowing, (Δ min diameter), median (IQR) | 9% (−14%, 32%) | 4% (−17%, 24%) | 40% (−19%, 60%) | 0.006 |
| Length of narrowing, mm | 4.2 ± 5.4 | 3.4 ± 5.1 | 7.3 ± 6.0 | 0.005 |
| Interarterial course, n (%) | 43 (42%) | 29 (67%) | 14 (33%) | 0.004 |
| Intramural, n (%) | ||||
| Not present | 64 (62%) | 56 (67%) | 8 (40%) | 0.004 |
| Present | 31 (30%) | 19 (23%) | 12 (60%) | |
| Indeterminate | 8 (8%) | 8 (10%) | 0 (0%) | |
| Acute angle take-off <45°, n (%) | 74 (72%) | 57 (69%) | 17 (85%) | 0.12 |
| Take-off level | ||||
| Below aortic valve commissure | 64 (62%) | 54 (65%) | 10 (50%) | 0.21 |
| At or above commissure | 39 (38%) | 29 (35%) | 10 (50%) | |
| Take-off type | ||||
| Separate ostia | 68 (66%) | 55 (66%) | 13 (65%) | 0.31 |
| Shared ostia | 18 (17%) | 13 (16%) | 5 (25%) | |
| Branch vessel | 15 (15%) | 14 (17%) | 1 (5%) | |
| Uninterpretable | 2 (2%) | 1 (1%) | 1 (5%) | |
| CAD severity, n (%) | ||||
| No CAD | 45 (44%) | 37 (45%) | 8 (40%) | 0.02 |
| Non-obstructive (1–49%) | 34 (33%) | 30 (36%) | 4 (20%) | |
| Moderate (50–69%) | 7 (7%) | 7 (8%) | 0 (0%) | |
| Severe (≥70%) | 14 (14%) | 7 (8%) | 7 (35%) | |
| Uninterpretable | 3 (3%) | 2 (2%) | 1 (5%) | |
| Any CAD ≥50% stenosis | 21 (20%) | 14 (17%) | 7 (35%) | 0.07 |
CAD, coronary artery disease; CTA, computed tomographic angiography; Revasc, revascularization of anomalous coronary artery arising from the opposite sinus (ACAOS). Values are mean ± standard deviation, or n (%), unless otherwise noted.
Table 3.
Odds ratio of ACAOS revascularization
| Variable | Odds ratioa | 95% CI | P-value |
|---|---|---|---|
| All adjusted for obstructive CAD (≥50% stenosis) | |||
| Age (per decile) | 0.90 | 0.75–1.08 | 0.25 |
| Any ischaemia on stress testing | 2.37 | 0.63–8.96 | 0.20 |
| CV symptomsb | 6.30 | 1.69–23.4 | 0.006 |
| CTA-identified ACAOS features | |||
| Intramural course | 1.71 | 0.81–3.64 | 0.16 |
| Acute angle <45° | 3.27 | 0.91–11.8 | 0.07 |
| ‘Slit-like’ ≥50% proximal narrowing | 4.37 | 1.44–13.3 | 0.009 |
| Interarterial course | 4.86 | 1.62–14.6 | 0.005 |
| Length of narrowing >5.4 mm | 5.54 | 1.85–16.6 | 0.002 |
aPer-patient, adjusted for CTA-identified obstructive CAD (n = 21/103, 20%).
bDefined as any of the following: history of aborted SCD, chest pain, presyncope, or syncope provoked by exertion.
Patient outcomes
Over a median follow-up of 5.8 years (IQR: 3.8–7.8), there were 20 surgical ACAOS revascularizations and 7 deaths (4 noncardiac, 3 CV) (Figure 7). No ACAOS revascularizations were performed in the patients who died (n = 4 retroaortic left circumflex, n = 3 interarterial ARCA patients). CV death occurred in two patients with an interarterial ARCA attributed to non-ischaemic cardiomyopathy (n = 1, at age 86 years) and severe aortic stenosis (n = 1, at age 86 years). There was 1 CV death in an 18-year-old female with a retroaortic left circumflex artery attributed to complications from aortic stenosis during pregnancy. Though our study was underpowered for hard outcome comparisons, there was no significant difference in all-cause mortality between patients with vs. without ACAOS revascularization (P = 0.17 between groups). No patients experienced sudden unexplained death or MI in follow-up.
Figure 7.
Outcomes of ACAOS patients following CTA. ACAOS, anomalous origin of the coronary artery arising from the opposite sinus; CABG, coronary artery bypass grafting; CAD, coronary artery disease; LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery.
Among 20 patients referred for ACAOS revascularization, 13 patients underwent CABG including 7 patients with obstructive CAD. In addition, 7 patients with an interarterial ARCA and no obstructive CAD underwent unroofing (n = 2) or reimplantation (n = 5). The 13 patients who underwent CABG had a mean age of 60 ± 7 years (range: 51–78 years) and were significantly older than the patients who underwent unroofing or reimplantation (mean age 36 ± 12 years; range: 13–52 years; P < 0.001).
Among patients undergoing ACAOS revascularization, there were no deaths related to surgery or during follow-up. One patient experienced post-operative transient vision loss without permanent sequelae. Remaining surgical complications were mild, including post-operative pericarditis (n = 5), coronary spasm (n = 2), anaemia requiring transfusion (n = 1), atrial fibrillation (n = 1), and acute kidney injury (n = 1).
In light of prior evidence suggesting subclinical ischaemia may persist following revascularization for interarterial ARCA,3 the subset of ARCA patients with no obstructive CAD referred for revascularization were examined for symptom status and ischaemic test results pre- and post-revascularization (Table 4). When ischaemic testing was performed, the majority of ARCA patients referred for revascularization had evidence of ischaemia (n = 7), and symptoms (n = 9) or prior aborted SCD (n = 2). After ARCA revascularization, no patients with follow-up testing demonstrated ischaemia, and no patients experienced CV symptoms. Additionally, two patients with dyspnoea undergoing aortic valve replacement had concomitant CABG for a subpulmonic left main coronary artery (Table 4).
Table 4.
Ischaemic testing and symptoms pre-/post-revascularization in patients with no obstructive CAD
| ID | History | Anatomy, % Narrowing | Pretest | Pre-revascularization test result | Time to post-test | Post-test | Post-test result | Symptoms (follow-up) |
|---|---|---|---|---|---|---|---|---|
| 30 | 27 M, chest pain, presyncope | ARCA, 50–70%a,b | ETT SPECT | Positive ETT, No ischaemia | 2.2 monthsc | ETT SPECT | Inconclusive ETT No ischaemia | None (67 months) |
| 33 | 52 M, chest pain | ARCA, 25–50% | ETT SPECT | Inconclusive ETT, Mild inferior ischaemia | 2.0 months | ETT SPECT | Normal ETT, No ischaemia | None (85 months) |
| 37 | 61 M, chest pain | ARCA, 50–70% | ETT SPECT | Positive ETT Mild inferolateral ischaemia | 8.7 months | ETT SPECT | Normal ETT No ischaemia | None (45 months) |
| 91 | 52 M, chest pain | ARCA, 50–70% | ETT SPECT | Normal ETT Mild inferior ischaemia | 8.4 months | ETT SPECT | Normal ETT No ischaemia | Noncardiac (56 months) |
| 3 | 60 F, chest pain | ARCA, >70% | ETT SPECT | Positive ETT Moderate inferior ischaemia | – | – | – | None (73 months) |
| 64 | 40 M, chest pain | ARCA, 50–70% | ETT SPECT | Positive ETT Moderate inferior ischaemia | – | – | – | None (1.5 months) |
| 94 | 69 F, chest pain | ARCA, 50–70% | ETT & PET | Positive ETT No ischaemia (pharm PET) | – | – | – | None (80 months) |
| 51 | 42 F, chest pain, palpitations | ARCA, 25–50% | ETT SPECT | Inconclusive ETT No ischaemia | – | – | – | No chest pain (104 months) |
| 84 | 13 M, chest pain | ARCA, 25–50% | – | – | 2.1 months | ESE | Normal ESE | Noncardiac (2.3 months) |
| 61 | 36 F, aborted SCD | ARCA, 25–50% | – | – | 10.1 months | ETT | Normal ETT | None pre/post (71 months) |
| 28 | 41 M, aborted SCD | ARCA, 50–70% | – | – | – | – | – | None pre/post (74 months) |
| 38 | 55 F, dyspnoea undergoing AVR | Subpulm. LM, <50% | ESE | Normal ETT No ischaemia | – | – | – | Dyspnoea (61 months) |
| 44 | 78 F, dyspnoea undergoing AVR | Subpulm LM, <50% | ETT SPECT | Normal ETT Mild anterior ischaemia | 32.0 months | ETT SPECT | Normal ETT, No ischaemia | None (94 months) |
ARCA, interarterial anomalous right coronary artery; COPD, chronic obstructive pulmonary disease; ESE, exercise stress echocardiogram; ETT, exercise treadmill test; PET, positron emission tomography; Pharm, pharmacologic stress; SCD, sudden cardiac death; SPECT, single photon emission computed tomography; Subpulm LM, subpulmonic left main coronary artery.
aDenotes % narrowing in proximal luminal diameter on CTA.
bAll patients with ARCA revascularization were RCA dominant.
cTime from coronary revascularization to post-test; (—) denotes no testing.
Discussion
The main findings of this study are as follows: (i) among patients referred for CTA, the prevalence of ACAOS was 1.7%; (ii) hard event rates were low with 3 CV deaths over 5.8-year follow-up, while 20 patients underwent ACAOS revascularization primarily attributed to CV symptoms, proximal ACAOS narrowing and/or obstructive CAD; and (iii) CTA-enabled detailed characterization of ACAOS vessels as well as features associated with subsequent revascularization.
By comparison with prior research,18,19,24–26 our study is among the largest to examine the prevalence and outcomes of patients with ACAOS undergoing CTA, and to our knowledge provides the longest follow-up after CTA. In comparison with prior studies, we performed the most detailed evaluation of CTA-identified ACAOS features and provide a novel association of these features with ACAOS revascularization. Additionally, we examined data regarding the presence and severity of myocardial ischaemia before and after interarterial ARCA revascularization—a subset of patients with the most uncertainty regarding their management.
Coronary CTA imaging of ACAOS features
Multiple autopsy studies have reported an association between interarterial ARCA and ALCA and an increased risk of sudden death.6,27,28 Despite a consistent finding that the relative risk of SCD is increased in patients with ARCA/ALCA, the absolute risk of SCD in these patients remains undefined. Furthermore, available data on the optimal management of ARCA/ALCA remain limited amid the variety of reported mechanisms for sudden death, ischaemia, and symptoms in ACAOS patients.29 Consequently, the absence of ischaemia and symptoms is not necessarily protective for the incidence of sudden death.1 Indeed, large autopsy studies have demonstrated that up to 50% of patients with anomalous coronary arteries have no reported symptoms prior to SCD.30
Recently, the potential to identify ‘high-risk’ ACAOS anatomical features with CTA has generated interest in the use of non-invasive imaging to risk stratify patients and guide management. As a robust non-invasive test to image coronary arteries, CTA offers detailed characterization of ACAOS features with high spatial and temporal resolution. In comparison with CTA, magnetic resonance angiography (MRA) avoids radiation and iodinated contrast exposure, at the expense of lower spatial resolution. Consequently, guidelines provide a Class I recommendation for CTA or MRA for imaging of ACAOS vessels—where the preference for either test depends on local expertise.1
Supporting the importance of the CTA to characterize ACAOS features in our study, we found that coronary revascularization was associated with CTA-identified ‘slit-like’ proximal vessel narrowing, length of narrowing >5.4 mm, and an interarterial course. Additionally, the frequency of an intramural course was increased among patients referred for revascularization compared with no revascularization (60 vs 23%, P = 0.004). These findings extend prior research demonstrating a high correlation of CTA use to identify an intramural course with direct anatomical confirmation at the time of surgical ACAOS revascularization.31 In addition, consistent with prior data demonstrating a correlation of symptoms with an intramural length of >5 mm,31 we found a significant association between a length of narrowing >5.4 mm and subsequent ACAOS revascularization. Consequently, our findings support that CTA offers the ability to non-invasively characterize ACAOS features (e.g., intramural course and severity of proximal luminal narrowing) that were previously only available with invasive techniques such as intravascular ultrasound.29 Importantly, invasive coronary angiography (ICA) has well-known limitations in characterizing ACAOS vessels, as large registry data have shown that the initial course of ACAOS vessels may not be accurately identified by ICA in up to 40% of cases.32 Supporting the challenges of diagnosing ACAOS with invasive angiography, in our study 44% of patients had prior invasive angiography and were referred for CTA to provide a more detailed characterization of ACAOS vessels.
ACAOS outcomes and the impact of revascularization
To date, several large surgical studies have reported on patient outcomes following ARCA/ALCA revascularization, demonstrating an improvement in symptoms33–35 and exercise without limitations in a majority of cases post-operatively.34,36 However, few studies with limited follow-up have examined the relationship of CTA-identified ACAOS features with patient outcomes.18,19,24–26 In a study by Opolski et al., 72 patients with ACAOS on CTA were retrospectively examined for ACAOS features and patient outcomes.18 During 15-month follow-up, only 2 patients out of 24 with an interarterial course (1 ARCA, 1 ALCA) underwent revascularization. In the remaining 70 ACAOS patients (97%) without revascularization, the authors reported symptom improvement in 73%, with 27% of patients experiencing new or worsening symptoms on follow-up. By comparison, in our study, 14/43 patients (33%) with an interarterial course underwent ACAOS revascularization, and none reported CV symptoms during a mean of 5 years following surgery.
While a majority of autopsy and surgical series appropriately focus on young patients with ACAOS, our cohort is predominantly older (mean age 52 years) reflecting the population typically referred for CTA. Though sudden death risk attributed to ACAOS appears greatest in patients aged <30 years with an interarterial course,6,37 we found that symptoms in ACAOS patients may present in older age groups. The mean age of symptomatic patients without obstructive CAD was 48 years (range: 36–61 years), including 7 ARCA patients with ischaemia, and 2 ARCA patients with aborted SCD at 36 and 41 years of age.
Currently, our understanding of the mechanisms and incidence of ischaemia in ACAOS patients remains limited. In a recent study by Brothers et al., investigators examined the results of ischaemic testing among 16 young patients (median age 12 years) during 15-month follow-up after ARCA revascularization.3 In that study, 9 patients were categorized as having a positive ischaemic test following ARCA revascularization. Importantly, the authors used a broad definition for a ‘positive ischaemia test’ that included a blunted blood pressure response to exercise (n = 2/9) and fixed defects on stress imaging with no reversible ischaemia (n = 2/9). By comparison, earlier small studies demonstrated no evidence of ischaemia after revascularization for ARCA.38,39 Despite a current Class I indication to revascularize patients with ALCA and ARCA with documented ischaemia,1 controversy remains in the ideal management of these complex patients. Consequently, future studies are needed to understand the potential for revascularization to improve ischaemia and outcomes in ALCA/ARCA patients.
Limitations
This is a retrospective analysis, where treatment decisions may result from a combination of factors, including CTA findings, patient symptoms, ancillary cardiac testing, patient preferences, and provider experience. Consequently, the incidence of ACAOS revascularization in our study should not be interpreted as synonymous with the need for revascularization based on CTA features. Nevertheless, the association between CTA-identified ACAOS findings and revascularization is important, as it may inform future studies incorporating features that may increase the risk of adverse outcomes. Given the expected low absolute risk of adverse cardiac events attributed to ACAOS, similar to all prior studies in this area, our study was underpowered to examine the impact of individual CTA-identified ACAOS features on CV death and MI. Reflecting the significant rarity of interarterial ALCA cases in clinical practice, our study focuses primarily on patients with an interarterial ARCA—a subset of ACAOS with the greatest equipoise in their management.1 Finally, our cohort has an inherent selection bias of CTA patients referred to two tertiary care centres with expertise in the management of ACAOS patients. Consequently, the observed prevalence of ACAOS on cardiac testing does not reflect the prevalence of ACAOS in the general population. However, our patients represent a population typically referred for CTA to evaluate symptoms concerning for ischaemic heart disease, and patients specifically referred for detailed CTA examination of previously known ACAOS vessels. Thus, our findings are highly applicable to current practice and consistent with the appropriate use of CTA40 and recommendations for imaging ACAOS vessels.1
Conclusion
In summary, our findings support the use of CTA to provide comprehensive anatomical assessment of ACAOS vessels that, when combined with clinical data, may be used to individualize treatment decisions for these complex patients.
Supplementary material
Supplementary material is available at European Journal of Echocardiography online.
Supplementary Material
Acknowledgements
Figure 4 courtesy of Dr Robert Padera, Department of Pathology, Brigham and Women's Hospital, Boston, MA.
Conflict of interest: Dr Cheezum and Dr Hulten declare the opinions and assertions contained herein are those of the authors' alone and do not represent the views of the United States Army, Office of the Surgeon General, Department of Defense, the United States Government, or Walter Reed National Military Medical Center. All other authors declare no conflicts of interest.
References
- 1. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA et al. . ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e143–263. [DOI] [PubMed] [Google Scholar]
- 2. Dodd JD, Ferencik M, Liberthson RR, Cury RC, Hoffmann U, Brady TJ et al. . Congenital anomalies of coronary artery origin in adults: 64-MDCT appearance. Am J Roentgenol 2007;188:W138–46. [DOI] [PubMed] [Google Scholar]
- 3. Brothers JA, McBride MG, Seliem MA, Marino BS, Tomlinson RS, Pampaloni MH et al. . Evaluation of myocardial ischemia after surgical repair of anomalous aortic origin of a coronary artery in a series of pediatric patients. J Am Coll Cardiol 2007;50:2078–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bittencourt MS, Hulten E, Ghoshhajra B, O'Leary D, Christman MP, Montana P et al. . Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging 2014;7:282–91. [DOI] [PubMed] [Google Scholar]
- 5. Maron BJ, Ackerman MJ, Nishimura RA, Pyeritz RE, Towbin JA, Udelson JE. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol 2005;45:1340–5. [DOI] [PubMed] [Google Scholar]
- 6. Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol 1992;20:640–7. [DOI] [PubMed] [Google Scholar]
- 7. Montagnana M, Lippi G, Franchini M, Banfi G, Guidi GC. Sudden cardiac death in young athletes. Intern Med 2008;47:1373–8. [DOI] [PubMed] [Google Scholar]
- 8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD et al. . Third universal definition of myocardial infarction. Circulation 2012;126:2020–35. [DOI] [PubMed] [Google Scholar]
- 10. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA et al. . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–51. [DOI] [PubMed] [Google Scholar]
- 11. Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF et al. . ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 2002;40:1531–40. [DOI] [PubMed] [Google Scholar]
- 12. Christman MP, Bittencourt MS, Hulten E, Saksena E, Hainer J, Skali H et al. . The yield of downstream tests after exercise treadmill testing: a prospective cohort study. J Am Coll Cardiol 2014;63:1264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Machac J, Bacharach SL, Bateman TM, Bax JJ, Beanlands R, Bengel F et al. . Positron emission tomography myocardial perfusion and glucose metabolism imaging. J Nucl Cardiol 2006;13:e121–51. [DOI] [PubMed] [Google Scholar]
- 14. Hansen CL, Goldstein RA, Berman DS, Churchwell KB, Cooke CD, Corbett JR et al. . Myocardial perfusion and function single photon emission computed tomography. J Nucl Cardiol 2006;13:e97–120. [DOI] [PubMed] [Google Scholar]
- 15. Hundley WG, Bluemke D, Bogaert JG, Friedrich MG, Higgins CB, Lawson MA et al. . Society for cardiovascular magnetic resonance guidelines for reporting cardiovascular magnetic resonance examinations. J Cardiovasc Magn Reson 2009;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al. . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- 17. Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L et al. . SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009;3:190–204. [DOI] [PubMed] [Google Scholar]
- 18. Opolski MP, Pregowski J, Kruk M, Witkowski A, Kwiecinska S, Lubienska E et al. . Prevalence and characteristics of coronary anomalies originating from the opposite sinus of Valsalva in 8,522 patients referred for coronary computed tomography angiography. Am J Cardiol 2013;111:1361–7. [DOI] [PubMed] [Google Scholar]
- 19. Nasis A, Machado C, Cameron JD, Troupis JM, Meredith IT, Seneviratne SK. Anatomic characteristics and outcome of adults with coronary arteries arising from an anomalous location detected with coronary computed tomography angiography. Int J Cardiovasc Imaging 2015;31:181–91. [DOI] [PubMed] [Google Scholar]
- 20. Virmani R, Chun PK, Goldstein RE, Robinowitz M, McAllister HA. Acute takeoffs of the coronary arteries along the aortic wall and congenital coronary ostial valve-like ridges: association with sudden death. J Am Coll Cardiol 1984;3:766–71. [DOI] [PubMed] [Google Scholar]
- 21. Miller JA, Anavekar NS, El Yaman MM, Burkhart HM, Miller AJ, Julsrud PR. Computed tomographic angiography identification of intramural segments in anomalous coronary arteries with interarterial course. Int J Cardiovasc Imaging 2012;28:1525–32. [DOI] [PubMed] [Google Scholar]
- 22. Mainwaring RD, Reddy VM, Reinhartz O, Petrossian E, Punn R, Hanley FL. Surgical repair of anomalous aortic origin of a coronary artery. Eur J Cardiothorac Surg 2014;46:20–6. [DOI] [PubMed] [Google Scholar]
- 23. Poynter JA, Bondarenko I, Austin EH, DeCampli WM, Jacobs JP, Ziemer G et al. . Repair of anomalous aortic origin of a coronary artery in 113 patients: a congenital heart surgeons’ society report. World J Pediatr Congenit Heart Surg 2014;5:507–14. [DOI] [PubMed] [Google Scholar]
- 24. Krupinski M, Urbanczyk-Zawadzka M, Laskowicz B, Irzyk M, Banys R, Klimeczek P et al. . Anomalous origin of the coronary artery from the wrong coronary sinus evaluated with computed tomography: ‘high-risk’ anatomy and its clinical relevance. Eur. Radiol. 2014;24:2353–9. [DOI] [PubMed] [Google Scholar]
- 25. Lee HJ, Hong YJ, Kim HY, Lee J, Hur J, Choi BW et al. . Anomalous origin of the right coronary artery from the left coronary sinus with an interarterial course: subtypes and clinical importance. Radiology 2012;262:101–8. [DOI] [PubMed] [Google Scholar]
- 26. Ashrafpoor G, Danchin N, Houyel L, Ramadan R, Belli E, Paul JF. Anatomical criteria of malignancy by computed tomography angiography in patients with anomalous coronary arteries with an interarterial course. Eur. Radiol. 2015;25:760–6. [DOI] [PubMed] [Google Scholar]
- 27. Cheitlin MD, De Castro CM, McAllister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva, A not-so-minor congenital anomaly. Circulation 1974;50:780–7. [DOI] [PubMed] [Google Scholar]
- 28. Mirchandani S, Phoon CK. Management of anomalous coronary arteries from the contralateral sinus. Int J Cardiol 2005;102:383–9. [DOI] [PubMed] [Google Scholar]
- 29. Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation 2007;115:1296–305. [DOI] [PubMed] [Google Scholar]
- 30. Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN et al. . Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med 2004;141:829–34. [DOI] [PubMed] [Google Scholar]
- 31. Kaushal S, Backer CL, Popescu AR, Walker BL, Russell HM, Koenig PR et al. . Intramural coronary length correlates with symptoms in patients with anomalous aortic origin of the coronary artery. Ann Thorac Surg 2011;92:986–92. [DOI] [PubMed] [Google Scholar]
- 32. Barriales-Villa R, Moris C, Sanmartin JC, Fernandez E, Pajin F, Ruiz Nodar JM. Anomalous coronary arteries originating in the contralateral sinus of Valsalva: registry of thirteen Spanish hospitals (RACES). Rev Esp Cardiol 2006;59:620–3. [PubMed] [Google Scholar]
- 33. Sharma V, Burkhart HM, Dearani JA, Suri RM, Daly RC, Park SJ et al. . Surgical unroofing of anomalous aortic origin of a coronary artery: a single-center experience. Ann Thorac Surg 2014;98:941–5. [DOI] [PubMed] [Google Scholar]
- 34. Turner II, Turek JW, Jaggers J, Herlong JR, Lawson DS, Lodge AJ. Anomalous aortic origin of a coronary artery: preoperative diagnosis and surgical planning. World J Pediatr Congenit Heart Surg 2011;2:340–5. [DOI] [PubMed] [Google Scholar]
- 35. Mainwaring RD, Reddy VM, Reinhartz O, Petrossian E, MacDonald M, Nasirov T et al. . Anomalous aortic origin of a coronary artery: medium-term results after surgical repair in 50 patients. Ann Thorac Surg 2011;92:691–7. [DOI] [PubMed] [Google Scholar]
- 36. Graham TP Jr, Driscoll DJ, Gersony WM, Newburger JW, Rocchini A, Towbin JA. Task Force 2: congenital heart disease. J Am Coll Cardiol 2005;45:1326–33. [DOI] [PubMed] [Google Scholar]
- 37. Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol 2000;35:1493–501. [DOI] [PubMed] [Google Scholar]
- 38. Erez E, Tam VK, Doublin NA, Stakes J. Anomalous coronary artery with aortic origin and course between the great arteries: improved diagnosis, anatomic findings, and surgical treatment. Ann Thorac Surg 2006;82:973–7. [DOI] [PubMed] [Google Scholar]
- 39. Romp RL, Herlong JR, Landolfo CK, Sanders SP, Miller CE, Ungerleider RM et al. . Outcome of unroofing procedure for repair of anomalous aortic origin of left or right coronary artery. Ann Thorac Surg 2003;76:589–96. [DOI] [PubMed] [Google Scholar]
- 40. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P et al. . ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr 2010;4:407.e1–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.