Abstract
The year 2014 was a turning point for the field of renal denervation (RDN) and its potential use to treat resistant hypertension. Tremendous enthusiasm shifted to sober reflection on the efficacy of a technology once touted as the cure to resistant hypertension. The following review highlights 2 major questions: First, does catheter-based RDN lower blood pressure and, second, will RDN using catheter-directed therapy for the treatment of resistant hypertension ever become more than an investigational technology.
Keywords: blood pressure, denervation, hypertension, treatment
The year 2014 marked a turning point for the field of renal denervation (RDN) as a potential treatment of resistant hypertension. Tremendous enthusiasm shifted to sober reflection on the efficacy of a technology once touted as the cure to resistant hypertension. Prior trials investigating RDN using catheter-directed technology resulted in marked decreases in blood pressure (BP) and set the stage for Medtronic’s (Medtronic, Santa Rosa, CA) US-based phase III sham-controlled RDN trial, SYMPLICITY–SYMPLICITY HTN-3.1 However, the lack of BP lowering difference between the intervention and sham control arms of SYMPLICITY–SYMPLICITY HTN-3 is well established1,2 and has been thoroughly dissected in multiple forums.
Proponents of RDN point to the technical limitations of SYMPLICITY–SYMPLICITY HTN-3 and ongoing and completed preclinical and clinical trials are designed to address these issues. In spite of this there are key issues precluding use of denervation for treatment of resistant hypertension, Table 1. Moreover, the answers to 2 critical questions are still loom large: Does RDN significantly lower BP and will RDN using catheter-directed therapy for the treatment of hypertension ever become more than an investigational technology worldwide.
Table 1.
Major barriers to adoption of renal denervation for the treatment of hypertension
| • Unproven efficacy in sham-controlled trials |
| • Potential for complications with updated and comprehensive renal denervation techniques |
| • Unknown long-term efficacy |
The answer to the first question is “yes,” with the caveat that is must be performed adequately and properly in a specific patient population. The physiological basis is sound and multiple preclinical and clinical studies demonstrate BP lowering effects with RDN.
However, the second question is more pertinent and is truly the crux of any argument for or against RDN. Given our current understanding, the answer to that question is “no.” RDN is not ready for “prime time” and many more questions remain than answers. The major barriers to our understanding and ultimate adoption of RDN beyond an investigational technology include: (i) unproven efficacy in sham-controlled trials; (ii) potential for higher rate of complications with updated and comprehensive RDN techniques; and (iii) the unknown long-term durability of RDN.
Procedural efficacy
Since the first surgical sympathectomy in humans was performed in the early 20th century, the medical community has become increasingly aware of the effect the sympathetic nervous system can have on systemic hypertension. These initial operations typically involved a splanchnicectomy3 and/or removal of lumbar and thoracic sympathetic ganglia.3–5 Generally, more extensive procedures were performed on younger, more symptomatic, patients. Focused and limited operations were reserved for the elderly.4 There was evidence of marked improvement in mortality and BP control in certain patients with severe hypertension treated in this fashion. However, the efficacy in all-comers was moderate at best.
Smithwick and Thompson3 reported that the severity of hypertension was attenuated in approximately half of 1,266 subjects. With the advent of antihypertensive medications, these techniques were predominantly abandoned in the 1970s.6 However, these procedures formed the early physiologic rationale for renal nerve ablation and were followed by preclinical models of RDN for the treatment hypertension.
Undoubtedly, the sympathetic nervous system plays a key role in the development of primary hypertension, and sympathetic activity is increased in many patients with resistant hypertension.7–9 This is supported by measurements of increased renal norepinephrine (NE) spillover in subjects with primary and resistant hypertension. Renal NE spillover is the only method currently available that provides a quantitative assessment of sympathetic outflow to the kidneys and is ultimately a marker for renal sympathetic nerve activity (RSNA). This suggests that renal nerves act as the unifying link between increased central sympathetic outflow and impairment of renal excretory function that leads to enduring hypertension.7,8,10
Increased sympathetic activity is a key part of the pathophysiology in many but not all animal models of hypertension. However, not all animal models respond to a reduced BP to denervation. For example, the most consistent BP lowering in response to RDN in rodent models of hypertension are seen in the spontaneously hypertensive rat, a model of hypertension in which increased RSNA has been documented.11–13 But, even in that animal model, RDN has minimal BP lowering effects in the advanced stages of hypertension when there is already target organ damage.13
In a sympathetically driven model of obesity hypertension induced by feeding dogs a high-fat diet, RDN eliminates hypertension before target organ damage.14 Alternatively, there is little evidence of sympathetic activation, or a contribution of the renal nerves to renal vascular hypertension or hypertension produced by chronic infusion of angiotensin II and aldosterone in canine models.15–21 Collectively, preclinical models reveal that the renal nerves do not contribute to all forms of hypertension, but do play a critical role in chronically increasing BP when RSNA is elevated. Thus, it is not surprising, that in SYMPLICITY HTN trials, RDN attenuated the severity of hypertension in some but not in all patients with resistant hypertension.22–25
As a proof of concept, the extent of RDN was determined in 10 subjects via measurement of renal NE spillover. Renal NE spillover was reduced 47% when measured 2–4 weeks days after RDN 22. In a study conducted in dogs with obesity hypertension, RDN lowered renal cortical NE levels 42% (a similar reduction to SYMPLICITY HTN-1).26 In response to a 42% reduction in renal tissue levels of NE, mean arterial pressure decreased 9mm Hg. However, this is only half of the decrease that occurs with surgical RDN, which achieves 95% reduction in renal cortical NE.14 Unfortunately, because of technical limitations in determining renal NE spillover, clinicians are unable to routinely assess the antihypertensive effects of RDN in relation to baseline RSNA in patients with resistant hypertension. Moreover, while the quantitative relationship between reductions in NE spillover and BP lowering is unknown, the SYMPLICITY HTN-1 investigators concluded, based on the impressive reductions in BP, that the extent of RDN performed was sufficient to achieve therapeutic efficacy.
Unfortunately, we now better understand the heterogeneity in the reduction in renal NE spillover achieved in patients subjected to catheter-based RDN, and it is likely that variable denervation has contributed to the inconsistent BP lowering in past clinical trials.8 Admittedly, this was a major criticism of SYMPLICITY HTN-3, based on the inexperience of many of the interventional cardiologists. More recent anatomical studies in an animal model demonstrate that ablations need to be performed more distally within the renal artery and affect all main renal arteries as well as branches to adequately ablate afferent and efferent pathways.27 This more extensive denervation approach will be used in Medtronic’s upcoming phase II trials of 100 patients each using a new multielectrode radiofrequency ablation catheter from Medtronic, the Spyral catheter. The trials are eponymously named SPYRAL HTN-ON (NCT02439775) and SPYRAL HTN-OFF (NCT02439749). They will not enroll patients with resistant hypertension, but instead Stage 2 hypertension.
Perhaps those that would benefit most from properly performed catheter-based RDN, based on the physiology on preclinical models, are those patients with the recently coined “refractory hypertension.” Defined by the investigators as uncontrolled BP on 4 or more antihypertensive medications, including chlorthalidone and a mineralocorticoid receptor antagonist, patients with “refractory hypertension” are younger, more likely to be female, have higher urinary normetanephrine levels, higher clinic heart rates, higher 24-hour ambulatory heart rates, higher systemic vascular resistance, and higher pulse wave velocity, in addition to reduced heart rate variability. Taken together, the uncontrolled nature of the hypertension appears more related to adrenergic overdrive than the more often seen resistant hypertension.28 However, this is a small fraction of patients.
Another critique of SYMPLICITY HTN-3 that warrants discussion is that the antihypertensive medication regimen was adjusted in approximately 40% of subjects,29 and presumably optimized, with a greater percentage being exposed to aldosterone antagonists, than in the first 2 SYMPLICITY trials.30 Additional BP lowering in SYMPLICITY HTN-3’s sham-control arm attributed to spironolactone is clearer after publication of the PATHWAY-2 trial comparing spironolactone to placebo, bisoprolol, and doxazosin for the treatment of resistant hypertension.31 Spironolactone was the most effective agent in lowering BP. Almost 60% of subjects achieved their BP goal when spironolactone was added to their antihypertensive regimen. This is compared to only about 40% with bisoprolol and doxazosin treatment.
The PRAGUE-15 trial adds further legitimacy to the HTN-3 data, especially when questioning the procedural efficacy of HTN-3. Six and 12-month follow-up have been published.32,33 Although not sham-controlled, 106 subjects with resistant hypertension were randomized to catheter-based RDN (using the SYMPLICITY catheter) with optimal antihypertensive treatment group or to an intensified pharmacological treatment group that included spironolactone. There were only 3 study centers and 6 total interventionalists with significant prior RDN experience. At 6 months, both groups experienced significant decreases in office and ambulatory BP from baseline; however, no difference between intensified medical therapy and RDN was demonstrated. At a year, both groups again demonstrated significant BP decline compared to baseline, but perprotocol analysis of 101 subjects exposed a significant difference of 24-hour systolic BP decline between complete RDN (6.3mm Hg) and the subgroup where spironolactone was added (15mm Hg). They conclude that spironolactone, when tolerated because there was an increased incidence of adverse events, is likely better at reducing BP in patients with resistant hypertension than RDN.
A single trial published following the release of HTN-3 data demonstrated effective BP lowering of RDN plus an optimized and standardized antihypertensive medication regimen, when compared to an optimized standardized antihypertensive regimen alone. This was the Renal Denervation for Hypertension (DENERHTN) trial.34 This trial did not have a sham arm thus limiting its wide applicability.
Aside from prospective clinical trials, a 2015 meta-analysis of RDN vs. pharmacotherapy for resistant hypertension35 revealed 9 studies meeting the authors’ inclusion criteria with a total number of subjects analyzed being 1,096. This included 6 randomized control trials and 3 controlled trials. Pooled analysis for all 9 studies suggested that catheter-based RDN significantly lowers systolic and diastolic BP compared to pharmacotherapy. However, when only including the 6 truly randomized control trials this significant difference is not present.
POTENTIAL COMPLICATIONS
The positive spin from SYMPLICITY HTN-3 was the demonstrated safety of the procedure, and the trial’s lack of complications allows continuation of further clinical examination and trial enrolment. Long-term follow-up of SYMPLICITY HTN-1 and 2 also reflect a positive safety profile.23,24 Subsequent single center publications using magnetic resonance imaging and computed tomography angiography to quantitatively assess the degree of renal artery stenosis seen after RDN came to similar conclusions.
Two separate cohorts using the original single electrode SYMPLICITY catheter evaluated for renal artery stenosis. In a 76-subject cohort found that at 6 months after RDN no stenosis greater than 70% was noted, with only 2 subjects having a stenosis greater than 50%.36 Another 51-subject cohort had magnetic resonance angiography at a median follow-up of 11 months and demonstrated no significant vascular narrowing.37
However, recent experimental studies of renal histology and renal NE tissue levels indicate that truly effective denervation may require delivery of ablative energy to the distal sections of the renal artery, near the bifurcation, rather than to just the more proximal regions of the main renal artery as performed in a significant percentage of SYMPLICITY patients.38,39 A porcine model demonstrated that increasing the number of ablations in the main renal artery was not sufficient to yield a clear dose–response relationship on NE content and axon density.39 However, targeted treatment of renal artery branches, or distal segments of the main renal artery, resulted in significantly less variability of response and greater reduction of both NE and axon density than treatment of only the main renal artery. The changes remained 28 days postprocedure and treatment of both the main renal arteries and branches produced the greatest change in renal NE and axon density. Thus, including the initial branches of the renal artery leads to more complete denervation, unfortunately this may be technically challenging to achieve, may increase the risk of arterial damage, and alter the safety profile of RDN as seen in SYMPLICITY HTN-3.
Aside from the possibility of structural damage from more extensive denervation, long-term postprocedure renal function itself is an important safety consideration in a patient population at high risk for chronic kidney disease. Although most renal function measurements conducted within a year of RDN in patients with resistant hypertension demonstrate that renal nerve ablation technology does not have adverse functional effects on kidney function.1,22,24,25
Loss of kidney function following denervation has been tracked in the SYMPLICITY registry. A loss in estimated glomerular filtration rate (eGFR) of 9.3ml/min per 1.73 m2 at 3 years was observed,23 a greater than 2-fold larger decline in eGFR than in the recent ACCOMPLISH trial, in which hypertensive patients at high cardiovascular risk were treated with combination pharmacotherapy.40 Undetermined is whether the greater decline in eGFR reported in the SYMPLICITY registry reflects adverse effects of denervation, impaired ability of the afferent arteriole to respond to a fall in arterial pressure or just the natural progression of resistant hypertension.
To provide insight into physiological mechanisms that may account for abnormal long-term reductions in GFR following suppression of RSNA (as seen in HTN-1), one can use the obesity model of hypertension and compare the renal hemodynamic response in canine models during a procedure the induces global reflex suppression of sympathetic activity (baroreceptor stimulation41 and renal specific sympathoinhibition by surgical RDN42).
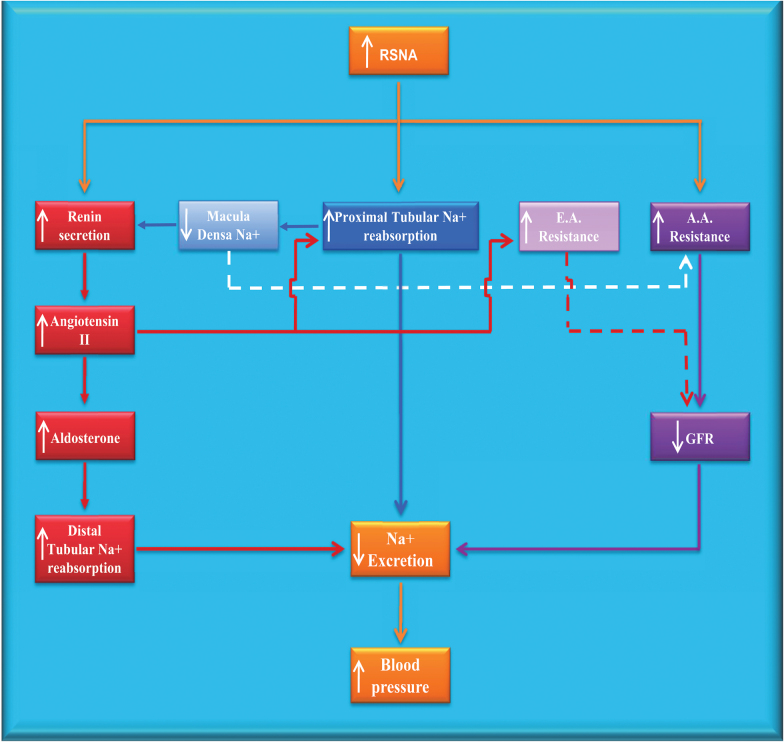
In obese subjects, precursors to progressive renal injury are hypertension and glomerular hyperfiltration. Hyperfiltration is attributed to both a high rate of neurally mediated sodium reabsorption in the proximal tubule and loop of Henle that decreases sodium delivery to the and stimulates of renin secretion.43 Consequently, a tubuloglomerular signal dilates the afferent arterioles, and concomitantly angiotensin II is generated in response to increased renin. This intensifies tubuloglomerular-mediated hyperfiltration by constricting the efferent arterioles. Presumably, suppression of sympathetic activation would diminish these renal hemodynamic changes (Figure 1).
Figure1.
Association of renal sympathetic activity with other regulatory systems for blood pressure. Increased RSNA leads to chronic increases in blood pressure by decreasing sodium excretion (orange boxes and lines). The 3 major pathways by which changes in RSNA affect sodium excretion are through alterations in activation of the renin–angiotensin system (red boxes and lines), sodium reabsorption (blue box and line), and glomerular hemodynamics (purple boxes and lines). Light blue and light purple lines represent mechanisms indirectly affected by RSNA. Continuous lines represent stimulatory effects; dotted lines reflect inhibitory effects. Abbreviations: A.A., afferent arteriole; E.A., efferent arteriole; GFR, glomerular filtration rate; RSNA, renal sympathetic nerve activity. (Adopted from (Iliescu R, Lohmeier TE, Tudorancea I, Laffin L, Bakris GL. Renal denervation for the treatment of resistant hypertension: review and clinical perspective. American Journal of Physiology-Renal Physiology 2015; 309(7):F583–F594).)
With chronic global suppression of central sympathetic outflow by baroreceptor stimulation in a canine model of obesity hypertension diminished hyperfiltration is in fact demonstrated, as is reduction in sodium reabsorption and plasma renin activity. Therefore, the decline in GFR is likely due to tubuloglomerular-mediated constriction of preglomerular vessels and reduction of angiotensin II-induced postglomerular arteriolar tone.42 This is supported by a reported decrease in eGFR of 8% after 6 months of baroreceptor stimulation in cohort of obese patients with resistant hypertension that did not worsen after 12 months of treatment.44 The nonprogressive reduction in GFR suggests the clinical benefit of lowering the renal arterial pressure and arresting hyperfiltration.
Whereas glomerular hyperfiltration diminished during baroreceptor stimulation in dogs with obesity hypertension, there were increases in GFR when measured 2 weeks after surgical RDN.42 It was presumed that complete surgical RDN decreases preglomerular resistance, as a result of reducing RSNA to levels lower than during the more physiologic reflex suppression of sympathetic activity by baroreceptor stimulation. It is thus, not surprising that studies show increases in renal plasma flow immediately following RDN in those with resistant HTN.45 Decreasing preglomerular resistance and in turn increasing glomerular pressure, RDN may ultimately predispose obese patients with resistant hypertension to glomerular injury due to glomerular hypertension, especially if the BP lowering response to renal nerve ablation is not pronounced. Therefore, in resistant hypertensive subjects with declining kidney function, additional reductions in preglomerular resistance in already hyperfiltering nephrons may actually accelerate the progression of renal injury (Figure 1).
Taken together, the findings reported in the SYMPLICITY registry indicate a relatively disproportionate reduction in eGFR after 36 months of RDN in patients with resistant hypertension. This reinforces the need for careful long-term determinations of intrinsic renal function in subjects with normal and impaired baseline renal function.
Related to procedural safety are new “noninvasive” technologies being developed that have the potential to supplant invasive catheter-based RDN. Most recently, investigators reported early safety and efficacy data of a noninvasive, externally delivered, focused ultrasound device that is thought to denervate the renal arteries and lower BP.46 Although early in its clinical testing, and without long-term safety profile, or sham-controlled efficacy trials, it is representative of a new wave of medical technologies that can heed the lessons learned in the relatively long history of catheter-based RDN, and perhaps capitalize on renal nerve ablations recent setbacks.
Long-term efficacy
Let us presume that, even in the face of the aforementioned barriers, catheter-based RDN technology advances in the coming years and the procedure successfully demonstrates significant BP lowering against a sham-controlled group in a large-scale randomized control trial. The next appropriate question is how durable will denervation be, i.e., does reinnervation of these arteries occur?
These questions will remain unanswered until that time, because the durability of effect would likely vary based on the complexity and extent of the procedure performed. One may glean insight however, from recent data using the SYMPLICITY RDN system (Medtronic). This catheter was used to investigate serial changes in the renal arteries after RF RDN in a porcine model.47 Forty-nine renal arteries from 28 animals, at 4 different time points (7, 30, 60, and 180 days), were examined. Histological assessment of the arterial medial circumferential injury was greatest at 7 days and least at 180 days. At 7 days, the nerve injury score was significantly greater in comparison to other time points. In fact, focal nerve regeneration at the sites of ablation was seen in 17% of renal arteries at 60 days and 71% at 180 days. Studies in multiple species including rats, dogs, and sheep all demonstrate anatomic and functional reinnervation of sympathetic and sensory fibers within months after RDN.48–50 However, there is limited data regarding nerve regeneration in humans following renal nerve ablation. Thus, the time course of functional reinnervation in patients with resistant hypertension and the importance of this mechanism in diminishing the procedure’s antihypertensive effects are currently unknown.
If reinnervation does in fact occur in humans, would patients be offered serial procedures to maintain successful denervation? Would serial ablations have similar BP lowering efficacy and still be safe to an already instrumented renal artery? These questions are particularly important if RDN is to be used in a fashion comparable to the current SPYRAL HTN-OFF trial (NCT02439749).
Unlike trials looking at patients with resistant hypertension and lack of other effective therapies, SPYRAL HTN-OFF is being undertaken in subjects with Stage 2 hypertension on no antihypertensive medications. It will require office systolic BP greater than 150mm Hg, a diastolic office BP greater than 90mm Hg, and 24-hour ambulatory BP monitoring with an average systolic BP between 140 and 170mm Hg. These subjects likely could have BP controlled on 2–3 antihypertensive medications, with greater than 25 years of data of known efficacy.
CONCLUSION
Catheter-based RDN remains an area of profound importance to many researchers and clinicians. Given expanding data suggesting medication compliance in the treatment of chronic diseases is less than ideal,51 device-based therapy for chronic conditions such as primary and/or resistant hypertension are logical areas of innovation and interest. Unfortunately, RDN as presently performed is not ready for “prime time.” A clear physiological basis for RDN BP lowering efficacy, in certain subsets of patients with hypertension, is established, and initial clinical studies were promising. Yet, the equivocal findings of the sham-controlled SYMPLICITY-HTN-3 resulted in a thorough re-examination of the physiology of renal nerve ablation, the technology used to disrupt renal sympathetic innervation, and the way in which efficacy of denervation is established and tested.
This re-examination is currently ongoing and answers to the many uncertainties posed in the above text will be based on forthcoming discoveries and clinical trials. The end result may be the widespread adoption of catheter-based RDN for resistant hypertension, primary hypertension, and other chronic conditions associated with increased sympathetic activity. However, given the current state of knowledge regarding RDN, one cannot confidently claim or endorse catheter-based RDN beyond an investigational technology.
DISCLOSURE
Bakris—Principal investigator-FIDELIO trial (funded by Bayer); Consultant to: Relypsa, Janssen, AbbVie, Bayer, Boehringer-Ingelheim, Astra-Zeneca, Vascular Dynamics, Medtronic, NxStage. The other author has no conflict of interest.
REFERENCES
- 1. Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL; SYMPLICITY HTN-3 Investigators A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 2. Bakris GL, Townsend RR, Liu M, Cohen SA, D’Agostino R, Flack JM, Kandzari DE, Katzen BT, Leon MB, Mauri L, Negoita M, O’Neill WW, Oparil S, Rocha-Singh K, Bhatt DL; SYMPLICITY HTN-3 Investigators Impact of renal denervation on 24-hour ambulatory blood pressure: results from SYMPLICITY HTN-3. J Am Coll Cardiol 2014; 64:1071–1078. [DOI] [PubMed] [Google Scholar]
- 3. Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152:1501–1504. [DOI] [PubMed] [Google Scholar]
- 4. Morrissey DM, Brookes VS, Cooke WT. Sympathectomy in the treatment of hypertension; review of 122 cases. Lancet 1953; 1:403–408. [DOI] [PubMed] [Google Scholar]
- 5. Newcombe CP, Shucksmith HS, Suffern WS. Sympathectomy for hypertension; follow-up of 212 patients. Br Med J 1959; 1:142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertog SC, Sobotka PA, Sievert H. Renal denervation for hypertension. JACC Cardiovasc Interv 2012; 5:249–258. [DOI] [PubMed] [Google Scholar]
- 7. Esler M. The sympathetic nervous system in hypertension: back to the future? Curr Hypertens Rep 2015; 17:11. [DOI] [PubMed] [Google Scholar]
- 8. Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 2015; 116:976–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grassi G, Seravalle G, Brambilla G, Pini C, Alimento M, Facchetti R, Spaziani D, Cuspidi C, Mancia G. Marked sympathetic activation and baroreflex dysfunction in true resistant hypertension. Int J Cardiol 2014; 177:1020–1025. [DOI] [PubMed] [Google Scholar]
- 10. Esler M. Renal denervation for hypertension: observations and predictions of a founder. Eur Heart J 2014; 35:1178–1185. [DOI] [PubMed] [Google Scholar]
- 11. Judy WV, Watanabe AM, Henry DP, Besch HR, Jr, Murphy WR, Hockel GM. Sympathetic nerve activity: role in regulation of blood pressure in the spontaenously hypertensive rat. Circ Res 1976; 38:21–29. [DOI] [PubMed] [Google Scholar]
- 12. Norman RA, Jr, Dzielak DJ. Role of renal nerves in onset and maintenance of spontaneous hypertension. Am J Physiol 1982; 243:H284–H288. [DOI] [PubMed] [Google Scholar]
- 13. Winternitz SR, Katholi RE, Oparil S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J Clin Invest 1980; 66:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 2012; 59:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldblatt H, Gross J, Hanzal RF. Studies on experimental hypertension: II. The effect of resection of splanchnic nerves on experimental renal hypertension. J Exp Med 1937; 65:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lohmeier TE, Dwyer TM, Hildebrandt DA, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension 2005; 46:1194–1200. [DOI] [PubMed] [Google Scholar]
- 17. Lohmeier TE, Iliescu R. The baroreflex as a long-term controller of arterial pressure. Physiology (Bethesda) 2015; 30:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lohmeier TE, Iliescu R. Lowering of blood pressure by chronic suppression of central sympathetic outflow: insight from prolonged baroreflex activation. J Appl Physiol (1985) 2012; 113:1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lohmeier TE, Liu B, Hildebrandt DA, Cates AW, Georgakopoulos D, Irwin ED. Global- and renal-specific sympathoinhibition in aldosterone hypertension. Hypertension 2015; 65:1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oparil S, Sripairojthikoon W, Wyss JM. The renal afferent nerves in the pathogenesis of hypertension. Can J Physiol Pharmacol 1987; 65:1548–1558. [DOI] [PubMed] [Google Scholar]
- 21. Wyss JM, Sripairojthikoon W, Oparil S. Failure of renal denervation to attenuate hypertension in Dahl NaCl-sensitive rats. Can J Physiol Pharmacol 1987; 65:2428–2432. [DOI] [PubMed] [Google Scholar]
- 22. Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 23. Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 2014; 383:622–629. [DOI] [PubMed] [Google Scholar]
- 24. Symplicity HTNI. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917. [DOI] [PubMed] [Google Scholar]
- 25. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 26. Henegar JR, Zhang Y, De Rama R, Hata C, Hall ME, Hall JE. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens 2014; 27:1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR, Kolodgie FD, Virmani R, Joner M. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643. [DOI] [PubMed] [Google Scholar]
- 28. Dudenbostel T, Acelajado MC, Pisoni R, Li P, Oparil S, Calhoun DA. Refractory hypertension: evidence of heightened sympathetic activity as a cause of antihypertensive treatment failure. Hypertension 2015; 66:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, Leon MB, Ma A, Massaro J, Mauri L, Oparil S, O’Neill WW, Patel MR, Rocha-Singh K, Sobotka PA, Svetkey L, Townsend RR, Bakris GL. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J 2015; 36:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Messerli FH, Bangalore S. Renal denervation for resistant hypertension? N Engl J Med 2014; 370:1454–1457. [DOI] [PubMed] [Google Scholar]
- 31. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society’s PATHWAY Studies Group Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosa J, Widimský P, Toušek P, Petrák O, Čurila K, Waldauf P, Bednář F, Zelinka T, Holaj R, Štrauch B, Šomlóová Z, Táborský M, Václavík J, Kociánová E, Branny M, Nykl I, Jiravský O, Widimský J., Jr Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 2015; 65:407–413. [DOI] [PubMed] [Google Scholar]
- 33. Rosa J, Widimský P, Waldauf P, Lambert L, Zelinka T, Táborský M, Branny M, Toušek P, Petrák O, Čurila K, Bednář F, Holaj R, Štrauch B, Václavík J, Nykl I, Krátká Z, Kociánová E, Jiravský O, Rappová G, Indra T, Widimský J., Jr Role of Adding Spironolactone and Renal Denervation in True Resistant Hypertension: One-Year Outcomes of Randomized PRAGUE-15 Study. Hypertension 2016; 67:397–403. [DOI] [PubMed] [Google Scholar]
- 34. Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier-Véhier C, Courand PY, Lantelme P, Denolle T, Dourmap-Collas C, Trillaud H, Pereira H, Plouin PF, Chatellier G; Renal Denervation for Hypertension (DENERHTN) investigators Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965. [DOI] [PubMed] [Google Scholar]
- 35. Sun D, Li C, Li M, Liu J, Wen S. Renal Denervation vs Pharmacotherapy for Resistant Hypertension: A Meta-Analysis. J Clin Hypertens (Greenwich) 2016; 18:733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lambert T, Nahler A, Reiter C, Schwarz S, Gammer V, Blessberger H, Kammler J, Saleh K, Grund M, Steinwender C. Frequency of renal artery stenosis after renal denervation in patients with resistant arterial hypertension. Am J Cardiol 2015; 115:1545–1548. [DOI] [PubMed] [Google Scholar]
- 37. Schmid A, Schmieder R, Lell M, Janka R, Veelken R, Schmieder RE, Uder M, Ott C. Mid-Term Vascular Safety of Renal Denervation Assessed by Follow-up MR Imaging. Cardiovasc Intervent Radiol 2016; 39:426–432. [DOI] [PubMed] [Google Scholar]
- 38. Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-Based Radiofrequency Renal Denervation: Location Effects on Renal Norepinephrine. Am J Hypertens 2015; 28:909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mahfoud F, Tunev S, Ewen S, Cremers B, Ruwart J, Schulz-Jander D, Linz D, Davies J, Kandzari DE, Whitbourn R, Böhm M, Melder RJ. Impact of Lesion Placement on Efficacy and Safety of Catheter-Based Radiofrequency Renal Denervation. J Am Coll Cardiol 2015; 66:1766–1775. [DOI] [PubMed] [Google Scholar]
- 40. Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, Jamerson K, Velazquez EJ, Staikos-Byrne L, Kelly RY, Shi V, Chiang YT, Weber MA; ACCOMPLISH Trial investigators Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet 2010; 375:1173–1181. [DOI] [PubMed] [Google Scholar]
- 41. Laffin LJ, Bakris GL. Carotid Baroreceptor Stimulation. Interventional Therapies for Secondary and Essential Hypertension. Springer International Publishing: Switzerland, 2016, pp 339–348. [Google Scholar]
- 42. Iliescu R, Lohmeier TE, Tudorancea I, Laffin L, Bakris GL. Renal denervation for the treatment of resistant hypertension: review and clinical perspective. Am J Physiol Renal Physiol 2015; 309:F583–F594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol 2004; 286:F8–15. [DOI] [PubMed] [Google Scholar]
- 44. Alnima T, de Leeuw PW, Tan FE, Kroon AA; Rheos Pivotal Trial Investigators Renal responses to long-term carotid baroreflex activation therapy in patients with drug-resistant hypertension. Hypertension 2013; 61:1334–1339. [DOI] [PubMed] [Google Scholar]
- 45. Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 2009; 361:932–934. [DOI] [PubMed] [Google Scholar]
- 46. Neuzil P, Ormiston J, Brinton TJ, Starek Z, Esler M, Dawood O, Anderson TL, Gertner M, Whitbourne R, Schmieder RE. Externally delivered focused ultrasound for renal denervation. JACC Cardiovasc Interv 2016; 9:1292–1299. [DOI] [PubMed] [Google Scholar]
- 47. Sakakura K, Tunev S, Yahagi K, O’Brien AJ, Ladich E, Kolodgie FD, Melder RJ, Joner M, Virmani R. Comparison of histopathologic analysis following renal sympathetic denervation over multiple time points. Circ Cardiovasc Interv 2015; 8:e001813. [DOI] [PubMed] [Google Scholar]
- 48. Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP, May CN. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension 2015; 65:393–400. [DOI] [PubMed] [Google Scholar]
- 49. Mogil RA, Itskovitz HD, Russell JH, Murphy JJ. Renal innervation and renin activity in salt metabolism and hypertension. Am J Physiol 1969; 216:693–697. [DOI] [PubMed] [Google Scholar]
- 50. Mulder J, Hökfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 2013; 304:R675–R682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hyman DJ, Pavlik V. Medication adherence and resistant hypertension. J Hum Hypertens 2015; 29:213–218. [DOI] [PubMed] [Google Scholar]