In this retrospective study of two large phase III studies of patients with metastatic castration-resistant prostate cancer, abiraterone acetate conferred benefit to patients regardless of Gleason score (<8 versus ≥8) at initial diagnosis.
Keywords: abiraterone acetate, chemotherapy-naïve, Gleason score, post-chemotherapy, prostate cancer
Abstract
Background
The usefulness of Gleason score (<8 or ≥8) at initial diagnosis as a predictive marker of response to abiraterone acetate (AA) plus prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC) was explored retrospectively.
Patients and methods
Initial diagnosis Gleason score was obtained in 1048 of 1195 (COU-AA-301, post-docetaxel) and 996 of 1088 (COU-AA-302, chemotherapy-naïve) patients treated with AA 1 g plus prednisone 5 mg twice daily by mouth or placebo plus prednisone. Efficacy end points included radiographic progression-free survival (rPFS) and overall survival (OS). Distributions and medians were estimated by Kaplan–Meier method and hazard ratio (HR) and 95% confidence interval (CI) by Cox model.
Results
Baseline characteristics were similar across studies and treatment groups. Regardless of Gleason score, AA treatment significantly improved rPFS in post-docetaxel [Gleason score <8: median, 6.4 versus 5.5 months (HR = 0.70; 95% CI 0.56–0.86), P = 0.0009 and Gleason score ≥8: median, 5.6 versus 2.9 months (HR = 0.58; 95% CI 0.48–0.72), P < 0.0001] and chemotherapy-naïve patients [Gleason score <8: median, 16.5 versus 8.2 months (HR = 0.50; 95% CI 0.40–0.62), P < 0.0001 and Gleason score ≥8: median, 13.8 versus 8.2 months (HR = 0.61; 95% CI 0.49–0.76), P < 0.0001]. Clinical benefit of AA treatment was also observed for OS, prostate-specific antigen (PSA) response, objective response and time to PSA progression across studies and Gleason score subgroups.
Conclusion
OS and rPFS trends demonstrate AA treatment benefit in patients with pre- or post-chemotherapy mCRPC regardless of Gleason score at initial diagnosis. The initial diagnostic Gleason score in patients with mCRPC should not be considered in the decision to treat with AA, as tumour metastases may no longer reflect the histology at the time of diagnosis.
Clinical trials number
COU-AA-301 (NCT00638690); COU-AA-302 (NCT00887198).
introduction
The Gleason scoring system enabled a standardised risk assessment for men with localised prostate cancer based on histology. It was developed in 1966 by Donald F. Gleason, and soon became the international standard by which prostate cancers were classified. Five cellular architectural patterns observed in prostatic tissue were characterised: 1, 2 and 3 representing normal prostate tissue, and 4 and 5 indicative of cancer or abnormal tissue. The score is the sum of the two most common patterns observed in tumour samples [1]. Since then, several refinements have been adopted to improve the consistency of scoring, the most recent of which occurred in 2005 under the auspices of the International Society of Urological Pathology [2], which tightened the definition of pattern 3 and widened the definition of pattern 4 prostatic adenocarcinomas. The change has resulted in greater inter-observer reproducibility among pathologists [1, 2].
Applied clinically in patients with clinically localised disease at diagnosis, the Gleason score, and in particular the modified system, has been shown to be prognostic for biochemical recurrence, the development of metastasis and overall survival (OS) [3]. The prognostic significance of the Gleason score of the primary tumour in later disease states is less certain. For example, the Gleason score is strongly prognostic of outcomes in early non-castrate disease [4], and weaker or absent in metastatic castration-resistant prostate cancer (mCRPC) [5–8] when the degree of differentiation is predominant high grade. In patients with mCRPC, metastatic biopsies are rarely performed outside of research indications, and if done Gleason grading is not applicable.
Abiraterone acetate (AA) plus prednisone (P) is approved for the treatment of mCRPC based on the significant radiographic progression-free survival (rPFS) and OS benefits in the phase III trials in patients with mCRPC post-docetaxel [9, 10], and in mCRPC chemotherapy-naïve patients [11–13]. In mCRPC, the predictive value of the Gleason score at initial diagnosis on patient outcomes following treatment with AA is unknown. We retrospectively evaluated efficacy outcomes in patients with mCRPC treated with AA + P versus placebo plus P in pivotal studies COU-AA-301 (post-docetaxel) and COU-AA-302 (chemotherapy-naïve) by Gleason score.
patients and methods
The phase III double-blind, randomised placebo-controlled study COU-AA-301 (ClinicalTrials.gov: NCT00638690) was conducted in patients with mCRPC who had been treated previously with docetaxel; the study methodology has been described in detail previously [9, 10]. Patients were stratified by Eastern Cooperative Oncology Group (ECOG) performance status (0–1 versus 2), worst pain over the past 24 h on the Brief Pain Inventory (Short Form) (0–3 for absent versus 4–10 for present), number of prior chemotherapy regimens (one versus two) and type of progression [prostate-specific antigen (PSA) progression versus radiographic progression with or without PSA progression]. Patients were randomised 2:1 to receive AA 1000 mg once daily by mouth plus P 5 mg twice daily by mouth, or placebo plus P.
The phase III double-blind, randomised placebo-controlled COU-AA-302 study (ClinicalTrials.gov: NCT00887198) was conducted in mildly symptomatic or asymptomatic patients with progressive mCRPC who were chemotherapy naïve [11, 12]. The study methodology has been described in detail previously [11, 12]. Briefly, patients were stratified by ECOG performance status score (0 versus 1) and randomised 1:1 to receive AA 1000 mg once daily by mouth plus P 5 mg twice daily by mouth, or placebo plus P.
Gleason scores at diagnosis were available for 88% (1048/1195) of patients with mCRPC post-docetaxel in study COU-AA-301 and for 92% (996/1088) of patients with mCRPC who were chemotherapy naïve in study COU-AA-302. For most patients in COU-AA-301, Gleason scores were determined before 2005, when the new scoring criteria were established [AA plus P, 70% (487/698); P, 65% (226/350)], whereas the distribution of patients with scores determined before and after 2005 in COU-AA-302 was different [before 2005, AA plus P, 50% (246/488); P, 48% (246/508)]; however, determination of Gleason scores was similar in both treatment groups at both time periods. The Gleason score at initial diagnosis was based on the interpretation at the site where the biopsy was performed and not verified by a central review.
Studies were done according to the Declaration of Helsinki, the International Conference on Harmonisation and the Guidelines for Good Clinical Practice.
statistical analysis
The distributions and medians were estimated by the Kaplan–Meier method; the hazard ratios (HR) and 95% confidence intervals (CI) were estimated by the Cox model. The stratified log-rank test was used for treatment comparison, and statistical significance was declared if the P value was <0.05, without adjustment for multiple testing in this retrospective analysis. To evaluate the effect of Gleason score on the OS end point in the AA plus P arm, a univariate analysis using the Cox model was used to obtain the estimate of the HR and its 95% CI. Although COU-AA-301 was not powered to discern treatment benefit in Gleason subgroups, 797 death events were estimated to provide 85% power to detect an HR = 0.80 at a two-tailed significance level of 0.05 [9]. In COU-AA-302, 378 planned progression-free events were planned to provide 91% power to detect an HR = 0.67 for rPFS at a two-tailed significance level of 0.01 and 773 death events to provide 85% power to detect an HR = 0.80 at a two-tailed significance level of 0.04 [12].
Data obtained from the final analysis are reported for both COU-AA-301 [10] and COU-AA-302 [13] at 97% and 96% of planned deaths, respectively, with a median follow-up for OS of 20.2 and 49.2 months, respectively. Interpretation of rPFS as an outcome is not equivalent for both trial datasets as the primary end point in COU-AA-301 [9, 10] was OS, and rPFS (based on investigator review) was a secondary end point. In COU-AA-302 [11–13], OS and rPFS were co-primary end points with rPFS a pre-established, centrally reviewed end point.
results
A total of 698 and 350 patients with mCRPC post-docetaxel and 488 and 508 mCRPC chemotherapy-naïve patients were treated with AA plus P versus P, respectively (supplementary Figure S1, available at Annals of Oncology online). The proportions of patients with Gleason score <8 or ≥8 were similar across treatment groups and studies (supplementary Table S1, available at Annals of Oncology online). Baseline disease characteristics were similar across treatment groups in each study and by Gleason score subgroup (Table 1).
Table 1.
Baseline patient and disease characteristics
| mCRPC post-docetaxel |
mCRPC chemotherapy-naïve |
|||||||
|---|---|---|---|---|---|---|---|---|
| GS <8 (N =
503) |
GS ≥8 (N = 545) |
GS <8 (N =
479) |
GS ≥8 (N = 517) |
|||||
| AA + P (n = 342) | P (n = 161) | AA + P (n = 356) | P (n = 189) | AA + P (n = 225) | P (n = 254) | AA + P (n = 263) | P (n = 254) | |
| Age, median (range), years | 70 (42–95) | 70 (39–87) | 68 (45–86) | 67 (43–90) | 71 (45–95) | 71 (50–90) | 69 (44–90) | 69 (44–90) |
| Extent of disease, n (%) | ||||||||
| Bone only | 123 (36) | 70 (43) | 130 (37) | 81 (43) | 122 (54) | 119 (47) | 121 (46) | 128 (50) |
| Bone, soft tissue | 219 (64) | 91 (57) | 226 (63) | 108 (57) | 103 (46) | 135 (53) | 142 (54) | 126 (50) |
| ECOG PS, n (%) | ||||||||
| 0 | – | – | – | – | 170 (76) | 190 (75) | 203 (77) | 198 (78) |
| 1 | – | – | – | – | 55 (24) | 64 (25) | 60 (23) | 56 (22) |
| 0–1 | 308 (90) | 147 (91) | 317 (89) | 166 (88) | – | – | – | – |
| 2 | 34 (10) | 14 (9) | 39 (11) | 23 (12) | – | – | – | – |
| Baseline PSA, median (range), ng/ml | 123.3 (0.7–8099.9) | 176.5 (0.6–3595.1) | 141.7 (0.4–9253.0) | 123.7 (3.8–10 114.0) | 40.5 (0.0–3927.4) | 36.7 (1.7–1782) | 40.1 (0.6–1715.7) | 36.3 (0.7–6606.4) |
| Baseline haemoglobin, median (range), g/dl | 11.9 (8.1–16.1) | 11.9 (8.4–15.7) | 11.6 (7.3–15.2) | 11.6 (7.2–16.5) | 12.9 (9.3–16.6) | 13.2 (9.3–15.7) | 13.0 (7.2–16.2) | 13.0 (7.0–15.6) |
| Baseline LDH, median (range), IU/l | 216.0 (84.0–3373.0) | 238.0 (143.0–2104.0) | 226.0 (97.0–2232.0) | 238.5 (123.0–1384.0) | 185.0 (60.0–600.0) | 184.0 (108.0–554.0) | 187.0 (103.0–871.0) | 181.0 (87.0–781.0) |
| Time from initial diagnosis to first dose, months | 94.8 (5.8–237.2) | 93.6 (21.1–267.8) | 54.2 (6.9–226.6) | 46.7 (2.0–215.7) | 89.4 (5.9–267.2) | 84.8 (8.8–331.5) | 42.9 (5.6–235.6) | 39.9 (3.0–217.9) |
AA, abiraterone acetate; ECOG PS, Eastern Cooperative Oncology Group performance status; GS, Gleason score; LDH, lactate dehydrogenase; mCRPC, metastatic castration-resistant prostate cancer; P, prednisone; PSA, prostate-specific antigen.
Separate univariate analyses confirmed that Gleason score did not significantly impact the OS for the AA plus P arm patients with either mCRPC post-docetaxel (HR = 1.14; 95% CI 0.95–1.38, P = 0.1653) or with chemotherapy-naïve mCRPC (HR = 1.28; 95% CI 0.96–1.72, P = 0.0986).
Patients with mCRPC post-docetaxel had significant improvement in rPFS with AA plus P compared with P, irrespective of Gleason score (<8: HR = 0.70; 95% CI 0.56–0.86, P = 0.0009; ≥8: HR = 0.58; 95% CI 0.48–0.72, P = 0.0001) (Figure 1A). Improvement in OS was not significant for patients with Gleason score <8, but was significant for those with Gleason score ≥8 (<8: HR = 0.82; 95% CI 0.64–1.04, P = 0.1041; ≥8: HR = 0.61; 95% CI 0.49–0.76, P < 0.0001) (Figure 1B). Similarly, improvement in time to PSA progression (TTPP) was not significant for patients with Gleason score <8 and was significant for patients with Gleason score ≥8 (<8: 8.6 versus 8.5 months, P = 0.1346; ≥8: 8.4 versus 5.6 months, P < 0.0001) (supplementary Figure S2A, available at Annals of Oncology online).
Figure 1.
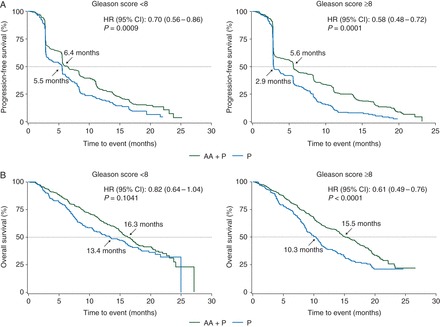
Radiographic progression-free survival (A) and overall survival (B) in post-docetaxel metastatic castration-resistant prostate cancer patients treated with abiraterone acetate (AA) plus prednisone (P) or placebo plus P as a function of Gleason score (<8 and ≥8) at initial diagnosis. AA, abiraterone acetate; CI, confidence interval; HR, hazard ratio; P, prednisone.
Chemotherapy-naïve patients with mCRPC had significant improvements in rPFS irrespective of Gleason score with AA plus P treatment compared with treatment with P (<8: HR = 0.50; 95% CI 0.4–0.62, P < 0.0001; ≥8: HR = 0.61; 95% CI 0.49–0.76, P < 0.0001) (Figure 2A). The subgroup of patients with Gleason score <8 who received AA plus P versus P had significant improvement in OS, while patients with Gleason score ≥8 showed a trend in improvement (<8: HR = 0.78; 95% CI 0.62–0.97, P = 0.0247; ≥8: HR = 0.82; 95% CI 0.67–1.01, P = 0.0603) (Figure 2B). The subgroups of chemotherapy-naïve patients with mCRPC, with Gleason score either <8 or ≥8, had significant improvement in TTPP with AA plus P treatment compared with treatment with P (<8: 11.1 versus 5.6 months, P < 0.0001; ≥8: 11.0 versus 6.5 months, P < 0.0001) (supplementary Figure S2B, available at Annals of Oncology online).
Figure 2.
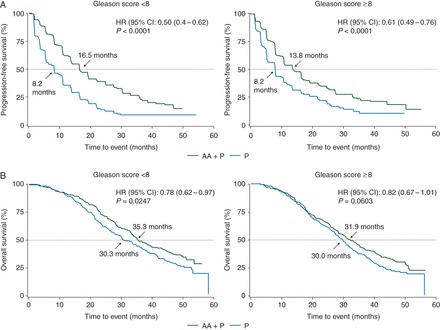
Radiographic progression-free survival (A) and overall survival (B) in chemotherapy-naïve metastatic castration-resistant prostate cancer patients treated with abiraterone acetate (AA) plus prednisone (P) or placebo plus P as a function of Gleason score (<8 and ≥8) at initial diagnosis. AA, abiraterone acetate; CI, confidence interval; HR, hazard ratio; P, prednisone.
The PSA response rate (≥50% decline in PSA from baseline) for patients with mCRPC post-docetaxel was greater in patients treated with AA plus P versus P regardless of Gleason score (<8: 34% versus 9%; ≥8: 26% versus 2%) (supplementary Table S2, available at Annals of Oncology online). In the subgroup of patients with measureable disease at baseline, the objective response defined according to Response Evaluation Criteria in Solid Tumors (RECIST) in this study was also greater with AA plus P versus P irrespective of Gleason score (<8: 17% versus 5%; ≥8: 19% versus 1%). The subgroup of mCRPC chemotherapy-naïve patients treated with AA plus P versus P had favourable PSA responses irrespective of Gleason score (<8: 64% versus 24%; ≥8: 59% versus 22%). Likewise, the objective response was better in the subgroup of patients treated with AA plus P versus P irrespective of Gleason score (<8: 44% versus 15%; ≥8: 42% versus 17%).
Exploratory multivariate analyses of OS adjusting for baseline prognostic factors including Gleason score as a co-variate was performed for both post-docetaxel and chemotherapy-naïve mCRPC patients (supplementary Table S3, available at Annals of Oncology online). Gleason score had prognostic value on OS in both post-docetaxel (HR = 1.17; 95% CI 1.01–1.37, P = 0.04) and chemotherapy-naïve (HR = 1.20; 1.03–1.39, P = 0.0221) mCRPC patients, although the determination of significance may be ascribed to the large sample sizes in the two cohorts and the level of significance was much less than that observed for the other common prognostic factors studied. Interaction tests for heterogeneity of treatment effect across Gleason score subgroups did not demonstrate a significant interaction effect (treatment × Gleason score) for OS in post-docetaxel and chemotherapy-naïve mCRPC patients (supplementary Table S3, available at Annals of Oncology online).
discussion
In this retrospective study of nearly 2000 patients with mCRPC who were either chemotherapy naïve or previously treated with docetaxel, we explored the predictive value of Gleason scores obtained at initial diagnosis on outcome after AA plus P therapy. In all cohorts assessed, a baseline Gleason score of <8 versus ≥8 was not predictive of treatment benefit of AA plus P versus P in post-docetaxel and chemotherapy-naïve patients with mCRPC; regardless of Gleason score, both groups benefited from treatment with AA plus P.
Treatment decisions for mCRPC are particularly challenging given the number of choices (e.g. chemotherapy, several androgen-signalling–targeted therapies, bone-targeted therapies and immunotherapy) along with a paucity of treatment decision recommendations based on prospective randomised studies. For mCRPC, recent models do not identify Gleason score as a prognostic indicator [5, 7, 8]. The usefulness of the Gleason score as a predictive factor for treatment efficacy in mCRPC has not been established for cabazitaxel [14] or ipilimumab [15].
Recent analyses of the pivotal clinical trials of AA plus P in mCRPC have retrospectively evaluated the impact of other patient and disease characteristics that could influence study outcomes. The results showed that mCRPC patients appear to benefit from AA plus P treatment regardless of the presence of visceral disease at baseline [16] or advanced age [17, 18]. Baseline serum androgens were not predictive factors for benefit from treatment with AA plus P [19]. Similarly, patients appear to benefit from AA plus P therapy regardless of favourable or unfavourable baseline circulating tumour cell counts, and regardless of the presence of TMPRSS2-ERG rearrangements [20, 21]. Preliminary data suggest that the presence of the androgen receptor splice variant-7, which lacks the ligand-binding domain required for abiraterone activity, may predict resistance to treatment with AA in patients with mCRPC, mostly in those pre-treated with enzalutamide [22].
The analyses presented here are important as they comprise large, well-defined study populations, but there are several caveats. These post hoc analyses were not powered to discern treatment benefit in Gleason subgroups, notwithstanding the remarkable change in the therapeutic landscape during the conduct of these two studies (COU-AA-301, COU-AA-302). Notably, patients with mCRPC post-docetaxel (COU-AA-301) were heavily pre-treated, and when the study was conducted patients had limited options for life-extending treatment. Yet, patients with higher Gleason score (≥8) benefitted from AA plus P therapy versus P. To our knowledge, study COU-AA-302 represents the longest treatment follow-up (>4 years) for chemotherapy-naïve mCRPC, with most receiving subsequent therapies; mCRPC patients are living longer with effective therapies, making comparisons to historical controls difficult. The COU-AA-302 final analysis confirmed that AA plus P OS clinical benefit was statistically significant in addition to previously established rPFS and TTPP benefits [11–13]. Thus, overall trends in OS, rPFS and TTPP provide compelling support for use of AA plus P regardless of Gleason score at initial diagnosis. It should be noted that Gleason score at initial diagnosis may not be a suitable predictive marker of the potential efficacy of AA in mCRPC patients, whose metastatic deposits may no longer be reflective of the histology at the time of diagnosis.
Another important consideration in the interpretation of the current results is that the Gleason score was not centrally recorded and reviewed but the system as defined has shown good inter-observer reproducibility and represents real world practice. The Gleason score definition has evolved over time and individual scores may have varied depending on when and where the patients were biopsied.
conclusion
We observed clinical benefit with AA plus P versus P in both post-docetaxel and chemotherapy-naïve mCRPC patients with a Gleason score of either <8 or ≥8 at initial diagnosis. Thus, the Gleason score at the time of diagnosis should not factor into the decision to prescribe or treat a patient with mCRPC with AA plus P.
funding
This work was supported by Janssen Research & Development (formerly Ortho Biotech Oncology Research & Development, unit of Cougar Biotechnology). Writing assistance was funded by Janssen Global Services, LLC (no grant number).
disclosure
KF has participated in advisory boards and has received honorarium from Janssen. TWF has a leadership role and holds stock options with Aurora Oncology, has served as a consultant and received honoraria from GTx and has received research funding from Novartis, Bavarian Nordic, Cougar Biotechnology, Dendreon, GlaxoSmithKline, GTx, Janssen Oncology, Medivation, Sanofi, Pfizer, Bristol-Myers Squibb, Amgen, Roche/Genentech and Exelixis. MS has no disclosure to report. HIS has served as an uncompensated consultant to Janssen Research & Development and reports support to Memorial Sloan Kettering Cancer Center from Janssen Research & Development related to this work. He has also served as an uncompensated consultant to AstraZeneca, Bristol-Myers Squibb, Celgene, Endocyte, Exelixis, Foundation Medicine, Genentech, Medivation, Novartis, Pfizer, Takeda-Millennium and Ventana—member of Roche; a consultant to Astellas, BIND Pharmaceuticals, Chugai Academy for Advanced Oncology, Endo/Orion Pharmaceuticals, OncologySTAT, Palmetto GBA, LLC, Sanofi Aventis and WCG Oncology; has received an honorarium from Chugai Academy for Advanced Science; and has received support for Memorial Sloan Kettering Cancer Center from BIND Pharmaceuticals, Epic Sciences, Exelixis and Medivation. JSB is a paid employee of The Institute of Cancer Research, which has a commercial interest in abiraterone, and has served as a paid consultant for Johnson & Johnson. DER has received research funding from Janssen Research & Development. CJR has received honoraria from Janssen Research & Development. TK, JL, MBT, TWG and AM are employees of Janssen Research & Development and hold stock options in Johnson & Johnson. CHO has served as a consultant and received honoraria and research funding from Janssen Cilag.
Supplementary Material
acknowledgements
Writing assistance was provided by Hajira Koeller and Ira Mills, of PAREXEL.
references
- 1. Lotan TL, Epstein JI. Clinical implications of changing definitions within the Gleason grading system. Nat Rev Urol 2010; 7: 136–142. [DOI] [PubMed] [Google Scholar]
- 2. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2005; 29: 1228–1242. [DOI] [PubMed] [Google Scholar]
- 3. Dong F, Wang C, Farris AB et al. . Impact on the clinical outcome of prostate cancer by the 2005 International Society of Urological Pathology modified Gleason grading system. Am J Surg Pathol 2012; 36: 838–843. [DOI] [PubMed] [Google Scholar]
- 4. Freedland SJ, Humphreys EB, Mangold LA et al. . Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294: 433–439. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong AJ, Tannock IF, de Wit R et al. . The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer 2010; 46: 517–525. [DOI] [PubMed] [Google Scholar]
- 6. Fizazi K, Massard C, Smith M et al. . Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol 2015; 68: 42–50. [DOI] [PubMed] [Google Scholar]
- 7. Halabi S, Lin CY, Small EJ et al. . Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst 2013; 105: 1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Halabi S, Lin CY, Kelly WK et al. . Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2014; 32: 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Bono JS, Logothetis CJ, Molina A et al. . Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fizazi K, Scher HI, Molina A et al. . Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13: 983–992. [DOI] [PubMed] [Google Scholar]
- 11. Rathkopf DE, Smith MR, de Bono JS et al. . Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 2014; 66: 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryan CJ, Smith MR, de Bono JS et al. . Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryan CJ, Smith MR, Fizazi K et al. . Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–160. [DOI] [PubMed] [Google Scholar]
- 14. Oudard S, Mercier M, Flechon A et al. . Efficacy of cabazitaxel and its relationship with predictors of poor response to second hormonal therapies (2d HT) in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2013; 31: 137. [Google Scholar]
- 15. Kwon ED, Drake CG, Scher HI et al. . Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodman OB Jr, Flaig TW, Molina A et al. . Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2014; 17: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulders PF, Molina A, Marberger M et al. . Efficacy and safety of abiraterone acetate in an elderly patient subgroup (aged 75 and older) with metastatic castration-resistant prostate cancer after docetaxel-based chemotherapy. Eur Urol 2014; 65: 875–883. [DOI] [PubMed] [Google Scholar]
- 18. Smith MR, Rathkopf DE, Mulders PF et al. . Efficacy and safety of abiraterone acetate in elderly (≥75 years) chemotherapy-naïve patients with metastatic castration-resistant prostate cancer. J Urol 2015; 194: 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryan CJ, Molina A, Li J et al. . Serum androgens as prognostic biomarkers in castration-resistant prostate cancer: results from an analysis of a randomized phase III trial. J Clin Oncol 2013; 31: 2791–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Attard G, de Bono JS, Logothetis CJ et al. . Improvements in radiographic progression-free survival stratified by ERG gene status in castration-resistant prostate cancer patients treated with abiraterone. Clin Cancer Res 2015; 21: 1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danila DC, Anand A, Sung CC et al. . TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. Eur Urol 2011; 60: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antonarakis ES, Lu C, Wang H et al. . AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014; 371: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


