Table of contents
Abbreviations and acronyms 191
- Introduction 191
- Health economic considerations 192
- Obesity 192
- General diet considerations 192
- Blood lipids and fish consumption 192
- What is the impact of blood lipids on risk of AF? 195
- Dietary fish consumption vs. studies with fish oil supplements 195
- Obstructive sleep apnoea 195
- Hypertension 197
- Diabetes mellitus 197
- Smoking 199
- Air pollution 201
- Caffeine 204
- Alcohol consumption 204
- Recreational drugs 205
- Medications 207
- Psychological distress 207
- Physical activity and inactivity 208
- Genetic predisposition 209
- Hyperthyroidism and other endocrine disorders 211
- Electrophysiological considerations 211
- Atrial premature beats triggering AF 211
- Supraventricular tachyarrhythmias causing AF 212
- Post-operative AF 213
- Upstream therapies to prevent AF 214
- Risk factors leading to AF development as risk factors for thromboembolic complications 214
- Patient values and preferences 218
Conclusions 218
References 219
Abbreviations and acronyms
- ACEI,
angiotensin converting enzyme inhibitors
- AF,
atrial fibrillation
- ARB,
angiotensin receptor blockers
- AVNRT,
atrioventricular nodal re-entry tachycardia
- BMI,
body mass index
- CHADS2,
cardiac failure, hypertension, age, diabetes, stroke (doubled)
- CHA2DS2-VASc,
congestive heart failure or left ventricular dysfunction, hypertension, age ≥75 (doubled), diabetes, stroke/transient ischaemic attack (doubled)-vascular disease, age 65–74, sex category (female)
- CI,
confidence interval
- FU,
follow-up
- HR,
hazard ratio
- HDL,
high-density lipoprotein cholesterol
- ICD,
implantable cardioverter defibrillators
- LA,
left atrium
- LDL,
low-density lipoprotein cholesterol
- LV,
left ventricle
- NOAC,
non-VKA oral anticoagulant
- OAC,
oral anticoagulation
- OR,
odds ratio
- OSA,
obstructive sleep apnoea
- n3-PUFA,
ω-3 polyunsaturated fatty acids
- RAAS,
renin–angiotensin–aldosterone system
- RR,
relative risk
- SBP,
systolic blood pressure
- SAMe-TT2R2,
sex (female), age (<60 years), medical history, treatment (interacting drugs, e.g. amiodarone for rhythm control), tobacco use (within 2 years) (doubled), Race (non-Caucasian) (doubled)
- SVT,
supraventricular tachyarrhythmia
- VKA,
vitamin K antagonist
Introduction
Atrial fibrillation (AF) is an important and highly prevalent arrhythmia, which is associated with significantly increased morbidity and mortality, including a four- to five-fold increased risk for stroke,1,2 a two-fold increased risk for dementia,3,4 a three-fold risk for heart failure,2 a two-fold increased risk for myocardial infarction,5,6 and a 40–90% increased risk for overall mortality.2,7 The constantly increasing number of AF patients and recognition of increased morbidity, mortality, impaired quality of life, safety issues, and side effects of rhythm control strategies with antiarrhythmic drugs, and high healthcare costs associated with AF have spurred numerous investigations to develop more effective treatments for AF and its complications.8 Although AF treatment has been studied extensively, AF prevention has received relatively little attention, while it has paramount importance in the prevention of morbidity and mortality, and complications associated with arrhythmia and its treatment. Current evidence shows a clear association between the presence of modifiable risk factors and the risk of developing AF.
By implementing AF risk reduction strategies aiming at risk factors such as obesity, hypertension, diabetes, and obstructive sleep apnoea (OSA), which are interrelated, we impact upon the escalating incidence of AF in the population and ultimately decrease the healthcare burden of associated co-morbidities of AF.
To address this issue, a Task Force was convened by the European Heart Rhythm Association and the European Association of Cardiovascular Prevention and Rehabilitation, endorsed by the Heart Rhythm Society and Asia-Pacific Heart Rhythm Society, with the remit to comprehensively review the published evidence available, to publish a joint consensus document on the prevention of AF, and to provide up-to-date consensus recommendations for use in clinical practice. In this document, our aim is to summarize the current evidence on the association of each modifiable risk factor with AF and the available data on the impact of possible interventions directed at these factors in preventing or reducing the burden of AF. While the evidence on AF prevention is still emerging, the topic is not fully covered in current guidelines and some aspects are still controversial. Therefore, there is a need to provide expert recommendations for professionals participating in the care of at-risk patients and populations, with respect to addressing risk factors and lifestyle modifications.
Health economic considerations
Atrial fibrillation is a costly disease, both in terms of direct, and indirect costs, the former being reported by cost of illness studies as per-patient annual costs in the range of US $2000–14 200 in North America and of €450–3000 in Europe.9
In individuals with AF or at risk of developing AF, any effective preventive measure, intervention on modifiable risk factors or co-morbidities, as well as any effective pharmacological or non-pharmacological treatment has the aim to reduce AF occurrence, thromboembolic events and stroke, morbidity and, possibly, mortality related to this arrhythmia. Apart from the clinical endpoints, achievement of these goals has economic significance, in terms of positive impact on direct and indirect costs and favourable cost–effectiveness at mid- or long-term, in the perspective of healthcare systems.10–12
In view of the epidemiological profile of AF and progressive aging of the population,13 an impressive increase of patients at risk of AF or affected by AF,14 also in an asymptomatic stage, is expected in the next decades, inducing a growing financial burden on healthcare systems, not only in Europe and North America, but also worldwide.15,16
In consideration of this emerging epidemiological threat due to AF, it is worth considering a paradigm shift, going beyond the conventional approach of primary prevention based on treatment of AF risk factors, but, instead, considering the potential for ‘primordial’ prevention, defined as prevention of the development of risk factors predisposing to AF in the first place.17 This approach, aimed at avoiding the emergence and penetration of risk factors into the population, has been proposed in general terms for the prevention of cardiovascular diseases17 and should imply combined efforts of policymakers, regulatory and social service agencies, providers, physicians, community leaders, and consumers, in an attempt to improve social and environmental conditions, as well as individual behaviours, in the pursuit of adopting healthy lifestyle choices.16 Since a substantial proportion of incident AF events can be attributable to elevated or borderline levels of risk factors for AF,18 this approach could be an effective way to reduce the financial burden linked to AF epidemiology. In terms of individual behaviour and adoption of a ‘healthy lifestyle’, it is worth considering that availability of full healthcare coverage (through health insurance or the healthcare system) may in some cases facilitate the unwanted risk of reducing, at an individual level, the motivation to adopt all the preventive measures that are advisable, in line with the complex concept of ‘moral hazard effect’.19 Patient education and patient empowerment are the correct strategies for avoiding this undesirable effect.
Obesity
Obesity is associated with the development of AF and has an important impact on AF-related clinical outcomes (Table 1).20–25 A strategy of weight control may reduce the increasing incidence of AF making it an important subject in the prevention of AF22,26,27 and long-term benefit for patients at risk for developing AF.28 The strongest evidence for adverse clinical outcomes comes from various large cohort studies (Table 1). The Framingham Heart Study23 revealed that obesity is an important predictor of development of AF in adults and demonstrated via echocardiographic data, that the relationship between body size and AF is mediated by left atrial enlargement and inflammation.29 A recent community-based study in the Netherlands confirmed that, in addition to the conventional risk factors for AF, body mass index (BMI) was strongly associated with AF with a 45% increased risk of AF with every five points of BMI increase.25 This study supports the notion that BMI should be regarded as a validated risk factor for incident AF.25 Indeed, obesity was the strongest contributor to incident AF in a number of studies, worldwide.20,21,25,30 In the Guangzhou Biobank Cohort Study, for example, both general and central obesity were associated with increased risk of AF in an Asian population with generally much lower levels of obesity compared with Western countries.21
Table 1.
Obesity and risk of AF in population cohorts. Incidences per total duration of follow-up
| Study | Design | Subjects | FU | BMI groups (kg/m2) | AF, % | Riska (95% CI) |
|---|---|---|---|---|---|---|
| Dublin et al.20 | Population based, case–control design |
1410 cases 2203 controls |
N/A | Obese: (BMI ≥30) | N/A | OR: 1.40 (1.15–1.71) |
| Long et al.21 | Nested case–control study |
5882 men 14 548 women |
N/A | Overweight (BMI 23 to <25) Obese (BMI ≥25) |
0.8 | Overweight: 1.18 (0.78–1.79), Obese: 1.47 (1.01–2.13) |
| Tedrow et al.22 Women's Health Study |
Prospective cohort study | 34 309 | 12.9 ± 1.9 yrs | Overweight (BMI 25 to <30) Obese (BMI ≥30) |
2.4 | Overweight: HR 1.22 (1.02 1.45) Obese: HR: 1.65 (1.36–2.00) |
| Wang et al.23 Framingham Heart Study |
Prospective cohort study |
5282 | 13.7 yrs | Normal (BMI 18.5 to <25) Overweight (BMI 25 to <30) Obese (BMI ≥30) |
10.0 | Obese: men 1.52 (1.09–2.13) women 1.46 (1.03–2.07) |
| Frost et al.24 | Prospective cohort study |
55 273 | 13.5 yrs | Underweight (BMI <18.5) Normal (BMI 18.5 to <25) Overweight (BMI 25 to <30) Obese (BMI ≥30) |
Men 3% (1669) Women 1.6% (912) |
1.29 (1.24–1.33) |
| Vermond et al.25 | Dutch community based cohort study | 8265 | 9.7 yrs | Continuous BMI | AF incidence 3.3 per 1000 person-year | BMI, per 5 kg/m2 HR: 1.45 (1.21–1.74) |
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; FU, follow-up; HR, hazard ratio; N/A, not available; OR, odds ratio; pts, patients; SD, standard deviation; yrs, years.
aHR per 1 sex-specific standard deviation (SD) or the adjusted HR for 1 sex-specific SD increment.
A large Danish prospective population-based cohort study,24 among 55 273 men and women aged 50–64 years of age at recruitment, also confirmed the association between obesity and incident AF. In addition, bioelectrical impedance derived measures of body composition and combinations of anthropometric measures of body fat distribution were associated with the increased risk of developing AF.24 Also, diabetes at baseline increased proportionally from 6.9% with a BMI <25 kg/m2 to 26% in those with a BMI >30 kg/m2.24 This is probably important since a meta-analysis has shown that patients with diabetes had an ∼40% greater risk of AF compared with those without diabetes.31
The potential implications of these findings are amplified by the fact that obesity has reached epidemic proportions worldwide.32 As both AF and obesity are increasing in low- and middle-income countries, the results should have significant public health implications. Importantly, obesity may contribute to the risk of AF-related complications. For example, another large cohort study from Denmark has shown that the combination of overweight and AF can increase the risk of stroke and death,33 demonstrating that being either overweight or obese increases the risk for ischaemic stroke, thromboembolism and death in patients with AF, even after adjustment for the CHADS2 and CHA2DS2–VASc risk scores. However, an obesity paradox exists. As an example, The Atrial Fibrillation Follow-up Investigation of Rhythm Management study, one of the largest multicentre trials of AF including 4060 patients, found that obese patients with AF appear to have better long-term outcomes than non-obese patients.34
A logical consequence of these studies is that overweight/obese patients should be informed that there is not only a risk for the commonly known consequences such as diabetes, hypertension, coronary artery disease, and heart failure, but also that there is a greater risk of developing AF and a subsequent risk of stroke and death.
General dietary considerations
There is currently a paucity of evidence on the effect of unhealthy or extreme weight-loss diets on the development of AF (Table 2),35–40 and therefore the association between specific dietary factors and AF is tenuous at this time. Only one study falls under this topic, by Al Suwaidi et al.42 which enrolled 465 outpatients who were fasting during the month of Ramadan. Of the ∼5% who had AF at enrolment, only one had to be hospital admitted. There were no reports on conversion to or from AF in other patients. All other studies refer to specific dietary habits or interventions,41 rather than to extreme diets. Other data are limited by virtue of selective reporting, multiple testing, and positive publication bias. Also, many studies are small, some are retrospective, and the effect sizes of dietary exposures are modest leading to potential residual confounding, especially since diet is inextricably linked with age, race, sex, socioeconomic status, etc.
Table 2.
Relation between diet and AF
| Study | Design | Subjects | FU | Intervention | AF risk (95% CI) | Comment |
|---|---|---|---|---|---|---|
| (a) Population cohorts | ||||||
| Shen et al.35 Framingham Heart Study |
Prospective | 4526 from original and off-spring cohort; participants without AF | 4 yrs | None | No association with alcohol, caffeine, fibre and fish-derived polyunsaturated fatty acids; limited attributable risk of AF>4 servings of dark fish/wk had HR 6.53 (2.65–16.06) vs. <1 serving |
Alcohol, caffeine, fibre, and fish-derived polyunsaturated fatty acids were not associated with AF risk |
| Khawaja et al.36 Physicians' Health Study |
Prospective | 21 054 men | 20 yrs (median 24 yrs) | None | - | No association between nut consumption and incident AF |
| Fretts et al.37 Cardiovascular Health Study |
Prospective | 4337 >65 years; no prevalent CHD or AF |
up to 19 yrs | None | - | No association between plasma phospholipid or dietary alpha linoleic acid and incident AF |
| Costanzo et al.38 | Prospective | 217; cardiac surgery | ICU stay +1 wk post-surgery unit | None | Highest tertile of dietary total antioxidant capacity vs. 2 lowest tertiles: OR 0.46 (0.22–0.95) |
Antioxidant-rich foods are associated with reduced incidence of post-operative AF |
| Mattioli et al.39 | Case–control | 800; 400 first detected AF episode | – | None | (a) OR 1.9 (1.58–2.81) (b) OR 1.8 (1.56–2.99) |
(a) Lower adherence to Mediterranean diet and lower antioxidant intake in patients with AF compared to control population; (b) Patients with arrhythmia who had higher Mediterranean score had higher probability of spontaneous conversion from AF to sinus rhythm |
| Pastori et al.40 | Prospective | 709 anticoagula-ted pts with AF | 39.9 months | None | – | Reduction in CV events; antioxidant effects such as down-regulation of NOX2 and decreased excretion of F2-isoprostanes |
| (b) Intervention studies | ||||||
| Martínez-González et al.41 PREDIMED- Prevención con Dieta Mediterránea |
Randomized primary prevention trial; post hoc analysis | 6705 | Median 4.7 yrs | Three diets: Mediterranean diet enriched with extra virgin olive oil, or mixed nuts; control group |
Mediterranean diet enriched with extra virgin olive oil vs. mixed nuts; HR 0.89 (0.65–1.2) Mediterranean diet enriched with extra virgin olive oil vs. control group: HR 0.62 (0.45–0.85) |
Mediterranean diet with olive oil reduced AF risk compared with control group; however, with no effect in a group with nuts Reduced incidence of stroke, myocardial infarction, and CV mortality; consumption of extra virgin olive oil but not nuts was associated with a lower risk of AF |
AF, atrial fibrillation; CHD, coronary heart disease; CI, confidence interval; CV, cardiovascular; FU, follow-up; HR, hazard ratio; ICU, intensive care unit; OR, odds ratio; pts, patients; wk, week; yrs, years.
Blood lipids and fish consumption
Among the modifiable risk factors that can be targeted for AF prevention, caloric intake, and physical activity are critical factors that significantly impact weight, blood pressure, risk of diabetes mellitus and atherosclerosis, and atrial structure/function.43
What is the impact of blood lipids on risk of AF?
Table 3A summarizes two recent cohort-based studies that evaluated the association of blood lipid components with the development of AF during follow-up.44,45 In both, with adjustments for age, sex, and race, but no adjustment for BMI, low levels of HDL cholesterol, and high levels of plasma triglycerides were associated with increased risk of AF. Low-density lipoprotein cholesterol levels (LDL) were not associated with AF risk in either study; elevated total cholesterol was associated with risk of AF in one study.44 Both studies note the impact of comorbid conditions confounding the association of blood lipid levels with AF risk. Thus, evidence for selectively targeting lower plasma LDL or total cholesterol as a means of reducing AF risk is weak.
Table 3.
Relationship of blood lipids, fish, and n-3 polyunsaturated fatty acids to incident AF risk per total duration of follow-up
| Study | Design | Subjects | FU, yrs | LDL/HDL, TG, TC levels | AF, n (%) | Risk HR (95% CI), P-value |
|---|---|---|---|---|---|---|
| (A) Blood lipids | ||||||
| Lopez et al.44 ARIC |
Community cohort study; baseline age: 45–64 yrs | 13 969 | 18.7 | HDL ≥60 mg/dL, vs. ≤40 mg/dL TC >240 mg/dL vs. <200 mg/dL TGs ≥200 mg/dL vs. ≤150 mg/dL LDL (not significant) |
1433 (10.25) | 0.63 (0.53–0.74)a, P < 0.0001 0.89 (0.77–1.02), P = 0.03 1.4 (1.21–1.62), P < 0.0001 |
| Alonso et al.45 MESA Framingham Heart Study |
Community cohorts; average baseline age 60.5 yrs (10) | 7142 | 9.6 | HDL ≥60 mg/dL, vs. ≤40 mg/dL TGs ≥200 mg/dL vs. ≤150 mg/dL TC, LDL not significant |
480 (6.7) | 0.64 (0.48–0.87) 1.6 (1.25, 2.05) |
| (B) Fish intake and plasma n-3 fatty acid levels | ||||||
| Gronroos et al.46 ARIC |
Community cohort study, baseline age 45–64 yrs |
14 222 | 17.6 | Intake of canned tuna/oily fish >2/week, vs. none Dietary DHA + EPA (Q4 vs. Q1) Plasma DHA + EPA (Q4 vs. Q1) Plasma DHA (Q4 vs. Q1) Plasma EPA (Q4 vs. Q1) |
1604 (11.3) | 0.86 (0.72–1.03), P = 0.09 0.95 (0.82–1.10)a, P = 0.42 0.79 (0.60, 1.03), P = 0.18 0.74 (0.57, 0.97), P = 0.10 1.12 (0.85, 1.49), P = 0.33 |
| Rix et al.47 Danish Diet, Cancer and Health cohort study |
Cohort study, baseline ages 50–64 yrs | 57 053 | 13.6 | Dietary intake: Q1 (<0.39 g/day) Q2 vs. Q1 Q3 vs. Q1 Q4 vs. Q1 Q5 vs. Q1 |
3345 (5.9) | 1 0.92 (0.82–1.03), P = 0.16 0.87 (0.78–0.98), P = 0.02 0.96 (0.86–1.08), P = 0.49 1.05 (0.93–1.18), P = 0.42 |
| Rix et al.48 Danish Diet, Cancer and Health cohort study |
Cohort study, baseline ages 50–64 yrs | 3440 with adipose tissue specimens | 13.6 | Total adipose n3-PUFA T2 vs. T1 T3 vs. T1 Adipose DHA T2 vs. T1 T3 vs. T1 Adipose EPA T2 vs. T1 T3 vs. T1 |
179 (5.2) | 0.87 (0.60–1.24) 0.77 (0.53–1.1) 1.03 (0.73–1.46) 0.73 (0.5–1.06) 0.67 (0.46–0.99) 0.86 (0.61–1.22) |
| Virtanen et al.49 Kuopio Ischemic Heart Disease Risk Factor Study |
Cohort study, baseline ages 42–60 yrs | 1941 with serum specimens | 17.7 | Plasma DHA + EPA + DPA Q2 vs. Q1 Q3 vs. Q1 Q4 vs. Q1 Plasma DHA (Q4 vs. Q1) Plasma EPA (Q4 vs. Q1) |
240 (11.0) | 0.65 (0.46–0.93) 0.82 (0.58–1.14) 0.65 (0.46–0.93) 0.64 (0.45–0.92) 0.93 (0.0.65–1.33) |
AF, atrial fibrillation; CI, confidence interval; DHA, docosahexaenoic acid; FU, follow-up; HDL, high-density lipoprotein cholesterol; HR, hazard ratio; EPA, eicosapentaenoic acid; LDL, low-density lipoprotein cholesterol; n3-PUFA, ω-3 polyunsaturated fatty acids; Q, quartile; T, tertile; TC, total cholesterol; TG, triglyceride; yrs, years.
acorrected only for age, sex, race.
Despite the uncertain association of lipids with incident AF, there is evidence that statins protect against AF in patients with chronic stable coronary artery disease, independently of reductions in plasma total cholesterol level.50 In experimental studies, statin use protected against electrical remodelling associated with atrial tachycardia pacing51 and decreased AF inducibility in a canine model of sterile pericarditis.52 Recent meta-analyses suggest that statins reduce new onset AF following cardiac surgery, a setting in which inflammatory processes are strongly implicated in AF onset.53,54 In contrast to the post-surgical setting, large meta-analyses have not demonstrated the efficacy of statins for the primary prevention of AF, whilst a heterogeneous benefit is reported for secondary AF prevention.55,56 Statins, which impact oxidant and inflammatory mechanisms in addition to lowering plasma LDL levels, most likely attenuate AF risk primarily due to effects independent of LDL reduction.
In recognition of this ‘uncoupling’, recent ACC/AHA guidelines for the prevention of coronary heart disease have changed from a primary focus on specific LDL target levels to one that focuses on the overall risk factor profile of the patient.57 A similar logic may apply to AF prevention as well.
Dietary fish consumption vs. studies with fish oil supplements
Older epidemiological studies have suggested that consumption of fatty fish is associated with significant health benefits, including reduced risk of AF.58 One recent study in the USA (Table 3B) noted a non-significant trend for a lower incidence of AF with higher intake of fatty fish (P = 0.09).46 Fish oil is enriched in ω-3 polyunsaturated fatty acids (ω3-PUFA), especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA). The Kuopio study found a trend for the highest vs. lowest quartile of plasma EPA + DHA + DPA to be associated with lower risk of AF (P = 0.07). This relationship was modestly significant for DHA (P = 0.02).49 A retrospective analysis of a large Danish cohort (n = 55 246), which was a population with high fish consumption, suggests that the relationship between fish consumption and AF risk is more complex and U-shaped, with both low- and high-levels of either fatty fish consumption or consumption of the individual ω-3-fatty acids associated with increased risk of AF.47 Also, in the Danish population (Table 3B), analysis of adipose DHA and EPA content identified non-significant trends for benefit with elevated levels of both DHA and EPA.48 An obvious and important confounding factor is the individual burden of adiposity.
While fish oil extracts have demonstrated significant effects on the development of atrial fibrosis in the setting of experimental heart failure,59 and on the inducibility of AF after experimental cardiac surgery,60 highly purified n3-PUFA supplements, often formulated as ethyl esters, have demonstrated either poor or no efficacy in randomized clinical trials for the prevention of new onset AF following cardiac surgery,61 or for the prevention of AF recurrence.62,63 It remains unclear if the lack of efficacy is related to differences in bioavailability,64 to loss of other components in fish that are functionally important, or to intrinsic differences between studies in younger experimental animals and those in older patients at greatest risk of AF. At present, there is no compelling argument for the use of commercially available fish oil supplements for either primary or secondary AF prevention.65,66
On the basis of the available epidemiological studies, the current AHA/ACC guidelines for individuals with elevated blood LDL levels now recommends consumption of a diet ‘that emphasizes intake of vegetables, fruits, and whole grains; includes low-fat dairy products, poultry, fish, legumes, non-tropical vegetable oils, and nuts; and limits intake of sweets, sugar-sweetened beverages, and red meats’.66
While quite reasonable, this and other similar guidelines do not specifically address diet in relation to AF risk. Lacking direct evidence, the above dietary suggestions coupled with an emphasis on physical activity and maintenance of a healthy lifestyle and weight seem reasonable as interim guidance for AF patients, and for those with significant risk of AF.
Obstructive sleep apnoea
Sleep related breathing disorders are common and ∼25% of adults are at risk for sleep apnoea of some degree,67 with OSA commonly seen in patients with cardiovascular diseases, especially in obese patients and those with Type 2 diabetes mellitus.68 Various studies have established that patients with OSA, particularly those with more severe disease, are significantly more likely to develop AF, and patients with AF have about twice the risk for developing OSA (Table 4).69,70
Table 4.
Incident risk of AF in obstructive sleep apnoea per total duration of follow-up
| Study | Design | Subjects | FU, yrs | OSA, n (%) | AF, % | Risk (95% CI) |
|---|---|---|---|---|---|---|
| Gami et al.69 | Olmsted County cohort study | 3542 | 4.7 | 2626 (74) | 14.0 | HR 2.18 (1.34–3.54) |
| Cadby et al.70 | Sleep-clinic cohort study | 6841 | 11.9 | 100% | 6.7 | HR 1.55 (1.21–2.00) |
AF, atrial fibrillation; CI, confidence interval; FU, follow-up; HR, hazard ratio; OSA, obstructive sleep apnoea; pts, patients; yrs, years.
Patients with AF and those with OSA share several similar characteristics. For example, hypertension is common (one-third of OSA) in both conditions, and both occur more frequently in men and increase with advancing age.68 Furthermore, increasing BMI plays an important role in the development of both OSA and AF.28,71
The mechanisms for this may be multifactorial, but autonomic dysregulation may connect sleep apnoea and AF, independent of other known risk factors. This has been confirmed experimentally in dogs72 and clinically.73 In a prospective cohort study,73 a relationship among the severity of sleep apnoea syndrome, cardiac arrhythmias, and autonomic imbalance was demonstrated.
These observations may have important clinical implications, and large observational studies suggest that OSA may be a modifiable risk factor for recurrent AF after cardioversion or ablation.74,75 Furthermore, some data support a role for continued positive airway pressure (CPAP) therapy in abolishing nocturnal ventricular asystole and improving other arrhythmias in patients with OSA.76–79 CPAP therapy was effective in several other studies,80–83 but not in heart failure patients.84
Based on the evidence, routine screening for OSA and other sleep-related breathing disorders in general practice and in cardiac rehabilitation programmes may be considered if clinically indicated. More data are needed to show the benefit of prevention and the treatment of OSA and associated improvement of AF incidence, recurrence rate and outcomes in patients with new onset or recurrent AF.
Hypertension
Hypertension is a major risk factor for AF (Table 5). In the Framingham Heart Study,85 the odds ratios for the development of AF in men and women with hypertension were 1.5 and 1.4, respectively. Data from the Atherosclerotic Risk in Communities Study18 show that approximately one-fifth of the risk of developing AF was attributable to hypertension. The optimal systolic blood pressure appears to be 120–130 mmHg with both higher and lower blood pressures associated with an increased incidence of AF.25,86,93
Table 5.
Hypertension and risk of AF
| Study | Design | Subjects | FU | BP levels, mmHg/treatment | AF | Risk (95% CI) |
|---|---|---|---|---|---|---|
| AF incidence trials | ||||||
| Benjamin et al.85 Framingham Heart Study |
Cohort | 2090 men 2641 women |
38 yrs | SBP >160 DBP >95 |
OR for AF Men 1.5 (1.2–2.0) Women 1.4 (1.1–1.8) |
|
| Huxley et al.18 ARIC Study |
Cohort | 14 598 | 17.1 yrs | SBP >140 DBP >90 |
21.6% (16.8–26.7) of risk of AF is attributable to HT | |
| Thomas et al.86 | Case–control | 433 pts with AF 899 controls |
20 yrs (median) |
SBP <120 120–129 130–139 140–149 150–159 160–169 >170 |
OR 1.99 (1.10–3.62) Reference 1.19 (0.78–1.81) 1.40 (0.93–2.09) 2.02 (1.30–3.15) 2.27 (1.31–3.93) 1.84 (0.89–3.80) |
|
| Vermond et al.25 | Dutch community-based cohort study | 8265 | 9.7 yrs | Per 10 mm SBP | AF incidence 3.3 per 1000 person-year | SBP, per 10 mmHg HR 1.11 (1.01–1.22) |
| Intervention trials | ||||||
| Primary prevention | ||||||
| Wachtell et al.87 LIFE Study |
Randomized, double blind comparison of losartan vs. atenolol | Losartan 4298 Atenolol 4182 |
4.8 yrs (mean) |
Losartan Atenolol |
New AF 150 New AF 221 |
RR 0.67 (0.55–0.83) |
| Marott er al.88 | Registry analysis: comparison of AF incidence in pts with HT treated with ACEI and ARB compared with BB, diuretics and CCB | 725 680 Danish pts treated with anti-HT monotherapy |
5.9–6.8 yrs depending on comparison | ACEI vs. BB ARB vs. BB ACEI vs. diuretic ARB vs. diuretic ACEI vs. CCB ARB vs. CCB |
0.12 (0.10–0.15) 0.10 (0.07–0.14) 0.51 (0.44–0.59) 0.43 (0.32–0.58) 0.97(0.81–1.16) 0.78 (0.56–1.08) |
|
| Okin et al.89 | Analysis of the effect of BP reduction using losartan or atenolol (randomly assigned) on the risk of new AF | 8831patients with HT, ECG evidence of LVH and no history of AF | 4.6 yrs | SBP <130 SBP 131–141 SBP >142 |
Overall new AF in 701 pts (7.9%) | Compared with SBP >142, SBP <130 is associated with 40% lower risk of AF (18–55%). Compared with SBP >131–141, SBP <130 is associated with 24% lower risk of AF (7–38%) |
| Secondary prevention | ||||||
| GISSI-AF90 | Randomized double blind comparison of valsartan vs. placebo for prevention of recurrent AF | 1442 pts Valsartan 722 Placebo 720 |
1 yr | Valsartan Placebo |
Recurrent AF 371 (51.4%) Recurrent AF 375 (52.1%) |
HR 0.97 (0.83–1.14) |
| ANTIPAF91 | Randomized double blind comparison of olmesartan vs. placebo for prevention of recurrent AF burden | 425 pts w/o structural heart disease; ∼49% with htn | 12 months | Olmesartan Placebo |
% of AF days 15.1% % of AF days 14.7% |
No difference (P = 0.77) |
| Lip et al.92 | Retrospective longitudinal analysis of participants in SPORTIF III and V trials. Comparison of clinical event rates and mortality in ACEI and ARB users compared with non-users in an anti-coagulated AF population | 4760 ACEI or ARB users 2569 ACEI or ARB non-users |
18.7 months ACEI ARB users 18.4 months ACEI ARB non-users |
ACEI-ARB users ACEI-ARB non-users |
No difference in stroke, systemic embolic event, or mortality in ACEI, ARB users compared with non-users in the entire cohort For age >75 years lower mortality in ACEI or ARB users compared with non-users: HR 0.71 (0.52–0.95) |
|
ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BB, β-blocker; BP, blood pressure; CCB, calcium channel blocker; CI, confidence interval; DBP, diastolic blood pressure; FU, follow-up; HR, hazard ratio; HT, hypertension; OR, odds ratio; pts, patients; RR, relative risk; SBP, systolic blood pressure; yrs, years.
Proposed mechanisms include sympathetic activation, activation of the renin–angiotensin–aldosterone system, atrial dilation, fibrosis, and left ventricular remodelling including diastolic dysfunction and left ventricular hypertrophy.43 Hypertension may also lead to coronary disease and myocardial infarction, subsequently increasing the risk for AF. Alcohol consumption is also a common predisposing factor to both AF and hypertension.
For the primary prevention of AF in a hypertensive population, the optimal on-treatment systolic BP goal appears to be <130 mmHg.89 Nevertheless, it remains unclear whether different antihypertensive medications affect the development of AF independent of blood pressure reduction. In the Losartan Intervention for End Point Reduction in Hypertension Study,87 for example, new onset AF occurred less frequently in patients treated with losartan compared with patients treated with atenolol, although blood pressure reduction was similar in both groups. In another study,88 ACE inhibitors and angiotensin II-receptor blocker (ARB) were superior to β-blockers and diuretics for the primary prevention of AF. These two studies suggest that the inhibition of the renin-angiotensin system may be associated with a decreased risk of new onset AF, incremental to the effect of BP reduction alone.
ARB therapy has also been studied for the secondary prevention of AF. For example, the GISSI-AF study90 evaluated the secondary prevention of AF using valsartan, but was not superior to placebo. Follow-up was only for 1 year and it remains possible that the beneficial effects of ARBs on atrial remodelling might be seen with a longer study duration.94 In the ANTIPAF trial,91 olmesartan did not decrease AF burden compared with placebo in patients without structural heart disease.
Additionally, Lip et al.,92 retrospectively analysing data from the SPORTIF III and SPORTIF V trials, found that ACEI and ARBs did not result in any difference in stroke or systemic embolism in a controlled, anticoagulated AF population. Mortality was lower in the AF population over 75 years of age treated with ACEI or ARBs.
The role of aldosterone antagonists in the treatment of AF has been evaluated in the setting of heart failure,95 but not in its absence. Given the increasing incidence of AF, additional well-conducted studies are needed to clarify the impact of renin–angiotensin–aldosterone system (RAAS) inhibitors on both the primary and secondary prevention of AF.8,96
Diabetes mellitus
Diabetes and elevated blood glucose have been recognized for several years as potential risk factors for AF, although there are conflicting results97 (Table 6). Multiple studies31,85,98–104 report an increased incidence of AF in patients with diabetes. However, there are methodological differences that make comparisons among studies difficult. In particular, some studies adjusted the results for confounding variables including obesity and hypertension, while others did not. When these other risk factors were considered, the risk attributable to the development of AF from diabetes was limited. In a meta-analysis of 7 cohort studies and 4 case–control studies including more than 1 600 000 subjects, Huxley et al.31 found that patients with diabetes had a 39% greater risk of developing AF compared with individuals without diabetes. In studies that adjusted the risk for confounding variables, the relative risk decreased to 1.24 (95% CI 1.06–1.44).
Table 6.
Diabetes and risk of AF
| Study | Design | Subjects | FU | FBG or HbA1c levels/DM duration | AF | Risk (95% CI) |
|---|---|---|---|---|---|---|
| Incidence | ||||||
| Benjamin et al.85 Framingham Heart Study |
Cohort | 2090 men 2641 women |
38 yrs | FBG >140 mg/dL Non-fasting BG >200 mg/dL |
OR for AF Men 1.4 (1.0–2.0) Women 1.6 (1.1–2.2) After adjustment for valve disease Men 1.1 (0.8–1.7) Women 1.5 (1.0–2.3) |
|
| Alonso et al.98 | Meta-analysis of 3 cohorts: ARIC, CVH and FHS | 18 556 pts | HR 1.27 (1.10, 1.46) for 5-year AF risk in pts with DM | |||
| Huxley et al.99 ARIC Study |
Cohort | 13 025 | 14.5 yrs | FBG >126 mg/dL or HbA1c >6.5% or use of diabetic meds | Diabetes is associated with increased incidence of AF: HR 1.35 (1.14–1.60) HbA1c levels are independently associated with AF: HR 1.13 (1.01–1.20) per 1% increase in HbA1c level |
|
| Ostgren et al.100 | Cohort | 171 HT + DM 147 DM only 597 HT only 825 no HT or DM |
FBG >6.6 mmol/L or 2 hr glucose after oral glucose tolerance test >11.0 mmol/L |
HT + DM: OR 3.3 (1.6–6.7) DM only: OR 2.0 (0.9–4.7) HT only: OR 0.7 (0.3–1.5) Reference no HT or DM: ORR 1.0 |
||
| Pfister et al.101 | Analysis of development of new AF in the PROactive trial | 5233 pt with DM | 36 months | Incidence of new AF at: 12 months—0.8% 24 months—1.5% 36months—2.4% |
||
| Schoen et al.102 Womens Health Study |
Cohort | 34 720 women health professionals | 16.4 yrs | At baseline 937 (2.75%) had DM | Compared with women without DM, women with DM had HR for new AF of 1.95 (1.49–2.56). In models that adjusted for HT, obesity (BMI) and inter-current cardiovascular events, HR for new AF decreased to 1.14 (0.93–1.40) |
|
| Dublin et al.103 | Case–control | 1410 new AF pts 2203 control pts |
21 yrs—AF pts 20 yrs—control pts |
HbA1c <7% HbA1c 7–8% HbA1c 8–9% HbA1c >9% |
252 (17.9%) AF pts had DM 311 (14.1%) control pts had DM |
OR for AF 1.40 (1.15–1.71) for pts with DM compared with those without DM Compared with pts without DM risk (OR): 1.06 (0.74–1.51) 1.48 (1.09–2.01) 1.46 (1.02–2.08) 1.96 (1.22–3.14) |
| Aksnes et al.104 VALUE Trial |
Prospective randomized trial comparing valsartan and amlodipine for treatment of htn | 15 245 total pts with htn 5250 diabetes at baseline 1298 developed diabetes during FU |
4.2 yrs | FBG >140 mg/dL | 551 pts developed AF during the trial | HR 1.49 (1.14, 1.94) new onset diabetes for development of AF HR 1.19 (0.99, 1.42) baseline diabetes for development of AF |
| Huxley et al.31 | Meta-analysis of cohort (7) and case control (4) studies | 1 686 097 subjects combined allstudies | RR of pts with DM for AF: 1.39 (1.10–1.75) Studies with adjustment for other risk factors, RR of pts with DM for AF: 1.24 (1.06–1.44) |
|||
| Intervention trials | ||||||
| Chang et al.105 | Registry | 645 710 pts with diabetes | 13 yrs | 9983 pts developed AF, incidence rate 1.5% (287/100 000 person/yrs) | Metformin use protected against the development of AF, HR 0.81 (0.76–0.86) |
|
| Overvad et al.106 | Registry | 137 222 pts with AF | No DM 120 204 DM 0–4 yrs 7922 DM 5–9 yrs 4781 DM 10–14 yrs 2435 DM >15 yrs 1880 |
Risk of thromboembolism or death No DM reference 1.0 HR 1.24 (1.20–1.29) HR 1.42 (1.37–1.48) HR 1.45 (1.37–1.53) HR 1.72 (1.62–1.82) |
||
ARIC, Atherosclerotic Risk in Communities; CVH, Cardiovascular Health Study; FHS, Framingham Heart Study; VALUE, Valsartan Anti-hypertensive Long-term Use Evaluation Trial; AF, atrial fibrillation; BG, blood glucose; BMI, body mass index; DM, diabetes mellitus; FBG, fasting blood glucose; FU, follow-up; HbA1c, glycated haemoglobin; HR, hazard ratio; HT, hypertension; OR, odds ratio; pts, patients; yrs, years.
Using a population based, case–control design, Dublin et al.103 found that patients with longer durations of diabetes had a greater risk of AF development. Specifically, the risk of AF was 3% higher for each year of diabetes treatment, and the risk of AF correlated with worsened glycemic control. Hence, better glycemic control (as measured by haemoglobin A1c) was associated with a lower risk of AF development. High basal haemoglobin A1c level, increased BMI and advanced age were also associated with higher recurrence of AF after catheter ablation in patients with diabetes.107
Recently, investigators using the Taiwan National Health Insurance Research Database developed a time-dependent Cox proportional hazard model to study the effects of metformin on the development of AF.105 The study population included 645 710 patients with diabetes taking metformin but not other diabetic medications. Over a 13-year follow-up, fewer patients taking metformin developed AF, suggesting that metformin had a protective effect on the development of AF in diabetic patients.
Additionally, the duration of diabetes appears to be related to a higher risk of thromboembolic events in patients with AF. Using data from multiple Danish registries, Overvad et al.106 identified 13 722 patients with AF, 12.4% of whom had diabetes. Compared with AF patients without diabetes, thromboembolism was more prevalent and this relationship was time-dependent with longer diabetes duration being associated with higher rates of thromboembolism and death. A longer diabetes duration was not associated with an increased risk of bleeding among AF patients treated with vitamin K antagonists.
In summary, diabetes appears to confer an increased risk for the development of AF, but this risk seems less than for other factors including hypertension, obesity, and smoking.18 Furthermore, a longer diabetes duration and worse glycemic control increases the risk for AF and its complications, and in one retrospective study,105 treatment with metformin appeared to reduce this risk.
Smoking
Smoking is reported to predict incident AF in individuals of European,98,108–111 African,108,112 and Japanese113 ancestry (Table 7). Risks of developing incident AF with smoking are similar in men and women,98,108–114 and in blacks and whites.108 Multivariable risk prediction models for AF indicate that compared with non-smokers, both current,109,110 and ever smokers110 have a higher risk of incident AF. Current smoking was responsible for ∼10% of the variability in AF risk.18 Some data also suggest a dose–response relationship, with the highest risk of AF observed in individuals with the greatest cigarette-years of smoking108 and current smokers with increasing number of cigarettes per day.114 However, not all studies have reported an adjusted association between smoking and AF,2,30,115–119 but the lack of association has been ascribed to several factors including modest numbers of cases of AF, combining current, and former smokers,122 adjusting for factors along the causal pathway such as myocardial infarction, heart failure, and lung disease114 and competing risks of death among smokers.108,122
Table 7.
Smoking and risk of AF
| Study | Design | Subjects | FU | Tobacco | AF, % |
Multivariable Risk (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| (a) Population cohorts | ||||||||
| Alonso et al.98 CHARGE-AF Study |
Meta-analysis 3 cohorts, replication 2 cohorts |
18 556 B and W; 1186 incident AF 7672 W; 585 incident AF |
5 yrs | Current smoking | HR 1.44 (1.20–1.72) | |||
| Chamberlain et al.108 ARIC |
Cohort Incident AF |
15 329 B and W 876 incident AF |
Mean 13.1 yrs |
Smoking status Never Ever Former Current Cigarette-years. 0 ≤300 >300 to ≤675 >675 Continued vs. quit smoking |
Age-sex adjust. incidence rate/10 000 py 28 41 36 48 28 28 41 55 |
Reference 1.58 (1.35–1.85) 1.32 (1.10–1.57 ) 2.05 (1.71–2.47) Reference 1.04 (0.83–1.30) 1.60 (1.30–1.95) 2.10 (1.74–2.53) 0.88 (0.65–1.17) |
||
| Pfister et al.109 EPIC Norfolk |
Cohort Incident AF |
24 020 W 236 incident hospitalized AF |
5 yrs | Current smoking Incident AF No Incident AF Yes |
11.6% 14.0% |
1.86 (1.28–2.69) Observed in EPIC cohort free of CVD, HT, DM: HR 2.03 (1.26, 3.27) |
||
| Friberg et al.110 Copenhagen City Heart Study |
Cohort Incident AF |
10 955 W 379 incident hospitalized AF |
7 yrs | Never smokers Current smoking Current or ex |
NA | Multivariable-adjusted Reference 2.0 (1.4–2.8) 1.8 (1.3–2.5) |
||
| Everett et al.111 Women's Health Study |
Cohort Incident AF |
20 822 mostly W women 616 incident AF |
Median 14.5 yrs | Never Ever smoker |
NA | Multivariable-adjusted Reference 1.29 (1.06–1.57) P = 0.01 |
||
| Rodriguez et al.112 Multi-Ethnic Study of Atherosclerosis |
Cohort Incident AF |
6721 Multi-ethnic 305 incident AF |
Mean 6.98 yrs | All races Never Former Current Chinese Hispanics Non-Hispanic B Non-Hispanic W |
AFb 42.9% 46.2% 10.9% NA |
No AFb 50.7% 36.1% 13.2% NA |
Age- and sex-adjusted population attributable fraction current smoking −0.7 (−17.7 to 46.9) −0.9 (−21.1 to 15.8) 27.0 (5.8 to 43.5) 6.9 (−1.3 to 14.4) |
|
| Heeringa et al.114 Rotterdam Study |
Cohort Incident AF |
5668 W 371 incident AF |
Median 7.2 yrs | Never smoker Current Former |
78/1280 160/2159 |
Multivariable adjusted 1.51 (1.07–2.12) 1.48 (1.12–1.96) |
||
| Huxley et al.18 Atherosclerosis Risk in Communities |
Cohort Incident AF |
14 598 B and W 1520 incident AF |
Mean 17.1 yrs |
Never Former Current |
Incidence rate/1000 py 4.23 5.76 7.45 |
Population attributable fraction 0 2.06 (−2.05 to 6.05) 9.78 (6.74 to 12.9) |
Relative hazard—adjusted Note reference is current smokers 0.55 (0.48–0.62) 0.60 (0.52–0.68) Reference |
|
| Schnabel et al.115 Framingham Heart Study |
Cohort Incident AF |
4764 W 457 incident AF |
Max 10 yrs |
Current | NA | Age- and sex-adjusted 1.08 (0.88–1.33) P = 0.47 Not included in multivariable risk prediction instrument |
||
| Psaty et al.116 Cardiovascular Health Study |
Cohort Incident AF |
4844 B and W 304 incident AF |
Mean 3.28 yrs |
Current smoking | NA | Did not enter multivariable model | ||
| Frost et al.117 Danish Diet, Cancer, and Health Study |
Cohort Incident AF |
47 589 W 553 incident AF |
Mean 5.7 yrs |
Never—Reference Former Current |
NA | Men 0.80 (0.62–1.04) 0.83 (0.64–1.07) |
Women 0.94 (0.65–1.36) 0.95 (0.66–1.35) |
|
| Wilhelmsen et al.118 Multifactor Primary Prevention Study, Göteborg |
Cohort Incident hospitalized AF |
7495 W Men 754 incident AF |
Mean 25.2 yrs |
Never + ex-smoker 1–14 cig/day >15 cig/day |
10.6 9.1 11.8 |
Referencea age-adjusted 0.83 (0.71–0.97) 1.16 (0.73–1.86) |
||
| Nyrnes et al.30 Tromsø study |
Cohort Incident AF |
22 815 W 822 incident AF |
Mean 11.1 yrs |
Current smoking No AF AF |
Men 37.1% 24.3% |
Women 36.7% 22.7% |
Not included in multivariable model | |
| Stewart et al.119 Renfrew/Paisley study |
Cohort Prevalent AF Incident AF |
15 406 W 100 prevalent AF 537 incident of 8532 in f/u |
20 yrs | Current or former Prevalent AF No AF (n = 15 306) AF (n = 100) |
Mena 81.2% 79.0% |
Womena 54.1% 65.8% |
aAge-adjusted prevalence Not significantly associated in age-adjusted analyses; not selected for inclusion in multivariable analyses for prevalent or incident AF |
|
| Hergens et al.120 Swedish cohort studies |
7 Cohort studies Incident AF |
127 907 W men never smoker 3494 incident AF |
Prevalence of Snus use 25% | Adjusted for age and BMI 1.07 (0.97–1.19) |
||||
| (b) Hospital-based | ||||||||
| Suzuki et al.113 Shinken database |
New patients attending Cardiovascular Institute Incident AF |
15 221 Japanese 190 incident AF |
Mean 2 yrs Max 8.1 yrs |
Nonsmokers Smokers Former Current Brinkman index ≥800 |
5.0/1000 py 9.0/1000 py 8.6/1000 py 9.8 /1000 py 10.6/1000 py |
Reference, adjusted analyses 1.47 (1.09–2.00) 1.33 (0.94–1.89) 1.81(1.17–2.79) 1.69 (1.05–2.70) |
||
| (c) Internet-based survey | ||||||||
| Dixit et al.121 Health eHeart Study |
Self-referred internet self-report Prevalent AF |
4976 ∼80% W 593 prevalent AF |
Cross-sectional | Never Past Current |
AF 52.7% 43.6% 3.8% |
No AF 66.5% 29.5% 4.0% |
Unadjusted P-value, P < 0.001 |
|
| Median yrs smoked, past and current smokers | 18 | 12 | Unadjusted P-value P < 0.001 |
|||||
| Secondhand smoke Smoking parent during gestation Residing with smoker |
AF 68% 39% |
No AF 51% 26% |
Multivariable adjustment OR 1.37 (1.08–1.73) P = 0.009 OR 1.40(1.10–1.79) P = 0.007 |
|||||
AF, atrial fibrillation; B, Black; BMI, body mass index; CI, confidence interval; cig., cigarette; CVD, cardiovascular disease; DM, diabetes mellitus; FU, follow-up; HR, hazard ratio; HT, hypertension; NA, not available; OR, odds ratio; pts, patients; py, person years; W, White; yrs, years.
aAF incidence not depicted by smoking status.
bPersonal communication Carlos J. Rodriguez, MD, MPH 10/26/2015.
Whether other forms of tobacco exposure are associated with AF is more equivocal. One case report of an elderly woman with several comorbidities suggests a possible temporal relation between electronic cigarettes and paroxysms of AF.123 To our knowledge, there is no published research linking electronic cigarettes with AF. Similarly, there are no prospective data regarding the relation of second-hand smoke to AF. However, one recent retrospective study suggested that being exposed to second-hand smoke gestationally or living with a smoker during childhood were associated with an increased risk of AF as an adult.121 In another study, AF risk was associated with the environmental tobacco use.124 There have also been case reports of AF associated with chewing nicotine gum.125–127 In contrast, a pooled analysis of Swedish studies found current use of snus, a powdered smokeless tobacco product, was not significantly associated with incident AF (RR, 1.07; 0.97–1.19).120 Whether nicotine per se, or other chemicals associated with smoking are responsible for the increased risk of AF is uncertain.
Both experimental and human studies support multiple mechanisms linking smoking to AF. Nicotine and cigarettes predispose to inflammation,128 atrial electrical alterations,129,130 atrial fibrosis,131–133 reduced lung function,134,135 myocardial infarction,108 and heart failure,108 all of which predispose to AF. Smoking also may be a marker of deprivation and unhealthy lifestyle.136,137 An inverse association between socioeconomic status and incident AF has been reported, which is partially mediated by other risk factors.138,139
In individuals with AF, most studies examining the risk of events such as stroke, dementia, heart failure, myocardial infarction,5,6 and death have included smoking as a covariate, but have not specifically identified risk factors for events.140 Smoking was not a risk factor for incident heart failure in individuals with AF.141,142 Neither the CHADS2 nor the CHA2DS2–VASc scores include smoking as a risk factor for stroke. However, smoking is a risk factor for stroke in AF, even accounting for coexisting risk factors,143,144 but this relationship was not evident in one study.145 Smoking has also been reported to predict an increased risk for intracranial bleeding, mortality,144,146 and the combined outcome of stroke or death145 in people with AF.
Although there are no randomized trials proving that smoking cessation reduces the risk of AF, the preponderance of evidence supports efforts to encourage individuals to avoid uptake or to quit smoking to reduce their risk. Mirroring population trends, smoking rates in individuals with AF have declined significantly over time.14 Current smoking was more strongly and consistently associated with AF compared with former smoking status in most,98,113 but not all114 studies (Table 7). In models excluding individuals with prior coronary heart disease and heart failure, former smoking was no longer significantly associated with incident AF.98 One biracial observational study noted a nonsignificant trend towards reduced rates of AF in individuals who had quit smoking.98
The results of smoking cessation interventions in AF have not been well studied. Despite the potential benefits of smoking cessation in AF, individuals with AF were less likely to be prescribed smoking cessation aids than those without AF.147 One randomized trial of aggressive risk factor reduction, which included smoking cessation in individuals post-AF catheter ablation, demonstrated that those randomized to risk factor reduction had lowered AF frequency, duration, and symptoms.148
Air pollution
Experimental and epidemiological studies have indicated that air pollution is related to an increased prevalence of cardiovascular risk factors, for example diabetes mellitus and hypertension, as well as cardiovascular disease.149–154 Fine particular matter (PM2.5) produced by burning fossil fuels may contribute to this relationship. The underlying pathophysiology has been attributed to an increased inflammatory response to high particle exposure, influencing the autonomous nervous system.153
Although fine particle pollution has been linked to stroke in several studies,155–157 it has not been found to be associated with the induction of AF. Likewise, epidemiological studies have failed to show a relationship between permanently higher fine particle exposure and AF incidence158,159 (Table 8). Short-term exposure may directly enhance AF susceptibility in patients with cardiac disease.160,161
Table 8.
Relation of air pollution to risk of AF
| Study | Design | Subjects | FU | Particle pollution | AF | Risk |
|---|---|---|---|---|---|---|
| Link et al.160 Tufts Medical Center Cardiac Arrhythmia Center |
Prospective cohort study; acute exposure 24 hrs prior | 176; ICD pts | 1.9 yrs | PM2.5, sulphate, NO2, SO2, O3 | 328 episodes of AF >30 s | Odds of AF increased by 26% for each 6.0 μg/m3 increase in PM2.5 in the 2 h prior to the event (P = 0.004) |
| Milojevic et al.158 Myocardial Ischaemia National Audit Project (MINAP) |
Case-cross-over design | 2 867 473 CV events; mean age 73 yrs |
6 yrs | CO, NO2, PM10, PM2.5, SO2, O3; Lags up to 4 days |
310 568 pts with AF | NO2 increased risk for AF 2.8% (0.3–5.4) |
| Bunch et al.159 Utah's Wasatch Front |
Case-crossover study design | 10 457 AF hospitalizations | 15 yrs | PM2.5; day Exposure and cumulative lagged exposures for up to 21 days | 100% | No association between PM2.5 and hospitalization for AF |
AF, atrial fibrillation; CV, cardiovascular; FU, follow-up; ICD, implantable cardioverter-defibrillator; PM2.5, particular fine particular matter; pts, patients; hrs, hours; yrs, years; s, seconds.
Caffeine
Caffeine is a methylxanthine compound that is chemically similar to theophylline. Caffeine is present in tea, coffee, cola, or energy drinks. It has several cardiovascular effects increasing neurohormonal and sympathetic nervous system stimulation.162 Therefore, caffeine has been addressed as a potential trigger for AF.
The acute effects of high caffeine loading or even intoxication show minor and overall inconsistent evidence for increased susceptibility to supraventricular arrhythmias.163–165 Habitual caffeine ingestion has been investigated in several prospective cohort studies (Table 9), but these failed to show any significant relationship to incident AF.168 Also, heavy coffee drinking167 failed to demonstrate a significant relationship between caffeine and AF or flutter even in very high consumers (10 cups, 1000 mg/day). Overall, caffeine consumption on a habitual and regular basis does not seem to increase the incidence of AF.35,166,167 However, other forms of caffeine ingestion such as energy drinks containing other stimulants such as taurine in combination with alcohol, may possibly contribute to an increase of risk, at least in case reports.169
Table 9.
Caffeine use and risk of AF
| Study | Design | Subjects | FU | Caffeine assessment | AF | Caffeine consumption in mg/dL (corresponding hazard ratio) |
|---|---|---|---|---|---|---|
| Conen et al.166 Women's Health Study |
Cohort, USA | 33 638 100% female mean age 53 yrs |
14.4 yrs | Food Frequency Questionnaire | n = 945 | Quintiles: 22 (1.0) 135 (0.88) 285 (0.78) 402 (0.96) 656 (0.89) |
| Shen et al.35 Framingham Heart Study |
Cohort, USA | 4 526 56% female mean age 62 yrs |
4 yrs | Food Frequency Questionnaire | n = 296 | Quartiles: 23 (1.0) 142 (0.84) 347 (0.87) 452 (0.98) |
| Frost et al.167 Danish Diet, Cancer, and Heart Study |
Cohort, Denmark | 47 949 54% female mean age 56 yrs |
5.7 yrs | Food Frequency Questionnaire | n = 555 | Quintiles: 248 (1.0) 475 (1.12) 584 (0.85) 769 (0.92) 997 (0.91) |
AF, atrial fibrillation; FU, follow-up; yrs, years.
Alcohol consumption
Alcohol as a cause of AF has been recognized in the setting of acute consumption, commonly described as the ‘holiday heart’.170 Binge drinking (>5 drinks on a single occasion) is associated with an increased risk of new onset AF.171
A variety of mechanisms has been proposed for the role of alcohol in contributing to AF as triggers or substrate for the arrhythmia including decreased vagal tone, hyper-adrenergic state, direct toxic effect on the cardiomyocytes, altered atrial conduction, and shortening of refractoriness.172–174
In evaluating the contribution of chronic alcohol consumption to the development of AF, an important limitation is that unlike the objective measures available for many of the established risk factors for AF, the quantification of alcohol consumption is based on self-reported levels. Most studies have found an association between heavy alcohol consumption and incident AF (Table 10). For example, the Copenhagen City Heart Study observed that men consuming >35 drinks/week had a high risk of AF.175 Similarly, the Framingham cohort study suggested that heavy alcohol consumption (>36 g/day) significantly increased the risk of AF.177 The Women's Health Study showed that consumption of >2 drinks/day was associated with an increased risk of AF.176 A consistent increase in risk of AF with chronic, heavy alcohol consumption was confirmed in a meta-analysis, which also demonstrated that the association between AF and alcohol consumption was linear.179
Table 10.
Risk of AF and alcohol consumption
| Study | Design | Subjects | FU | Alcohol, drinks/day (week) | AF, n | Risk (95% CI) |
|---|---|---|---|---|---|---|
| (a) Population cohorts | ||||||
| Mukamal et al.175 Copenhagen City Heart study |
Prospective cohort | 16 415 men and women free of AF at baseline |
26 yrs | Men Multivariable risk <1 drinks/week ≥35 drinks/week: Adjusted for CHD, CHF, BP Women Multivariable risk <1 drinks/week 21–27 drinks/week |
1071 | Reference (risk in HR) 1.45 (1.02–2.04) HR 1.63 (1.15–2.31) In men 5% of incident AF is attributable for heavy drinking Reference (risk in HR) 1.04 (0.64–1.70) P = 0.87 for trend |
| Conen et al.176 Women Health Study |
Prospective cohort | 34 715 women <45 yrs free of AF |
12.4 yrs median |
0 drinks/day ≥2 drinks/day |
653 | Reference (risk in HR) 1.6 (1.13–2.25) |
| Djousse et al.177 Framingham Heart Study |
Prospective cohort Case–control analysis |
1055 who developed AF 4672 controls men and women |
>50 yrs | 0 g/day >36 g/day |
1055 | Reference (risk in OR) 1.34 (1.01–1.78) |
| Larsson et al.178 Swedish Cohort Study |
Prospective cohort | 79 019 men and women free of AF at baseline | 12 yrs | Dose responsea <1 drink/week 15–21 drinks/week >21 drinks/week Binge drinking (>5 drinks/single occasion) Type of drinks Liquor 7–14 drinks/week >14 drinks/week Wine >14 drinks/week Beer |
7245 | Reference (risk—RR) 1.14 (1.01–1.28) 1.39 (1.22–1.58) 1.13 (1.05–1.32) 1.13 (1.01–1.28) 1.43 (1.14–1.74) 1.30 (1.06–1.61) NS |
| Kodama et al.179 | Meta-analysis 14 observational cohort and case–control studies |
14 studies 130 820 participants 7558 cases 9 studies 126 051 participants 6341 cases |
2.5–44 yrs | Overall Highest vs. lowest alcohol intake Dose–response (4–86.4 g/day) |
7558 6341 |
Pooled OR/RR 1.51 (1.31–1–74) RR 1.8 (1.05–1.10) per 10 g alcohol per day |
| Larsson et al.178 | Meta-analysis 7 prospective cohort studies | 206 073 participants 12 554 cases men, women |
4.7 to >50 yrs | 0 drinks/daya 1 drink/day 2 drinks/day 3 drinks/day 4 drinks/day 5 drinks/day Overall |
12 554 | Reference (risk in RR) 1.08 (1.06–1.10) 1.17 (1.13–1.21) 1.26 (1.19–1.33) 1.36 (1.27–1.46) 1.47 (1.34–1.61) 1.08 (1.06–1.10) 8% (6–10%) increase in AF risk per 1 drink/day increment |
| (b) Intervention studies | ||||||
| Pathak et al.148 ARREST-AF |
Prospective cohort study | 281 pts with AF undergoing catheter ablation 68 pts RFM 88 pts controls |
2 yrs | RFM—alcohol <30 g/week + BP, lipids and glycemic control, weight reduction, smoking cessation vs. control |
– | RFM predictor of arrhythmia free survival HR 4.8 (2.04–11.4) |
AF, atrial fibrillation; BP, blood pressure; CHD, coronary heart disease; CHF, chronic heart failure; CI, confidence interval; FU, follow-up; HR, hazard ratio; OR, odds ratio; RR, relative risk; RFM, risk factor modification; pts, patients; yrs, years.
aStandard drinks = 12 g alcohol. One standard drink corresponds to ∼40 mL liquor, 80 mL strong wine, 150 mL wine, 330 mL class III beer (alcohol by volume, >3.5%), 50 mL Class II beer (2.8–3.5%), or 660 mL class I beer (<2.25%).
Although these large epidemiological datasets have confirmed the association of heavy alcohol consumption with AF, recent studies have implicated a contributory role of even small quantities of alcohol with an increased risk of AF. Data from 2 large prospective Swedish cohorts comprising 79 000 individuals show that, when compared with <1 drink per week, the consumption of 15–21 and >21 drinks per week conferred significant risks of developing AF on multivariable analysis.178 This study identified that the risk for AF may be most pronounced with liquor; modest for wine and no excess risk was detected with beer. In addition, one meta-analysis of seven prospective studies suggested that there was a greater risk of AF with even low levels of alcohol consumption.178 In both men and women, each drink of alcohol was associated with an 8% increase in relative risk of AF.
The consistent epidemiological relationship between alcohol and AF has led to the suggestion that lowering alcohol consumption may be an effective AF preventive strategy.180 Recent studies have also highlighted the importance of aggressive risk factor management, including reducing alcohol consumption, in maintaining sinus rhythm in patients with established AF. In obese and overweight individuals, these studies have established an ultimate goal of reducing alcohol consumption to ≤30 g/week.148 In the context of a directed management of risk factors, reducing alcohol consumption has contributed to short-term improvements in AF burden26 and AF ablation outcomes,148 as well as long-term maintenance of sinus rhythm.28 The above evidence perhaps confirms some atrial toxicity related to alcohol consumption. Thus, physicians must not neglect obtaining a detailed history on alcohol consumption and providing appropriate counselling to reduce alcohol intake, when necessary, in patients with AF.
Recreational drugs
There are numerous reports on the effects on myocardial infarction, ventricular arrhythmias, and sudden cardiac death caused by recreational (illicit) drugs such as amphetamine, cocaine, and cannabis.181 However, data on these drugs as risk factors for AF per se are sparse. AF has not been reported to be associated with amphetamine, heroin, or LSD abuse and there are limited reports on the abuse of cannabis, cocaine, ecstasy, and anabolic–androgenic steroids with AF.
Cannabis is the most commonly used recreational drug, which is increasing in Europe. A systematic review and a case series with literature review reported that all cases of cannabis-related AF were among young people without co-morbidities.182,183 The underlying mechanism is probably adrenergic stimulation and disturbance in microvascular flow facilitating AF development by increased pulmonary vein ectopy. Cannabis abuse leading to AF is not benign in young and healthy subjects as it may contribute to atrial remodelling long-term.182 AF caused by cannabis abuse may be more malignant in older patients having other risk factors for thromboembolism. The burden of this problem is probably underestimated, given that most illicit cannabis users avoid seeking medical care unless serious disease is present.
Physicians should carefully examine for recreational drug abuse in young new onset AF patients without known predisposing factors. One case report describes AF in a healthy adolescent who had used ecstasy.184 Anabolic–androgenic steroids are often used by young athletes to increase their capacity. Thus AF in a young healthy athlete should raise the suspicion that illicit drugs may be a possible cause and lead to careful search for drug abuse in order to prevent AF and more serious cardiac consequences.185,186
Medications
A number of cardiovascular and non-cardiovascular drugs have been associated with increased risk of AF (Table 11). Drug-induced AF has received relatively little attention, and the exact incidence is not known.
Table 11.
Medications associated with risk of incident AF
Many cardiovascular (adenosine, dobutamine, ivabradine) and non-cardiovascular [non-steroidal anti-inflammatory drugs (NSAIDS), high-dose corticosteroids, and respiratory medications as aminophylline] drugs can induce AF.187,189,193 Adenosine is reported to induce AF when used for terminating supraventricular tachycardia with atrioventricular nodal involvement. Many patients undergoing cardiac surgery and treated with the inotrope dobutamine may develop post-operative AF. However, AF is usually transient and of short duration. Evidence of chemotherapy-induced AF has been summarized.187,188 Anthracyclines, melphalan, interleukin-2, and cisplatin appear to be associated with AF, in addition to cancer itself that creates an inflammatory arrhythmogenic milieu.194 Several case reports of antipsychotic drugs associated with AF have been published,192 include with olanzapine (used for the treatment of schizophrenia and bipolar disorder). The antiemetic drug ondansetron is probably related to AF.187
Whether bisphosphonate drugs against osteoporosis are associated with AF remains somewhat controversial. A systematic review and meta-analysis from 2014 concluded that AF risk is increased by 40% with intravenous use and 22% by oral use.190 A more recent meta-analysis stated that bisphosphonates may modestly increase the risk of AF, but given the large reduction in fractures with these drugs, the authors did not recommend changes in treatment.191
Drug-induced AF can occur through pharmacological stimulation promoting ectopic impulses or by modulating the underlying substrate. Further research is perhaps needed to determine the incidence and risk factors of drug-induced AF, and particularly whether specific medications increase the risk of thromboembolism or mortality. In patients with a new-onset AF, it is reasonable to review the pharmacological history to identify whether any of the prescribed drugs may be responsible for the arrhythmia and make a balanced judgement on the risks and benefits of the drug use. Drug-induced AF may appear in healthy patients, but occurs more frequently in the elderly, after cardiac surgery, and if comorbidities and risk factors associated with AF are present. These risk factors include polypharmacy, hypertension, major heart disease, chronic obstructive pulmonary disease, and sleep apnoea.
Psychological distress
Psychological distress is prevalent among AFpatients;195–199 ∼25–50% have symptoms of anxiety and/or depression and fear and worry are common.195–202 There is some evidence from ICD patients that acute emotional distress (particularly anger and anxiety)197,203,204 and depression205 may be antecedents to ventricular arrhythmias but there are no data in ICD patients regarding atrial arrhythmias. Only three studies have specifically examined the impact of psychological distress on incident AF.206–208
The Framingham Offspring Study examined the association between Type A behaviour, anger, and hostility and incident AF. In age-adjusted analyses, anger-out predicted incident AF in women, while trait anger, symptoms of anger, and hostility predicted onset of AF in men206 (Table 12). On multivariable analyses, symptoms of anger, hostility, and trait-anger predicted the 10-year incidence of AF in men but not in women.206 Another analysis of this cohort investigated the effect of tension and anxiety on the development of AF.207 In age-adjusted analyses, tension, and anxiety predicted development of AF in men only. After adjustment for confounders, only tension was an independent predictor of incident AF but only among men.207
Table 12.
Psychological distress and risk of AF
| Study | Design | Subjects n (% women) | FU, yrs | Psychological distress measures | AF, n (%) | Age-adjusted risk RR (95% CI) | Multivariable-adjusted risk RR (95% CI) |
|---|---|---|---|---|---|---|---|
| Eaker et al.206 Framingham Offspring Study |
Prospective, observational cohort | 3682 (52%) Mean age 48.5 (10.1) yrs |
10 | Type A behaviour Anger Hostility |
Women: 62/1908 (3.2%) Men: 132/1750 (7.5%)b |
Women: Anger-out 1.3(1.0–1.6); P < 0.05 Men: Trait anger 1.2 (1.0–1.4); P < 0.05 Symptoms of anger 1.2 (1.1–1.4); P < 0.05 Hostility 1.3 (1.1–1.6); P < 0.05 |
Womena: NS Mena: Trait anger 1.1 (1.0–1.4); P = 0.04 Symptoms of anger 1.2 (1.1–1.4); P = 0.008 Hostility 1.3 (1.1–1.5); P = 0.03 |
| Eaker et al.207 Framingham Offspring Study |
Prospective, observational cohort | 3682 (52%) Mean age 48.5 (10.1) yrs |
10 | Tension Anxiety |
Women: 62/1908 (3.2%) Men: 132/1750 (7.5%)b |
Women:c Men: Tension 1.28 (1.08–1.52) Anxiety 1.16 (1.01–1.33) |
Womena: Tension 0.83 (0.63–1.11) Anxiety 1.03 (0.81–1.31) Mena: Tension 1.24 (1.04–1.48) Anxiety 1.10 (0.95–1.27) |
| Whang et al.208 Women's Health Study |
RCT, plus observational follow-up | 30 746 women without CVD at baseline Age: ≥45 yrs |
10.5 | MHI-5d MHI-5 score: 86–100 76–85 53–75 <53 |
359 235 129 48 |
Reference 0.86 (0.73–1.02) 0.91 (0.74–1.11) 1.08 (0.80–1.47) P-value for trend 0.61 |
Reference 0.87 (0.73–1.03) 0.89 (0.72–1.09) 0.99 (0.72–1.35) P-value for trend 0.34 |
AF, atrial fibrillation; CI, confidence interval; CVD, cardiovascular disease; FU, follow-up; MHI-5, Mental Health Inventory 5-items; NS, not significant in multivariable analyses; RCT, randomized controlled trial; RR, relative risk; SD, standard deviation; yrs, years.
aAdjusted for age, diabetes, hypertension, history of myocardial infarction or history of congestive heart failure, and valvular heart disease (defined as any diastolic murmur or ≥3 out of 6 systolic murmur).
bNot reported by each psychological measure.
cNot reported for women.
dScore <53 indicates significant global distress.
The absence of an association between psychological distress and the development of AF in women was confirmed in the Women's Health Study.208 In this cohort of 30 746 female health professionals aged ≥45 years who were free from cardiovascular disease at baseline, 771 (2.51%) developed AF over a median 10-year follow-up period. Psychological distress was not associated with incident AF in age-adjusted or multivariable analyses.208 These findings require replication in other more diverse populations since these cohorts were predominantly white, middle-class, and middle-aged204–208 and the effect sizes in the Framingham Offspring study were modest.207,208
Psychological distress, particularly depression, is more commonly associated with adverse lifestyle choices (smoking, excessive alcohol intake, poor diet, physical inactivity), poorer adherence to medication, etc., all of which may increase the likelihood of development of other risk factors for AF, and hence predispose people to incident AF. It is also plausible that the autonomic nervous system may be the conduit by which AF is linked with psychological distress and vice versa. The current evidence is therefore limited and equivocal, and future research is needed.
Physical activity and inactivity
Physical activity has profound benefits on lowering cardiovascular morbidity and mortality and physical inactivity is a major risk factor for cardiovascular disease. The effects of physical activity on the development of AF are less well documented and intervention studies on physical activity and the development of AF are lacking (Table 13).
Table 13.
Physical activity and risk of AF
| Study | Design | Subjects | Age, yrs | FU, yrs | Physical activity | AF, % | Risk |
|---|---|---|---|---|---|---|---|
| Population cohorts | |||||||
| Qureshi et al.209 (FIT project) patients referred for treadmill |
Retrospective | 69 885 | 54.5 | 5.4 | Graded by treadmill | 7 | 1 Met higher decreases AF risk by 7% |
| Drca et al.210 Swedish Mammography Cohort Healthy |
Prospective | 36 513 women | 60 | 10 | Level of leisure activity | 7.9 | AF risk decreases with increased level of activity |
| Mozaffarian et al.211 Cardiovascular Health Study |
Prospective | 5446 men and women | Over 65 | 10 | Exercise intensity | 19 | AF less with low to moderate exercise |
| Grimsmo et al.212 Cross country skiers |
Prospective | 122 and 117 | Over 54 | 28–30 | High in all | 12.8 | Endurance training increases AF |
| Myrstad et al.213 Male, cross country skiers |
Retrospective | 3712 | Over 53 | High in all | 12.5 | Endurance training increases AF | |
| Lee et al.214 Leisure-time running |
Longitudinal cohort study | 309 540 men and women | 40–45 | 4 | Leisure time activity | 0.4 | AF increases with self-reported activity in men |
| Thelle et al.215 Walkers and runners |
Proportional hazards analysis of | 14 734 | All ages | 6.2 | Walking or running | 1.9–2.7 (arrhythmia) | AF similar in walkers and runners Arrhythmia decreases per MET |
| Aizer et al.216 Physicians Health Study Healthy men |
Prospective | 16 921 | 40–84 | 12 | Degree of physical activity | 9.8 | Vigorous activity increases AF |
AF, atrial fibrillation; FU, follow-up; MET, metabolic equivalent task; pts, patients.
The risk of AF depends on the interaction between individual susceptibility, environment, and the degree of physical activity.217 Vigorous exercise may increase risk of sudden cardiac death, and even AF in some instances; however, habitual moderate physical activity may have several benefits that can reduce the incidence of AF. Lowering heart rate, blood pressure, better glucose and lipid control, weight loss, improved endothelial function, and lower systemic inflammation are some of the benefits of exercise that may decrease the development of AF.97 On the other hand, vigorous activity can cause acute cathecholamine fluxes, autonomic tone changes, and atrial stretch, all contributing to AF risk.218–223 Autonomic influences should also be taken into consideration to decrease aggravation of AF.218,224
The Euro Heart Survey on AF showed that an autonomic trigger pattern, either adrenergic, vagal, or mixed was present in 33% of patients; however, physicians did not choose rhythm or rate control medications according to those triggers,224 and inappropriate therapy in vagal AF patients enhanced progression of AF.
As stated earlier, obesity begets AF, and increased cardiorespiratory fitness is protective against incident AF. Indeed, the CARDIO-FIT study showed that arrhythmia free time was greatest in obese patients with high cardiorespiratory fitness. In this study, AF burden and symptom severity significantly decreased in the group with cardiorespiratory fitness gain over two metabolic equivalent tasks (METs).27
Different studies have suggested a possible relationship between endurance training and the development of AF, although this has not been confirmed in all studies or a Cochrane meta-analysis.212,214,225–230 Most studies have looked at the effects of endurance training and vigorous exertion in young and middle-aged adults. In a study of 44 410 men, intense endurance training at age 30 increased risk of AF later in life whereas moderate intensity decreased AF risk.231 Similar findings were reported in older athletes.211 A meta-analysis of several small studies showed that risk of AF development in athletes was more than in non-athletes, but referents were not age matched and there was variance in the level of endurance across studies.232 Age, years of training, and type of sport will all affect the outcome, therefore it is not possible to deduct a net conclusion from these studies except that vigorous endurance exercise may have a possible and small facilitating effect on AF.
In older adults, prospective epidemiological studies have shown a U-shaped relationship between level of physical activity and risk of AF. For example, the Cardiovascular Health Study demonstrated that leisure time activity was associated with lower AF incidence in a graded manner with lower risk as the intensity increased.211 AF incidence was lower in those with moderate exercise compared with no exercise (HR 0.72, 95% CI 0.58–0.89). However, high-intensity exercise was not associated with a significantly reduced risk of AF (HR 0.87, 95% CI 0.64–1.19). There is also a graded inverse relationship between cardiorespiratory fitness and incident AF especially in obese patients.209 In a large population-based Swedish cohort, the risk of AF decreased with increased leisure time exercise in middle aged and elderly women.210 Inactivity and obesity may lead to diastolic dysfunction and left atrial enlargement, and therefore increased AF risk whereas exercise training improves diastolic function and reduces left atrial volume.233
Current evidence would suggest that moderate physical activity is associated with better cardiovascular health, decreased mortality and decreased risk of AF. The on-going Routine vs. Aggressive upstream rhythm Control for prevention of Early atrial fibrillation in moderate heart failure (RACE 3) trial is investigating whether the combination of RAAS modulators, statins, and cardiac rehabilitation interventions to promote a better lifestyle including physical activity, weight reduction, and a healthy diet, may reduce progression of AF.234
Genetic predisposition and risk of AF
About 5% of patients with AF and 15% with lone AF referred for the evaluation of arrhythmias have family history of arrhythmias.235 Population-based studies demonstrated association between family history and risk of AF development236–241 (Table 14), which become stronger with increased numbers of affected first degree relatives and younger age. Several genes and loci linked to AF and its substrate were identified in families, individuals, and different populations,242–244 still there are genes in development state with unknown effects and risk associated with AF.245,246 AF with genetic predisposition is defined as monogenic when related to inherited cardiomyopathies and as polygenic in the presence of common gene variants associated with early AF onset in population.247,248
Table 14.
Genetic predisposition and risk of AF—population-based studies
| Study | Design | Subjects | FU | Familial AF history | AF, % | Risk* (95% CI) |
|---|---|---|---|---|---|---|
| Fox et al.236 Framngham Heart Study |
Prospective cohort Population-based epidemiological study |
2243 O 1165 women 1078 men At least 30 yrs |
16 yrs | 681—at least 1 parent had documented AF | n = 70 | Parental AF vs. no FH |
| OR 1.85 (1.12–3.06; P = 0.02) | ||||||
| Parental AF vs. no FH <75 years (O and P) | ||||||
| OR 3.23 (1.87–5.58; P < 0.001) | ||||||
| Parental AF vs. no FH <75 years (O w/o overt clinical heart disease) | ||||||
| OR 3.17 (1.71–5.86; P < 0.001) | ||||||
| Arnar et al.237 Iceland cohort |
Population-based cohort | 5269 pts with AF | – | AF risk in first to fifth degree relatives | – | First degree relative |
| RR 1.77 (1.67 = 1.88 P = 0.001) | ||||||
| First degree relative <60 years old | ||||||
| RR 4.67 (3.57–6.08, P = 0.001) | ||||||
| Gundlund et al.238 Denmark cohort |
Population-based study | New-onset AF | AF screening: | RR compared with general Denmark population | ||
| 67 310 mothers—64 yrs | 133 516 maternal O | 2536 (1.9%) | 3.37 (3.21–3.53) | |||
| 103 822 fathers—70 yrs | 221 774 paternal O | 2906 (1.3%) | 2.81 (2.69–2.93) | |||
| 11 800 siblings—46 yrs | 21 448 sibling O | 292 (1.4%) | 5.20 (4.61–5.85) | |||
| Zoller et al.239 Sweden cohort |
Population-based case-controlled study | 300 586 individuals with AF/AFl multiplex families |
Case vs. control | |||
| 1 parent | 22.6 vs. 13.6% | OR 1.95 (1.89–2.00) | ||||
| ≤49 yrs | 22.8 vs. 11.9% | OR 2.33 (2.23–2.44) | ||||
| 2 parents | 2.0 vs. 0.2% | OR 3.6 (3.3–3.92) | ||||
| ≤49 yrs | 2.1 vs. 0.5% | OR 5.04 (4.36–5.28) | ||||
| ≥1 sibling | 14.7 vs. 5.6% | OR 3.08 (3.0–3.16) | ||||
| ≤49 yrs | 8.1 vs. 2.3% | OR 4.06 (3.79–4.41) | ||||
| ≥2 siblings | 2.9 vs. 0.6% | OR 5.72 (5.28–6.19) | ||||
| ≤49 yrs | 1.4 vs. 0.2% | OR 8.51 (6.49–11.15) | ||||
| Lubitz et al.240 Framingham Heart Study |
Prospective cohort | 4421 participants | – | Familial AF—1185 Premature familial AF (<65 yrs) −351 |
Overall 440 Familial AF vs. no FH 5.8 vs. 3.1% |
Presence of any first degree familial AF vs. no |
| HR 1.4 (1.13–1.74, P = 0.002) | ||||||
| Presence of premature familial AF (<65 years) | ||||||
| HR 2.01 (1.49–2.71, P < 0.001) | ||||||
| Number of first degree relative with AF—risk per each additional affected member | ||||||
| HR 1.24 (1.05–1.46, P=0.01) | ||||||
| Oyen et al.241 Denmark cohort |
Prospective cohort | 3 985 446 individuals Lone AF—9507 subjects <60 yrs |
31 yrs | First degree relative | n = 269 | IRR 3.48 (3.08–3.93) |
| Second degree relative | n = 19 | IRR 1.64 (1.04–2.59) | ||||
| Number of affected first degree relatives | ||||||
| 1 affected | n = 264 | IRR 3.45 (3.05–3.9) | ||||
| ≥2 affected | n = 5 | IRR 6.24 (2.59–15.0) | ||||
| Age at onset of lone AF for cohort member and first degree relative | ||||||
| <30 yrs for both | N/A | IRR 8.53 (3.82–19.0) | ||||
| <40 yrs for both | n = 31 | IRR 5.42 (3.8–7.72) |
AF, atrial fibrillation; CI, confidence interval; FH, family history; FU, follow-up; HR, hazard ratio; IRR, incidence rate ratio; O, offspring; OR, odds ratio; P, parent; pts, patients; RR, relative risk; yrs, years.
The evidence of genetic predisposition to AF is evolving, and more studies are needed to clarify the role of various genes in AF development and as the genetic predisposition is a non-modifiable risk factor more studies are needed to establish whether intervention on modifiable risk factors can decrease risk of AF in populations with genetic predisposition.
Hyperthyroidism and other endocrine disorders
Among endocrine disorders, hyperthyroidism and diabetes mellitus (see above) are commonly associated with risk of developing AF,31,103,249,250 while hypothyroidism poses no or reduces risk for arrhythmia.249,251,252
Observational cohort and registry studies (Table 15) reported AF incidence rates of 4.6–13.8% in overt hyperthyroidism, 8.5–12.7% in subclinical hyperthyroidism, and 7.3% in high-normal euthyroidism [based on thyroid stimulating hormone (TSH) level].249–251,253–257
Table 15.
Risk of AF in thyroid dysfunction
| Study | Design | Subjects | FU | Thyroid function | AF, % | Risk (95%CI) |
|---|---|---|---|---|---|---|
| Selmer et al.249 | Cohort | 586 460 | 5.5 yrs | Euthyroid | 2.9 | Reference |
| Overt Hyperthyroid | 4.6 | IRR 1.42 (1.22–1.63) | ||||
| Subclinical Hyperthyroid | – | IRR 1.31 (1.19–1.44) | ||||
| Overt Hypothyroid | 2.5 | IRR 0.67 (0.5–0.9) | ||||
| Subclinical Hypothyroid | – | IRR 0.87 (0.7–0.97) | ||||
| TSH levels | ||||||
| Reduced TSH | – | IRR 1.16 (0.99–1.36) | ||||
| Suppressed TSH | – | IRR 1.41 (1.35–1.89) | ||||
| High-normal Euthyroid (TSH levels) | – | IRR 1.12 (1.03–1.21) | ||||
| Cappola et al.251 Cardiovascular Health study |
Cohort | 3233 >65 yrs |
13 yrs | Euthyroid | 5.2 | Reference |
| Subclinical Hyperthyroid | 8.5 | HR 1.98 (1.29–3.03)a | ||||
| Overt Hypothyroid | 4.8 | HR 0.96 (0.52–1.79)a | ||||
| Subclinical Hypothyroid | 3.9 | HR 1.13 (0.94–1.36)a | ||||
| Frost et al.250 | Cohort | 40 628 | 30 days | Overt Hyperthyoid | 8.3 | – |
| Auer et al.253 | Retrospective | 23 638 elderly | – | Euthyroid | 2.3 | – |
| Overt Hyperthyroid | 13.8 | – | ||||
| Subclinical Hyperthyroid | 12.7 | RR 5.2 (2.1–8.7) | ||||
| Gammage et al.254 | Cohort | 5860 >65 yrs |
– | Euthyroid | 4.7 | Reference |
| Subclinical Hyperthyroid | 9.5 | OR 1.87(1.01–3.57)b | ||||
| Subclinical Hypothyroid | 4.2 | – | ||||
| Serum free T4 | – | OR 1.09 (1.03–1.15) | ||||
| Sawin et al.255 Framingham Heart study |
Cohort | 2007 | 10 yrs | Euthyroid | 8.4 | |
| Reduced TSH 0.1–0.4 μU/L | 12.2 | RR 1.6 (1.0–2.5) | ||||
| Suppressed TSH <0.1 μU/L | 21.3 | RR 3.8 (1.7–8.3) | ||||
| Colett et al.256 Thyroid studies collaborators |
Meta-analysis | 52 674 | 8.8 yrs | Subclinical Hyperthyroid | – | HR 1.68 (1.16–2.43) |
| Reduced TSH | – | HR 1.63 (1.1–2.4) | ||||
| Suppressed TSH | – | HR 2.54 (1.08–5.99) | ||||
| Heeringa et al.257 | Registry | 1426 | 8 yrs | High-normal Euthyroid (TSH levels) | 7.3 | HR 1.94 (1.13–3.34)c |
| TSH - 0.4–1.04 mU/L | ||||||
| Kim et al.252 Framingham Heart study |
Cohort | 5055 | 10 yrs | TSH 0.45–4.5 μU/L | 5.4 | Reference |
| TSH 4.5–10.0 μU/L | 7.0 | HR 1.23 (0.77–1.97) | ||||
| TSH 10.0–19.9 μU/L | 4.0 | HR 0.57 (0.21–1.54) |
Definitions of thyroid dysfunction.249
Euthyroidism: TSH 0.2–5.0 mIU/L; free thyroxine 9–22 pmol/L; total thyroxine 60–140 mmol/L.
Overt hypothyroidism: TSH >5.0 mIU/L; free thyroxine <9 pmol/L; total thyroxine <60 mmol/L.
Subclinical hypothyroidism: TSH >5.0 mIU/L; free thyroxine 9–22 pmol/L; total thyroxine 60–140 mmol/L.
Overt hyperthyroidism: TSH <0.2 mIU/L; free thyroxine >22 pmol/L; total thyroxine >140 mmol/L.
Subclinical hyperthyroidism: TSH <0.2 mIU/L; free thyroxine 9–22 pmol/L; total thyroxine 60–140 mmol/L.
TSH level dependent thyroid dysfunction.249
Euthyroidism: TSH 0.4–5.0 MiU/L; free thyroxine 9–22 pmol/L; total thyroxine 60–140 mmol/L.
High normal euthyroidism: TSH 0.2–0.4 mIU/L; free thyroxine 9–22 pmol/L; total thyroxine 60–140 mmol/L.
Subclinical hyperthyroidism (reduced TSH): TSH 0.1–0.2 mIU/L; free thyroxine 9–22 pmol/L; total thyroxine 60–140 mmol/L.
Subclinical hyperthyroidism (suppressed TSH): TSH <0.1 mIU/L; free thyroxine 9–22 pmol/L; total thyroxine 60–140 mmol/L.
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; d, days; DM, diabetes mellitus; HF, heart failure; HR, hazard ratio; HT, hypertension; IRR, incidence rate ratio; LVF, left ventricular function; MI, myocardial infarction; OR, odds ratio; pts, patients; RR, relative risk; SBP, systolic blood pressure; TSH, thyroid stimulating hormone; VHD, valvular heart disease; yrs, years.
aAdjusted for age, sex, CVD, thyroid medication use, atrial size, SBP, fasting glucose. VHD, β-blockers and diuretics use.
bAdjusted for male, age >70, DM, HF, HT.
cAdjusted for age, sex, smoking, BMI, SBP, HT, HF, MI, LVF, DM.
The risk of new-onset AF in hyperthyroidism depends on the level of thyroid dysfunction. AF is increased by 42% in overt hyperthyroidism, by 31% in subclinical hyperthyroidism, and by 12% in high-normal euthyroidism.249 Patients with subclinical forms are 1.68-fold more likely to develop AF during long-term follow-up, and those with suppressed TSH values have been shown to possess 2.54-fold higher risk of incident AF compared with euthyroid populations.249,251,253,255,256 Though the evidence on risk of AF in individuals with high-normal euthyroidism is limited, the Rotterdam study demonstrated an increased risk of AF in individuals with high-normal thyroid function (based on TSH level)257 and in subjects <65 years old with higher free thyroxine levels within normal range.258 Nonetheless the evidence on demographic and cardiovascular disease risk factors associated with AF in thyroid dysfunction is scarce. In overt hyperthyroidism, age >65 years, male sex, comorbidities like coronary artery disease, chronic heart failure, and valvular heart disease were reported as predictors of arrhythmia.259 In the subclinical form, age and sex were shown to affect the incident risk of AF, being significant in all age categories in women, and young male individuals, except in the older (>65 years) male population.249 In a recent meta-analysis,256 the risk of AF in subclinical hyperthyroidism was associated with male sex, but was not altered by the presence of cardiovascular disease or its risk factors. In another study, subclinical hyperthyroidism was shown to be a predictor of AF in elderly individuals, along with advanced age category (>75 years), male sex, diabetes mellitus, hypertension, and heart failure.257
AF risk diminishes during antithyroid treatment,249 with spontaneous restoration of sinus rhythm in ∼76% of patients260 and reduction of arrhythmia on long-term monitoring.259 Sinus rhythm restoration rates are also higher in elderly patients with overt and subclinical hyperthyroidism without cardiovascular disease and its risk factors, when compared with those with comorbidities.253 After restoration of an euthyroid state and electrical cardioversion or catheter ablation for persistent AF, long-term sinus rhythm maintenance rates have been shown to be either higher in patients with hyperthyroidism261 or did not differ from those without history of thyroid dysfunction.262,263
Hyperthyroidism had been long considered to be associated with higher thromboembolic risk,65 but recent studies demonstrated that thyroid disease is not an independent predictor of AF-related complications such as thromboembolism and stroke.264–266
Thus, prevention of AF in overt and subclinical hyperthyroidism should include measures, such as controlling thyroid function, treatment of associated cardiovascular diseases, and modification of risk factors. More research is needed regarding risk factors and prevention of AF in populations with high-normal euthyroidism based on TSH level and normal thyroid function with higher free thyroxine levels within normal range.
Electrophysiological considerations
Atrial premature beats triggering AF
Atrial fibrillation can be maintained by rapid focal firing or by reentrant activity. The actual mechanism by which triggers (ectopic beats) initiate AF is unclear, but an important topic of research. Prior reports have mapped spontaneous ectopic triggers for AF and demonstrated their spatial diversity in both atria and prematurity in rate.267 Several mechanisms produce abnormal impulse formation that can cause focal ectopic activity: abnormal automaticity and triggered activity. Abnormal automaticity relies on an increased Phase 4 depolarization in cells that normally have a flat Phase 4. The (upregulation of the) pacemaker current If (funny current) may play an important role in this mechanism.
Triggered activity consists of depolarizations occurring after the action potential: delayed after depolarizations (DADs) or within the action potential: late Phase 3 early after depolarizations. These triggers often originate from predilected sites in the atria, such as the ostia of the pulmonary vein sleeves.267 DADs are thought the most common cause of focal atrial ectopic firing and are caused by diastolic Ca++ leak from the sarcoplasmic reticulum via SR Ca++-release channels (RyR2) and the Na+/Ca++ exchange (NCX).268
To maintain AF, these ectopic beats must be sustained to produce rapid driver activity or form the trigger to initiate reentry in a vulnerable substrate. AF remodels the atrial electrical properties to promote both initiation and propagation. It is well known that electrical remodelling consists of shortening of the duration of the action potential and depressed intracellular Ca++ transients. Besides the involvement of the regular ion channels, also the INa late current plays a possible role.
Structural remodelling plays another important role in the initiation and maintenance of AF.269 Various pathways play a role including the RAAS, inflammation, and fat deposition leading to enlarged atria, hypertrophy, fibrosis, and myolysis.270–276 Indeed, the first manifestation of AF usually occurs after years of atrial remodelling.273 Once AF develops, it causes marked changes in atrial electrophysiology (electrical remodelling) in addition to further deterioration of the structural remodelling processes, constituting a vicious cycle in which ‘AF begets AF’,271 making it challenging to restore and maintain sinus rhythm.273,274
Molecular mechanisms
Abnormal cellular Ca++ handling is typically seen in AF patients. Defective Ca++ handling promotes spontaneous ryanodine receptor (RyR2)-mediated Ca++ release in atrial cells of patients with AF. Phosphorylation of RyR2 and CAMKII is increased in AF. Increases in NCX expression/activity are also common noted in AF.
Supraventricular tachyarrhythmias causing AF
Supraventricular tachyarrhythmias (SVT) and pre-excitation may associate with AF.275–278 In 169 paroxysmal SVT outpatients, AF incidence was 19% over 2.5 years, assessed by remote monitoring (Figure 1).277 Atrial flutter and AF coexist even more often, one arrhythmia potentially reinforcing the other.279 Finally, flutter is frequently accompanied by atrioventricular nodal re-entry tachycardia (AVNRT).280
Figure 1.
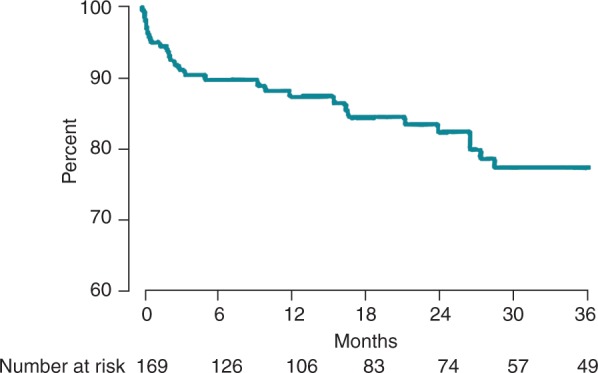
Graph showing time to occurrence of symptomatic atrial fibrillation in all 169 patients with paroxysmal supraventricular tachycardia. Y-Axis reflects the percentage of patients free from atrial fibrillation. (Reprinted from reference 277: J Am Coll Cardiol Vol.25, Hamer ME, Wilkinson WE, Clair WK, Page RL, McCarthy EA, Pritchett EL. Incidence of symptomatic atrial fibrillation in patients with paroxysmal supraventricular tachycardia. number, p. 984–8, Copyright 1995, with permission from Elsevier.)
Causal mechanisms include tachycardia-related atrial ischaemia or dispersion of conduction and refractoriness, which can be facilitated by background atrial remodelling. Enhanced vagal tone is another mechanism.281 Digitalis may cause shortening of atrial refractoriness282 and also associate SVT or atrial flutter with AF. The same may hold for adenosine, which may elicit AF when given for the termination of SVT, and potentially cause haemodynamic deterioration.283 Due to conduction slowing, flutter may emerge under drug treatment for AF through activation of a sleeping circuit, seen especially with flecainide or propafenone (class-Ic flutter).284 Late onset AVNRT may occur upon cardiovascular ageing, in turn producing triggers and substrate for both AVNRT, as well as AF and flutter.285 Similarly, atrial remodelling (e.g. in the setting of hypertension) may connect atrial tachycardia and atrial flutter to AF. Last, but not least, AF and SVT may also simply associate due to the presence of both arrhythmia mechanisms including frequent pulmonary vein ectopy, as part of paroxysmal AF, but triggering the SVT substrate meanwhile.
In pre-excitation syndrome, the very presence of the accessory atrioventricular pathway (i.e. in the absence of atrial remodelling like in ‘classic’ AF) has been associated with local atrial arrhythmogenesis and hence AF. Conduction dispersion emerges during retrograde pathway conduction after ventricular premature beats or during orthodromic tachycardia. Asymptomatic pre-excitation usually is not associated with AF, although younger patients as well as those with inducible SVT or AF and those with a short anterograde refractory period may be at risk.286 AF and pre-excitation, together with premature conduction disease, may occur in a rare genetic form of hypertrophic cardiomyopathy due to AMP kinase gene mutation deregulating cellular energy homoeostasis.287
When PAF and SVT associate, medical (including upstream anti-remodelling) therapy may apply for both although ablation of both mechanisms seems most appropriate. Ablation of SVT or flutter may abolish AF or make it better amenable to rhythm control, although frequently electrophysiologists will perform pulmonary vein isolation at the same time. Ablation of the accessory pathway, in patients with overt pre-excitation suffering from AF, may prevent further AF attacks288 and is the preferred treatment also to prevent rare sudden death due to ventricular fibrillation. If these patients refuse ablation or complications are expected (e.g. atriovenricular block), then medical therapy may be indicated.286,289 Usually flecainide or propafenone are prescribed and amiodarone may be needed in the presence of concurrent cardiac disease. After ablation of class Ic flutter it is advocated to continue drug treatment for suppression of the initial AF although after isthmus ablation AF attacks may subside spontaneously. To avoid repeat procedures, SVT mechanisms should be checked electrophysiologically during any AF ablation, especially in the younger non-remodelled AF patients (Figure 2).
Figure 2.
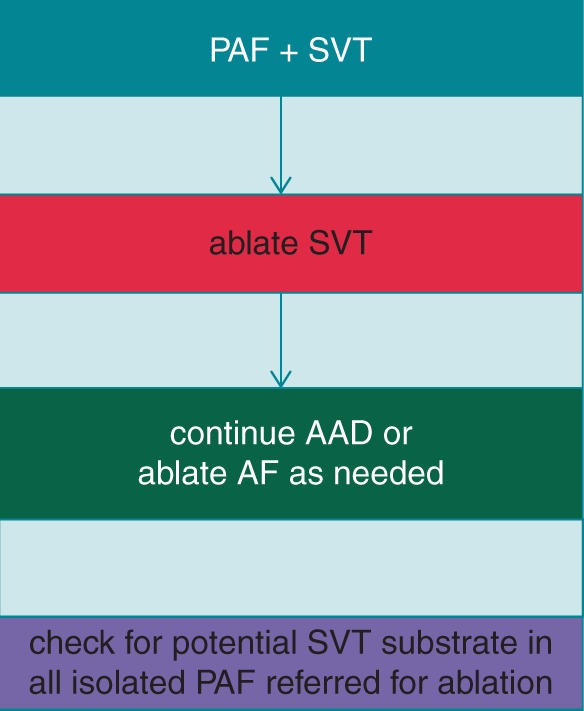
Management of supraventricular tachycardias causing AF. AF, atrial fibrillation; AAD, antiarrhythmic drug; PAF, paroxysmal AF; SVT, supraventricular tachycardia.
Post-operative atrial fibrillation
AF after cardiac surgery occurs in ∼30% of patients,290 and is also frequent after thoracic surgery. This arrhythmia is associated with higher occurrence of heart failure and stroke, both resulting in increased hospitalization and healthcarecosts,291 and also correlating with a higher rate of other serious complications [increased risk of in-hospital morbidity and mortality, and increased long-term risk of stroke].292 Post-operative AF usually is developed between Days 1 and 4 after surgical intervention. The mechanisms underlying the development of AF after cardiac surgery are not completely understood, but are thought to be multifactorial.291 Numerous predisposing factors such as advanced age, hypertension, diabetes, left atrial enlargement, left ventricular hypertrophy, type of intervention, and the presence of cardiac valvular disease, intra-operative and post-operative factors such as atrial injury or ischaemia, can favour the development of post-operative AF.293
Different drugs have been investigated to prevent post-operative AF. Centrally acting β-adrenergic receptor-blocking agents tend to reduce sympathetic efferent activity and promote cardiac vagal outflow.294 Current guidelines strongly recommend using β-blockers to reduce post-operative AF incidence65 and for that reason, preoperative β-blocker administration is standard in all patients without contraindications. Indeed, the European guidelines recommend that treatment should be started at least 1 week before surgery with a β1-blocker without intrinsic sympathomimetic activity.65 A large meta-analysis of 27 randomized controlled trials with 3 840 patients, reported that the incidence of post-operative AF in control patients was 33% compared with 19% in those taking β-blockers, although an inexplicable and marked heterogeneity was found between trials.295 The importance of β-blockers is also affirmed by the two- to five-fold increase in AF after cardiac surgery, when β-blockers are discontinued post-operatively.296
The effectiveness of sotalol vs. placebo and sotalol vs. conventional β-blockers in preventing AF after surgery has been analysed in several clinical trials. A recent meta-analysis297 analysed 8 trials (1294 patients in total) evaluating the effect of sotalol to reduce post-operative AF, and demonstrated a reduction in AF incidence (37% in placebo group vs. 17% in sotalol group) with no significant heterogeneity between trials. Sotalol and other β-blockers were compared directly in 4 trials including 900 patients.295 Once again, sotalol reduced the incidence of post-operative AF from 22% in the other β-blocker group to 12% in the sotalol group with no significant heterogeneity. However, the use of sotalol places patients at risk of bradycardia and torsade de pointes, especially in those with electrolyte disturbances, reason why its use in post-operative AF is limited.65
Several studies have analysed the impact of amiodarone on post-operative AF, with more than 10 randomized placebo-controlled trials. In a recent meta-analysis,297 prophylactic amiodarone decreased the incidence of post-operative AF (OR 0.43; 95% CI 0.34–0.54) and significantly shortened the duration of hospital stay, reduced the incidence of stroke and of post-operative ventricular tachyarrhythmia, but not post-operative mortality.298 European guidelines recommend considering preoperative amiodarone for patients at high risk for post-operative AF.65
It is recognized that the use of statins is associated with a 22–34% lower risk of post-operative AF.65 The largest and most robust trial of atorvastatin carried out to date, the Atorvastatin for Reduction of Myocardial Dysrhythmia After cardiac surgery study (ARMYDA-3),299 demonstrated that atorvastatin treatment conferred a 61% reduction in risk of post-operative AF in multivariable analyses. A recent large randomized trial did not show beneficial effects of rosuvastatin on incidence of complications or AF after cardiac surgery.300
Other drugs have been studied,297,301 but most show conflicting results. For example, no significant effect of RAAS-related medications on the occurrence of AF following cardiac surgery291 and safety concerns about the potential risk of associated renal dysfunction. A meta-analysis demonstrated a significant reduction in post-operative AF using corticosteroids,302 but we should take into account the potential adverse effects on glucose metabolism, wound healing, and infection. Other drugs explored included magnesium supplements, colchicine, non-steroidal anti-inflammatory drugs, and antioxidant agents (i.e. polyunsaturated fatty acids or N-acetylcysteine).301
Current European guidelines recommend β-blockers and amiodarone as prophylactic therapies for post-operative AF. However, new pharmacological agents, with anti-inflammatory, and remodelling properties could take a place in the prevention of post-operative AF. Further research in this field is needed.
Upstream therapies to prevent AF
Upstream therapy refers to the use of non-ion-channel antiarrhythmic drugs that modify the atrial substrate upstream of AF to prevent new-onset AF (i.e. primary prevention) or recurrent AF (i.e. secondary prevention). It includes treatment with RAAS blockers [ACEIs, ARBs, and mineralocorticoid receptor antagonists (MRAs)], statins, and possibly n3-PUFAs.303,304 RAAS blockers may prevent or reduce atrial structural remodelling by decreasing fibrosis and improving haemodynamics. Interestingly, recent data support the favourable effects of physical activity, i.e. moderate exercise on AF burden.211
Upstream therapy has been encouraging in animal experiments, hypothesis-generating small clinical studies, and primary prevention studies.303,304 However, only few data support its beneficial effect for secondary prevention of AF. ACEIs and ARBs seem valuable, especially when added to amiodarone.274,305 Mineralocorticoid receptor antagonists may be even more effective in preventing AF recurrences but few data are available.306,307
Statins, known for their lipid-lowering capacities, have pleiotropic properties such as reduction of inflammation and oxidative stress. Through these properties, statins may play a protective role against AF development. However, results regarding effectiveness of statins have been inconclusive.304
The effects of PUFAs have been well demonstrated in animal model, but limited evidence in secondary prevention of AF is available.303,304
Favourable effects of lifestyle changes, including moderate exercise, have been demonstrated in selected patients.26,27,148,201 In a recent randomized trial, in obese AF patients, weight management, including physical activity and counselling, was compared with general lifestyle advice.26 In addition to a significant reduction of BMI, AF symptoms and burden were significantly reduced in the aggressive weight management group. This finding was confirmed in the Long-term Effect of Goal directed weight management on AF Cohort: a 5 Year follow-up (LEGACY) trial, again in obese AF patients.28 Progressive weight loss was associated with a reduced AF burden and symptoms and, interestingly, left atrial volume.
Overall, upstream therapy may be effective in primary prevention. The disappointing results regarding secondary prevention of AF may have been caused by inclusion of patients in whom the extent of remodelling was too severe and irreversible due to a long history of AF and underlying diseases.273,274 Inclusion of patients, in whom remodelling processes are less advanced, may improve outcome, in addition to tailoring certain upstream therapies to distinct patient groups (e.g. lifestyle changes in obese inactive patients).
Risk factors leading to AF development as risk factors for thromboembolic complications
Stroke prevention is central to the management of AF,308 and many of the risk factors leading to AF development are also risk factors for its thromboembolic complications. Whilst AF increases the risk of stroke five-fold, this risk is not homogeneous and depends on the presence of various stroke risk factors.309 Some risk factors are independent predictors of stroke risk, and have been used to formulate various stroke risk stratification schemes, such as the CHA2DS2-VASc score, which is now recommended in guidelines.310 There are also various stroke risk modifiers, such as OSA311 and renal impairment,312 that have been associated with an increased stroke risk per se, although their additive predictive (and practical) value over and above validated stroke risk scores is less certain. Whether treatment of sleep apnoea with continuous positive airway pressure reduces stroke risk is unproved.311
Some risk factors within the CHA2DS2-VASc score, such as age, prior stroke, or thromboembolism, vascular disease, and female sex, are non-modifiable. Also, prior heart failure especially if associated with a hospital admission with decompensation, confers an excess of stroke risk.313 Hence, efforts to minimize hospitalizations and decompensation of heart failure may help. Diabetes mellitus is less modifiable, but the duration of diabetes may predispose to an even higher risk of stroke and thromboembolism 107).
In a systematic review of stroke risk factors, a history of hypertension or uncontrolled hypertension conferred an increase in stroke risk,309 but clearly, well-controlled hypertension has a lower risk of stroke compared with uncontrolled hypertension.314 Hypertension is also the commonest comorbidity associated with AF. Thus, patients with AF should have blood pressures ∼130/80 mmHg, reflecting the fact that AF could be considered a manifestation of hypertensive target organ damage, and given that stroke risk starts to rise beyond SBPs of 130 mmHg.314
Other potentially modifiable risk factors such as obesity, smoking, and alcohol excess have been related to an increased risk of stroke and mortality,33,315,316 although intervention studies to show how these would successfully decrease the risk of stroke in AF are lacking. Data from cohort studies very recently indicated that weight reduction and improvement in physical fitness may reduce the recurrence of AF.27 Also, rhythm control measures, such as cardioversion and ablation, may help in symptom management, and improve functional status, but randomized trials, clearly showing that such interventions reduce stroke in a broad range of unselected AF cohorts are lacking.317 Observational data, in selected cohorts, suggest that successful catheter ablation may be associated with a lowered stroke risk318 but, given that asymptomatic recurrences and late recurrence are recognized phenomena, guidelines recommend continuation of oral anticoagulation (OAC), in patients with a CHA2DS2-VASc score of ≥2, irrespective of apparent success of rhythm control.317
Modifiable factors to reduce the risk of stroke can include attention to quality of anticoagulation control for a patient taking a VKA (e.g. warfarin). The quality of anticoagulation control is usually quantified by the average time in therapeutic range (TTR) and a TTR of >70% is recommended.319 However, TTR can be influenced by various clinical risk factors, especially in inception cohorts where warfarin is introduced.320 Thus, in newly diagnosed and previously anticoagulated naïve AF patients, a ‘trial of warfarin’ prior to considering a non-VKA oral anticoagulant (NOAC) is not recommended given that TTR is likely to be subtherapeutic in the early phase of warfarin initiation, leading to an increased risk of stroke.321 The SAMe–TT2R2 score322 has been proposed to help decision-making between patients who are likely to do well on a VKA with high TTR (i.e. SAMe-TT2R2 score 0–2) and those unlikely to do well on a VKA with poor TTR (SAMe-TT2R2 score >2), where a NOAC would be a better first option.323,324 Thus, simple clinical decision-making, based on clinical risk factors that influence poor TTR as a stroke risk factor (within the SAMe–TT2R2 score), can help inform treatment decisions that would reduce the likelihood of labile INRs, and its adverse consequences such as stroke, bleeding, and death.325
Patient values/preferences
Many of the risk factors for the development of AF are to a certain extent preventable and/or modifiable via lifestyle choices such as diet, smoking, alcohol, recreational drug use, physical activity, maintenance of a healthy weight, and adherence to medication to control concomitant conditions (hypertension, diabetes, hyperthyroidism, etc.) and therefore potentially under individuals' conscious control.326 In addition, risk factors are likely to be cumulative in increasing risk of incident AF.98,111,115 However, an individual's ability to ‘control’ these factors may be limited by socioeconomic circumstances, access to healthcare and medications, health literacy, etc. Therefore, primary prevention of disease requires greater public awareness of the causes and consequences of the disease and how a person can modify his/her own risk of developing it. Thus, improving the general populations' understanding and perception of AF (what it is, how it develops, associated stroke risk), of how their lifestyle impacts their risk of developing AF, and identifying strategies to change their health beliefs and health behaviours to reduce their risk of progressing to AF, requires both an individual approach plus global public health campaigns. Since lifestyle choices have significant impacts on all diseases, healthcare professionals should utilize contacts with patients to discuss diet, smoking, alcohol/drug use, and exercise, offer appropriate education, advice, and intervention(s), and support people to adopt and maintain health-promoting behaviours to help reduce their risk of developing AF (and other diseases) Tables 16 and 17.
Table 16.
Consensus statements on AF prevention I: risk factors and lifestyle modification
| Risk factor/trigger | Recommendations for clinical practice | Recommendations for research |
|---|---|---|
| Obesity | Inform overweight and obese patients of greater risk of developing AF and a subsequent risk of stroke and death. Assess BMI and start lifestyle programmes if BMI is overweight or obese |
More studies are needed on how to effectively prevent weight gain and promote weight loss in individuals who are overweight or obese More randomized controlled studies with long-term follow-up (>5 years) are needed to clarify the obesity paradox |
| General dietary considerations | Recommend healthy nutrition and lifestyle to reduce risk of AF Mediterranean diet enriched with olive oil may reduce risk of AF and its complications |
More studies are needed on: the effect of unhealthy nutrition on risk of AF Whether modification of diet reduces risk of arrhythmia |
| Blood lipids, fish consumption | Inform patients with low HDL (≤40 mg/dL) and high triglyceride (TGs ≥200 mg/dL) levels of risk of AF and its complications Recommend to patients with abnormal blood lipids to consume of a diet ‘that emphasizes intake of vegetables, fruits, and whole grains; includes low-fat dairy products, poultry, fish, legumes, non-tropical vegetable oils, and nuts; and limits intake of sweets, sugar-sweetened beverages, and red meats’66 Recommend combination of diet with moderate physical activity and maintenance of a healthy lifestyle and weight |
Lacking direct evidence, more studies are needed to define whether modification of blood lipids reduces the risk of AF. |
| Obstructive sleep apnoea | Inform patients with obstructive sleep apnoea that there is a greater risk of developing AF and their subsequent risk of stroke and death. Assess by anamnesis (snoring, daytime fatigue) the possibility of OSA. Refer to specialised clinic, as needed. |
More studies are needed: To investigate how comorbidity in patients with obstructive sleep apnoea affects the risk of AF. To show the benefit of diagnostic efforts and the effect of treatment with CPAP. On adequate assessment of presence of OSA in AF population. To show reduced risk of AF in well powered RCTs using systematic therapeutic approach together with other lifestyle changes |
| Hypertension | Uncontrolled blood pressure is associated with AF risk Adequately assess patients at risk Control BP to reduce AF risk |
Additional well-conducted secondary AF prevention trials will be important to define target SBP optimal to prevent AF Implement in RCTs together with other lifestyle management |
| Diabetes mellitus | Longer duration of diabetes and worse glycemic control are associated with increased AF risk Control diabetes to reduce AF risk |
More research is needed on the effect of glycemic control on AF risk in patients with diabetes |
| Tobacco smoking | Intensively encourage children, young and older adults not to begin smoking. In individuals who smoke support smoking cessation to prevent AF incidence, recurrence, symptoms, and complications. Primordial prevention. Support efforts to prevent the uptake of tobacco smoking. Primary prevention. Encourage individuals to quit smoking. Secondary prevention. In individuals with AF promote efforts to quit smoking to improve AF frequency, duration, and symptoms |
Investigate whether electronic cigarettes and second hand smoke are associated with an increased risk of new-onset AF, and in individuals with prevalent AF, whether electronic cigarettes and second hand smoke are associated with AF recurrence and AF symptoms. In individuals with AF, examine the efficacy and effectiveness of smoking cessation interventions to decrease the risk of stroke, myocardial infarction, chronic kidney disease, dementia, and all-cause mortality. |
| Air pollution | No association with chronic exposure; patients prone to AF should refrain from severe pollution exposure. | Overall data are scarce and should be increased specifically aimed at incidence of AF in patients with known cardiac disease. |
| Caffeine | No increase in risk, rather a reduced association, even for heavy consumption. | Data should be extended to randomized intervention studies addressing caffeine consumption in patients with paroxysmal AF |
| Alcohol | Moderate-heavy and binge drinking increases AF risk To reduce AF risk: Recommend to avoid binge drinking (>4 drinks in women and >5 drinks in men on a single occasion) Recommend to refrain consumption to no more than 2 drinks per day for men and 1 drink per day for women Obtain a detailed history on alcohol consumption Provide appropriate counselling to reduce alcohol consumption in patients with AF |
More intervention studies are needed on the effect of alcohol consumption reduction on AF risk |
| Medications | Many drugs increase AF risk: common (>20 %) - dobutamine, cisplatin; infrequent (5–20 %) - anthracyclines, melphalan, interleukin, NSAIDS, bisphosphonates; rare (<5 %) - adenosine, corticosteroids, aminophylline, antipsychotics, ivabradin, ondansetron. In patients with new-onset AF, review the pharmacological history to identify whether any of the prescribed drugs may cause the arrhythmia. |
More research on the effects on AF incidence for drug induced new-onset AF is needed, as many studies show conflicting results. Also more research is needed on which medications cause increased risk of AF. |
| Recreational drugs | Recreational drugs (cannabis, ecstasy and anabolic–androgenic steroids) may increase risk of AF. Examine for recreational drug abuse in new-onset AF Encourage avoidance of recreational drugs. |
More research is needed on the effect of illicit drugs, particularly cannabis, on new-onset AF, as most of the evidence is from case reports |
| Psychological distress | Identify significant psychological distress, particularly depression and anxiety, and treat appropriately to reduce the likelihood of adverse lifestyle choices (smoking, excessive alcohol intake, poor diet, physical inactivity) and poorer adherence to medication and lifestyle modification, all of which may increase the likelihood of development of other risk factors for AF, and hence predispose people to incident AF and other chronic diseases. | Further investigation of the impact of psychological distress on the development of AF in more diverse populations is warranted since the current limited evidence is based predominantly on white, middle-class, and middle-aged cohorts, and is only evident in men. |
| Physical activity | Recommend daily moderate exercise to reduce risk of AF | Role of physical activity clearly warrants further research, plus genetics involved in AF in excessive sports |
AF, atrial fibrillation; BMI, body mass index; BP, blood pressure; CPAP, continuous positive airway pressure; HDL, high-densiy lipoprotein cholesterol; OSA, obstructive sleep apnoea; RCT, randomised controlled trial; SBP, systolic blood pressure.
Table 17.
Consensus statements on AF prevention II: management of associated conditions
| Risk factor/trigger | Recommendations for clinical practice | Recommendations for research |
|---|---|---|
| Hyperthyroidism | Overt and subclinical hyperthyroidism increase AF risk Control thyroid function in patients at risk of AF Treat associated cardiovascular diseases and consider modification of risk factors |
More research is needed regarding risk factors and prevention of AF in populations with high-normal thyroid function (based on TSH level) and individuals with higher level of free thyroxin within normal range. |
| Supraventricular tachyarrhythmias and paroxysmal AF | In patients with SVT and paroxysmal AF: Ablate SVT, continue antiarrhythmic drugs or ablate AF as needed. Checking for potential SVT substrate should be considered in patients with isolated PAF referred for ablation |
Additional studies on prevention of AF in patients with SVT are needed |
| Post-operative AF | β-Blockers and amiodarone are indicated for prophylaxis of post-operative AF | More research is needed on use of pharmacological agents with anti-inflammatory and anti-remodelling properties, statins and other possible drugs for prevention of post-operative AF |
| Upstream therapies | – | Investigation of the long term effects of sustained secondary prevention with upstream therapies starting before AF in people at risk and early after AF diagnosis are required |
AF, atrial fibrillation; PAF, paroxysmal atrial fibrillation; SVT, supraventricular tachycardia; TSH, thyroid stimulating hormone.
Conclusions
In the present document, the determinants and triggers of atrial fibrillation (AF) are extensively discussed and it appears clear that prevention of this disorder requires a tailored approach to the individual patient. Moreover, certain modifiable risk factors, such smoking, alcohol abuse, and lack of physical activity, are deemed important components of a preventive strategy.33,315,316
In order to reduce the risk of AF, both an individual approach and global public health campaigns are required.
Many of the risk factors for AF are preventable and/or modifiable via lifestyle choices. As explained, modifying an inappropriate diet, quitting smoking, abstaining from alcohol and recreational drugs, and participating in regular physical activity programmes are efficient strategies under the patient's control.
A lifetime approach to cardiovascular risk modification is required (Figure 3). General physicians have a relevant role in this strategy, by monitoring their patients closely and adopting a lower threshold for educational intervention. A particular relevance to the scope is assigned to the implementation of nutritional interventions and to promote regular exercise programmes and sport participation. However, the greatest effort should be paid by policy makers in order to improve the population's capability to achieve and maintain a healthy cardiovascular lifestyle. The most adverse risk profile is actually prevalent among individuals with low-socioeconomic status, poorer educational attainment, and limited access to healthcare.
Figure 3.
Lifetime approach to primary prevention of AF. AF, atrial fibrillation.
The prevention of AF, more than other cardiovascular disorders, requires an approach that targets the global population, and a new political vision in the management of the healthcare system. In a society with available limited financial resources, it appears wise to modify the risk factors and quality of life of the largest majority of general population, more than developing sophisticated devices to shortly prolong the life of a few terminal patients.
Finally, special attention should be paid to the adolescent and young generations, who paradoxically are not at low cardiac risk, because of the epidemic incidence of obesity, inappropriate nutritional behaviour, smoking and alcohol abuse, and a widespread sedentary lifestyle.
Acknowledgments
Prof. Gregory Lip (chair), Prof. Bulent Gorenek (co-chair), Prof. Christian Sticherling, Prof. Laurent Fauchier, Prof. A. Goette, Prof. Werner Jung, Prof. Marc A Vos, Dr Michele Brignole, Dr. Christian Elsner, Prof. Gheorghe-Andrei Dan, Dr Francisco Marin, Prof. Giuseppe Boriani, Dr Deirdre Lane, Prof. Carina Blomstrom Lundqvist, Dr Irina Savelieva.
Conflict of interest: none declared.
Contributor Information
EHRA Scientific Committee Task Force::
Bulent Gorenek, Antonio Pelliccia, Emelia J. Benjamin, Giuseppe Boriani, Harry J. Crijns, Richard I. Fogel, Isabelle C. Van Gelder, Martin Halle, Gulmira Kudaiberdieva, Deirdre A. Lane, Torben Bjerregaard Larsen, Gregory Y. H. Lip, Maja-Lisa Løchen, Francisco Marín, Josef Niebauer, Prashanthan Sanders, Lale Tokgozoglu, Marc A. Vos, and David R. Van Wagoner
Document reviewers::
Laurent Fauchier, Irina Savelieva, Andreas Goette, Stefan Agewall, Chern-En Chiang, Márcio Figueiredo, Martin Stiles, Timm Dickfeld, Kristen Patton, Massimo Piepoli, Ugo Corra, Pedro Manuel Marques-Vidal, Pompilio Faggiano, Jean-Paul Schmid, and Ana Abreu
References
- 1. Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham Study. Neurology 1978;28:973–7. [DOI] [PubMed] [Google Scholar]
- 2. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-up Study. Am J Med 1995;98:476–84. [DOI] [PubMed] [Google Scholar]
- 3. Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study: the Rotterdam Study. Stroke 1997;28:316–21. [DOI] [PubMed] [Google Scholar]
- 4. Miyasaka Y, Barnes ME, Petersen RC, Cha SS, Bailey KR, Gersh BJ et al. . Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a community-based cohort. Eur Heart J 2007;28:1962–7. [DOI] [PubMed] [Google Scholar]
- 5. Soliman EZ, Lopez F, O'Neal WT, Chen LY, Bengtson L, Zhang ZM et al. . Atrial fibrillation and risk of ST-segment-elevation versus non-ST-segment-elevation myocardial infarction: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2015;131:1843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA et al. . Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 2014;174:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–52. [DOI] [PubMed] [Google Scholar]
- 8. Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A et al. . Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation 2009;119:606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda JG, Van Gelder IC. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace 2011;13:1375–85. [DOI] [PubMed] [Google Scholar]
- 10. Boriani G, Maniadakis N, Auricchio A, Müller-Riemenschneider F, Fattore G, Leyva F et al. . Health technology assessment in interventional electrophysiology and device therapy: a position paper of the European Heart Rhythm Association. Eur Heart J 2013;34:1869–74. [DOI] [PubMed] [Google Scholar]
- 11. Maniadakis N, Vardas P, Mantovani LG, Fattore G, Boriani G. Economic evaluation in cardiology. Europace 2011;13(Suppl 2):ii3–8. [DOI] [PubMed] [Google Scholar]
- 12. Fattore G, Maniadakis N, Mantovani LG, Boriani G. Health technology assessment: what is it? Current status and perspectives in the field of electrophysiology. Europace 2011;13(Suppl 2):ii49–53. [DOI] [PubMed] [Google Scholar]
- 13. Boriani G, Diemberger I, Martignani C, Biffi M, Branzi A. The epidemiological burden of atrial fibrillation: a challenge for clinicians and health care systems. Eur Heart J 2006;27:893–4. [DOI] [PubMed] [Google Scholar]
- 14. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD et al. . 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ et al. . Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boriani G, Diemberger I. Globalization of the epidemiologic, clinical, and financial burden of atrial fibrillation. Chest 2012;142:1368–70. [DOI] [PubMed] [Google Scholar]
- 17. Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC Jr, Hayman LL et al. . Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation 2011;124:967–90. [DOI] [PubMed] [Google Scholar]
- 18. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ et al. . Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2011;123:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dave D, Kaestner R. Health insurance and ex ante moral hazard: evidence from Medicare. Int J Health Care Finance Econ 2009;9:367–90. [DOI] [PubMed] [Google Scholar]
- 20. Dublin S, French B, Glazer NL, Wiggins KL, Lumley T, Psaty BM et al. . Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med 2006;166:2322–8. [DOI] [PubMed] [Google Scholar]
- 21. Long MJ, Jiang CQ, Lam TH, Xu L, Zhang WS, Lin JM et al. . Atrial fibrillation and obesity among older Chinese: the Guangzhou Biobank Cohort Study. Int J Cardiol 2011;148:48–52. [DOI] [PubMed] [Google Scholar]
- 22. Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE et al. . The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (Women's Health Study). J Am Coll Cardiol 2010;55:2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang TJ, Parise H, Levy D, D'Agostino RB Sr, Wolf PA, Vasan RS et al. . Obesity and the risk of new-onset atrial fibrillation. JAMA 2004;292:2471–7. [DOI] [PubMed] [Google Scholar]
- 24. Frost L, Benjamin EJ, Fenger-Grøn M, Pedersen A, Tjønneland A, Overvad K. Body fat, body fat distribution, lean body mass and atrial fibrillation and flutter. A Danish cohort study. Obesity (Silver Spring) 2014;22:1546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL et al. . Incidence of atrial fibrillation and relation with cardiovascular events, heart failure and mortality – a community-based study from the Netherlands. J Am Coll Cardiol 2015;66:1000–7. [DOI] [PubMed] [Google Scholar]
- 26. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME et al. . Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–60. [DOI] [PubMed] [Google Scholar]
- 27. Pathak RK, Elliot A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R et al. . Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT study. J Am Coll Cardiol 2015;66:985–96. [DOI] [PubMed] [Google Scholar]
- 28. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX et al. . Long-term Effect of Goal-Directed Weight Management in an Atrial Fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 29. Rienstra M, Sun JX, Lubitz SA, Frankel DS, Vasan RS, Levy D et al. . Plasma resistin, adiponectin, and risk of incident atrial fibrillation : the Framingham Offspring Study. Am Heart J 2012;163:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nyrnes A, Mathiesen EB, Njolstad I, Wilsgaard T, Lochen ML. Palpitations are predictive of future atrial fibrillation. An 11-year follow-up of 22,815 men and women: the Tromso study. Eur J Prev Cardiol 2013;20:729–36. [DOI] [PubMed] [Google Scholar]
- 31. Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coromilas J. Obesity and atrial fibrillation: is one epidemic feeding the other? JAMA 2004;292:2519–20. [DOI] [PubMed] [Google Scholar]
- 33. Overvad TF, Rasmussen LH, Skjøth F, Overvad K, Lip GY, Larsen TB. Body mass index and adverse events in patients with incident atrial fibrillation. Am J Med 2013;126:640.e9–17. [DOI] [PubMed] [Google Scholar]
- 34. Badheka AO, Rathod A, Kizilbash MA, Garg N, Mohamad T, Afonso L et al. . Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med 2010;123:646–51. [DOI] [PubMed] [Google Scholar]
- 35. Shen J, Johnson VM, Sullivan LM, Jacques PF, Magnani JW, Lubitz SA et al. . Dietary factors and incident atrial fibrillation: the Framingham Heart Study. Am J Clin Nutr 2011;93:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khawaja O, Gaziano JM, Djousse L. Nut consumption and risk of atrial fibrillation in the Physicians' Health Study. Nutr J 2012;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fretts AM, Mozaffarian D, Siscovick DS, Heckbert SR, McKnight B, King IB et al. . Associations of plasma phospholipid and dietary alpha linoleic acid with incident atrial fibrillation in older adults: The Cardiovascular Health Study. J Am Heart Assoc 2013;2:e003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Costanzo S, De Curtis A, di Niro V, Olivieri M, Morena M, De Filippo CM et al. . on behalf of the Polyphemus Observational Study Investigators. Postoperative atrial fibrillation and total dietary antioxidant capacity in patients undergoing cardiac surgery: the Polyphemus Observational Study. J Thorac Cardiovasc Surg 2015;149:1175–82. [DOI] [PubMed] [Google Scholar]
- 39. Mattioli AV, Miloro C, Pennella S, Pedrazzi P, Farinetti A. Adherence to Mediterranean diet and intake of antioxidants influence spontaneous conversion of atrial fibrillation. Nutr Metab Cardiovasc Dis 2013;23:115–21. [DOI] [PubMed] [Google Scholar]
- 40. Pastori D, Carnevale R, Barimoccia S, Nocella C, Tanzilli G, Cangemi R et al. . Does Mediterranean diet reduce cardiovascular events and oxidative stress in atrial fibrillation? Antioxid Redox Signal 2015;23:682–7. [DOI] [PubMed] [Google Scholar]
- 41. Martínez-González MA, Toledo E, Arós F, Fiol M, Corella D, Salas-Salvadó J et al. . Extra-virgin olive oil consumption reduces risk of atrial fibrillation. The PREDIMED (Prevención con Dieta Mediterránea) Trial. Circulation 2014;130:18–26. [DOI] [PubMed] [Google Scholar]
- 42. Al Suwaidi J, Zubaid M, Al-Mahmeed WA, Al-Rashdan I, Amin H, Bener A et al. . Impact of fasting in Ramadan in patients with cardiac disease. Saudi Med J 2005;26:1579–83. [PubMed] [Google Scholar]
- 43. Van Wagoner DR, Piccini JP, Albert CM, Anderson ME, Benjamin EJ, Brundel B et al. . Progress toward the prevention and treatment of atrial fibrillation: a summary of the Heart Rhythm Society Research Forum on the Treatment and Prevention of Atrial Fibrillation, Washington, DC, December 9–10, 2013. Heart Rhythm 2015;12:e5–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lopez FL, Agarwal SK, Maclehose RF, Soliman EZ, Sharrett AR, Huxley RR et al. . Blood lipid levels, lipid-lowering medications, and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities study. Circ Arrhythm Electrophysiol 2012;5:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alonso A, Yin X, Roetker NS, Magnani JW, Kronmal RA, Ellinor PT et al. . Blood lipids and the incidence of atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis and the Framingham Heart Study. J Am Heart Assoc 2014;3:e001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gronroos NN, Chamberlain AM, Folsom AR, Soliman EZ, Agarwal SK, Nettleton JA et al. . Fish, fish-derived n-3 fatty acids, and risk of incident atrial fibrillation in the Atherosclerosis Risk in Communities (ARIC) study. PLoS One 2012;7:e36686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rix TA, Joensen AM, Riahi S, Lundbye-Christensen S, Tjønneland A, Schmidt EB et al. . A U-shaped association between consumption of marine n-3 fatty acids and development of atrial fibrillation/atrial flutter-a Danish cohort study. Europace 2014;16:1554–61. [DOI] [PubMed] [Google Scholar]
- 48. Rix TA, Joensen AM, Riahi S, Lundbye-Christensen S, Overvad K, Schmidt EB. Marine n-3 fatty acids in adipose tissue and development of atrial fibrillation: a Danish cohort study. Heart 2013;99:1519–24. [DOI] [PubMed] [Google Scholar]
- 49. Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation 2009;120:2315–21. [DOI] [PubMed] [Google Scholar]
- 50. Young-Xu Y, Jabbour S, Goldberg R, Blatt CM, Graboys T, Bilchik B et al. . Usefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery disease. Am J Cardiol 2003;92:1379–83. [DOI] [PubMed] [Google Scholar]
- 51. Shiroshita-Takeshita A, Schram G, Lavoie J, Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation 2004;110:2313–9. [DOI] [PubMed] [Google Scholar]
- 52. Kumagai K, Nakashima H, Saku K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res 2004;62:105–11. [DOI] [PubMed] [Google Scholar]
- 53. Elgendy IY, Mahmoud A, Huo T, Beaver TM, Bavry AA. Meta-analysis of 12 trials evaluating the effects of statins on decreasing atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 2015;115:1523–8. [DOI] [PubMed] [Google Scholar]
- 54. Jacob KA, Nathoe HM, Dieleman JM, van Osch D, Kluin J, van Dijk D. Inflammation in new-onset atrial fibrillation after cardiac surgery: a systematic review. Eur J Clin Invest 2014;44:402–28. [DOI] [PubMed] [Google Scholar]
- 55. Rahimi K, Emberson J, McGale P, Majoni W, Merhi A, Asselbergs FW et al. . Effect of statins on atrial fibrillation: collaborative meta-analysis of published and unpublished evidence from randomised controlled trials. BMJ 2011;342:d1250. [DOI] [PubMed] [Google Scholar]
- 56. Fauchier L, Clementy N, Babuty D. Statin therapy and atrial fibrillation: systematic review and updated meta-analysis of published randomized controlled trials. Curr Opin Cardiol 2013;28:7–18. [DOI] [PubMed] [Google Scholar]
- 57. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH et al. . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 58. Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF et al. . Fish intake and risk of incident atrial fibrillation. Circulation 2004;110:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sakabe M, Shiroshita-Takeshita A, Maguy A, Dumesnil C, Nigam A, Leung TK et al. . Omega-3 polyunsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation 2007;116:2101–9. [DOI] [PubMed] [Google Scholar]
- 60. Mayyas F, Sakurai S, Ram R, Rennison JH, Hwang ES, Castel L et al. . Dietary omega3 fatty acids modulate the substrate for post-operative atrial fibrillation in a canine cardiac surgery model. Cardiovasc Res 2011;89:852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mozaffarian D, Wu JH, de Oliveira Otto MC, Sandesara CM, Metcalf RG, Latini R et al. . Fish oil and post-operative atrial fibrillation: a meta-analysis of randomized controlled trials. J Am Coll Cardiol 2013;61:2194–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Darghosian L, Free M, Li J, Gebretsadik T, Bian A, Shintani A et al. . Effect of omega-three polyunsaturated fatty acids on inflammation, oxidative stress, and recurrence of atrial fibrillation. Am J Cardiol 2015;115:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A et al. . Fish oil for the reduction of atrial fibrillation recurrence, inflammation and oxidative stress. J Am Coll Cardiol 2014;64:1441–8. [DOI] [PubMed] [Google Scholar]
- 64. Visioli F, Rise P, Barassi MC, Marangoni F, Galli C. Dietary intake of fish vs. formulations leads to higher plasma concentrations of n-3 fatty acids. Lipids 2003;38:415–8. [DOI] [PubMed] [Google Scholar]
- 65. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S et al. . Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Europace 2010;12:1360–420. [DOI] [PubMed] [Google Scholar]
- 66. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS et al. . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2960–84. [DOI] [PubMed] [Google Scholar]
- 67. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A et al. . Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008;118:1080–111. [DOI] [PubMed] [Google Scholar]
- 69. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T et al. . Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007;49:565–71. [DOI] [PubMed] [Google Scholar]
- 70. Cadby G, McArdle N, Briffa T, Hillman DR, Simpson L, Knuiman M et al. . Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest 2015;148:945–52. [DOI] [PubMed] [Google Scholar]
- 71. Arias MA, Sánchez AM, Alonso-Fernández A, García-Río F. Atrial fibrillation, obesity, and obstructive sleep apnea. Arch Intern Med 2007;167:1552–3. [DOI] [PubMed] [Google Scholar]
- 72. Ghias M, Scherlag BJ, Lu Z, Niu G, Moers A, Jackman WM et al. . The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol 2009;54:2075–83. [DOI] [PubMed] [Google Scholar]
- 73. Roche F, Xuong AN, Court-Fortune I, Costes F, Pichot V, Duverney D et al. . Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin Electrophysiol 2003;26:669–77. [DOI] [PubMed] [Google Scholar]
- 74. Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K et al. . Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013;62:300–5. [DOI] [PubMed] [Google Scholar]
- 75. Neilan TG, Farhad H, Dodson JA, Shah RV, Abbasi SA, Bakker JP et al. . Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013;2:e000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Grimm W, Hoffmann J, Menz V, Köhler U, Heitmann J, Peter JH et al. . Electrophysiologic evaluation of sinus node function and atrioventricular conduction in patients with prolonged ventricular asystole during obstructive sleep apnea. Am J Cardiol 1996;77:1310–4. [DOI] [PubMed] [Google Scholar]
- 77. Simantirakis EN, Schiza SI, Marketou ME, Chrysostomakis SI, Chlouverakis GI, Klapsinos NC et al. . Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J 2004;25:1070–6. [DOI] [PubMed] [Google Scholar]
- 78. Naruse Y, Tada H, Satoh M, Yanagihara M, Tsuneoka H, Hirata Y et al. . Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013;10:331–7. [DOI] [PubMed] [Google Scholar]
- 79. Li L, Wang ZW, Li J, Ge X, Guo LZ, Wang Y et al. . Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace 2014;16:1309–14. [DOI] [PubMed] [Google Scholar]
- 80. Khan A, Latif F, Hawkins B, Tawk M, Sivaram CA, Kinasewitz G. Effects of obstructive sleep apnea treatment on left atrial volume and left atrial volume index. Sleep Breath 2008;12:141–7. [DOI] [PubMed] [Google Scholar]
- 81. Maeno K, Kasagi S, Ueda A, Kawana F, Ishiwata S, Ohno M et al. . Effects of obstructive sleep apnea and its treatment on signal-averaged P-wave duration in men. Circ Arrhythm Electrophysiol 2013;6:287–93. [DOI] [PubMed] [Google Scholar]
- 82. Arias MA, García-Río F, Alonso-Fernández A, Mediano O, Martínez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation 2005;112:375–83. [DOI] [PubMed] [Google Scholar]
- 83. Shukla A, Aizer A, Holmes D, Fowler S, Park DS, Bernstein S et al. . Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC-CEP 2015;1:41–51. [DOI] [PubMed] [Google Scholar]
- 84. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E et al. . Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015;373:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA et al. . Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. JAMA 1994;271:840–4. [PubMed] [Google Scholar]
- 86. Thomas MD, Dublin S, Kaplan RC, Glazer NL, Lumley T, Longstreth WT Jr et al. . Blood pressure control and risk of incident atrial fibrillation. Am J Hypertens 2008;21:1111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlöf B et al. . Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention for End Point Reduction in Hypertension (LIFE) Study. J Am Coll Cardiol 2005;45:712–9. [DOI] [PubMed] [Google Scholar]
- 88. Marott SCW, Nielsen SF, Benn M, Nordestgaard BG. Antihypertensive treatment and risk of atrial fibrillation: a nationwide study. Eur Heart J 2014;35:1205–14. [DOI] [PubMed] [Google Scholar]
- 89. Okin PM, Hille DA, Larstorp ACK, Wachtell K, Kjeldsen SE, Dahlof B et al. . Effect of lower on-treatment systolic blood pressure on the risk of atrial fibrillation in hypertensive patients. Hypertension 2015;66:368–73. [DOI] [PubMed] [Google Scholar]
- 90. The GISSI-AF Investigators. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med 2009;360:1606–17. [DOI] [PubMed] [Google Scholar]
- 91. Goette A, Schon N, Kirchhof P, Breithardt G, Fetsch T, Hausler KG et al. . Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circ Arrhythm Electrophysiol 2012;5:43–51. [DOI] [PubMed] [Google Scholar]
- 92. Lip GY, Frison L, Grind M. Angiotensin converting enzyme inhibitor and angiotensin receptor blockade use in relation to outcomes in anticoagulated patients with atrial fibrillation. J Intern Med 2007;261:577–86. [DOI] [PubMed] [Google Scholar]
- 93. Emdin CA, Callender T, Cao J, Rahimi K. Effect of antihypertensive agents on risk of atrial fibrillation: a meta-analysis of large-scale randomized trials. Europace 2015;17:701–10. [DOI] [PubMed] [Google Scholar]
- 94. Gillis AM. Angiotensin-receptor blockers for prevention of atrial fibrillation – a matter of timing or target? N Engl J Med 2009;360:1669–71. [DOI] [PubMed] [Google Scholar]
- 95. Mayyas F, Alzoubi KH, Van Wagoner DR. Impact of aldosterone antagonists on the substrate for atrial fibrillation: aldosterone promotes oxidative stress and atrial structural/electrical remodeling. Int J Cardiol 2013;168:5135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin-angiotensin-aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 2013;165:17–24. [DOI] [PubMed] [Google Scholar]
- 97. Menezes AR, Lavie CJ, DiNicolantonio JJ, O'Keefe J, Morin DP, Khatib S et al. . Atrial fibrillation in the 21st century: a current understanding of risk factors and primary prevention strategies. Mayo Clin Proc 2013;88:394–409. [DOI] [PubMed] [Google Scholar]
- 98. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB et al. . Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Huxley RR, Alonso A, Lopez FL, Filion KB, Agarwal SK, Loehr LR et al. . Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart 2012;98:133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ostgren CJ, Merlo J, Råstam L, Lindblad U. Atrial fibrillation and its association with type 2 diabetes and hypertension in a Swedish community. Diabetes Obes Metab 2004;6:367–74. [DOI] [PubMed] [Google Scholar]
- 101. Pfister R, Michels G, Cairns R, Schneider CA, Erdmann E. Incidence of new onset bundle branch block and atrial fibrillation in patients with type 2 diabetes and macrovascular disease: an analysis of the PROactive study. Int J Cardiol 2011;153:233–4. [DOI] [PubMed] [Google Scholar]
- 102. Schoen T, Pradhan AD, Albert CM, Conen D. Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J Am Coll Cardiol 2012;60:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins KL et al. . Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med 2010;25:853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Aksnes TA, Schmieder RE, Kjeldsen SE, Ghani S, Hua TA, Julius S. Impact of new onset diabetes mellitus on development of atrial fibrillation and heart failure in high risk hypertension (from the VALUE Trial). Am J Cardiol 2008;101:634–8. [DOI] [PubMed] [Google Scholar]
- 105. Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CF et al. . Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol 2014;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Overvad TF, Skjøth F, Lip GYH, Lane DA, Albertsen IE, Rasmussen LH et al. . Duration of diabetes mellitus and risk of thromboembolism and bleeding in atrial fibrillation: nationwide cohort study. Stroke 2015;46:2168–74. [DOI] [PubMed] [Google Scholar]
- 107. Anselmino M, Matta M, D'ascenzo F, Pappone C, Santinelli V, Bunch TJ et al. . Catheter ablation of atrial fibrillation in patients with diabetes mellitus: a systematic review and meta-analysis. Europace 2015;17:1518–25. [DOI] [PubMed] [Google Scholar]
- 108. Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M et al. . Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm 2011;8:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pfister R, Bragelmann J, Michels G, Wareham NJ, Luben R, Khaw KT. Performance of the CHARGE-AF risk model for incident atrial fibrillation in the EPIC Norfolk cohort. Eur J Prev Cardiol 2015;22:932–9. [DOI] [PubMed] [Google Scholar]
- 110. Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. Rising rates of hospital admissions for atrial fibrillation. Epidemiology 2003;14:666–72. [DOI] [PubMed] [Google Scholar]
- 111. Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J 2013;34:2243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC Jr et al. . Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: The Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol 2015;25:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Suzuki S, Otsuka T, Sagara K, Kano H, Matsuno S, Takai H et al. . Association between smoking habits and the first-time appearance of atrial fibrillation in Japanese patients: evidence from the Shinken database. J Cardiol 2015;66:73–9. [DOI] [PubMed] [Google Scholar]
- 114. Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam study. Am Heart J 2008;156:1163–9. [DOI] [PubMed] [Google Scholar]
- 115. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB et al. . Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet 2009;373:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP et al. . Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997;96:2455–61. [DOI] [PubMed] [Google Scholar]
- 117. Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health study. Am J Med 2005;118:489–95. [DOI] [PubMed] [Google Scholar]
- 118. Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: Morbidity and risk factors. J Intern Med 2001;250:382–9. [DOI] [PubMed] [Google Scholar]
- 119. Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 2001;86:516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hergens MP, Galanti R, Hansson J, Fredlund P, Ahlbom A, Alfredsson L et al. . Use of Scandinavian moist smokeless tobacco (snus) and the risk of atrial fibrillation. Epidemiology 2014;25:872–6. [DOI] [PubMed] [Google Scholar]
- 121. Dixit S, Pletcher MJ, Vittinghoff E, Imburgia K, Maguire C, Whitman IR et al. . Second hand smoke and atrial fibrillation: data from the health Eheart study. Heart Rhythm 2016;13:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Okumura Y. Smoking and the risk of the perpetuation of atrial fibrillation: under debate in large cohort studies. Heart Rhythm 2011;8:1167–8. [DOI] [PubMed] [Google Scholar]
- 123. Monroy AE, Hommel E, Smith ST, Raji M. Paroxysmal atrial fibrillation following electronic cigarette use in an elderly woman. Clin Geriatr 2012;20:28–32. [Google Scholar]
- 124. O'Neal WT, Qureshi WT, Judd SE, McClure LA, Cushman M, Howard VJ et al. Environmental tobacco smoke and atrial fibrillation: The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. J Occup Environ Med 2015;57:1154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rigotti NA, Eagle KA. Atrial fibrillation while chewing nicotine gum. JAMA 1986;255:1018. [PubMed] [Google Scholar]
- 126. Stewart PM, Catterall JR. Chronic nicotine ingestion and atrial fibrillation. Br Heart J 1985;54:222–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Choragudi NL, Aronow WS, DeLuca AJ. Nicotine gum-induced atrial fibrillation. Heart Dis 2003;5:100–1. [DOI] [PubMed] [Google Scholar]
- 128. Levitzky YS, Guo CY, Rong J, Larson MG, Walter RE, Keaney JF Jr et al. . Relation of smoking status to a panel of inflammatory markers: the Framingham offspring. Atherosclerosis 2008;201:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tuan TC, Chang SL, Tai CT, Lin YJ, Hu YF, Lo LW et al. . Impairment of the atrial substrates by chronic cigarette smoking in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2008;19:259–65. [DOI] [PubMed] [Google Scholar]
- 130. Hayashi H, Omichi C, Miyauchi Y, Mandel WJ, Lin SF, Chen PS et al. . Age-related sensitivity to nicotine for inducible atrial tachycardia and atrial fibrillation. Am J Physiol Heart Circ Physiol 2003;285:H2091–8. [DOI] [PubMed] [Google Scholar]
- 131. Goette A. Nicotine, atrial fibrosis, and atrial fibrillation: do microRNAs help to clear the smoke? Cardiovasc Res 2009;83:421–2. [DOI] [PubMed] [Google Scholar]
- 132. Goette A, Lendeckel U, Kuchenbecker A, Bukowska A, Peters B, Klein HU et al. . Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart 2007;93:1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shan H, Zhang Y, Lu Y, Zhang Y, Pan Z, Cai B et al. . Downregulation of mir-133 and mir-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res 2009;83:465–72. [DOI] [PubMed] [Google Scholar]
- 134. Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in the Copenhagen city heart study. Eur Respir J 2003;21:1012–6. [DOI] [PubMed] [Google Scholar]
- 135. Li J, Agarwal SK, Alonso A, Blecker S, Chamberlain AM, London SJ et al. . Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2014;129:971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bosdriesz JR, Willemsen MC, Stronks K, Kunst AE. Socioeconomic inequalities in smoking cessation in 11 European countries from 1987 to 2012. J Epidemiol Community Health 2015;69:886–92. [DOI] [PubMed] [Google Scholar]
- 137. Hitchman SC, Fong GT, Zanna MP, Thrasher JF, Chung-Hall J, Siahpush M. Socioeconomic status and smokers' number of smoking friends: Findings from the international tobacco control (itc) four- country survey. Drug Alcohol Depend 2014;143:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zoller B, Li X, Sundquist J, Sundquist K. Neighbourhood deprivation and hospitalization for atrial fibrillation in Sweden. Europace 2013;15:1119–27. [DOI] [PubMed] [Google Scholar]
- 139. Misialek JR, Rose KM, Everson-Rose SA, Soliman EZ, Clark CJ, Lopez FL et al. . Socioeconomic status and the incidence of atrial fibrillation in whites and blacks: the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc 2014;3: e001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ et al. . Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J 2014;35:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Schnabel RB, Rienstra M, Sullivan LM, Sun JX, Moser CB, Levy D et al. . Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail 2013;15:843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Lip GY. Predictors and prognostic implications of incident heart failure following the first diagnosis of atrial fibrillation in patients with structurally normal hearts: the Belgrade atrial fibrillation study. Eur J Heart Fail 2013;15:415–24. [DOI] [PubMed] [Google Scholar]
- 143. Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke 2010;41:2731–8. [DOI] [PubMed] [Google Scholar]
- 144. Nakagawa K, Hirai T, Ohara K, Fukuda N, Numa S, Taguchi Y et al. . Impact of persistent smoking on long-term outcomes in patients with nonvalvular atrial fibrillation. J Cardiol 2015;65:429–33. [DOI] [PubMed] [Google Scholar]
- 145. Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D'Agostino RB et al. . A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA 2003;290:1049–56. [DOI] [PubMed] [Google Scholar]
- 146. Angoulvant D, Villejoubert O, Bejan-Angoulvant T, Ivanes F, Saint Etienne C, Lip GY et al. . Effect of active smoking on comparative efficacy of antithrombotic therapy in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest 2015;148:491–8. [DOI] [PubMed] [Google Scholar]
- 147. Huang Y, Britton J, Hubbard R, Lewis S. Who receives prescriptions for smoking cessation medications? An association rule mining analysis using a large primary care database. Tob Control 2013;22:274–9. [DOI] [PubMed] [Google Scholar]
- 148. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D et al. . Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–31. [DOI] [PubMed] [Google Scholar]
- 149. Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K et al. . ESC Working Group on Thrombosis, European Association for Cardiovascular Prevention and Rehabilitation; ESC Heart Failure Association. Expert position paper on air pollution and cardiovascular disease. Eur Heart J 2015;36:83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes 2012;61:3037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Brook RD, Rajagopalan S, Pope CA, Bhatnagar A, Diez-Roux AV, Holguin F et al. . Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–78. [DOI] [PubMed] [Google Scholar]
- 152. Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens 2009;3:332–50. [DOI] [PubMed] [Google Scholar]
- 153. Pope CA III, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR et al. . Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res 2015;116:108–15. [DOI] [PubMed] [Google Scholar]
- 154. Peters A, Frohlich M, Doring A, Immervoll T, Wichmann HE, Hutchinson WL et al. . Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J 2001;22:1198–204. [DOI] [PubMed] [Google Scholar]
- 155. Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P et al. . Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med 2012;172:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. O'Donnell MJ, Fang J, Mittleman MA, Kapral MK, Wellenius GA. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology 2011;22:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke 2005;36:2549–53. [DOI] [PubMed] [Google Scholar]
- 158. Milojevic A, Wilkinson P, Armstrong B, Bhaskaran K, Smeeth L, Hajat S. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart 2014;100:1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bunch TJ, Horne BD, Asirvatham SJ, Day JD, Crandall BG, Weiss JP et al. . Atrial fibrillation hospitalization is not increased with short-term elevations in exposure to fine particulate air pollution. Pacing Clin Electrophysiol 2011;34:1475–9. [DOI] [PubMed] [Google Scholar]
- 160. Link MS, Luttmann-Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR et al. . Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol 2013;62:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttmann-Gibson H, Catalano PJ et al. . Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect 2006;114:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Rashid A, Hines M, Scherlag BJ, Yamanashi WS, Lovallo W. The effects of caffeine on the inducibility of atrial fibrillation. J Electrocardiol 2006;39:421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Newcombe PF, Renton KW, Rautaharju PM, Spencer CA, Montague TJ. High-dose caffeine and cardiac rate and rhythm in normal subjects. Chest 1988;94:90–4. [DOI] [PubMed] [Google Scholar]
- 164. Strubelt O, Diederich KW. Experimental treatment of the acute cardiovascular toxicity of caffeine. J Toxicol Clin Toxicol 1999;37:29–33. [DOI] [PubMed] [Google Scholar]
- 165. Donnerstein RL, Zhu D, Samson R, Bender AM, Goldberg SJ. Acute effects of caffeine ingestion on signal-averaged electrocardiograms. Am Heart J 1998;136(4 Pt 1):643–6. [DOI] [PubMed] [Google Scholar]
- 166. Conen D, Chiuve SE, Everett BM, Zhang SM, Buring JE, Albert CM. Caffeine consumption and incident atrial fibrillation in women. Am J Clin Nutr 2010;92:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Frost L, Vestergaard P. Caffeine and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr 2005;81:578–82. [DOI] [PubMed] [Google Scholar]
- 168. Caldeira D, Martins C, Alves LB, Pereira H, Ferreira JJ, Costa J. Caffeine does not increase the risk of atrial fibrillation: a systematic review and meta-analysis of observational studies. Heart 2013;99:1383–9. [DOI] [PubMed] [Google Scholar]
- 169. Di R Jr, During A, Morelli PJ, Heyden M, Biancaniello TA. Atrial fibrillation in healthy adolescents after highly caffeinated beverage consumption: two case reports. J Med Case Rep 2011;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ettinger PO, Wu CF, De La Cruz C Jr, Weisse AB, Ahmed SS, Regan TJ. Arrhythmias and the ‘Holiday Heart’: alcohol-associated cardiac rhythm disorders. Am Heart J 1978;95:555–62. [DOI] [PubMed] [Google Scholar]
- 171. Liang Y, Mente A, Yusuf S, Gao P, Sleight P, Zhu J et al. . Alcohol consumption and the risk of incident atrial fibrillation among people with cardiovascular disease. CMAJ 2012;184:E857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Mandyam MC, Vedantham V, Scheinman MM, Tseng ZH, Badhwar N, Lee BK et al. . Alcohol and vagal tone as triggers for paroxysmal atrial fibrillation. Am J Cardiol 2012;110:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Laszlo R, Eick C, Schwiebert M, Schreiner B, Weig HJ, Weretka S et al. . Alcohol-induced electrical remodeling: effects of sustained short-term ethanol infusion on ion currents in rabbit atrium. Alcohol Clin Exp Res 2009;33:1697–703. [DOI] [PubMed] [Google Scholar]
- 174. Maki T, Toivonen L, Koskinen P, Naveri H, Harkonen M, Leinonen H. Effect of ethanol drinking, hangover, and exercise on adrenergic activity and heart rate variability in patients with a history of alcohol-induced atrial fibrillation. Am J Cardiol 1998;82:317–22. [DOI] [PubMed] [Google Scholar]
- 175. Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation 2005;112:1736–42. [DOI] [PubMed] [Google Scholar]
- 176. Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA 2008;300:2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Djousse L, Levy D, Benjamin EJ, Blease SJ, Russ A, Larson MG et al. . Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol 2004;93:710–3. [DOI] [PubMed] [Google Scholar]
- 178. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol 2014;64:281–9. [DOI] [PubMed] [Google Scholar]
- 179. Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y et al. . Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol 2011;57:427–36. [DOI] [PubMed] [Google Scholar]
- 180. Conen D, Albert CM. Alcohol consumption and risk of atrial fibrillation: how much is too much? J Am Coll Cardiol 2014;64:290–2. [DOI] [PubMed] [Google Scholar]
- 181. Devlin R, Henry JA. Clinical review: major consequences of illicit drug consumption. Crit Care 2008;12:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Korantzopoulos P, Liu T, Papaioannides D, Li G, Goudevenos JA. Atrial fibrillation and marijuana smoking. Int J Clin Pract 2008;62:308–13. [DOI] [PubMed] [Google Scholar]
- 183. Krishnamoorthy S, Lip GY, Lane DA. Alcohol and illicit drug use as precipitants of atrial fibrillation in young adults: a case series and literature review. Am J Med 2009;122:851–6.e3. [DOI] [PubMed] [Google Scholar]
- 184. Madhok A, Boxer R, Chowdhury D. Atrial fibrillation in an adolescent – the agony of ecstasy. Pediatr Emerg Care 2003;19:348–9. [DOI] [PubMed] [Google Scholar]
- 185. Furlanello F, Serdoz LV, Cappato R, Ambroggi LD. Illicit drugs and cardiac arrhythmias in athletes. Eur J Cardiovasc Prev Rehab 2007;14:487–94. [DOI] [PubMed] [Google Scholar]
- 186. Lau DH, Stiles MK, Shashidhar BJ, Glenn D, Young GD, Sanders P. Atrial fibrillation and anabolic steroid abuse. Int J Cardiol 2007;117:e86–7. [DOI] [PubMed] [Google Scholar]
- 187. Kaakeh Y, Overholser BR, Lopshire JC, Tisdale JE. Drug-induced atrial fibrillation. Drugs 2012;72:1617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis AB. Introducing a new entity: chemotherapy-induced arrhythmia. Europace 2009;11:1579–86. [DOI] [PubMed] [Google Scholar]
- 189. Schjerning Olsen AM, Fosbøl EL, Pallisgaard J, Lindhardsen J, Lock Hansen M, Køber L et al. . NSAIDs are associated with increased risk of atrial fibrillation in patients with prior myocardial infarction: a nationwide study. Eur Heart J Cardiovasc Pharmacother 2015;1:107–14. [DOI] [PubMed] [Google Scholar]
- 190. Sharma A, Einstein AJ, Vallakati A, Arbab-Zadeh A, Walker MD, Mukherjee D et al. . Risk of atrial fibrillation with use of oral and intravenous bisphosphonates. Am J Cardiol 2014;113:1815–21. [DOI] [PubMed] [Google Scholar]
- 191. Kim DH, Rogers JR, Fulchino LA, Kim CA, Solomon DH, Kim SC. Bisphosphonates and risk of cardiovascular events: a meta-analysis. PLoS One 2015;10:e0122646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Yalaci S, Tamer A, Kocayigit I, Gunduz H. Atrial fibrillation due to olanzapine overdose. Clin Toxicol 2011;49:440. [DOI] [PubMed] [Google Scholar]
- 193. Martin RI, Pogoryelova O, Koref MS, Bourke JP, Teare MD, Keavney BD. Atrial fibrillation associated with ivabradine treatment: meta-analysis of randomised controlled trials. Heart 2014;100:1506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol 2014;63:945–53. [DOI] [PubMed] [Google Scholar]
- 195. McCabe PJ. Psychological distress in patients diagnosed with atrial fibrillation: the state of the science. J Cardiovasc Nurs 2010;25:40–51. [DOI] [PubMed] [Google Scholar]
- 196. Thrall G, Lip GY, Carroll D, Lane D. Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest 2007;132:1259–64. [DOI] [PubMed] [Google Scholar]
- 197. Habibović M, Versteeg H, Pelle AJ, Theuns DA, Jordaens L, Pedersen SS. Poor health status and distress in cardiac patients: the role of device therapy vs. underlying heart disease. Europace 2013;15:355–61. [DOI] [PubMed] [Google Scholar]
- 198. von Eisenhart Rothe A, Hutt F, Baumert J, Breithardt G, Goette A, Kirchhof P et al. . Depressed mood amplifies heart-related symptoms in persistent and paroxysmal atrial fibrillation patients: a longitudinal analysis--data from the German Competence Network on Atrial Fibrillation. Europace 2015;17:1354–62. [DOI] [PubMed] [Google Scholar]
- 199. von Eisenhart Rothe AF, Goette A, Kirchhof P, Breithardt G, Limbourg T, Calvert M et al. . Depression in paroxysmal and persistent atrial fibrillation patients: a cross-sectional comparison of patients enrolled in two large clinical trials. Europace 2014;16:812–9. [DOI] [PubMed] [Google Scholar]
- 200. Gehi AK, Sears S, Goli N, Walker TJ, Chung E, Schwartz J et al. . Psychopathology and symptoms of atrial fibrillation: implications for therapy. J Cardiovasc Electrophysiol 2012;23:473–8. [DOI] [PubMed] [Google Scholar]
- 201. Patel D, Mc Conkey ND, Sohaney R, McNeil A, Jedrzejczyk A, Armaganijan L. A systematic review of depression and anxiety in patients with atrial fibrillation: the mind-heart link. Cardiovasc Psychiatry Neurol 2013;2013:159850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lip GY, Laroche C, Boriani G, Cimaglia P, Dan GA, Santini M et al. . Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace 2015;17:24–31. [DOI] [PubMed] [Google Scholar]
- 203. Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation 2002;106:1800–5. [DOI] [PubMed] [Google Scholar]
- 204. Burg MM, Lampert R, Joska T, Batsford W, Jain D. Psychological traits and emotion-triggering of ICD shock-terminated arrhythmias. Psychosom Med 2004;66:898–902. [DOI] [PubMed] [Google Scholar]
- 205. Whang W, Albert CM, Sears SF Jr, Lampert R, Conti JB, Wang PJ et al. . Depression as a predictor for appropriate shocks among patients with implantable cardioverter-defibrillators: results from the Triggers of Ventricular Arrhythmias (TOVA) study. J Am Coll Cardiol 2005;45:1090–5. [DOI] [PubMed] [Google Scholar]
- 206. Eaker ED, Sullivan LM, Kelly-Hayes M, D'Agostino RB Sr, Benjamin EJ. Anger and hostility predict the development of atrial fibrillation in men in the Framingham Offspring Study. Circulation 2004;109:1267–71. [DOI] [PubMed] [Google Scholar]
- 207. Eaker ED, Sullivan LM, Kelly-Hayes M, D'Agostino RB Sr, Benjamin EJ. Tension and anxiety and the prediction of the 10-year incidence of coronary heart disease, atrial fibrillation, and total mortality: the Framingham Offspring Study. Psychosom Med 2005;67:692–6. [DOI] [PubMed] [Google Scholar]
- 208. Whang W, Davidson KW, Conen D, Tedrow UB, Everett BM, Albert CM. Global psychological distress and risk of atrial fibrillation among women: The Women's Health Study. J Am Heart Assoc 2012;1:e001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Qureshi WT, Alirhayim Z, Blaha MJ, Juraschek SP, Keteyian SJ, Brawner CA et al. . Cardiorespiratory fitness and risk of incident atrial fibrillation: results from the Henry Ford Exercise Testing (FIT) Project. Circulation 2015;131:1827–34. [DOI] [PubMed] [Google Scholar]
- 210. Drca N, Wolk A, Jensen-Urstad M, Larsson SC. Physical activity is associated with a reduced risk of atrial fibrillation in middle-aged and elderly women. Heart 2015;101:1627–30. [DOI] [PubMed] [Google Scholar]
- 211. Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the Cardiovascular Health Study. Circulation 2008;118:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Grimsmo J, Grundvold I, Maehlum S, Arnesen H. High prevalence of atrial fibrillation in long-term endurance cross-country skiers: echocardiographic findings and possible predictors-a 28–30 years follow-up study. Eur J Cardiovasc Prev Rehabil 2010;17:100–5. [DOI] [PubMed] [Google Scholar]
- 213. Myrstad M, Nystad W, Graff-Iversen S, Thelle DS, Stigum H, Aarønæs M et al. . Effect of years of endurance exercise on risk of atrial fibrillation and atrial flutter. Am J Cardiol 2014;114:1229–33. [DOI] [PubMed] [Google Scholar]
- 214. Lee DC, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol 2014;64:472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Thelle DS, Selmer R, Gjesdal K, Sakshaug S, Jugessur A, Graff-Iversen S et al. . Resting heart rate and physical activity as risk factors for lone atrial fibrillation: a prospective study of 309,540 men and women. Heart 2013;99:1755–60. [DOI] [PubMed] [Google Scholar]
- 216. Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol 2009;103:1572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–68. [DOI] [PubMed] [Google Scholar]
- 218. Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J 1994;15(Suppl A):9–16. [DOI] [PubMed] [Google Scholar]
- 219. D'Ascenzi F, Cameli M, Padeletti M, Lisi M, Zacà V, Natali B et al. . Characterization of right atrial function and dimension in top-level athletes: a speckle tracking study. Int J Cardiovasc Imaging 2013;29:87–94. [DOI] [PubMed] [Google Scholar]
- 220. D'Andrea A, Riegler L, Cocchia R, Scarafile R, Salerno G, Gravino R et al. . Left atrial volume index in highly trained athletes. Am Heart J 2010;159:1155–61. [DOI] [PubMed] [Google Scholar]
- 221. Brugger N, Krause R, Carlen F, Rimensberger C, Hille R, Steck H et al. . Effect of lifetime endurance training on left atrial mechanical function and on the risk of atrial fibrillation. Int J Cardiol 2014;170:419–25. [DOI] [PubMed] [Google Scholar]
- 222. Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC et al. . Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 2011;123:13–22. [DOI] [PubMed] [Google Scholar]
- 223. Lindsay MM, Dunn FG. Biochemical evidence of myocardial fibrosis in veteran endurance athletes. Br J Sports Med 2007;41:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. De Vos CB, Nieuwlaat R, Crijns HJ, Camm AJ, LeHeuzey JY, Kirchhof CJ et al. . Autonomic trigger patterns and antiarrhythmic treatment of paroxysmal atrial fibrillation: data from the Euro Heart Survey. Eur Heart J 2008;29:632–9. [DOI] [PubMed] [Google Scholar]
- 225. O'Keefe JH, Schnohr P, Lavie CJ. The dose of running that best confers longevity. Heart 2013;99:588–90. [DOI] [PubMed] [Google Scholar]
- 226. Schnohr P, Marott JL, Lange P, Jensen GB. Longevity in male and female joggers: the Copenhagen City Heart Study. Am J Epidemiol 2013;177:683–9. [DOI] [PubMed] [Google Scholar]
- 227. Ofman P, Khawaja O, Rahilly-Tierney CR, Peralta A, Hoffmeister P, Reynolds MR et al. . Regular physical activity and risk of atrial fibrillation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2013;6:252–6. [DOI] [PubMed] [Google Scholar]
- 228. Kwok CS, Anderson SG, Myint PK, Mamas MA, Loke YK. Physical activity and incidence of atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol 2014;177:467–76. [DOI] [PubMed] [Google Scholar]
- 229. Mont L, Elosua R, Brugada J. Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter. Europace 2009;11:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Calvo N, Ramos P, Montserrat S, Guasch E, Coll-Vinent B, Domenech M et al. . Emerging risk factors and the dose-response relationship between physical activity and lone atrial fibrillation: a prospective case-control study. Europace 2016;18:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Drca N, Wolk A, Jensen-Urstad M, Larsson SC. Atrial fibrillation is associated with different levels of physical activity levels at different ages in men. Heart 2014;100:1037–42. [DOI] [PubMed] [Google Scholar]
- 232. Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace 2009;11:1156–9. [DOI] [PubMed] [Google Scholar]
- 233. Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R et al. . Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 2011;58:1780–91. [DOI] [PubMed] [Google Scholar]
- 234. Alings M, Smit MD, Moes ML, Crijns HJ, Tijssen JG, Brugemann J et al. . Routine versus aggressive upstream rhythm control for prevention of early atrial fibrillation in heart failure: Background, aims and design of the RACE 3 study. Neth Heart J 2013;21:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh PG, Shen WK et al. . Familial AF is a genetically heterogeneous disorder. J Am Coll Cardiol 2003;41:2185–92. [DOI] [PubMed] [Google Scholar]
- 236. Fox CS, Parise H, D'Agostino RB Sr, Lloyd-Jones DM, Vasan RS, Wang TJ et al. . Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA 2004;291:2851–5. [DOI] [PubMed] [Google Scholar]
- 237. Arnar DO, Thorvaldsson S, Manolio TA, Thorgeirsson G, Kristjansson K, Hakonarson H et al. . Familial aggregation of atrial fibrillation in Iceland. Eur Heart J 2006;27:708–12. [DOI] [PubMed] [Google Scholar]
- 238. Gundlund A, Christiansen MN, Hansen ML, Olesen JB, Zahir D, Køber L et al. . Familial clustering and subsequent incidence of atrial fibrillation among first-degree relatives in Denmark. Europace 2016;18:658–64. [DOI] [PubMed] [Google Scholar]
- 239. Zöller B, Ohlsson H, Sundquist J, Sundquist K. High familial risk of atrial fibrillation/atrial flutter in multiplex families: a nationwide family study in Sweden. J Am Heart Assoc 2012;2:e003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M et al. . Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA 2010;304:2263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Oyen N, Ranthe MF, Carstensen L, Boyd HA, Olesen MS, Olesen SP et al. . Familial aggregation of lone atrial fibrillation in young persons. J Am Coll Cardiol 2012;60:917–21. [DOI] [PubMed] [Google Scholar]
- 242. Brugada R, Tappscot T, Czernuszewicz GZ, Marian AJ, Iglesias A, Mont L et al. . Identification of a genetic locus for familial atrial fibrillation. New Engl J Med 1997;336:905–11. [DOI] [PubMed] [Google Scholar]
- 243. Tucker NP, Ellinor PT. Emerging directions in genetics of atrial fibrillation. Circ Res 2014;114:1462–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Sinner MF, Tucker NR, Lunetta KL, Ozaki K, Smith JG, Trompet S et al. . Integrating genetic, transcriptional, and functional analyses to identify 5 novel genes for atrial fibrillation. Circulation 2014;130:1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Smith JG, Almgren P, Engstrom G, Hedblad B, Platonov PG, Newton-Cheh C et al. . Genetic polymorphisms for estimating risk of atrial fibrillation: a literature-based meta-analysis. J Intern Med 2012;272:573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Mohanty S, Santangeli P, Bai R, Di Biase L, Mohanty P, Pump A et al. . Variant rs2200733 on chromosome 4q25 confers increased risk of atrial fibrillation: evidence from a meta-analysis. J Cardiovasc Electrophysiol 2013;24:155–61. [DOI] [PubMed] [Google Scholar]
- 247. Kirchhof P, Breithardt G, Aliot E, Al Khatib S, Apostolakis S, Auricchio A et al. . Personalized management of atrial fibrillation: proceedings from the fourth atrial fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace 2013;15:1540–56. [DOI] [PubMed] [Google Scholar]
- 248. Fabritz L, Guasch E, Antoniades C, Bardinet I, Benninger G, Betts TR et al. . Expert consensus document: defining the major health modifiers causing atrial fibrillation: a roadmap to underpin personalized prevention and treatment. Nat Rev Cardiol 2016;13:230–7. [DOI] [PubMed] [Google Scholar]
- 249. Selmer C, Olesen JB, Hansen ML, Lindhardsen J, Schjerning Olsen AM, Clausager J et al. . The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ 2012;345:e7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter. a population-based study. Arch Intern Med 2004;164:1675–8. [DOI] [PubMed] [Google Scholar]
- 251. Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke JL et al. . Thyroid status, cardiovascular risk and mortality in older adults. JAMA 2006;295:1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Kim EJ, Lyass A, Wang N, Massaro JM, Fox CS, Benjamin EJ et al. . Relation of hypothyroidism and incident atrial fibrillation (from the Framingham Heart Study). Am Heart J 2014;167:123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Auer J, Scheibner P, Mische T, Langsteger W, Eber O, Eber B. Subclinical hypothyroidism as a risk factor for atrial fibrillation. Am Heart J 2001;142:838–42. [DOI] [PubMed] [Google Scholar]
- 254. Gammage MD, Parle JV, Holder RL, Roberts LM, Hobbs FDR, Wilson S et al. . Association between free thyroxine concentration and atrial fibrillation. Arch Intern Med 2007;167:928–34. [DOI] [PubMed] [Google Scholar]
- 255. Sawin CT, Geller A, Wolf PA, Belander AJ, Baker E, Bacharach P et al. . Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 1994;331:1249–52. [DOI] [PubMed] [Google Scholar]
- 256. Collet TH, Gussekloo J, Bauer DC, den Elzen WPJ, Wendy PJ, Cappola AR et al. . Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med 2012;172:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Heeringa J, Hoogendoorn EH, van der Deure WM, Hofman A, Peeters RP, Hop WCJ et al. . High-normal thyroid function and risk of atrial fibrillation. Arch Intern Med 2008;168:2219–24. [DOI] [PubMed] [Google Scholar]
- 258. Chaker L, Heeringa J, Dehghan A, Medici M, Visser WE, Baumgartner C et al. . Normal thyroid function and the risk of atrial fibrillation: the Rotterdam Study. J Clin Endocrinol Metab 2015;100:3718–24. [DOI] [PubMed] [Google Scholar]
- 259. Von Olshausen K, Bischoff S, Kahaly G, Mohr-Kahaly S, Erbel R, Beyer J et al. . Cardiac arrhythmias and heart rate in hyperthyroidism. Am J Cardiol 1989;63:930–3. [DOI] [PubMed] [Google Scholar]
- 260. Nakazawa HK, Sakurai K, Hamada N, Momotani N, Ito K. Management of atrial fibrillation in the post-thyrotoxic state. Am J Med 1982;72:903–6. [DOI] [PubMed] [Google Scholar]
- 261. Siu CW, Jim MH, Zhang X, Chan YH, Pong V, Kwok J et al. . Comparison of atrial fibrillation recurrence rates after successful electrical cardioversion in patients with hyperthyroidism-induced versus non-hyperthyroidism-induced persistent atrial fibrillation. Am J Cardiol 2009;103:540–3. [DOI] [PubMed] [Google Scholar]
- 262. Machino T, Tada H, Sekiguchi Y, Yamasaki H, Kuroki K, Igarashi M et al. . Prevalence and influence of hyperthyroidism on the long-term outcome of catheter ablation for drug-refractory atrial fibrillation. Circ J 2012;76:2546–51. [DOI] [PubMed] [Google Scholar]
- 263. Wongcharoen W, Lin YJ, Chang SL, Lo LW, Hu YF, Chung FP et al. . History of hyperthyroidism and long-term outcome of catheter ablation of drug-refractory atrial fibrillation. Heart Rhythm 2015;12:1956–62. [DOI] [PubMed] [Google Scholar]
- 264. Chan PH, Hai J, Yeung CY, Lip GY, Lam KS, Tse HF et al. . Benefit of anticoagulation therapy in hyperthyroidism-related atrial fibrillation. Clin Cardiol 2015;38:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500–10. [DOI] [PubMed] [Google Scholar]
- 266. Bruere H, Fauchier L, Bernard Brunet A, Pierre B, Simeon E, Babuty D et al. . History of thyroid disorders in relation to clinical outcomes in atrial fibrillation. Am J Med 2015;128:30–7. [DOI] [PubMed] [Google Scholar]
- 267. Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G et al. . Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 268. Voigt N, Dobrev D. Cellular and molecular correlates of ectopic activity in patients with atrial fibrillation. Europace 2012;14(Suppl 5):v97–v105. [DOI] [PubMed] [Google Scholar]
- 269. Kirchhof P, Lip GY, Van Gelder IC, Bax J, Hylek E, Kaab S et al. . Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options–a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 2012;14:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res 2011;89:754–65. [DOI] [PubMed] [Google Scholar]
- 271. Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 272. Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F et al. . Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipofibrokines. Eur Heart J 2015;36:795–805. [DOI] [PubMed] [Google Scholar]
- 273. Cosio FG, Aliot E, Botto GL, Heidbüchel H, Geller CJ, Kirchhof P et al. . Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace 2008;10:21–7. [DOI] [PubMed] [Google Scholar]
- 274. Nattel S, Guasch E, Savelieva I. Early management of atrial fibrillation to prevent cardiovascular complications. Eur Heart J 2014;35:1448–56. [DOI] [PubMed] [Google Scholar]
- 275. Wellens HJ, Durrer D. Wolff-Parkinson-White syndrome and atrial fibrillation. Relation between refractory period of accessory pathway and ventricular rate during atrial fibrillation. Am J Cardiol 1974;34:777–83. [DOI] [PubMed] [Google Scholar]
- 276. Campbell RW, Smith RA, Gallagher JJ, Pritchett EL, Wallace AG. Atrial fibrillation in the preexcitation syndrome. Am J Cardiol 1977;40:514–22. [DOI] [PubMed] [Google Scholar]
- 277. Hamer ME, Wilkinson WE, Clair WK, Page RL, McCarthy EA, Pritchett EL. Incidence of symptomatic atrial fibrillation in patients with paroxysmal supraventricular tachycardia. J Am Coll Cardiol 1995;25:984–8. [DOI] [PubMed] [Google Scholar]
- 278. Ozcan C, Strom JB, Newell JB, Mansour MC, Ruskin JN. Incidence and predictors of atrial fibrillation and its impact on long-term survival in patients with supraventricular arrhythmias. Europace 2014;16:1508–14. [DOI] [PubMed] [Google Scholar]
- 279. Waldo AL. Mechanisms of atrial flutter and atrial fibrillation: distinct entities or two sides of a coin? Cardiovasc Res 2002;54:217–29. [DOI] [PubMed] [Google Scholar]
- 280. Lin CH, Chang SL, Huang HK, Lo LW, Lin YJ, Chiang CH et al. . Novel electrophysiological characteristics of atrioventricular nodal continuous conduction curves in atrioventricular nodal re-entrant tachycardia with concomitant cavotricuspid isthmus-dependent atrial flutter. Europace 2015;pii: euv345. [DOI] [PubMed] [Google Scholar]
- 281. Chen YJ, Chen SA, Tai CT, Wen ZC, Feng AN, Ding YA et al. . Role of atrial electrophysiology and autonomic nervous system in patients with supraventricular tachycardia and paroxysmal atrial fibrillation. J Am Coll Cardiol 1998;32:732–8. [DOI] [PubMed] [Google Scholar]
- 282. Sticherling C, Oral H, Horrocks J, Chough SP, Baker RL, Kim MH et al. . Effects of digoxin on acute, atrial fibrillation-induced changes in atrial refractoriness. Circulation 2000;102:2503–8. [DOI] [PubMed] [Google Scholar]
- 283. Crijns HJGM, Lie KI. Hemodynamic deterioration after treatment with adenosine. Br Heart J 1995;73:103. [PubMed] [Google Scholar]
- 284. Nabar A, Rodriguez LM, Timmermans C, Van den Dool A, Smeets JLRM, Wellens HJJ. Observations in four patient groups having type I atrial flutter with or without associated atrial fibrillation. Circulation 1999;99:1441–5. [DOI] [PubMed] [Google Scholar]
- 285. Pentinga ML, Meeder JG, Crijns HJGM, De Muinck ED, Wiesfeld ACP, Lie KI. Late onset atrioventricular nodal tachycardia. Int J Cardiol 1993;38:293–8. [DOI] [PubMed] [Google Scholar]
- 286. Wellens HJ. When to perform catheter ablation in asymptomatic patients with a Wolff-Parkinson-White electrocardiogram. Circulation 2005;112:2201–16. [DOI] [PubMed] [Google Scholar]
- 287. McKenna W. Hypertrophic cardiomyopathy. Lancet 2004;363:1881–91. [DOI] [PubMed] [Google Scholar]
- 288. Haissaguerre M, Fischer B, Labbé T, Lemétayer P, Montserrat P, d'Ivernois C et al. . Frequency of recurrent atrial fibrillation after catheter ablation of overt accessory pathways. Am J Cardiol 1992;69:493–7. [DOI] [PubMed] [Google Scholar]
- 289. Pappone C, Santinelli V. Catheter ablation should be performed in asymptomatic patients with Wolff-Parkinson-White syndrome. Circulation 2005;112:2207–16. [PubMed] [Google Scholar]
- 290. McKeown PP, Gutterman D. Executive summary: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest 2005;128:1S–5S. [DOI] [PubMed] [Google Scholar]
- 291. Shariff N, Zelenkofske S, Eid S, Weiss MJ, Mohammed MQ. Demographic determinants and effect of pre-operative angiotensin converting enzyme inhibitors and angiotensin receptor blockers on the occurrence of atrial fibrillation after CABG surgery. BMC Cardiovasc Disord 2010;8:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Shantsila E, Watson T, Lip GY. Atrial fibrillation post-cardiac surgery: changing perspectives. Curr Med Res Opin 2006;22:1437–41. [DOI] [PubMed] [Google Scholar]
- 293. Sánchez-Quiñones J, Marín F, Roldán V, Lip GY. The impact of statin use on atrial fibrillation. QJM 2008;101:845–61. [DOI] [PubMed] [Google Scholar]
- 294. Nair SG. Atrial fibrillation after cardiac surgery. Ann Card Anaesth 2010;13:196–205. [DOI] [PubMed] [Google Scholar]
- 295. Crystal E, Connolly SJ, Sleik K, Ginger TJ, Yusuf S. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: a meta-analysis. Circulation 2002;106:75–80. [DOI] [PubMed] [Google Scholar]
- 296. Jidéus L, Blomström P, Nilsson L, Stridsberg M, Hansell P, Blomström-Lundqvist C. Tachyarrhythmias and triggering factors for atrial fibrillation after coronary artery bypass operations. Ann Thorac Surg 2000;69:1064–9. [DOI] [PubMed] [Google Scholar]
- 297. Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM et al. . Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev 2013;1:CD003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Bagshaw SM, Galbraith PD, Mitchell LB, Sauve R, Exner DV, Ghali WA. Prophylactic amiodarone for prevention of atrial fibrillation after cardiac surgery: a meta-analysis. Ann Thorac Surg 2006;82:1927–37. [DOI] [PubMed] [Google Scholar]
- 299. Patti G, Chello M, Candura D, Pasceri V, D'Ambrosio A, Covino E et al. . Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation 2006;114:1455–61. [DOI] [PubMed] [Google Scholar]
- 300. ESC Press Release Office. STICS - Short-term peri-operative statin treatment does not reduce complications after cardiac surgery. 02 Sep 2014. http://www.escardio.org/The-ESC/Press-Office/Press-releases/Last-5-years/STICS-Short-term-peri-operative-statin-treatment-does-not-reduce-complications (6 December 2015, date last accessed).
- 301. Orenes-Piñero E, Montoro-García S, Banerjee A, Valdés M, Lip GYH, Marín F. Pre and post-operative treatments for prevention of atrial fibrillation after cardiac surgery. Mini Rev Med Chem 2012;12:1419–31. [DOI] [PubMed] [Google Scholar]
- 302. Ho KM, Tan JA. Benefits and risks of corticosteroid prophylaxis in adult cardiac surgery: a dose–response meta-analysis. Circulation 2009;119:1853–66. [DOI] [PubMed] [Google Scholar]
- 303. Savelieva I, Kakouros N, Kourliouros A, Camm JA. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace 2011;13:308–28. [DOI] [PubMed] [Google Scholar]
- 304. Savelieva I, Kakouros N, Kourliouros A, Camm JA. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: secondary prevention. Europace 2011;13:610–25. [DOI] [PubMed] [Google Scholar]
- 305. Madrid AH, Bueno MG, Rebollo JM, Marín I, Peña G, Bernal E et al. . Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation 2002;106:331–6. [DOI] [PubMed] [Google Scholar]
- 306. Swedberg K, Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Shi H et al. . Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol 2012;59:1598–603. [DOI] [PubMed] [Google Scholar]
- 307. Liu T, Korantzopoulos P, Shao Q, Zhang Z, Letsas KP, Li G. Mineralocorticoid receptor antagonists and atrial fibrillation: a meta-analysis. Europace 2016;18:672–8. [DOI] [PubMed] [Google Scholar]
- 308. Lip GYHL, Lane D. Stroke prevention in atrial fibrillation. A systematic review. JAMA 2015;313:1950–62. [DOI] [PubMed] [Google Scholar]
- 309. Pisters R, Lane DA, Marin F, Camm AJ, Lip GY. Stroke and thromboembolism in atrial fibrillation. Circ J 2012;76:2289–304. [DOI] [PubMed] [Google Scholar]
- 310. Lip GYHL, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 311. Lamberts M, Nielsen OW, Lip GY, Ruwald MH, Christiansen CB, Kristensen SL et al. . Cardiovascular risk in patients with sleep apnoea with or without continuous positive airway pressure therapy: follow-up of 4.5 million Danish adults. J Intern Med 2014;276:659–66. [DOI] [PubMed] [Google Scholar]
- 312. Friberg L, Benson L, Lip GY. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J 2015;36:297–306. [DOI] [PubMed] [Google Scholar]
- 313. Agarwal M, Apostolakis S, Lane DA, Lip GY. The impact of heart failure and left ventricular dysfunction in predicting stroke, thromboembolism, and mortality in atrial fibrillation patients: a systematic review. Clin Ther 2014;36:1135–44. [DOI] [PubMed] [Google Scholar]
- 314. Lip GYHL, Frison L, Grind M. Effect of hypertension on anticoagulated patients with atrial fibrillation. Eur Heart J 2007;28:752–9. [DOI] [PubMed] [Google Scholar]
- 315. Overvad TF, Rasmussen LH, Skjoth F, Overvad K, Albertsen IE, Lane DA et al. . Alcohol intake and prognosis of atrial fibrillation. Heart (Br Card Soc) 2013;99:1093–9. [DOI] [PubMed] [Google Scholar]
- 316. Albertsen IE, Rasmussen LH, Lane DA, Overvad TF, Skjoth F, Overvad K et al. . The impact of smoking on thromboembolism and mortality in patients with incident atrial fibrillation: insights from the Danish Diet, Cancer, and Health study. Chest 2014;145:559–66. [DOI] [PubMed] [Google Scholar]
- 317. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA et al. . 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 318. Bunch TJ, May HT, Bair TL, Weiss JP, Crandall BG, Osborn JS et al. . Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm 2013;10:1272–7. [DOI] [PubMed] [Google Scholar]
- 319. De Caterina R, Husted S, Wallentin L, Andreotti F, Arnesen H, Bachmann F et al. . Vitamin K antagonists in heart disease: current status and perspectives (Section III). Position paper of the ESC Working Group on Thrombosis--Task Force on Anticoagulants in Heart Disease. Thromb Haemost 2013;110:1087–107. [DOI] [PubMed] [Google Scholar]
- 320. Gallego P, Roldan V, Marin F, Romera M, Valdes M, Vicente V et al. . Cessation of oral anticoagulation in relation to mortality and the risk of thrombotic events in patients with atrial fibrillation. Thromb Haemost 2013;110:1189–98. [DOI] [PubMed] [Google Scholar]
- 321. Azoulay L, Dell'Aniello S, Simon TA, Renoux C, Suissa S. Initiation of warfarin in patients with atrial fibrillation: early effects on ischaemic strokes. Eur Heart J 2014;35:1881–7. [DOI] [PubMed] [Google Scholar]
- 322. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT(2)R(2) score. Chest 2013;144:1555–63. [DOI] [PubMed] [Google Scholar]
- 323. Proietti M, Lip GY. Simple decision making between a vitamin K Antagonist and Non-Vitamin K Antagonist Oral Anticoagulant (NOACs): using the SAMe-TT2R2 Score. Eur Heart J Cardiovasc Pharmacother 2015;1:150–2. [DOI] [PubMed] [Google Scholar]
- 324. Dogliotti A, Giugliano RP. A novel approach indirectly comparing benefit–risk balance across anti-thrombotic therapies in patients with atrial fibrillation. Eur Heart J Cardiovasc Pharmacother 2015;1:15–28. [DOI] [PubMed] [Google Scholar]
- 325. Lip GY, Haguenoer K, Saint-Etienne C, Fauchier L. Relationship of the SAME-TT2R2score to poor quality anticoagulation, stroke, clinically relevant bleeding and mortality in patients with atrial fibrillation. Chest 2014;146:719–26. [DOI] [PubMed] [Google Scholar]
- 326. Lane DA, Aguinaga L, Blomström-Lundqvist C, Boriani G, Dan GA, Hills MT et al. . Cardiac tachyarrhythmias and patient values and preferences for their management: the European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2015;17:1747–69. [DOI] [PubMed] [Google Scholar]